Abstract
Anoxia, specifically sulfate (SO42−) reduction, in the hypolimnia of stratified lakes is a prerequisite for monomethylmercury (HgCH3+) formation in sediments and subsequent efflux into the water column. A whole-lake pilot project used liquid calcium nitrate (LCN) to suppress formation of HgCH3+ by raising hypolimnetic redox in a dimictic, mesotrophic lake in Minnesota. A nearby lake of similar morphology and trophic status served as a reference. In the experimental lake, the HgCH3+ concentration at the onset of LCN application was 0.34 ng/L and 0.30 ng/L at the point of nitrate (NO3−) depletion. The NO3− concentration peaked at 17.7 mg/L (as ion) 16 days after application and declined to 0.5 mg/L at day 64. A principal NO3− sink was anaerobic oxidation of sulfidic compounds, but dissimilatory NO3− reduction to ammonium (NH4+) may also have been a major sink. SO42− at the time of the LCN dose was 0.99 mg/L and rose to 4.0 mg/L at the point of NO3− depletion (<0.5 mg/L). Thereafter, NO3−-induced SO42− enrichment of the hypolimnion induced HgCH3+ to increase to 1.09 ng/L by SO42− reduction. In both lakes, we observed strong redox functional relationships in the hypolimnia with unambiguous breakpoints (thresholds) for SO42− reduction and sediment efflux of iron, manganese, orthophosphate, NH4+, and HgCH3+. Study results suggest that LCN application be done in frequent, small doses with redox monitoring to prevent SO42− reduction until turnover. This method would minimize downstream NO3− loading at turnover while avoiding inadvertent SO42− enrichment that can boost HgCH3+ production in SO42− limited lakes.
Atmospheric deposition of mercury (Hg) is the root cause of widespread accumulation of methylmercury in freshwater fish tissue (Watras Citation2009). Sediment bacteria convert inorganic Hg under anoxic conditions into monomethylmercury (HgCH3+), making it bioavailable to primary and secondary producers (Ullrich et al. Citation2001, Hamelin et al. 2011). Biomagnification makes HgCH3+ highly toxic to top predators in food webs (Boening Citation2000). Elevated methylmercury content in fish tissue of 0.3 mg/kg wet weight causes designation of lakes as impaired or promulgation of fish consumption advisories (Watras et al. Citation1998, US EPA 2001, Monson and Heiskary Citation2008, Evers et al. Citation2011, Zananski et al. Citation2011).
Source control of Hg emissions is ultimately the most effective solution to atmospheric Hg pollution (Johansson et al. Citation2001, Morrison Citation2011), but Hg deposited to terrestrial areas will continue to load receiving lakes into the future, depending on site-specific factors (Scherbatskoy et al. Citation1998, Driscoll et al. Citation2007). Response of lakes to Hg loading is not instantaneous. Rather, there is potential for many years, perhaps decades, before Hg methylation reaches a steady state from mercury loading (Paterson et al. Citation2006). Effective Hg source control will depend on effective implementation of the Minamata Convention on Mercury, which itself will take decades to substantially reduce atmospheric Hg emissions (UNEP Citation2013). Consequently, Hg source control is unlikely to adequately remediate near- to intermediate-term Hg contamination problems for many Hg-impaired lakes that have received Hg from atmospheric loading.
The scale of Hg contamination severely limits a lake-by-lake approach to remediation of lakes impaired by Hg. A small subset of these lakes or reservoirs, however, will warrant the expense of site-specific remediation, particularly if technologies that suppress Hg methylation also suppress internal loading of nutrients in eutrophic lakes.
Anoxic hypolimnia can be important sources of HgCH3+ in lakes (Eckley et al. Citation2005, Watras Citation2009). In these lakes, relief from hypolimnetic anoxia offers potential remediation of Hg-impairment (Beutel et al. 2008b, Todorova et al. Citation2009, Dent et al. Citation2014, Matthews et al. Citation2013).
Sulfate (SO42−) reducing bacteria (SRB) are the principal producers of HgCH3+ in sediments (Compeau and Bartha Citation1985, Benoit et al. Citation2003, Effler and Matthews Citation2008, Corrales et al. Citation2011), but other bacteria, including iron (Fe)-reducing bacteria and methanogens, possess genes for Hg methylation (Gilmour et al. Citation2013, Parks et al. Citation2013). Elevated redox conditions at the sediment surface, too oxidizing for these reductive processes to occur, suppress HgCH3+ formation (Regnell and Tunlid Citation1991, Boszke et al. Citation2003, DeLaune et al. Citation2004). Thus, concentrations of nitrate ion (NO3−; Beutel et al. Citation2008b, Todorova et al. Citation2009, Shih et al. Citation2011) or dissolved oxygen (DO; Beutel et al. Citation2008b, Dent et al. Citation2014) above critical concentrations suppress HgCH3+ formation by suppression of SRB activity in surficial sediments.
Engineering methods can elevate hypolimnetic redox by adding NO3− or DO. The design practice for DO is well-understood (Beutel and Horne Citation1999, Cooke et al. Citation2005, Gantzer et al. Citation2009b) and has also demonstrated suppression of HgCH3+ production (Beutel et al. Citation2008b, Dent et al. Citation2014).
The so-called Riplox method injects NO3− in the hypolimnia to control internal loading of phosphate (PO43−; Ripl Citation1978, Willendbring et al. Citation1983, Noonan Citation1986). Although our engineering team was also engaged in hypolimnetic oxygen projects, we investigated NO3− addition because of its low infrastructure needs and potential to control both HgCH3+ and internal nutrient loading. Almost all hypolimnetic oxygenation projects occur in large reservoirs for utility clients. There is a need for remediation technologies appropriate at small scales and for clients with limited capacity to operate remediation infrastructure.
Internal loading of PO43− is largely a function of SO42− and Fe reduction in surficial sediments (Mortimer Citation1942, Holmer and Storkholm Citation2001, Christophoridis and Fytianos Citation2006), which elevated NO3− will suppress. Todorova et al. (Citation2009) observed suppression of HgCH3+ by residual NO3− in the hypolimnion of Lake Onondaga; the source of NO3− was wastewater discharge. Production of HgCH3+ returned after depletion of hypolimnion NO3−. Subsequently, but after the present study, Matthews et al. (2013) demonstrated suppression of HgCH3+ through injection of liquid calcium nitrate (LCN) to the hypolimnion of Lake Onondaga.
Peer reviewed literature or publically available engineering reports available at the time of the present study (2009–2010) did not provide a sufficient engineering design basis to assess the efficacy and practical application of NO3− addition for suppression of SRB activity in anoxic hypolimnia. We resolved, therefore, to conduct a lake-scale pilot project to investigate the design basis for HgCH3+ management.
This study documents a pilot project that investigated NO3− addition to control internal loading of HgCH3+ in the hypolimnion of a mesotrophic lake in Minnesota listed for Hg impairment. The study needed to address basic engineering issues:
evaluation of the efficacy of NO3− addition to suppress HgCH3+ formation;
exploration of a rational basis for establishing NO3− dosing criteria;
development of dosing methods;
determination of dominant NO3− sinks and rate of NO3− demand;
evaluation of operational parameters indicative of treatment efficacy; and
exploration of regulatory concerns over NO3− toxicity and nutrient enrichment, which are intrinsic issues surrounding NO3− addition.
