Abstract
Sia ME, Doyle RM, Moser KA. 2020. Recent trends in mountain lake primary production: evaluating the response to fish stocking relative to regional environmental stressors. Lake Reserv Manage. XX:XXX–XXX.
Although mountain lakes are often remote, they are impacted by a myriad of stressors, including species introductions, atmospheric fertilization, and climate change. These stressors have the potential to increase primary production, which can threaten mountain lakes by decreasing water quality, reducing species richness, and lowering dissolved oxygen concentrations. The relative importance of these stressors as drivers of production is not well understood. We therefore investigated the importance of fish stocking, relative to other stressors, as a driver of primary production in mountain lakes. Our research focused on Uinta Mountain lakes, which, like many lakes in the western United States, were stocked with salmonid fish in the 1950s. In 2 stocked lakes and one unstocked lake, we reconstructed a record of ∼300 yr of primary production from lake sediments using changes in percentage of organic matter and spectrally inferred concentrations of chlorophyll a and its derivatives. With long-term trends in primary production, we determined that all 3 study lakes, including the unstocked lake, show unprecedented increases in primary production beginning in the 1950s. We attribute recent increases in primary production mainly to atmospheric deposition of nitrogen and climate warming, but do not rule out future impacts of fish stocking. Our article demonstrates how breakpoint analysis can be used to identify the most serious threats to mountain lakes, even if those lakes are impacted by multiple stressors or stressors originating from distant places.
Nitrogen (N) and phosphorus (P) inputs to lakes have increased dramatically around the world (Galloway et al. Citation2008, Mahowald et al. Citation2008, Stoddard et al. Citation2016, Mahowald et al. Citation2017). Such nutrient inputs, as well as climate warming and fish introductions, enhance algal growth and drive lake eutrophication, threatening freshwater ecosystems (Schindler et al. Citation2001, Smol et al. Citation2005, Smol Citation2008). Eutrophication, or excessive primary production, can result in decreased light penetration, lower dissolved oxygen concentrations, loss of biodiversity, more frequent algal blooms, increased toxicity, and fish kills (Schindler et al. Citation2008). Since eutrophication is caused by multiple drivers working simultaneously and synergistically, reversing the damage of eutrophication is difficult (Jeppesen et al. Citation2000, Coveney et al. Citation2005, Søndergaard et al. Citation2007, Gąsiorowski and Sienjiewicz Citation2013). Consequently, studies identifying the most important drivers of eutrophication on lakes are urgently needed so lake managers can develop efficient strategies for addressing this problem.
Our research investigates the importance of fish stocking on primary production relative to other drivers of eutrophication, such as atmospheric deposition of nutrients and climate warming. We focus on mountain lakes since they are found worldwide and are typically oligotrophic, making them particularly sensitive to increases in nutrient inputs (Moser et al. Citation2019). Owing to the remote locations of many mountain lakes, increased nutrient inputs and primary production at these sites is often attributed to atmospheric transport and deposition of nutrients (Bergström and Jansson Citation2006, Hundey et al. Citation2014, Brahney et al. Citation2015). Climate warming can increase primary production at mountain sites through complex interactions between air temperatures, snow pack, water temperatures, and nutrients (Sadro et al. Citation2018, Jiménez et al. Citation2019). Local perturbations, including fish stocking, can also increase primary production (Carpenter et al. Citation1985, Leavitt et al. Citation1994). High mountain lakes are often naturally fishless because they are isolated from lower elevation streams by physical barriers (e.g., waterfalls), preventing natural fish colonization. In western North America, it is estimated that ∼95% of mountain lakes were historically fishless. Over time, 60% of western mountain lakes and 95% of sites deeper than 3 m and larger than 2 ha were stocked with nonnative fish (Bahls Citation1992). Fish stocking can increase algal production through 2 mechanisms: (1) indirectly through size-selective grazing (Carpenter and Kitchell Citation1993, Carlisle and Hawkins Citation1998, Schabetsberger et al. Citation2009), and (2) directly through the transfer of nutrients from benthic and terrestrial sources to the pelagic zone (Leavitt et al. Citation1994, Schindler et al. Citation2001, Vanni Citation2002). In the first case, primary production is increased by size-selective predation by fish on large herbivorous zooplankton, which reduces grazing of phytoplankton (Carpenter and Kitchell Citation1993, Carlisle and Hawkins Citation1998). In the second case, fish predation on benthic and terrestrial prey followed by excretion increases the supply of nutrients for pelagic algae (Carpenter et al. Citation1992, Schindler et al. Citation1993, Vanni Citation1996, Schaus et al. Citation1997, Schindler et al. Citation2001). Because algal production in oligotrophic lakes is likely more strongly limited by nutrient supply than by the intensity of size-selective grazing, we focus our research on the former, the addition of new nutrient sources (Neill and Peacock Citation1980, Elser et al. Citation1990, Schindler et al. Citation2001).
Although fish stocking, atmospheric deposition, and climate change have been shown to increase primary production in mountain lakes, the relative importance of these factors remains unclear. For example, in the Sierra Nevada, P-loading from trout was 7.4 mg/m2/yr and P-loading in rain was 3.5 mg/m2/yr, meaning that fish would contribute twice as much P as was derived from atmospheric loading (Schindler et al. Citation2001). However, as Schindler et al. (Citation2001) report, when atmospheric P inputs arriving via the catchment were included and assumed to be completely transferred to the lake, the catchment-scale P-regeneration by trout was only 13% of the total P-load. Furthermore, these estimates did not include P in dust, which in the Sierra Nevada was estimated to be 14 mg/m2/yr (Vicars et al. Citation2010). Less information is available for comparison between different sources of N, but fish contributions to N-load are likely less important than they are to P-load (Vanni Citation2002, Lyons et al. Citation2016).
We investigated the relative influence of fish stocking, climate warming, and atmospheric deposition on temporal changes in algal production. Primary production over 300 yr in 3 lakes in the Uinta Mountains, Utah, USA, was reconstructed using paleolimnological methods. The 3 lakes include: (1) a deep (17 m) stocked lake; (2) a shallow (3 m) stocked lake; and (3) a shallow (3 m) unstocked lake. With the paleolimnological reconstruction of algal production and a historic record of consequential local and regional disturbances, we addressed the following important questions: (1) Has fish stocking increased primary production in the studied mountain lakes? (2) Have other stressors, including climate change and atmospheric deposition, altered primary production? (3) How important is fish stocking compared to other stressors in affecting primary production? (4) Does primary production differ between shallow and deep stocked lakes? Addressing these questions contributes knowledge on the causes of eutrophication necessary for lake managers to make informed decisions in developing effective strategies and targeting lakes most at risk.
Study site
The Uinta Mountains
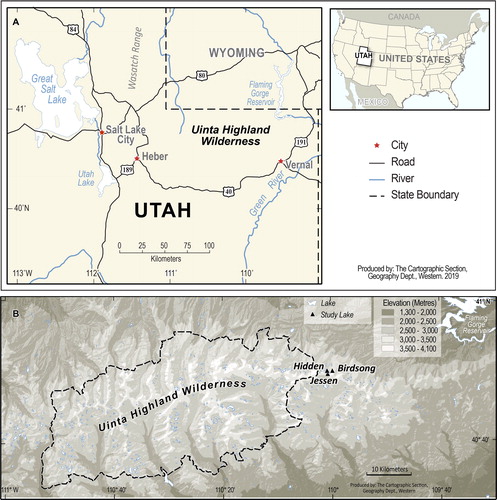
The Uinta Mountain range extends east–west 150 km, from northeastern Utah to northwestern Colorado (). The range reaches a maximum elevation of >4000 m above sea level. The geology of the Uinta Mountains is relatively consistent and comprised predominantly of late Precambrian quartz arenites (Condie et al. Citation2001). The Uinta Mountain range has a continental climate (hot, dry summers and cold, wet winters) and hundreds of alpine and subalpine lakes formed during the retreat of Pleistocene glaciations (Munroe et al. Citation2007).
Atmospheric deposition of nutrients in Uinta Mountain lakes
Atmospheric deposition of N is occurring in many mountain regions of the western United States, including the Uinta Mountains (Baron et al. Citation2000, Nanus et al. Citation2008, Nanus et al. Citation2012, Spaulding et al. Citation2015, Hundey et al. 2016). Because N can be the limiting nutrient in these regions, increased N led to higher primary production (Hundey et al. 2014). A study of 6 alpine lakes showed that N deposition increased in the Uinta Mountains as early as ca. 1850 and increased rapidly after ca. 1950 (Hundey et al. Citation2014). Seventy percent of the N transported to these alpine lakes arrived atmospherically from anthropogenic activities, mainly agriculture (Hundey et al. 2016). Through regional increases in phosphate mining and agriculture, P deposition in dust may have contributed to increased P in mountain lakes, which can also lead to increased primary production (Psenner Citation1999, Sickman et al. Citation2003, Ballantyne et al. Citation2011, Brahney et al. Citation2014, Citation2015, Stoddard et al. Citation2016). As settlement in the western United States expanded in the late 19th century, P increased in 2 Uinta Mountain lakes, owing to increased dust production (Reynolds et al. Citation2010).
History of fish stocking in Uinta Mountain lakes
Some lakes in the Uinta Mountains may have been unofficially stocked using horses before the 1950s, and aerial stocking in the Uinta Mountains began in 1955 (Hallows T. Utah DWR, 2009, unpubl. data). In the Uinta Mountains, many mountain lakes have been stocked with trout, mainly brook trout (Salvelinus fontinalis), cutthroat trout (Oncorhynchus clarkii), and rainbow trout (Oncorhynchus mykiss). Trout species are opportunistic feeders, consuming benthic (e.g., chironomids) and terrestrial (e.g., insects) organisms. Through these feeding patterns, trout excretion transfers P nutrients from benthic and terrestrial ecosystems to pelagic ecosystems (Schindler et al. Citation1993, Vanni Citation1996, Citation2002, Schaus et al. Citation1997).
Study lakes
All study lakes are located below treeline, near the subalpine/alpine transition, within the Middle Fork Sheep Creek drainage basin on the north slope of the Uinta Mountains (). The area immediately adjacent to the lakes is closed coniferous forest, but for all 3 lakes much of the catchment is comprised of steep talus slopes. This drainage basin was chosen because the fish stocking histories of all study lakes were relatively well known. To examine the effect of lake depth on primary production in response to atmospheric deposition and fish stocking, 2 shallow lakes (∼3 m), Birdsong and Hidden, and one deep lake (17 m), Jessen, were chosen (). Birdsong Lake and Hidden Lake do not stratify during summer and are well mixed, whereas Jessen Lake is dimictic. All 3 lakes have similar ratios of catchment to lake area (, ).
Figure 2. Plan view of the area within the Middle Fork Sheep Creek drainage basin where Hidden, Birdsong, and Jessen lakes are located. Gray shading surrounding the lake area indicates the estimated catchment area for the lake (40 ft is approximately 12 m).
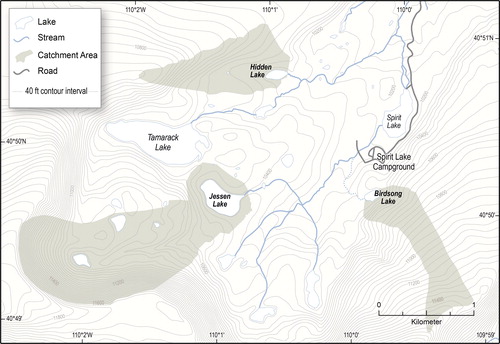
Table 1. Location and physical variables of study lakes.
The control study lake, unofficially named Birdsong Lake, has never been stocked, and past fish surveys conducted by the Utah Division of Wildlife indicated no fish were found in the lake. Birdsong Lake has a spring that intermittently flows into it just below the talus slope on the south side and no or intermittent outflow. It was classified as oligo-mesotrophic and N-limited ().
Table 2. Water chemistry variables for study lakes.
Based on Utah Division of Wildlife records, Hidden Lake was fishless before the 1950s. It has been officially stocked since at least 1966 (; High Uinta Lake Survey, Utah DWR, Aug. 1966, unpubl. data). Hidden Lake has a small outflow channel, and water can be heard entering the lake at the western end under a hummocky talus feature. This lake was meso-eutrophic and N-limited ().
Figure 3. History of fish stocking in Hidden and Jessen lakes in the Sheep Creek drainage (High Uintas Lake Survey, unpubl. data).
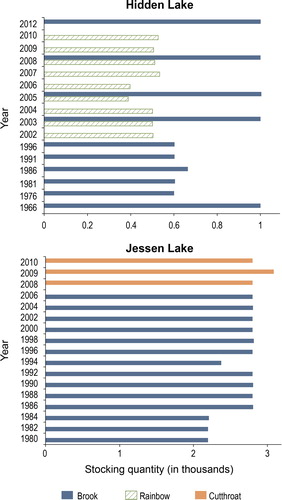
Fish surveys conducted at Jessen Lake before official stocking dates indicated the presence of cutthroat and rainbow trout in 1958 (High Uinta Lake Survey, Utah DWR, June 1958, unpubl.). Jessen Lake was stocked since at least 1980 (; High Uinta Lake Survey, Utah DWR, June 1980, unpubl. data). It is a headwater lake with significant outflow. Jessen Lake was oligotrophic and N-limited in summer, but co-limited in spring and fall following overturn (). Owing to Jessen Lake’s depth, it was well stratified during summer, resulting in anoxic conditions and elevated total phosphorus (TP) in the hypolimnion.
Materials and methods
Sediment core collection and sample preparation
Lake sediment cores were retrieved at the maximum depth from Birdsong, Hidden, and Jessen lakes using a Kajak–Brinkhurst (KB) gravity corer (Glew et al. Citation2001) in the summers of 2013, 2013, and 2012, respectively. The sediment core lengths were 36 cm, 33 cm, and 34 cm, respectively. Birdsong Lake sediments between 25 and 42 cm can be described as greenish-brown-gray clay, from 3 to 25 cm as light-brown clay with organics, and the top 3 cm as flocculent, greenish-brown organics. Sediments from Hidden and Jessen lakes were more homogeneous in color and texture throughout the core length; the upper ∼5 cm consisted of brown organic material, and sediments below ∼5 cm were tan-colored clay with organics. Each sediment core was subsectioned in the field using an extruder (Glew et al. Citation2001) in 0.25 cm intervals for the upper 15 cm, and 0.5 cm from 15 cm to the bottom of the core. Sediments were individually stored into labeled Whirl-Pak bags. In the field, the collected samples were kept in a cooler for no longer than 3 d before returning to the US Forest Service office in Vernal, Utah. Upon returning from the field, the samples were stored in a refrigerator under dark conditions at 4 C.
For each core, 15–16 samples were prepared for 210Pb dating and 37–47 samples were prepared for spectrally inferred chlorophyll a analysis. Sample wet weights of 2–3 cm3 were recorded and individually placed into 20 mL plastic scintillation vials prior to freeze-drying (Labconco Triad) for 24–48 h, until constant sample dry weights were reached. After freeze-drying, samples were homogenized and ground using a mortar and pestle until sediments passed through a 125 μm sieve. This process ensured each sample had uniform particle diameter for the spectrally inferred chlorophyll a analysis.
210Pb dating and age-depth model
Sediment samples were sent to MyCore Scientific, Inc. (Deep River ON), for 210Pb dating. For this dating method, the amount of atmospheric 210Pb incorporated into the upper lake sediment layers was measured and compared to background 210Pb levels to estimate the age of the sediment layer and sediment accumulation rate (Appleby Citation2001). To estimate 210Pb concentrations, alpha spectroscopy was used to estimate 210Po, a decay product of 210Pb (Cornett et al. Citation1984). Ages were determined from the 210Pb concentrations using the constant rate of supply (CRS) model (Appleby Citation2001).
Using ages determined by MyCore Scientific, Inc., an age-depth model was generated using the rBacon package 2.4.2 (Blaauw et al. Citation2020) in R (R Core Team Citation2020). Bacon uses Bayesian statistics to reconstruct sediment accumulation histories for sediment records (Blaauw and Christen Citation2011). Similar to previously dated Uinta Mountain lakes, no terrestrial organic carbon was found for 14C-dating sediments deposited before 1850 (Munroe Citation2007, Hundey et al. Citation2014). Because 210Pb dating is only suitable for dating sediments <150 yr old, Bacon was used to determine ages for sediments >150 yr old and for sediment intervals not selected for 210Pb dating. The dates of more recent sediments (upper 10–15 cm) are accurate. However, an absence of dates for the deeper (>15 cm) sediments means that both the chronology and sedimentation accumulation rate data in this core section should be considered with caution, especially when they are interpreted with sediment proxies.
Organics and spectrally inferred chlorophyll a
In-lake changes in primary production were determined by measuring the percentage of organic matter (%OM) and sedimentary chlorophyll a. The %OM was determined using the loss-on-ignition (LOI) method (Dean Citation1974, Heiri et al. Citation2001). For each lake, sample wet weights of 1 cm3 for 20–25 samples were recorded before placing sediment samples in the oven at 90 C for 24 h to remove water content. Samples were then heated at 550 C in a muffle furnace (Lindberg/Blue 1100 C Box Furnace) for 1 h to burn off the organic matter. After each heating step, sample dry weights were recorded. Changes in dry weight between successive heating steps provided estimates of %OM (i.e., in-lake production) in the sediment sample.
Sedimentary chlorophyll a and its degradation products (i.e., chlorophyll a and all chlorophyll a isomers and pheophytin a and pheophorbide a; Chl-a(s)) were measured using visible-range spectroscopy (VRS). VRS is a reliable proxy for paleoproduction (i.e., inferred lake primary production, mainly algal production; Das et al. Citation2005, Wolfe et al. Citation2006, Michelutti et al. Citation2010, Michelutti and Smol Citation2016). A review by Michelutti and Smol (Citation2016) evaluated the accuracy of this method by (1) comparing high-performance liquid chromoatography (HPLC) and VRS measurements of Chl-a(s); (2) comparing VRS measures of Chl-a(s) to measurements of chlorophyll a in the water column; and (3) comparing Chl-a(s) to the timing of known drivers of increasing chlorophyll a trends. Their research included studies from the Arctic to the Andes, and in all cases showed that Chl-a(s) measured with VRS provided accurate measures of lake production.
Spectrally inferred Chl-a(s) was measured using visible reflectance spectroscopy (Michelutti et al. Citation2010). Using a spectroradiometer (Rapid Content Analyzer) at the Paleoecological Environmental Assessment and Research Laboratory (PEARL, Queen’s University ON), the 400–2500 nm spectral range was measured for each sediment sample. The area under the peak between 650 and 700 nm for calibration standards of known Chl-a(s) concentrations (Das et al. Citation2005, Wolfe et al. Citation2006) was measured, and linear regression was used to develop a calibration model to estimate Chl-a(s) (Michelutti et al. Citation2010). The following linear relationship was used to infer the concentration of Chl-a(s) (Michelutti et al. Citation2010):
Chl-a(s) = 0.0919 * peak area 650–700 nm + 0.0011 (1)
Chl-a(s) flux (mg/m2/yr) was determined using the calculated Chl-a(s) concentration and estimated sediment accumulation rates from the rBacon package in the R Statistical Software (Blaauw et al. Citation2020, R Core Team Citation2020).
Nutrient limitation
Fish stocking primarily increases P to lakes, so it was important to consider potential temporal variations of nutrient limitation (Vanni Citation2002). Using meaurements from and , we calculated how much atmospheric deposition of N would need to increase to cause a shift from N- to P-limitation in the study lakes (). We assumed that P concentrations remained unchanged.
Historical data and statistical analyses
In order to investigate the influence of different stressors, including climate and atmospheric deposition of N, on algal production, we used breakpoint analysis. Piecewise linear regression was used to identify “breakpoints,” or abrupt thresholds (Toms and Lesperance Citation2003), in 3 time series of primary production (Chl-a(s)). We report the results from the breakpoint analysis using Chl-a(s) concentration instead of the Chl-a(s) flux data because of the potential inaccuracies in the chronology below 15 cm. Breakpoints were determined using generalized fluctuation tests, which test the null hypothesis that the regression coefficients remain constant against the alternative that at least one coefficient varies over time (Zeileis et al. Citation2003). Following this method, multiple breakpoints were possible and the optimal number of breakpoints was determined using Bayesian information criteria (BIC; Schwarz Citation1978) and the residual sum of squares (RSS; Bai and Perron Citation2003, Zeileis et al. Citation2003; ). For each breakpoint date, 95% confidence intervals were calculated (Bai and Perron Citation2003). All breakpoint analyses were completed using the strucchange package (Zeileis et al. Citation2019) in R (R Core Team Citation2020).
We also used breakpoint analysis to determine abrupt shifts in a record of annual temperature for nearby Duchesne County, Utah (National Oceanic and Atmospheric Administration [NOAA] Citation2020). We chose this record as it was the most proximal and continuous. We have compared it to other records from Utah and the US southwest and found the trends to be comparable. N deposition data were available from the National Atmospheric Deposition Program (NADP Citation2020). We used modeled total inorganic N (TNr) from the most proximal NADP site with the longest and most continuous record (1983–2018), the Logan (UT-01) site, to provide an estimate of reactive N deposition at our sites. Previous research showed that most of the N deposited in the Uinta Mountains was from agriculture (Hundey et al. 2016). Therefore, to extend the N record further back in time, we utilized commercial fertilizer use data for Utah available from the US Agriculture Census (1950–2012) (LaMotte Citation2015). Previous research showed that in the United States the use of N fertilizer was very low from 1850–1940, and then sharply increased (Cao et al. Citation2018). Thus, the main breakpoint in nitrogen fertilizer use occured after 1940, but was not identifiable using the methods we describe here because the record was too short to capture the breakpoint (Andersen et al. Citation2009). Therefore, we have assumed that the breakpoint in N deposition was between 1950 and 1960.
Results
210Pb dating and age-depth model
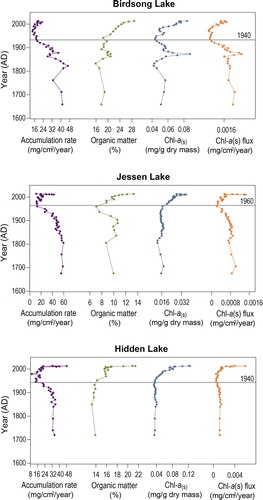
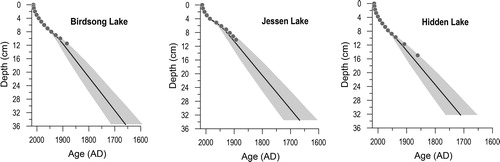
The total 210Pb activity in all 3 study lakes decreased exponentially with depth, reaching background levels by 12.25 cm for Hidden and Jessen lakes and by 17.5 cm for Birdsong Lake. Basal sediments in all 3 cores were deposited in the late 17th century, providing a record of ∼300 yr (). Sediment accumulation rates show similar trends in all 3 lakes, with generally higher values before the early 20th century, generally lower values after the onset of the 20th century, and increasing trends after ca. 1950 ().
Figure 4. Chronology for Birdsong, Jessen, and Hidden lakes. The chronology for all 3 lakes is based on 210Pb dating, and the age–depth model was determined using the rBacon package 2.4.2 (Blaauw et al. Citation2020) in R (R Core Team Citation2020).
Organics and spectrally inferred Chl-a(s)
The %OM was <30% for sediments from all 3 lakes, but %OM was lower (<15%) at Jessen Lake than at the other 2 lakes (, ). The overall averages of Chl-a(s) for Hidden and Birdsong lakes were similar, whereas Jessen Lake values were lower (). All 3 measures of algal production (%OM, Chl-a(s), and Chl-a(s) flux) showed increasing trends after 1940–1960 (). In all 3 lakes, post-1950 values of %OM, Chl-a(s), and Chl-a(s) flux were higher than pre-1850 values, with the exception of Chl-a(s) flux at Birdsong Lake (). At Birdsong Lake, however, the higher values of Chl-a(s) flux pre-1850 are due to higher sediment accumulation rates (), which should be viewed with caution owing to poor dating control prior to 1900 (). The increasing trend in primary production over the last ∼80 yr was unprecedented in all 3 lake records, but based on Chl-a(s), was greatest at Hidden Lake (100% increase from pre-1850 to post-1950) and lowest at Birdsong Lake (44%; ). In Birdsong Lake, we observed a notable peak in all 3 proxies of algal production in the late 19th century that was not related to sediment accumulation rate. This peak was not evident in the other 2 lakes ().
Table 3. Summary statistics for primary production proxies.
Nutrient limitation
Our results indicate that if atmospheric deposition of nitrogen increased between 0.05 to 2 kg/ha/yr our study lakes would shift from N- to P-limited (Table S1).
Statistical analyses
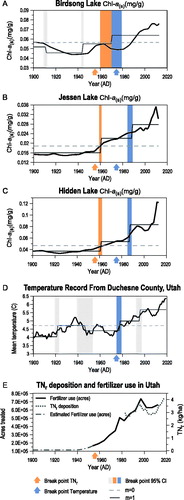
Three breakpoints were identified in the Chl-a(s) data from Birdsong Lake, at 1909–1912, 1943–1945, and 1960–1979 (, Table S2). Two breakpoints were identified in Jessen and Hidden lakes, at 1959–1962 and 1984–1989 (; Tables S3, S4). In the climate data, 4 breakpoints were identified (1919–1923, 1939–1953, 1975–1979, and 1992–1997; , Table S5). A notable warming trend began as early as 1970 and extends to the present. The onset of this warming trend was identified by the breakpoint occurring between 1975 and 1979. This breakpoint overlapped with a breakpoint in Chl-a(s) at Birdsong Lake (1960–1979) and slightly predated the breakpoints in Chl-a(s) at Hidden and Jessen lakes (1984–1989; ). The assumed breakpoint in N deposition that occurred between 1950 and 1960 () overlapped with the initial increases in Chl-a(s) identified by breakpoints at Birdsong Lake at 1960–1979 and at Hidden and Jessen lakes at 1959–1962 ().
Figure 6. Nonlinear changes identified in trends of algal production (Chl-a(s)) from (A) Birdsong Lake, (B) Jessen Lake, and (C) Hidden Lake. (D) Annual temperature from Duchesne County, Utah (NOAA Citation2020). (E) Total wet inorganic N deposition (TNr) (NADP Citation2020), with fertilizer use in Utah (LaMotte Citation2015). Vertical bands depict 95% confidence intervals (CI) surrounding breakpoints. The lighter (orange) arrows represent the years when we would expect to observe a breakpoint in the Chl-a(s) data based on changes in TNr deposition (E). The darker (blue) arrows represent the years when we would expect to observe a breakpoint in the Chl-a(s) data based on breakpoints in the temperature data (D). Observed breakpoints in the Chl-a(s) data that are closest to these arrows are colored light (orange) and dark (blue), respectively. The lightest gray bands indicate breakpoints that are not consistent across plots. The dashed horizontal lines show the intercept for the null model (m = 0) and the solid horizontal lines show the intercept for m = 1, 2 … n breakpoints.
Discussion
Has fish stocking increased primary production in mountain lakes?
Our data indicate that primary production began increasing in all 3 study lakes, whether stocked or unstocked, between 1940 and 1960. This timing roughly coincides with the timing of fish stocking (late 1950s); however, since the increasing trend in primary production is present in all 3 lakes, including fishless and unstocked lakes, fish stocking cannot be the only mechanism driving the increase. Similar increasing trends in primary production beginning in 1950 were reported from previously studied Uinta Mountain lakes; however, trends in the δ15N of lake sediments indicated that fish stocking was not the primary driver of increasing primary production in these systems (Hundey et al. 2014). Although our findings suggest that other factors are causing the increases in primary production, our results do not negate the effects on algal production by fish stocking. If other mechanisms are the main factor causing the increase in primary production, but fish stocking still has some effect, then primary production in stocked lakes should be increasing more than in the unstocked lake. This is, in fact, the case. The unstocked lake, Birdsong Lake, shows the smallest percent increase in primary production.
What other stressors have led to increased primary production?
Atmospheric deposition of nutrients, particularly N and P, could have resulted in increased primary production at all of the study lakes. Nutrients can enter mountain lakes through atmospheric deposition (Holtgrieve et al. Citation2014, Brahney et al. Citation2015) directly or via catchment events, such as runoff and spring snowmelt (McKnight et al. Citation1990). Depending on which nutrient is limiting algal growth, an influx of N and P can promote algal production (Elser et al. Citation2009). The timing of increased primary production in the study lakes coincides with increasing fertilizer use adjacent to the Wasatch Range, upwind of the Uinta Mountains (), and phosphate production in the United States (Hundey et al. Citation2014). Past studies on Uinta Mountain lake sediments attributed an increase in P since ∼1950 to dry atmospheric deposition from intensifying agricultural and phosphate mining activities (Reynolds et al. Citation2010). The study lakes, however, are generally N-limited, so N deposition has greater influence in driving increased algal production compared to P deposition ().
Similar increasing trends in algal production were reported from alpine lakes on the south slope of the Uinta Mountains, and these trends were attributed to atmospheric N-fertilization (Hundey et al. 2014, 2016). Analysis of diatom community composition showed an increase in Asterionella formosa, a nitrophilous diatom, coinciding with the increase in algal production (Hundey et al. 2014). Sediment trap samples show that Asterionella formosa is the most abundant diatom in Jessen Lake (Ngai Citation2014). The study lakes, which are similar to the lakes in Hundey et al. (2014, 2016), are N-limited or co-limited. It is likely, therefore, that the increase in algal production in all study lakes is mainly due to increased atmospheric N deposition.
Fertilizer use beginning between 1940 and 1960 has been shown to strongly influence reactive N deposition (; Anderson and Downing Citation2006). The breakpoint in Chl-a(s) at Birdsong Lake overlapped with this range, while breakpoints in the Chl-a(s) data collected from Hidden and Jessen lakes slightly postdated this period (). This evidence supports atmospheric deposition triggering the initial increase in primary production at all 3 study lakes.
Temperature data showed rapid warming beginning 1970, and our statistical analyses indicate a breakpoint at 1975–1979. This overlaps with the breakpoint in Chl-a(s) at 1960–1979 at Birdsong Lake and predates the breakpoint at 1984–1989 at Jessen and Hidden lakes (). This suggests that although the initial trigger increasing primary production was nitrogen deposition, warming temperatures have enhanced this trend.
It is also possible that increases in precipitation could enhance nutrient delivery from the lake catchment or the atmosphere, resulting in greater algal production (Schindler et al. Citation1996, Parker et al. Citation2008); however, analysis of precipitation records from this area indicates no long-term trends similar to the increasing trend in primary production (MacDonald and Tingstad Citation2007).
Local disturbances, such as fire or landslides, may deliver nutrients from the terrestrial ecosystem to lakes (Wright Citation1976, McColl and Grigal Citation1977, Enache and Prairie Citation2000, Kim et al. Citation2016); however, the effects of such disturbances on nutrient loads and primary production are typically local, relatively minor, and short-lived (Schindler et al. Citation1980, Bayley et al. Citation1992), so probably did not drive the decades-long increases in primary production observed in all 3 study lakes. A local disturbance, however, may have caused a relatively short-lived but large peak in algal production at Birdsong Lake in the mid-19th century. This peak is likely related to a local disturbance since it was not observed in the other records; however, a specific disturbance has not been identified.
What is the relative importance of fish stocking compared to other stressors in controlling primary production?
Although our results show that there is relatively little response of algal production to fish stocking compared to atmospheric deposition of nutrients, past evidence suggests that nutrient loading from fish stocking is an important contributor to phytoplankton nutrient requirements (Schindler et al. