ABSTRACT
The feasibility and safety of brain-computer interface (BCI) systems for patients with acute/subacute stroke have not been established. The aim of this study was to firstly demonstrate the feasibility and safety of a bedside BCI system for inpatients with acute/subacute stroke in a small cohort of inpatients. Four inpatients with early-phase hemiplegic stroke (7–24 days from stroke onset) participated in this study. The portable BCI system showed real-time feedback of sensorimotor rhythms extracted from scalp electroencephalograms (EEGs). Patients attempted to extend the wrist on their affected side, and neuromuscular electrical stimulation was applied only when the system detected significant movement intention-related changes in EEG. Between 120 and 200 training trials per patient were successfully and safely conducted at the bedside over 2–4 days. Our results clearly indicate that the proposed bedside BCI system is feasible and safe. Larger clinical studies are needed to determine the clinical efficacy of the system and its effect size in the population of patients with acute/subacute post-stroke hemiplegia.
Introduction
Brain-computer interface (BCI) technology has already been used successfully to control an external device with the user’s brain activity, and it is expected to be used on patients with strokes, spinal cord injuries, and neuromuscular intractable diseases, to assist their motor functions. In addition, the BCIs are investigated on healthy subjects with regard to human augmentation. Recently, several research groups have shown that BCI can also be used as a tool for promoting neural plasticity, leading to functional recovery from hemiplegia/hemiparesis after stroke (Shindo et al., Citation2011; Ushiba & Soekadar, Citation2016). The clinical application of such rehabilitative BCI-based neurofeedback in patients with stroke is a fast-growing area of research, and its effectiveness in patients with chronic stroke who have hemiplegia/hemiparesis has recently been confirmed (Broetz et al., Citation2010; Mukaino et al., Citation2014).
In the acute/subacute phase, the risk of stroke recurrence is higher than that in chronic stroke (Burn et al., Citation1994; Moroney et al., Citation1998), and the spontaneous reorganization of the nervous system is unstable. In most cases, patients with acute/subacute stroke cannot perform sitting exercises or transfer to/from a wheelchair because they cannot control their bodies. For all of these reasons, it is difficult for inpatients with acute/subacute stroke to train on a BCI system at regular intervals in a rehabilitation laboratory or a rehabilitation room.
Meanwhile, animal studies show critical neural recovery during early rehabilitative training, although the effect decreases with time (Biernaskie et al., Citation2004; Yang et al., Citation2003). Furthermore, most evidence suggests that early rehabilitation leads to better outcomes in humans than in animals (Horn et al., Citation2005; Maulden et al., Citation2005; Murphy & Corbett, Citation2009). Therefore, it is clinically important to establish an earlier (bedside) rehabilitative intervention protocol. The current study thus aimed to demonstrate the safety and feasibility of a bedside BCI system for inpatients with acute/subacute stroke. A number of compact and portable embedded BCI systems that have been developed by industry and academia are now available for neurorehabilitation, some of which can potentially be used for bedside treatment in acute stroke. However, from the viewpoint of a clinical-phase approach for the development of rehabilitation evidence (Whyte et al., Citation2009), the lack of phase 1 or 2 clinical trials has hindered progress in BCI intervention. To test the safety and feasibility of BCI interventions, here, we conducted a case-series study without a control group and conducted trials with our custom-designed BCI system in patients with acute/subacute hemiparetic stroke. We expect our results to encourage larger phase 3 clinical trials in the future.
Methods
Participant recruitment and intervention design
The potential risks of acute/subacute-phase interventions include (1) stroke recurrence caused by a rise in the blood pressure due to excessive engagement during training, (2) epileptic seizures, (3) headaches and other adverse nervous system effects, and (4) insufficient acceptance and commitment to the BCI training. To minimize these risks, we carefully selected participants and developed an appropriate intervention design.
We used the following criteria for patient selection: (1) ability to understand and follow our instructions and expressed commitment to the training; (2) sufficient cognitive functioning (Mini-Mental State Examination score >27 points); (3) no bilateral motor deficits; (4) no history of epilepsy or medication for epilepsy; (5) no visual deficits; and (6) first-ever stroke.
We recruited four inpatients (one female and three males; aged 67.4 ± 14.7 years) who fulfilled these criteria in the early phase (less than 1 month since stroke onset) at Asahikawa Medical University Hospital. The average time from stroke onset to the first intervention was 9.5 days (range 7–13 days) (). The experiment was repeated two to four times on separate days. All patients were right-handed and received conventional rehabilitation (physical, occupational, and speech-language therapy) for 1.5 hours/day on average.
Table 1. Patient information and clinical evaluation.
The study was conducted in accordance with the Declaration of Helsinki, and all patients gave written informed consent for participation and publication of their individual data, which was approved by the local ethics committee of Asahikawa Medical University (Number: 15119–2). The trial was retrospectively registered with the UMIN Clinical Trials Registry, number UMIN000023167, on July 14, 2016. Each experiment was completed within one hour, including the EEG setup and disassembly. The patients’ blood pressure was continuously monitored. If a patient felt fatigued or abnormal blood pressure was observed during the training, all experimental procedures were immediately halted.
System settings
For ease of use, the BCI system we developed has only two scalp-EEG signals and employs a head-mounted display (HMD) for visual feedback that allows patients to watch the feedback signal while reclining. Previous clinical BCI studies (Kasashima-Shindo et al., Citation2015; Ono et al., Citation2014) have shown that two-channel EEG recordings are sufficient for estimating sensorimotor cortical excitability. In our study, patients lay on their hospital bed in a reclining position and were equipped with an HMD (Wrap1200, Vuzix Corporation, Rochester, NY, U.S.). The main computer display was mirrored on the HMD to show the experimental instructions and visual feedback ().
Figure 1. The BCI training system and experimental setup.
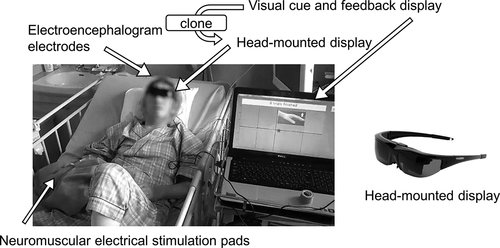
In the current study, the extensor carpi radialis (ECR) muscle of the affected side was chosen as the target muscle. A gelled self-adhesive electrode pad was placed on the belly of the ECR muscle on the affected side, and an identical pad was also placed on the distal side, 6 cm away. Electrical stimulation was delivered through a microcomputer-based stimulator that was connected to the two pads.
Neuromuscular electrical stimulation consisted of biphasic 0.300-ms rectangular impulses of 33.3 Hz (inter-stimulus interval 30.0 ms). Other research groups have reported that 30-Hz stimulation can induce longer cortical facilitation compared with low frequency (3 Hz) (Pitcher et al., Citation2003), while higher-frequency (>35 Hz) stimulation can cause rapid muscle fatigue and affect patient comfort (Naaman et al., Citation2000). Before each training day, the stimulus amplitude was adjusted so that overt wrist extension could be observed.
To record bipolar EEG on the scalp, two pairs of Ag/AgCl electrodes (each 9 mm in diameter) were placed at FC3-C3 and FC4-C4 of the 10/10 system (Klem et al., Citation1999), which are close to the hand representation motor area. The reference and ground channels were positioned on the right earlobe and forehead, respectively. A biosignal amplifier (g.USBamp, g.tec medical engineering GmbH, Graz, Austria) amplified 2–50 Hz EEG and sent the signal to the main computer, digitizing it at 256 Hz. The recorded data were used online but also stored on the main computer for offline analysis.
Interventions
After the system setting, interventions started. Each experimental day consisted of three stages: an EEG assessment before training (Stage 1), a BCI training with neurofeedback and neuromuscular electrical stimulation (Stage 2), and a second EEG assessment (Stage 3). These three stages were completed within one hour.
Stage 1 was performed to set up a nu-support vector regression (nu-SVR) model (model calibration) and to assess the pre-training condition of patients’ EEG. Nu-SVR was calibrated to classify EEG signals into “rest” or “intention to extend the wrist” in Stage 2. In a single trial of Stage 1, patients observed a star-shaped cursor moving from the left to the right on the HMD over a period of 9 seconds, at a constant speed with an update rate of 32 Hz (update interval 31 ms), and an instruction (“cue”) regarding an upper square (“motor intention/try to extend the affected-side wrist”) or a lower square (“rest”) appeared at the cue timing on the HMD (). Then, after a 2-second preparation period, participants performed the instructed task for the next 3 seconds. Stage 1 consisted of 20 motor-intention trials and 20 rest trials in random order. The procedure was the same for Stage 3, and the results of Stage 1 and 3 were compared to assess the effects of Stage 2. During Stages 1 and 3, EEG signals were recorded but no EEG feedback was given to patients. After Stage 1, a nu-SVR model (EEG classifier) was immediately created using EEG signals recorded in Stage 1. The details of feature extraction and nu-SVR are described in the subsection of “Feature extraction and classification”.
Figure 2. Movement instruction display.
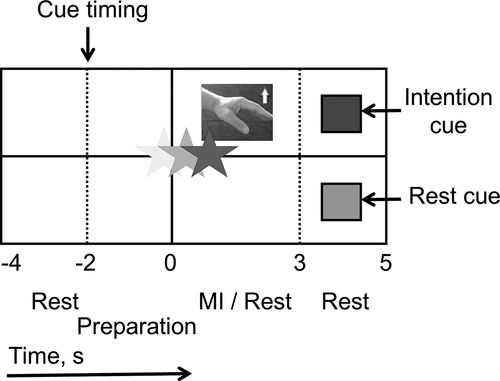
In Stage 2 (BCI training), we employed the same instruction display as in Stage 1 and 3, but here the star moved vertically in relation to the objective function of the EEG classifier. The star moved upward when the classifier output a positive objective function (motor intention) and downward when it output a negative objective function (rest). Similar EEG feedback has been employed in previous studies (Hashimoto et al., Citation2014; Shindo et al., Citation2011). The feedback period lasted from time 0 to 3 seconds in each trial. If the final height of the star represented a positive value in motor-intention trials when the feedback period finished, patients received 2 seconds of neuromuscular electrical stimulation. Patients were instructed to observe the star moving and to control the star height along with the motor intention and rest cues, which were presented in random order. In motor-intention trials, patients controlled their brain activity so that the star moved upward while trying to extend the wrist on the affected side. In rest trials, patients controlled their brain activity so that the star moves downward without any motor intention. A training session of Stage 2 consisted of 40 trials and the inter-session intervals was 1–2 minutes. The target number of trials was set at 120–200 (3–5 sessions) within 50 minutes.
We employed the “intention to extend the wrist” as a means to increase motor activity over the sensorimotor cortex. It might have been possible to replace the wrist movement with finger tapping or gripping. We chose the simple wrist movement because it was easy for the patients to understand. And the wrist extension is also the same movement as is induced by the neuromuscular electrical stimulation that we used. Since we focused on the outcome of finger function rather than wrist joint, we did not assess the motion range of the wrist joint.
Feature extraction and classification
To extract features from the EEG, we used the logarithms of the band power of mu (8–13 Hz) and beta (16–26 Hz) rhythms over the bilateral hemispheres (four types of features in total). The software we developed filtered EEG with a digital fourth-order Butterworth filter (with cutoff frequencies of 8–13 Hz for mu and 16–26 Hz for beta rhythms), fully rectified the filtered EEG and smoothed it with a moving averaging window of 1 second. To classify these EEG feature signals into two classes (motor intention and rest), we used nu-SVR with a radial basis function as the EEG classifier. Nu-SVR was established based on LIBSVM software version 3.22 (Chang & Lin, Citation2011). These extraction procedures were programmed and automated to enable the procedure to be administered by non-BCI experts such as therapists.
Support vector machine (SVM) analysis is a well-known and effective machine learning tool for classification and regression, first proposed by Vapnik et al. in 1992 (Vapnik, Citation1995). SVM that extends the objective variable to a continuous value is called support vector regression (SVR). SVR is widely used because it can solve nonlinear regression problems with relatively high accuracy.
A new version of SVR, nu-SVR, proposed by Schölkopf et al. (Citation2000), it uses a constant ν in the range (0, 1]. Nu-SVR formulation is similar to the original SVR, with some minor changes. It can automatically minimize ε, which is a certain error threshold used in the original SVR, and realizes robust regression with high classification accuracy.
In the current study, the same method of feature extraction and classifier is used for both offline analysis for EEG signals obtained in Stage 1 and Stage 3 and online BCI in Stage 2. One of the basic concepts of BCI technology is to decode the user’s intention from the brain activity in real time and operate external computers or devices. In particular, BCI research that aimed at promoting neural plasticity showed that it is important to perform all the processing online, and to create a closed loop including the brain, body and BCI system (Xu et al., Citation2014). In Stage 2 of the current study, EEG features were extracted online to create this closed loop.
Classification accuracy
To evaluate the performance of the BCI system, classification accuracy was calculated offline using nu-SVR, as in the online analysis. In this procedure, we estimated the single-trial classification accuracy for each experimental day and for each patient using leave-one-out cross-validation. Statistical significance levels for accuracy were also estimated by randomizing the order of data labels and calculating the accuracy of randomized data. Randomization and recalculation were repeated 10,000 times. Consequently, the 5% and 1% significance levels were over 70.0% and 75.0%, respectively.
Contribution rate of EEG features
For SVR with a nonlinear kernel, it was impossible to weigh the contribution of each EEG feature for classification, because the weight vector was not produced. Therefore, we used SVR with a linear kernel and calculated the weight vector for each EEG feature. If an EEG feature was more critical for classification, the greater absolute value of its weight was used.
Before the linear-SVR calculation, we applied the whitening procedure for each EEG feature to remove the first- and second-order statistical effects, and then conducted the discrimination to acquire the weight vectors so that the sum of the absolute values was 1 (100%). The weight vectors for the four EEG features (amplitudes of the mu and beta rhythms from each hemisphere) were converted to absolute values and normalized. We defined the average of the converted values during motor intentions (0–3 seconds) as the “contribution rate”.
All analyses described above were carried out using MATLAB 2015b and SIMULINK 2015b software (MathWorks, US) with custom-made programs.
Outcome assessment
To validate the practicality of our system, we set a primary training endpoint, as the point where patients performed 120–200 trials within 40 minutes on each experimental day. EEG classification accuracy was also checked daily. The number of trials was determined taking into consideration the average number of days that inpatients spend in bed and the average time taken for treatment and other rehabilitation procedures.
The other outcome assessment of the BCI training system was related to the recovery of voluntary movements in the affected upper limb, especially wrist extension. For the assessment, we used the finger and knee-mouth tests (motor function, upper extremity) from the Stroke Impairment Assessment Set (SIAS, see Chino et al., Citation1994 for details). The finger test is used to determine a clinical score from 0 to 5 in terms of finger flexion, extension and coordination. The knee mouth test confirms whether the patient can touch the contralateral knee with her/his affected hand and can also bring that hand back to her/his mouth. The score for the knee mouth test is also expressed on a scale of 0 to 5. We used these SIAS scores as the outcome measure because they reflect motor recovery in the two body parts trained with the BCI. However, as the inpatients who participated in our study would have most probably improved regardless of the BCI intervention, and as we did not include a control group, it would be unreasonable to consider voluntary movement as a major outcome.
Results
We validated that our system safely enabled BCI training in patients with acute/subacute stroke. Before and after training, patients verbally reported whether they experienced dizziness, nausea, sweating, or chest pain. We also checked the patients’ vital signs at rest using the following criteria: heart rate of 40–120 bpm; systolic and diastolic blood pressures of <220 mmHg and <120 mmHg, respectively, for infarction and <180 mmHg and <110 mmHg, respectively, for hemorrhage. These blood pressure criteria are based on the Japanese Guidelines for the Management of Stroke 2015.
Using our portable BCI system, all four patients successfully performed the experiments according to our training protocols in their beds with no aggravation of paralysis, no abnormal muscle tone or spasticity, and no other adverse effects. No patient reported any pain over the course of the study. The variations in blood pressure recorded before and after the experiments were also within the safe range, based on the Japanese clinical guidelines described in the Methods section. During most experiments, the patients achieved the objective number of BCI training trials (120–200 trials) within 40 minutes. The average number of experimental days was 3.3 days (range 2–4 days), and no patient reported anxiety during the experiment.
The finger-function test results indicated recovery of the distal function of the upper extremity in three patients who could not contract those muscles at initial enrollment. A reduction in abnormal muscle synergies was observed in the remaining patient. The knee-mouth test showed improved proximal contraction of the affected elbow flexor muscle in two patients, and no change in the other two patients ().
Classification accuracy of EEG
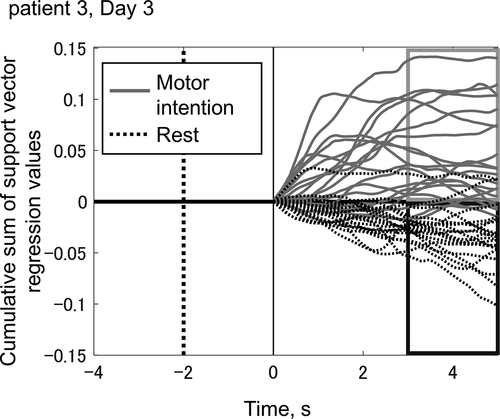
Forty trajectories of visual feedback during one BCI training session are overlaid in . This session shows the best classification accuracy for patient 3. The trajectories consist of 20 motor-intention trials and 20 rest trials. The discrimination of these two goals by our BCI system was successful in 35 of 40 trials (87.5%) within 3 seconds (P < .05 [70%] and P < .01 [75%]). In the motor-intention trials, if the discrimination was correct, the subsequent neuromuscular electrical stimulation would have generated extension of the wrist in the 3–5-second interval. While no session showed higher classification accuracy than the example shown in , the system succeeded in detecting motor intention with higher than chance-level accuracy on most experimental days.
Figure 3. Trajectories of visual feedback in a typical training session (patient 3, training day 3).
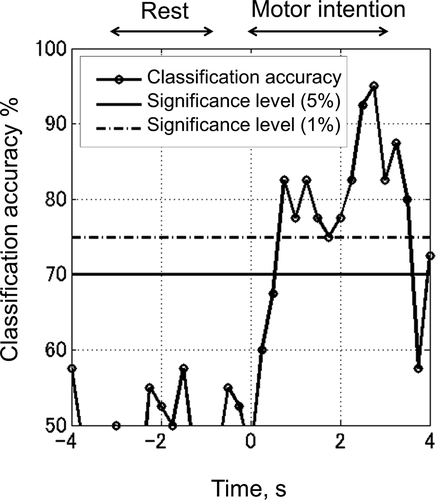
depicts an example of classification accuracy evaluation calculated event-related EEG. These data were recorded pre-training from patient 3. The accuracy reached 95% at its maximum. Motor intention should have occurred in the 0–3-second interval. The maximum classification accuracy during motor intention for each patient and each stage (Stage 1 and 3) is summarized in . Statistical significance levels were 70% (P < .05) and 75% (P < .01), respectively, as indicated in and . Stage 1 and 3 were recorded 26 times in total, 16 of which exceeded the 5% level and 11 of which exceeded the 1% level. Overall, accuracy decreased from 78.4% Stage 1 (pre-training) to 72.7% Stage 3 (post-training).
Table 2. Summary of classification accuracy (%).
Figure 4. Example of classification accuracy evaluation.
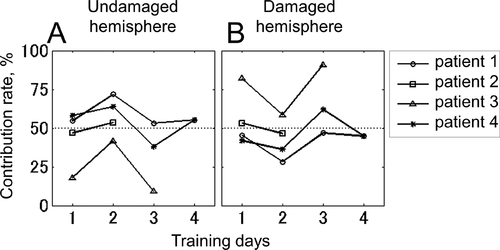
The contribution rate of each EEG feature is shown in . Panel A shows the summation of contribution rates of mu- and beta-band EEG amplitudes recorded from the undamaged hemisphere, and panel B shows those from the damaged side. The contribution rates of the features from the damaged side were not lower than those for the undamaged side.
Discussion
Portability and feasibility of bedside BCI system
We provided a simple and portable neuromuscular electrical stimulation system with a BCI for inpatients with acute/subacute hemiplegic stroke. Using this system, we are the first to show that patients can safely complete BCI training lying on their reclining beds.
Portability is an important factor for bedside BCIs. For good portability, we adopted a light-weight HMD as a visual feedback device. We found that an HMD, unlike an external computer screen used in conventional BCI research (Hashimoto et al., Citation2014), allows subjects to observe feedback in a comfortable reclining position. Another possible option for portability is the use of headphones for feedback. A previous study on portable P300-based BCI proposed a system that uses headphones for auditory stimulation, and such a system also does not require an external computer screen (Käthner et al., Citation2013).
A patient’s home or bedside is always noisier than a medical laboratory hence, the EEG measurements are expected to be less accurate in such environments. Therefore, there is a need to confirm the feasibility of BCI in such environments. Annen et al. (Citation2018) proposed a P300-based BCI for communication in patients with disorders of consciousness. In their study, a commercially available bedside BCI system developed by g.tec (mindBEAGLE, Graz, Austria) was employed. We adopted the same EEG recorder used in their system. This study, however, is the first to demonstrate the feasibility of bedside BCI that uses real-time feedback of sensorimotor rhythms.
BCI training for stroke patients
For patients with chronic stroke, studies of BCI-driven neuromuscular electrical stimulation (Mukaino et al., Citation2014) and BCI training combined with other physical therapy (Broetz et al., Citation2010) have already suggested that BCI systems may induce activity-dependent cortical plasticity and promote functional recovery. The functional improvement induced by the BCI training can continue for 6–12 months after the intervention (Biasiucci et al., Citation2018). When electrical stimulation is applied in the form of pulse trains to the motor point of the muscle, peripheral afferent activity is elicited and fed back to the central nervous system as sensation. This activity is believed to form a closed loop including the brain, body and BCI system, and to promote neural plasticity (Xu et al., Citation2014). This mechanism may contribute to the recovery of motor function from hemiplegia after stroke. In fact, a recent BCI meta-analysis (Bai et al., Citation2020) showed that BCIs combined with neuromuscular electrical stimulation may be a better combination for functional recovery than other kinds of neurofeedback.
Participation in BCI research as an outpatient in the chronic phase is safer than as an inpatient in the early stroke phase as it avoids the various risks of early-phase intervention. Moreover, in the acute/subacute-phase of stroke (within 90 days of stroke), vitals are sometimes unstable. Thus, to avoid clinical risks, experimenters should monitor the patients’ vital signs during the training. Additionally, most patients in the early-phase have little stamina and have difficulty moving to a rehabilitative laboratory or rehabilitation room even if it is in the same building as the inpatient ward. These factors make it difficult for researchers to conduct large-scale studies investigating the effects of BCI training on patients with acute/subacute-phase stroke.
Accuracy of EEG classification
In the current study, EEG classification accuracy was rather poor: 61% of Stage1 and 3 (16 out of 26 sessions) had accuracies above chance level (P < .05). This does not mean that our BCI is flawed; it means that patients with early stroke are not able to activate the sensorimotor cortex enough to affect their EEGs.
When the sensorimotor area is activated by motor intentions and shows proper patterns of cortical activity, positive visual feedback is given by the BCI system. When the sensorimotor area shows improper patterns despite motor intentions, feedback becomes negative. This process is part of the neural operant conditioning during BCI use. Because patients with acute stroke show smaller EEG changes (Event-related desynchronization, ERD) over the sensorimotor area in the affected hemisphere than control groups, despite motion intentions (Stępień et al., Citation2011), ERD enhancement induced by BCI use might be able to shift the patients’ EEG toward normal values. Previous studies also reported ERD enhancement via BCI use in patients with chronic stroke, as well as increased EEG classification accuracy (Ono et al., Citation2014).
Comparing classification accuracy before and after training showed that post-training accuracy was actually lower than pre-training accuracy in three of the four patients (excluding patient 2). One possible explanation is loss of concentration due to mental fatigue. Another is the difference in measurement conditions during Stage 1 and Stage 3. When the Stage 2 (BCI training) was completed and Stage 3 started, the visual feedback moved horizontally at a constant speed and did not move vertically; only the periods of rest, preparation, and intention were shown. The patients may thus have been perplexed, and the effects of daily training on classification accuracy could have been measured incorrectly.
Study limitations
In the four patients, finger function improved from 0, 0, 0, and 1B to 1A, 1B, 1B, and 2 (SIAS, finger-function test), and proximal arm function improved from 0, 0, 1, and 2 to 0, 2, 1, and 3 (SIAS, knee-mouth test). However, we cannot say for sure whether this functional recovery primarily resulted from using our system or whether it was part of the normal post-stroke recovery process.
Furthermore, the current study cannot directly speak to the effectiveness of BCI use for patients with acute/subacute stroke or to what the proper training intensity might be. What we can say is that our study showed that bedside training with a portable BCI is a practical and safe intervention in these cases. We can also say that patients with acute/subacute stroke can change their EEG patterns and that our system is capable of measuring such changes.
To learn more about functional improvements in the upper extremity through training, large-scale and long-term follow-up studies are needed. Open questions such as whether more training can change EEG patterns or improve hand function or whether functional improvement can be continued after discharge still persist. Additionally, next-phase clinical studies are necessary and should employ standard motor evaluations such as Fugl-Meyer assessment, electromyography, and range of motion measurements in the upper limb joints. Though this study is a short-term case series without control participants and without long-term detailed clinical evaluations, our findings should encourage future randomized controlled trials or larger-scale BCI studies in patients with acute stroke.
Conclusions
The present study shows that bedside training with our portable BCI is a feasible and safe intervention in cases of acute/subacute stroke. Four inpatients safely and successfully executed the training while reclining on their beds within 7–24 days of stroke onset, using our BCI system that includes neuromuscular electrical stimulation. Further trials are needed to determine an appropriate level of training intensity as well as to investigate the efficacy of introducing this training procedure early in the rehabilitation and recovery process.
Declaration of Interest
M.L. and J.U. are the founding scientists of Connect Inc., a commercial company for the development of rehabilitation devices since May 2018. J.U. is the Chief Executive Officer, and M.L. is the Executive Officer of Connect Inc. They have received a salary from Connect Inc., and have held shares in Connect Inc. This company does not have any relationship with the device or setup used in the current study.
Acknowledgments
We wish to thank the rehabilitation team in Asahikawa Medical University Hospital, Hokkaido, Japan, for their support in patients’ recruitment.
Additional information
Funding
References
- Annen, J., Blandiaux, S., Lejeune, N., Bahri, M. A., Thibaut, A., Cho, W., … Laureys, S. (2018). BCI performance and brain metabolism profile in severely brain-injured patients without response to command at bedside. Frontiers in Neuroscience, 12, 370. https://doi.org/https://doi.org/10.3389/fnins.2018.00370
- Bai, Z., Fong, K. N. K., Zhang, J. J., Chan, J., & Ting, K. H. (2020). Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: A systematic review and meta-analysis. Journal of Neuroengineering and Rehabilitation, 17(1), 57. https://doi.org/https://doi.org/10.1186/s12984-020-00686-2
- Biasiucci, A., Leeb, R., Iturrate, I., Perdikis, S., Al-Khodairy, A., Corbet, T., … Millán, J. D. R. (2018). Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nature Communications, 9(1), 2421. https://doi.org/https://doi.org/10.1038/s41467-018-04673-z
- Biernaskie, J., Chernenko, G., & Corbett, D. (2004). Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(5), 1245–1254. https://doi.org/https://doi.org/10.1523/JNEUROSCI.3834-03.2004
- Broetz, D., Braun, C., Weber, C., Soekadar, S. R., Caria, A., & Birbaumer, N. (2010). Combination of brain-computer interface training and goal-directed physical therapy in chronic stroke: A case report. Neurorehabilitation and Neural Repair, 24(7), 674–679. https://doi.org/https://doi.org/10.1177/1545968310368683
- Burn, J., Dennis, M., Bamford, J., Sandercock, P., Wade, D., & Warlow, C. (1994). Long-term risk of recurrent stroke after a first-ever stroke. The Oxfordshire community stroke project. Stroke, 25(2), 333–337. https://doi.org/https://doi.org/10.1161/01.str.25.2.333
- Chang, C.-C., & Lin, C.-J. (2011). LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems and Technology, 2(3), 27. https://doi.org/https://doi.org/10.1145/1961189.1961199 1–27:27
- Chino, N., Sonoda, S., Domen, K., Saitoh, E., & Kimura, A. (1994). Stroke Impairment Assessment Set (SIAS): A new evaluation instrument for stroke patients. The Japanese Journal of Rehabilitation Medicine, 31(2), 119–125. https://doi.org/https://doi.org/10.2490/jjrm1963.31.119
- Hashimoto, Y., Ota, T., Mukaino, M., Liu, M., & Ushiba, J. (2014). Functional recovery from chronic writer’s cramp by brain-computer interface rehabilitation: A case report. BMC Neuroscience, 15(1), 103. https://doi.org/https://doi.org/10.1186/1471-2202-15-103
- Horn, S. D., DeJong, G., Smout, R. J., Gassaway, J., James, R., & Conroy, B. (2005). Stroke rehabilitation patients, practice, and outcomes: Is earlier and more aggressive therapy better? Archives of Physical Medicine and Rehabilitation, 86(12 Suppl 2), S101–S114. https://doi.org/https://doi.org/10.1016/j.apmr.2005.09.016
- Kasashima-Shindo, Y., Fujiwara, T., Ushiba, J., Matsushika, Y., Kamatani, D., Oto, M., … Liu, M. (2015). Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: Proof of concept study. Journal of Rehabilitation Medicine, 47(4), 318–324. https://doi.org/https://doi.org/10.2340/16501977-1925
- Käthner, I., Ruf, C. A., Pasqualotto, E., Braun, C., Birbaumer, N., & Halder, S. (2013). A portable auditory P300 brain-computer interface with directional cues. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 124(2), 327–338. https://doi.org/https://doi.org/10.1016/j.clinph.2012.08.006
- Klem, G. H., Lüders, H. O., Jasper, H. H., & Elger, C. (1999). The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalography and Clinical Neurophysiology, 52, 3–6.
- Maulden, S. A., Gassaway, J., Horn, S. D., Smout, R. J., & DeJong, G. (2005). Timing of initiation of rehabilitation after stroke. Archives of Physical Medicine and Rehabilitation, 86(12 Suppl 2), S34–S40. https://doi.org/https://doi.org/10.1016/j.apmr.2005.08.119
- Moroney, J. T., Bagiella, E., Paik, M. C., Sacco, R. L., & Desmond, D. W. (1998). Risk factors for early recurrence after ischemic stroke: The role of stroke syndrome and subtype. Stroke, 29(10), 2118–2124. https://doi.org/https://doi.org/10.1161/01.str.29.10.2118
- Mukaino, M., Ono, T., Shindo, K., Fujiwara, T., Ota, T., Kimura, A., … Ushiba, J. (2014). Efficacy of brain-computer interface-driven neuromuscular electrical stimulation for chronic paresis after stroke. Journal of Rehabilitation Medicine, 46(4), 378–382. https://doi.org/https://doi.org/10.2340/16501977-1785
- Murphy, T. H., & Corbett, D. (2009). Plasticity during stroke recovery: From synapse to behaviour. Nature Reviews. Neuroscience, 10(12), 861–872. https://doi.org/https://doi.org/10.1038/nrn2735
- Naaman, S. C., Stein, R. B., & Thomas, C. (2000). Minimizing discomfort with surface neuromuscular stimulation. Neurorehabilitation and Neural Repair, 14(3), 223–228. https://doi.org/https://doi.org/10.1177/154596830001400308
- Ono, T., Shindo, K., Kawashima, K., Ota, N., Ito, M., Ota, T., … Ushiba, J. (2014). Brain-computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Frontiers in Neuroengineering, 7, 19. https://doi.org/https://doi.org/10.3389/fneng.2014.00019
- Pitcher, J. B., Ridding, M. C., & Miles, T. S. (2003). Frequency-dependent, bi-directional plasticity in motor cortex of human adults. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(7), 1265–1271. https://doi.org/https://doi.org/10.1016/s1388-2457(03)00092-0
- Schölkopf, B., Smola, A. J., Williamson, R. C., & Bartlett, P. L. (2000). New Support Vector Algorithms. Neural Computation, 12(5), 1207–1245. https://doi.org/https://doi.org/10.1162/089976600300015565
- Shindo, K., Kawashima, K., Ushiba, J., Ota, N., Ito, M., Ota, T., … Liu, M. (2011). Effects of neurofeedback training with an electroencephalogram-based brain-computer interface for hand paralysis in patients with chronic stroke: A preliminary case series study. Journal of Rehabilitation Medicine, 43(10), 951–957. https://doi.org/https://doi.org/10.2340/16501977-0859
- Stępień, M., Conradi, J., Waterstraat, G., Hohlefeld, F. U., Curio, G., & Nikulin, V. V. (2011). Event-related desynchronization of sensorimotor EEG rhythms in hemiparetic patients with acute stroke. Neuroscience Letters, 488(1), 17–21. https://doi.org/https://doi.org/10.1016/j.neulet.2010.10.072
- Ushiba, J., & Soekadar, S. R. (2016). Brain-machine interfaces for rehabilitation of poststroke hemiplegia. Progress in Brain Research, 228, 163–183. https://doi.org/https://doi.org/10.1016/bs.pbr.2016.04.020
- Vapnik, V. N. (1995). The nature of statistical learning theory. New York: Springer-Verlag. https://doi.org/https://doi.org/10.1007/978-1-4757-2440-0
- Whyte, J., Gordon, W., & Rothi, L. J. G. (2009). A phased developmental approach to neurorehabilitation research: The science of knowledge building. Archives of Physical Medicine and Rehabilitation, 90(11 Suppl), S3–10. https://doi.org/https://doi.org/10.1016/j.apmr.2009.07.008
- Xu, R., Jiang, N., Mrachacz-Kersting, N., Lin, C., Asín Prieto, G., Moreno, J. C., … Farina, D. (2014). A closed-loop brain–computer interface triggering an active ankle–foot orthosis for inducing cortical neural plasticity. IEEE Transactions on Biomedical Engineering, 61(7), 2092–2101. https://doi.org/https://doi.org/10.1109/TBME.2014.2313867
- Yang, Y.-R., Wang, R.-Y., & Wang, P. S.-G. (2003). Early and late treadmill training after focal brain ischemia in rats. Neuroscience Letters, 339(2), 91–94. https://doi.org/https://doi.org/10.1016/s0304-3940(03)00010-7