Study location
The 3 study lakes, Round, Ann, and Riley, are all located in the Riley Purgatory Bluff Creek Watershed District in the western suburbs of Minneapolis, Minnesota (). The water sources for all study lakes are suburban runoff and groundwater with no known industrial discharges. All study lakes are dimictic, stratifying immediately after ice thaws in late April or early May. All lakes have a May onset of hypolimnetic anoxia that continues until fall turnover. Lake basin morphology is similar, characterized by a deep (11–16 m) central basin bordered by a littoral fringe.
Table 1 Study lake descriptions. Addition lake descriptions available at http://www.dnr.state.mn.us/lakefind/index.html
Table 2 Wet chemistry parameters and analytical methods applied in this study.
Materials and methods
Round Lake was the focus of this study. It received a dose of LCN with the intent of suppressing HgCH3+ production. Lake Ann served as a reference lake monitored with the same protocol as Round Lake. Riley Lake was lumped with Round Lake and Lake Ann in the correlation of sulfide (H2S) odor with redox. We used 2008 (Jun–Oct) and 2009 (Apr–Oct) monitoring data for method development, as described below, and conducted the LCN application pilot study in 2010 (Mar–Oct). We used a Hydrolab DS5 multi-probe to obtain DO (ion selective electrode), redox (platinum band electrode), pH, conductivity, and temperature at 1.0 m intervals from surface to the bottom. We calibrated the redox probe (single point) to +428 mV in Zobell's solution at 25 C to the standard hydrogen reference electrode (SHE) scale. Field monitoring protocol allowed 3–5 minutes of equilibration of probes before recording values manually and to a data logger.
We used Van Dorn bottles to obtain wet samples from the surface, mid-metalimnion, and 1.0 m from the bottom (). Data reported as the hypolimnion are from samples 1.0 m from the bottom. We obtained all samples from a boat with the exception of the March 2010 HgCH3+ sample obtained from a hole in the ice. Surficial sediments in both Round Lake and Lake Ann were flocculent. Starting in early summer, inadvertent impact of the bottom with the van Dorn bottle produced plumes of bubbles a few meters in diameter. Field crews were careful not to entrain sediments into near-bottom samples and to shift sampling location if there was contact with the bottom.
Production of H2S within the hypolimnia of study lakes was monitored through depletion of SO42− and by a field odor assessment of sample bottle contents using a scale of 1-nondetect (nose close to sample), 2-presence (detection with nose close to sample), 3-moderate (distinct sulfide odor near van Dorn bottle at opening), and 4-strong (distinct sulfide odor across the boat upon opening van Dorn bottle). This method is similar to the serendipitous sniff test (Barbash Citation2002) but developed independently for this study to distinguish more than presence or absence of sulfide in the sample. The field monitoring team always had a member with experience in the scale of comparison.
The NO3− application to Round Lake was a slug dose of LCN [Ca(NO3)2• 4H2O], specific gravity 1.452. By mass, LCN total nitrogen (N) and total calcium (Ca) were 8.0 and 12.3%, respectively.
A weighted hose discharged LCN to the deepest point of the hypolimnion, pumped directly from tanker trucks. The small size of the pelagic area (3.64 ha) and simple, bowl-like morphology of Round Lake were ideal for this method of application. Two 20,412 kg (45,000 lb) tanker trucks of LCN each provided 3266 kg of NO3− (as ion) to the hypolimnion. The tanker trucks were cleaned before picking up the LCN for delivery to avoid contamination of unwanted compounds.
Regulatory concerns and NO3− depletion rates calculated from sediment oxygen demand (SOD) informed calculation of the LCN dose. The Minnesota Pollution Control Agency (MPCA) expressed concern that an NO3− ion concentration >30 mg/L could be toxic to invertebrates, and the US EPA had not promulgated a NO3− standard at that time. This project was informed instead by the promulgated Canadian standard of 13 mg/L NO3− (as ion; Environment Canada 2003), an order of magnitude lower than the lowest observed effect concentration (LOEC) toxicity threshold. The northern leopard frog (Rana pipiens) had a 56-day LOEC of 133 mg/L NO3−, and a water flea (Ceriodaphnia dubia) had a 7-day LOEC of 189 mg/L NO3−. Acute toxicity thresholds (96 hr-LC50, 120 hr-LC50) are at least an order of magnitude higher, except for the caddis fly (Hydropsyche occidentalis), which has a 120-hour LC50 of 290 mg/L NO3−.
The final criteria developed for an LCN dose maximum were 2-fold: (1) the initial NO3− concentration needed to be less than the lowest known LOEC, and (2) the NO3− concentration would not exceed the MPCA informal threshold of 30 mg/L NO3− for more than 30 days. Per Canadian standards, these criteria would fall well below known LOEC for NO3−.
Determination of the initial dose provisionally assumed that the hypolimnion of Round Lake is well mixed. This assumption simplified dose calculation by avoiding uncalibrated modeling of plume dispersion and dissolution rates from the pool of LCN resting on the lake bottom. The nominal initial dose concentration would be the mass of the NO3− dose divided by the hypolimnion volume (253,354 m3) determined from lake bathymetry for depths <3.8 m, which is the approximate location of the top of the thermocline.
NO3− is an alternative electron acceptor to O2 for bacterial respiration. There is a well-known equivalence of NO3− demand to O2 demand in wastewater engineering (Metcalf and Eddy Citation2003); therefore, hypolimnetic oxygen demand (HOD) can be used to approximate NO3− demand. Because 77.4% of NO3− is oxygen on a mass basis, we calculated the hypolimnetic NO3− demand as HOD/0.774.
Determination of HOD was problematic. Although DO profile data were available from late April or early May through September in 1996, 1997, 2001–2004, 2008, and 2009, in all years, the hypolimnion was anoxic (<1.0 mg/L DO) from the onset of spring monitoring. Calculation of HOD from DO depletion curves was not possible.
Direct measurement of HOD as the sum of SOD and suspended community oxygen demand (SCOD) provides an alternative to oxygen depletion curves. In 2009, the SOD was 2.16 g/m2/d after fall turnover (temperature 15 C), using the EPA in situ method of (diver-placed) isolation chambers on the sediment surface (Murphy and Hicks Citation1986, US EPA 2009). Site conditions prevented deployment of the diver support boat, forcing deployment of isolation chambers from the shore in 4.0 m of water rather than 11.0 m at deepest depth. This depth was in the thermocline, but anoxia extends to the top of the thermocline in Round Lake. The 50th percentile of July–August DO (n = 26) at 4.0 depth in 1997, 2001, 2002, 2003, 2008, and 2009 was 0.5 mg/L, with only the 75% percentile >2.0 mg/L. At depths of 10 m or greater (n = 27) during the same period, all DO values were <0.5 mg/L. Given the stronger anoxia at deepest depth, the SOD may be higher than at 4.0 m. The SCOD was assumed to be 1.04 g/m2/d O2 based on a 2010 winter diel curve analysis (Odum and Hoskins Citation1958) study completed on nearby lakes of similar trophic status in the Bluff Creek Watershed District (CH2M HILL unpubl. data). The hypolimnion area (AH) under the 3.8 m depth curve is 47,299 m2. Thus HOD = AH(SCOD + SOD)/d = 151 kg/d O2. Expressed as NO3− demand, HODasNO3 = 195 kg/d NO3−. Expressed as area, this rate is 4.1 g/m2/d NO3−.
Two truckloads of LCN provided a total dose 6532 kg NO3−. Divided by the hypolimnion volume, the resulting nominal NO3− concentration in the hypolimnion would be 26 mg/L. Per the areal depletion rate, NO3−at this concentration would fully deplete in ∼43 days. A third truckload would have raised the NO3− concentration to >30 mg/L. Assuming a linear depletion rate, 2 truckloads of LCN would meet the Canadian criterion within 3 weeks. Finally, a longer period would run the risk of not achieving complete NO3− depletion prior to thermal erosion of the hypolimnion in September. Complete depletion of NO3− was essential to our study goals, and therefore the dose was 6532 kg NO3− (2 truckloads of LCN).
Graphic analysis of data used Excel and Kaleidagraph software. Nonparametric statistical analyses of data employed Wilcoxon–Mann–Whitney rank sum tests using Kaleidagraph software.
Table 3 Free energy for selected potentially competing equations for nitrate reduction at standard conditions.
Gibbs free energy (kJ/mol) for likely completing reactions for NO3− () were obtained from thermodynamic references at standard conditions (Woods and Garrels Citation1987, Lide Citation2007) using:
(1)
Results
On 15 June 2010, 2 semi-trailer truckloads discharged the LCN dose to the hypolimnion of Round Lake. The NO3− hypolimnion concentration peaked 15 days after application at 17.7 mg/L NO3− (). By 18 August (64 d), the NO3− concentration had dropped to 0.34 mg/L. Depletion of NO3− from the concentration peak was linear at a rate of 102 kg/d, which is 2.8 g/m2/d, 67% of the estimated depletion rate.
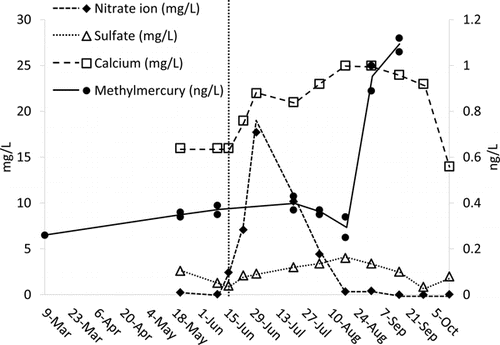
The HgCH3+ hypolimnetic concentration of Round Lake was 0.35 ng/L before the application of LCN and remained at this value with minor fluctuations until depletion of NO3− (). Addition of LCN produced SO42− (). After depletion of NO3−, the HgCH3+ concentration rose to 1.09 ng/L by 15 September (). DO in the hypolimion during the study was <0.1 mg/L, leaving no other oxidation mechanism for sulfidic compounds (e.g., HS−, H2S, FeS, FeS2) other than anaerobic oxidation via NO3−.
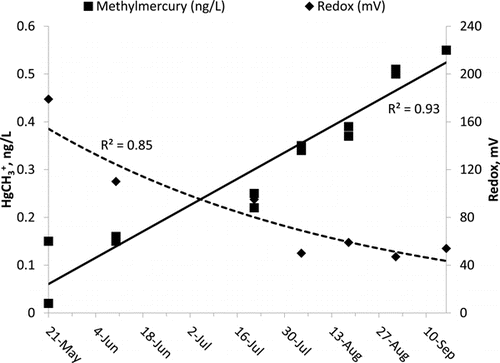
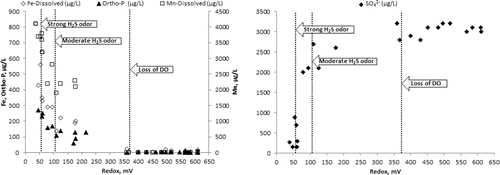
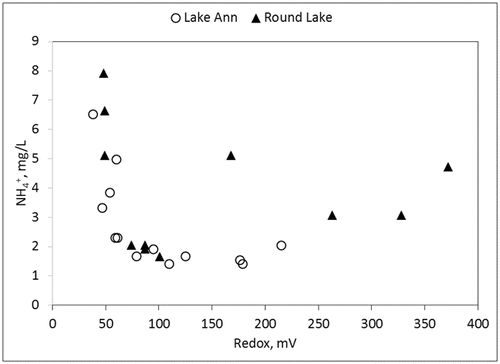
Lake Ann hypolimnetic DO was <0.1 mg/L May–September 2010. Redox values tracked production of HgCH3+ and geochemical shifts of solute species. HgCH3+ increased monotonically from 0.085 to 0.55 ng/L through the same period in inverse proportion to redox values (). Decreasing redox values tracked SO42− reduction and accompanying solubilization of Fe, Mn, and Ortho-P from the sediments (). A similar redox dynamic occurred in the hypolimnion of Round Lake ().
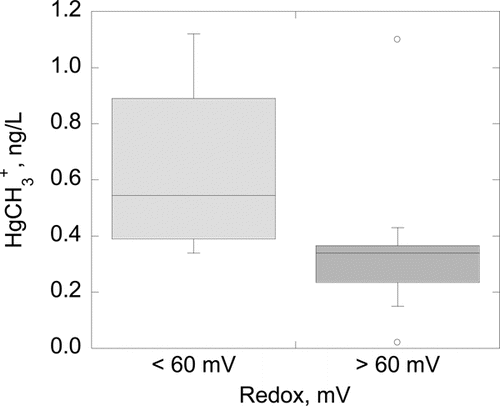
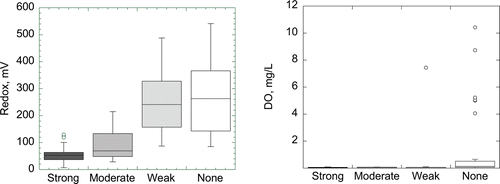
There were significant differences between redox values and H2S odor intensity () that corresponded to redox solubilization thresholds observed in Round Lake and Lake Ann (). The median redox value of +53 mV for samples with a strong H2S odor was significantly different (P = 0.015) from samples with a moderate odor (+70 mV). The median redox value of +263 mV for samples of weak or no odor was significantly different than moderate odor samples (P < 0.0001) and strong odor samples (P < 0.0001). By contrast, there was no statistically significant difference between median DO of strong and moderate sulfide odors (P = 0.48) or moderate and weak odors (P = 0.9; ). In the trivial case of no odor, there was a significant difference (P < 0.012) between median redox values of other odor categories. Across all lakes, +60 mV was the approximate threshold below which strong H2S odors where detected and median HgCH3+ concentrations were significantly higher (). Sharp increases in Ortho-P solubilization began in the upper quartile (∼+200 mV) of the moderate H2S odor redox range.
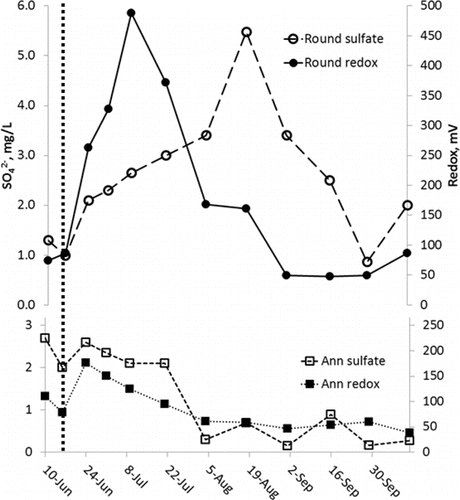
Both Lake Ann and Round Lake hypolimnia were strongly anoxic. Lake Ann redox was in the manganese (Mn) and Fe reduction redox range from March to early July when it dropped into SO42−-reducing conditions, where it remained until early September (). Round Lake redox conditions were already in the SO42−-reducing range when LCN was placed in the hypolimnion. Redox values rose from +45 mV prior to application on 15 June, to +85 mV on 16 June, to +138 mV by 17 June. By 01 July, redox values were in a range (>+300 mV) typically associated with oxic conditions (). By mid-August, redox values dropped to SO42−-reducing values with complete depletion of NO3−.
The dynamics of SO42− production in Lake Ann and Round Lake indicated that sulfidic compounds were important, possibly dominant, electron donors in NO3− reduction (). Whereas there was steady depletion of SO42− with dropping redox in Lake Ann, in Round Lake SO42− rose from 0.99 mg/L at the time of LCN addition to 4.0 mg/L just after depletion of NO3−.
Assuming that SO42− concentrations were uniform in the hypolimnion, SO42− mass increased by 760 kg. Per H2S oxidation by NO3− (), as much as 784 kg NO3− was consumed, or 12% of the NO3− dose. This calculation assumes a uniform hypolimnetic NO3− concentration of 0.34 mg/L (86 kg NO3− residual mass).
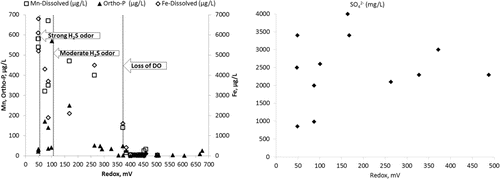
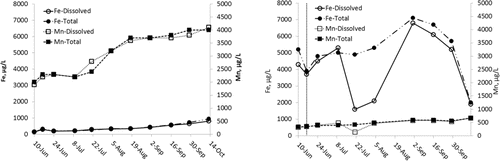
After addition of NO3−, soluble, but not total, Fe temporarily decreased in Round Lake (), but there was no significant effect on Mn concentration. In Lake Ann, soluble and total Fe and Mn were equal and increased throughout the study (). Assuming that the drop in Round Lake hypolimnetic Fe(II) was due to uniform oxidation by NO3− throughout the hypolimnion, ∼963 kg of Fe(II) oxidized to Fe(III). Oxidation of Fe(II) in the hypolimnion would account for up to 214 kg NO3− (), ∼3% of the NO3− dose.
There may have been dissimilatory NO3− reduction to NH4+ (DNRA) in Round Lake, which is thermodynamically favorable in both heterotrophic and autotrophic bacteria (). The NH4+ concentration increased 2.0 mg/L on 16 June to 5.5 mg/L on 18 August. Assuming a uniform distribution of NH4+ concentration in the hypolimnion, on a mass ratio basis of 3.4 g NO3−:1.0 g NH4+, the 881 kg increase in hypolimnetic NH4+ would consume 3034 kg of NO3−, which is 46% of the dose.
For Round Lake hypolimnetic NH4+ concentrations after 15 June, there was a significant difference (P = 0.019) between the 2010 median N of 4.0 mg/L (min 2.4, max 6.2, n = 8) and the 2008–2009 median N of 2.2 mg/L (min 0.4, max 4.6, n = 9). By comparison, there was no significant difference (P = 0.08) between the median concentration in Lake Ann (1.6 mg/L) and Round Lake (2.4 mg/L). NH4+ concentrations in both lakes had similar responses to redox () that followed redox-active solubilization of Ortho-P, Fe, and Mn ( and 4). The hypolimnetic NH4+ concentration rose in Round Lake from 4.4 mg/L on 18 August to 6.3 mg/L on 16 September, but there was no NO3− to produce NH4+ during this period.
Discussion
After addition of LCN to Round Lake, formation of HgCH3+ in the hypolimnion did not increase until depletion of NO3−. By contrast, HgCH3+ production continued unabated throughout the same period in Lake Ann. There are no baseline HgCH3+ data for either lake to evaluate the potential impacts of interannual variability. Suppression of HgCH3+ formation by NO3− addition was consistent with findings by Todorova et al. (Citation2009) and Matthews et al. (Citation2013) in Lake Onondaga, New York. NO3− concentrations >0.5 mg/L suppressed HgCH3+ production in a cold (<10 C) hypolimnia in both the current study and that of Lake Onondaga. In Lake Onondaga, mechanisms of NO3− control of HgCH3+ formation were suppression of SRB activity, decrease in methylation/demethylation ratios, and increased sorption to Fe and Mn hydroxides.
Data from this study implicate SO42− reduction in the production of HgCH3+. Strong H2S odors were unambiguously associated with the lowest recorded redox values (, 4, and 5) and with HgCH3+ production ( and 6).
Addition of NO3− immediately raised redox values above those associated with SO42− reduction (, 4, and 7). The mechanism of redox elevation was NO3− oxidation of sulfidic compounds, as seen in the increase in SO42− concentrations after the introduction of NO3− in Round Lake. Lake Ann, by contrast, exhibited monotonic SO42− depletion and decrease of redox values during the same period.
Nitrate sinks
Results from the present study and thermodynamics support anaerobic sulfide oxidation as an important, possibly primary, sink for NO3− depletion. The NO3− dose of 6532 kg was more than sufficient to produce the apparent Round Lake hypolimnetic increase of 760 kg SO42−. Free energy for potential denitrification stoichiometry in the hypolimnion reveals that chemolithotrophic bacterial (utilizing inorganic C for respiration) oxidation of H2S and FeS2 is energetically favored over heterotrophic bacterial (utilizing organic C for respiration) reactions (). Organic carbon, therefore, may not be a primary sink for NO3−.
The pH data support anaerobic H2S oxidation via NO3−. Chemolithotrophic denitrification produces acid (), DNRA neither produces nor consumes acid, and heterotrophic denitrification consumes acid. There was a drop in pH of 0.2–0.3 throughout the hypolimnion from LCN addition (15 Jun) until depletion of NO3− (18 Aug). Lake Ann did not undergo a midsummer depression in pH. There was a significant difference (P < 0.0001) between the Round 2010 median hypolimnetic pH (6.4) and the historic median (7.8) during 16 June–18 August 1997, 2001, 2002, 2003, 2004, 2008, and 2009.
DNRA may also have been an NO3− sink. Under conditions of NO3− limitation, bacteria cell growth yield and free energy available for respiration is higher for DNRA than for heterotrophic denitrification (Strohm et al. Citation2007). Four Round Lake hypolimnion samples of NH4+ were 2.6 mg/L or greater at redox values of +175 mV or higher () on dates between NO3− dose and depletion. Otherwise, all high concentration NH4+ values followed the asymptotic functional relationship with low redox values exhibited by Fe, Mn, and Ortho-P (, 4, and 9).
Although results suggest DNRA, it is difficult to distinguish in Round Lake from similar hypolimnetic NH4+ efflux in Lake Ann, in which there was no NO3− to drive DNRA. In Lake Ann, patterns of change in hypolimnion soluble and total Fe and Mn were identical throughout the study period (), indicating that the source for both was solubilization from sediments. In Round Lake, the drop in soluble Fe as total Fe increased might suggest NO3− oxidation of Fe(II) (Benz et al. Citation1998). Round Lake hypolimnetic Mn(II) did drop slightly concurrently with the drop in Fe(II), but oxidation of Mn(II) is strictly aerobic (Frankel and Bazylinski Citation2003, Burgin and Hamilton Citation2008) and can be ruled out. Co-precipitation of Mn(II) with Fe(III)is the likely cause of Mn(II) loss (Davison Citation1993, Hongve Citation1997). No storm or other high wind velocity event occurred that could have entrained hypolimnion waters and oxidized Fe or Mn. Regardless of mechanisms, results and thermodynamics () indicate anaerobic Fe(II) oxidation was a minor sink for NO3−.
Redox dynamics
Production of HgCH3+ and in Lake Ann occurs as SO42− nears depletion at low redox values ( and 3). There is an obvious inverse relationship between SO42− depletion and solubilization of Fe, Mn, and Ortho-P (). Using Fe as a model, reduction of SO42− produces H2S, lowering redox values (). In turn, H2S will abiotically reduce Fe and Mn, reducing competition to SRB from Fe- and Mn-reducing bacteria, which produces more H2S, reducing yet more Fe and Mn, and so on, until depletion of SO42− limits H2S production. Observed Fe, Mn, and Ortho-P solubilization is a classic positive feedback relationship. In Lake Ann, hypolimnetic SO42− reached maximum depletion by early August (), creating enriched H2S conditions at the sediment surface that drove HgCH3+ production for the rest of the summer (). Based on these data, observed HgCH3+ production was apparently part of a larger redox system characterized by strong depletion of SO42− and functionally linked to nutrient and metals mobilization from sediments. In Round Lake, the sharp jump in HgCH3+ concentrations (0.35–1.1 ng/L) occurred after depletion of NO3− and as SO42− reduction recommenced ( and 7). Thus, the NO3− dose to the hypolimnion apparently had the ultimate effect of stimulating HgCH3+ production via SO42− enrichment of the hypolimnion by NO3− oxidation of sulfidic compounds. Experimental design required NO3− depletion prior to turnover to observe NO3− sinks. To suppress hypolimnetic HgCH3+ production, however, study findings indicate that it is essential either to maintain hypolimnetic NO3− concentrations above a minimum value until turnover elevates hypolimnetic DO or to prevent hypolimnetic SO42− reduction by other means (e.g., oxygenation or chemical oxidant injection).
Evidence from this study and the literature indicate sediment SRB rapidly utilize NO3− as a terminal electron acceptor. Application of NO3− to sewers is a well-known method to suppress odors via NO3− oxidation of H2S (Mathioudakis et al. Citation2006), where it must act rapidly to be effective. In a laboratory simulation of sewer mains, Mohanakrishnan et al. (2009) observed induction of anaerobic H2S oxidation within 10 hours of exposure to NO3−. In Round Lake, redox increased at an approximate linear rate of +1.9 mV/h in the first 48 hours after application, which demonstrates a similarly rapid system response to NO3−.
Nitrate dose
Timing of the NO3−dose was more than a month later than intended. Ice-out in early May presented the first opportunity to dose NO3−, but logistical problems delayed application. It is not clear if timing would have changed results because limited winter data suggest that Round Lake may be permanently anoxic at deepest depths.
The observed rate of NO3− depletion of 2.8 g/m2/d was 67% of the estimated rate, suggesting that the dose was conservative. Potential causes of the underestimated error merit attention. SCOD may have been lower than assumed, isolation chamber placements in the SOD study may not have accurately captured overall SOD, or the difference may be an artifact of the isolation chamber method itself. Mixers circulate water in the chambers at a mean velocity of 3 cm/s. Velocity of water over sediments increases observed SOD by shear of diffusion boundary layers (Murphy and Hicks Citation1986, Gantzer et al. 2009b). There was no measurement of benthic boundary layer velocities in Round Lake. If seiche-driven velocities across the bottom averaged <3 cm/s, then the actual SOD may be lower than measured SOD.
The present study partially achieved a central goal of accurate determination of the NO3− dose. Matthews et al. (Citation2013) determined dosing criterion by analysis of NO3− depletion curves observed in the hypolimnion of Lake Onondaga. Source of the NO3− was discharge of nitrified effluent from a regional wastewater treatment facility. Where an NO3− depletion rate is unavailable, the SOD method of determining the NO3− dose seems to be conservative.
From a management perspective, it is essential to know how much NO3− is sufficient to suppress formation of HgCH3+. Although NO3− concentrations were >0.5 mg/L (15 Jun–18 Aug), redox values were substantially greater than values associated with HgCH3+ production and detection of H2S odors (, 5, 6, and 7). Maintenance of NO3− at 0.5 mg/L or higher, however, is not necessarily high enough to prevent formation of HgCH3+.
Rates of SO42− reduction are highly temperature dependent, approximately doubling from 0 to 12 C and every 10 C thereafter (Ingvorsen et al. Citation1981). During the study, the temperature in the hypolimnion of Round Lake was approximately 8 C. At higher water temperatures, higher NO3− concentrations in the water column may be necessary to suppress HgCH3+. In addition, SO42− in Round Lake and Lake Ann was <5 mg/L. Lakes with higher SO42− concentrations may require higher NO3− concentrations to suppress SRB.
These qualifications suggest that the NO3− concentration needed to suppress SRB, and thus HgCH3+ production, is likely be specific to geochemistry and physics of individual lakes (e.g., weak vs. strong seiches, sediment pore water vertical transport via ebullition, stratification dynamics, SOD, etc.). Mass flux of NO3− (or O2) from the water column into surficial sediments, whether diffusive or advective in nature, must overcome mass flux of H2S from sediments to the water column to maintain an oxidizing environment in the lake bottom water. A “rule of thumb” NO3− concentration to suppress SRB is not advisable.
Redox monitoring for methylmercury management
Redox monitoring may aid determination of NO3− concentration thresholds critical for lake managers to suppress HgCH3+ production. Redox probes figured prominently in assessing geochemical state conditions within the hypolimnia of Round Lake and Lake Ann and merit detailed discussion. A redox probe is a millivolt meter. Snoeyink and Jenkins (Citation1980) contend that a millivolt meter measures little, if anything, of significance in water chemistry. The US Geological Survey states that use of redox electrodes in water quality monitoring “is not recommended in general because of the difficulties inherent in its theoretical concept and its practical measurement” (Nordstrom and Wilde Citation2005). A redox probe does not measure water chemistry; rather it measures the effect of water chemistry on the millivolt probe, namely electron flow between redox couples; therefore, redox values have little meaning without corroborating data.
Interpretation of redox monitoring is further complicated by a wide range of response times across various probe types (Jang et al. Citation2005). Moreover, dependent on time of exposure, the drift in probe readings is sufficient to create large differences between in situ and manually deployed probes of the same type and in the same location (YSI Environmental 2005). The technical literature further confounds interpretation of redox data when it fails to state reference electrodes and calibration points.
Despite these issues, redox probes are important tools in wastewater operations as inputs to automated reactor controls for denitrifying systems (Wareham et al. 1993b, Demoulin et al. Citation1997, Zhao et al. Citation1999). Operational research establishes reliable empirical relationships between redox operating points and process goals. The NO3− breakpoint is a redox value associated with the disappearance of NO3− commonly and reliably used for real-time controls in wastewater operations (Wareham et al. 1993a, Chen et al. Citation2002, Kim et al. Citation2004).
These principles extend to hypolimnion management. Mere anoxia does not necessarily indicate HgCH3+ production or SO42− reduction ( and 4), both of which functionally relate to redox and not DO ( and 5). Redox is the only real-time monitoring tool that can assess state conditions within the hypolimnion that generate or suppress HgCH3+.
As demonstrated by this study, there are clear breakpoints for HgCH3+ production and SO42− reduction. A positive feedback process as function of redox characterizes solubilization of Mn, Fe, and Ortho-P production ( and ). The probable feedback mechanism comes from abiotic reduction of Mn(IV) and Fe(III) by H2S produced in deeper sediment layers (Thamdrup et al. Citation1993), which then removes substrate competition for SRB, which then produce more H2S/HS−, and so on, until SO42− is close to depletion ( and 4). Redox monitoring would allow real-time management of NO3− dosing to prevent HgCH3+ production by keeping the hypolimnion above the HgCH3+ production breakpoint or higher (e.g., redox values such as the SO42− reduction breakpoint), preventing positive feedback.
Per the redox monitoring protocols of this study, the HgCH3+ production breakpoint was apparently +60 mV (), suggesting that the HgCH3+ production redox breakpoint may be higher. Detection of mild H2S− odor began at approximately +200 mV and strong odor at about +130 mV. These numerical redox values will change with differing redox protocols of a particular study and the mix of redox-active solutes in a given hypolimnion. What is unlikely to change is the existence of functionally similar breakpoints for HgCH3+ production. Lake or reservoir managers can establish protocol and site-specific redox values for these breakpoints to monitor or manage water quality.
Biogeochemical system dynamics
The asymptotic increase in solubilization of NH4+, Ortho-P, Fe(II), and Mn(II) as function of drop in redox merits closer attention (, 4, and 9). As a function of time, the increase in these solutes is exponential (). As Lake Ann hypolimnetic anoxia intensified over the summer, there was a nonlinear increase of redox-active solutes.
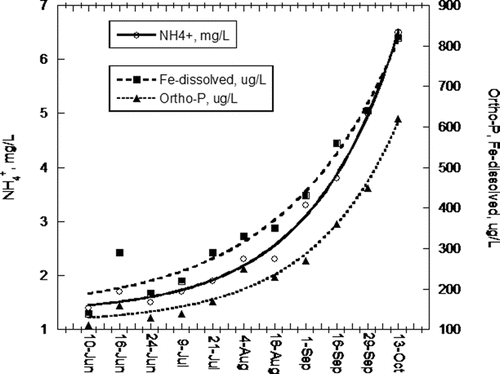
The increases in Fe(II) and Ortho-P concentrations follow established trends in anoxic hypolimnia as a consequence of sediment–water interaction (Mortimer Citation1942). Insoluble Fe(III) PO43− dissolves and releases PO43− previously bound in Fe(OH)3 complexes. The positive feedback dynamics of this process as controlled by SO42−, such as in the present study, have been observed since the mid-20th century (Hupfer and Lewandowski Citation2008).
An explanation for the same pattern of NH4+ efflux is not as straightforward because there is no shift in redox or solubility of N species. NH4+ is highly soluble, and there was obviously no pool of NO3− in sediments that might reduce to NH4+ via DNRA. A strong linear correlation (R2 = 0.97) between hypolimnetic Fe(II) and NH4+ seems to link Fe(III) reduction and Fe(II) efflux to NH4+ effluent ().
A reasonable hypothesis for the NH4+ efflux from sediments is ion exchange. A large fraction of NH4+ in sediments is adsorbed to organic material and highly exchangeable (Rosenfeld Citation1979, Seitzinger et al. Citation1991). Fe(II) and Mn(II) are divalent cations produced by low redox conditions that will displace loosely bound monovalent cations such as NH4+. Thus, the strong correlation of Fe(II) and Ortho-P efflux to NH4+ efflux () is probably causal rather than coincidental. Investigation of this hypothesis would entail measurement of sediment pore-water chemistry under study conditions.
The potential insight that NH4+ efflux provides to HgCH3+ efflux lies in similar redox-influenced ion exchange dynamics. Mass effects and charge are fundamental to ion exchange. When salinity is low, small increases in salinity dramatically increase efflux of NH4+ from sediments (Hou et al. Citation2003). Like ammonium, HgCH3+ preferentially binds to organic matter in sediments (Feyte et al. Citation2010). As monovalent cations, both are subject to similar ion-exchange dynamics. Salinity, as measured by specific conductivity (mS/cm), increased in the hypolimnion of Lake Ann as redox decreased. From +353 to +108 mV, salinity significantly (P < 0.002) increased by 1.9%, and by 2.8% (P < 0.0002) from +108 to +38 mV. The increase in salinity may seem small. Assuming a standard conversion factor of 0.67 (mg/L)/(mS/cm), these changes in salinity are 6.2 and 9.3 mg/L, respectively. Note the salinity concentration scale is parts per million, and the scale for HgCH3+ is parts per trillion; a concentration difference on the order of 106 has a powerful effect on ion exchange. If ion exchange substantially drives NH4+ efflux at low redox, then similar physical chemistry is likely significant to HgCH3+ efflux.
There seems to be an operational balance point for LCN application. Although there are legitimate concerns from the regulatory community that excess hypolimnion NO3− at fall turnover would flow downstream, evidence from this study indicates the need to maintain a sufficient hypolimnetic NO3− residual until fall turnover raises DO to the sediment surface. Without this residual, the net effect of LCN treatment in waters where HgCH3+ production is SO42− limited might be to enrich, rather than suppress, hypolimnetic HgCH3+ concentrations. NO3− oxidation of sulfidic minerals increases the hypolimnetic SO42− concentration, stimulating SRB activity if not suppressed by NO3− or DO. Hypolimnetic redox monitoring and small, frequent doses of LCN seem to be a reasonable management method to maintain this balance.
Although only peripherally within the scope of the present study, LCN control of Ortho-P efflux from sediments merits brief mention. Redox dynamics drive metal (Fe, Mn), nutrient (Ortho-P, NH4+), and HgCH3+ efflux from sediments. Maintaining the hypolimnion above a critical redox breakpoint will suppress these effluxes, strongly evidenced by hypolimnetic oxygenation projects (Beutel et al. Citation2008a, 2008b, 2014, Gantzer et al. 2009a, Dent et al. Citation2014). From a management perspective, redox controls sufficient to suppress HgCH3+ would benefit nutrient and metals management.
This study demonstrated that dosing of NO3− suppresses HgCH3+ production within an anoxic hypolimnion. Maintaining NO3− below known LOEC values while suppressing HgCH3+ production was readily accomplished. Whether suppression of hypolimnetic HgCH3+ formation translates into reduced Hg content of fish tissue is an important question that future studies should address. For lakes where HgCH3+ of hypolimnion origin is an important source of Hg in fish tissue, NO3− dosing of the hypolimnetic merits investigation as a means to remediate Hg impairment.
Funding
This study was funded by the Riley Purgatory Bluff Creek Watershed District.
References
- Barbash J. 2002. Methods for collecting data on hydrogen sulfide in ground water technical memorandum. Technical Memorandum, United States Geological Survey, September 2, 2002. http://ar.water.usgs.gov/nawqa/hydrogen.sulfide/H2S_coding+method_descr.doc.
- Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL. 2003. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In: Biogeochemistry of environmentally important trace elements. Washington (DC): American Chemical Society. p. 262–297.
- Benz M, Brune A, Schink B. 1998. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch Microbiol. 169:159–165.
- Beutel M, Dent S, Reed B, Marshall P, Gebremariam S, Moore B, Cross B, Gantzer P, Shallenberger E. 2014. Effects of hypolimnetic oxygen addition on mercury bioaccumulation in Twin Lakes, Washington, USA. Sci Total Environ. 496:688–700.
- Beutel MW, Horne AJ. 1999. A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality. Lake Reserv Manage. 5:285–297.
- Beutel MW, Horne AJ, Taylor WD, Losee RF, Whitney RD. 2008a. Effects of oxygen and nitrate on nutrient release from profundal sediments of a large, oligo-mesotrophic reservoir, Lake Mathews, California. Lake Reserv Manage. 24:18–29.
- Beutel MW, Leonard TM, Dent SR, Moore BC. 2008b. Effects of aerobic and anaerobic conditions on P, N, Fe, Mn, and Hg accumulation in waters overlaying profundal sediments of an oligo-mesotrophic lake. Water Res. 42:1953–1962.
- Boening DW. 2000. Ecological effects, transport, and fate of mercury: a general review. Chemosphere. 40:1335–1351.
- Boszke L, Kowalski A, Gosiska G, Szarek R, Siepak J. 2003. Environmental factors affecting the speciation of mercury in the bottom sediments; an overview. Polish J Environ Studies. 12:5–13.
- Burgin AJ, Hamilton SK. 2008. NO3−-driven SO42− production in freshwater ecosystems: implications for N and S cycling. Ecosystems. 11:908–922.
- Chen K-C, Chen C-Y, Peng J-W, Houng J-Y. 2002. Real-time control of an immobilized-cell reactor for wastewater treatment using ORP. Water Res. 36:230–238.
- Christophoridis C, Fytianos K. 2006. Conditions affecting the release of phosphorus from surface lake sediments. J Environ Qual. 35:1181–1192.
- Compeau GC, Bartha R. 1985. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 50:498–502.
- Cooke GD, Welch EB, Peterson SA, Nichols SA. 2005. Restoration and management of lakes and reservoirs, 3rd ed. New York (NY): Taylor & Francis.
- Corrales J, Naja GM, Dziuba C, Rivero RG, Orem W. 2011. Sulfate threshold target to control methylmercury levels in wetland ecosystems. Sci Total Environ. 409:2156–2162.
- Davison W. 1993. Iron and manganese in lakes. Earth-Sci Rev. 34:119–163.
- DeLaune RD, Jugsujinda A, Devai I, Patrick WH. 2004. Relationship of sediment redox conditions to methyl mercury in surface sediment of Louisiana lakes. J Environ Sci Heal A. 39:1925–1933.
- Demoulin G, Goronszy MC, Wutscher K, Forsthuber E. 1997. Co-current nitrification/ denitrification and biological p-removal in Cyclic Activated Sludge plants by redox controlled cycle operation. Water Sci Technol. 35:215–224.
- Dent SR, Beutel MW, Gantzer P, Moore BC. 2014. Response of methylmercury, total mercury, iron and manganese to oxygenation of an anoxic hypolimnion in North Twin Lake, Washington. Lake Reserv Manage. 30:119–130.
- Driscoll CT, Han Y-J, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK. 2007. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience. 57:17–28.
- Eckley CS, Watras CJ, Hintelmann H, Morrison K, Kent AD, Regnell O. 2005. Mercury methylation in the hypolimnetic waters of lakes with and without connection to wetlands in northern Wisconsin. Can J Fish Aquat Sci. 62:400–411.
- Effler SW, Matthews DA. 2008. Implications of redox processes for the rehabilitation of an urban lake, Onondaga Lake, New York. Lake Reserv Manage. 24:122–138.
- Environment Canada. 2003. Canadian water quality guidelines for the protection of aquatic life - nitrate ion. Hull (Quebec): Environment CCoMot.
- Evers D, Wiener J, Basu N, Bodaly R, Morrison H, Williams K. 2011. Mercury in the Great Lakes region: bioaccumulation, spatiotemporal patterns, ecological risks, and policy. Ecotoxicology. 20:1487–1499.
- Feyte S, Tessier A, Gobeil C, Cossa D. 2010. In situ adsorption of mercury, methylmercury and other elements by iron oxyhydroxides and organic matter in lake sediments. Appl Geochem. 25:984–995.
- Frankel RB, Bazylinski DA. 2003. Biologically induced mineralization by bacteria. Rev Mineral Geochem. 54:95–114.
- Gantzer PA, Bryant LD, Little JC. 2009a. Controlling soluble iron and manganese in a water-supply reservoir using hypolimnetic oxygenation. Water Res. 43:1285–1294.
- Gantzer PA, Bryant LD, Little JC. 2009b. Effect of hypolimnetic oxygenation on oxygen depletion rates in two water-supply reservoirs. Water Res. 43:1700–1710.
- Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA, Bailey KL, Elias DA. 2013. Mercury methylation by novel microorganisms from new environments. Environ Sci Technol.
- Hamelin Sp, Amyot M, Barkay T, Wang Y, Planas D. 2011. Methanogens: principal methylators of mercury in Lake Periphyton. Environ Sci Technol.
- Holmer M, Storkholm P. 2001. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshwater Biol. 46:431–451.
- Hongve D. 1997. Cycling of iron, manganese, and phosphate in a meromictic lake. Limnol Oceanogr. 42:635–647.
- Hou L, Liu M, Jiang H, Xu S, Ou D, Liu Q, Zhang B. 2003. Ammonium adsorption by tidal flat surface sediments from the Yangtze Estuary. Environ Geol. 45:72–78.
- Hupfer M, Lewandowski J. 2008. Oxygen controls the phosphorus release from lake sediments – a long-lasting paradigm in limnology. Int Rev Hydrobiol. 93:415–432.
- Ingvorsen K, Zeikus JG, Brock TD. 1981. Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl Environ Microbiol. 42:1029–1036.
- Jang A, Lee J-H, Bhadri PR, Kumar SA, Timmons W, Beyette FR, Papautsky I, Bishop PL. 2005. Miniaturized redox potential probe for in situ environmental monitoring. Environ Sci Technol. 9:6191–6197.
- Johansson K, Bergbäck B, Tyler G. 2001. Impact of atmospheric long range transport of lead, mercury and cadmium on the Swedish forest environment. Water Air Soil Pollut Focus. 1:279–297.
- Kim J-H, Chen M, Kishida N, Sudo R. 2004. Integrated real-time control strategy for nitrogen removal in swine wastewater treatment using sequencing batch reactors. Water Res. 38:3340–3348.
- Lide DR. 2007. Standard thermodynamic properties of chemical substances. CRC Handbook of Chemistry and Physics. 5.
- Mathioudakis VL, Vaiopoulou E, Aivasidis A. 2006. Addition of nitrate for odor control in sewer networks: laboratory and field experiments. Global NEST J. 8:37–42.
- Matthews DA, Babcock DB, Nolan JG, Prestigiacomo AR, Effler SW, Driscoll CT, Todorova SG, Kuhr KM. 2013. Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake, NY. Environ Res. 125:52–60.
- Metcalf and Eddy. 2003. Wastewater Engineering, Treatment and Reuse, 4th ed. Boston (MA): McGraw Hill.
- Mohanakrishnan J, Gutierrez O, Sharma KR, Guisasola A, Werner U, Meyer RL, Keller J, Yuan Z. 2009. Impact of nitrate addition on biofilm properties and activities in rising main sewers. Water Res. 43:4225–4237.
- Monson B, Heiskary SA. 2008. Minnesota national lakes assessment project:water mercury concentrations in Minnesota lakes. St. Paul (MN): Minnesota Pollution Control Agency.
- Morrison H. 2011. The Canadian clean air regulatory agenda mercury science program. Ecotoxicology. 20:1512–1519.
- Mortimer CH. 1942. The exchange of dissolved substances between mud and water in lakes. J Ecol. 30:147–201.
- Murphy PJ, Hicks DB, editors. 1986. In-situ method for measuring sediment oxygen demand. Athens (GA): University of Georgia, Institute of Natural Resources.
- Noonan TA. 1986. Water quality in long lake, Minnesota, following riplox sediment treatment. Lake Reserv Manage. 2:131–137
- Nordstrom DK, Wilde FD. 2005. Reduction-oxidation potential (electrode method). US Geological Survey.
- Odum HT, Hoskins CM. 1958. Comparative studies on the metabolism of marine waters. Institute of Marine Science. p. 65–96.
- Parks JM, Johs A, Podar M, Bridou R, Hurt RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC. 2013. The genetic basis for bacterial mercury methylation. Science. 339:1332–1335.
- Paterson MJ, Blanchfield PJ, Podemski C, Hintelmann HH, Gilmour CC, Harris R, Ogrinc N, Rudd JW, Sandilands KA. 2006. Bioaccumulation of newly deposited mercury by fish and invertebrates: an enclosure study using stable mercury isotopes. Can J Fish Aquat Sci. 63:2213–2224.
- Regnell O, Tunlid A. 1991. Laboratory study of chemical speciation of mercury in lake sediment and water under aerobic and anaerobic conditions. Appl Environ Microbiol. 57:789–795.
- Ripl W. 1978. Oxidation of lake sediments with nitrate - a restoration method for former recipients. Lund (Sweden): Lund University.
- Rosenfeld JK. 1979. Ammonium adsorption in nearshore anoxic sediments. Limnol Oceanogr. 24:356–364.
- Scherbatskoy T, Shanley J, Keeler G. 1998. Factors controlling mercury ttransport in an upland forested catchment. Water Air Soil Pollut. 105:427–438.
- Seitzinger S, Gardner W, Spratt A. 1991. The effect of salinity on ammonium sorption in aquatic sediments: implications for benthic nutrient recycling. Estuaries. 14:167–174.
- Shih R, Robertson WD, Schiff SL, Rudolph DL. 2011. Nitrate controls methyl mercury production in a streambed bioreactor. J Environ Qual. 40:1586–1592.
- Snoeyink VL, Jenkins D. 1980. Water Chemistry. New York (NY): John Wiley & Sons.
- Strohm TO, Griffin B, Zumft WG, Schink B. 2007. Growth yields in bacterial denitrification and nitrate ammonification. Appl Environ Microbiol. 73:1420–1424.
- Thamdrup B, Finster K, Hansen JW, Bak F. 1993. Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron or manganese. Appl Environ Microbiol. 59:101–108.
- Todorova SG, Driscoll CT, Matthews DA, Effler SW, Hines ME, Henry EA. 2009. Evidence for regulation of monomethyl mercury by nitrate in a seasonally stratified, eutrophic lake. Environ Sci Technol. 43:6572–6578.
- Ullrich SM, Tanton TW, Abdrashitova SA. 2001. Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Env Sci Tech. 31:241–293.
- [UNEP] United Nations Environment Programme. 2013. Report of the intergovernmental negotiating committee to prepare a global legally binding instrument on mercury on the work of its fifth session. Program UNE, New York (NY).
- [US EPA] US Environmental Protection Agency. 2001. Water quality criteria for the protection of human health: methylmercury. Washington (DC): Office of Science and Technology, US EPA, Office of Water.
- [US EPA] US Environmental Protection Agency. 2009. Sediment oxygen demand.
- Wareham D, Hall K, Mavinic D. 1993a. Real‐time control of aerobic–anoxic sludge digestion using ORP. J Environ Eng. 119:120–136.
- Wareham DG, Hall KJ, Mavinic DS. 1993b. Real-time control of wastewater treatment systems using ORP. Water Sci Technol. 28:273–282.
- Watras CJ, Backa RC, Halvorsen S, Hudson RJMA, Morrisona KA, Wente SP. 1998. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 219:183–208.
- Watras CJ. 2009. Mercury pollution in remote freshwaters. In: Encyclopedia of inland waters. p. 100–109.
- Willendbring P, Miller M, Weidenbacherm W. 1983. Reducing sediment phosphorus release rate in Long Lake through the use of calcium nitrate Third Nation Conference of the North American Lake Management Society. Knoxville (TN): US Environmental Protection Agency.
- Woods TL, Garrels RM. 1987. Thermodynamic values at low temperature for natural inorganic materials: an uncritical summary. New York: Oxford University Press.
- YSI Environmental. 2005. Measuring ORP on YSI 6-Series Sondes: tips, cautions and limitations.
- Zananski T, Holsen T, Hopke P, Crimmins B. 2011. Mercury temporal trends in top predator fish of the Laurentian Great Lakes. Ecotoxicology. 20:1568–1576.
- Zhao HW, Mavinic DS, Oldham WK, Koch FA. 1999. Controlling factors for simultaneous nitrification and denitrification in a two-stage intermittent aeration process treating domestic sewage. Water Res. 33:961–970.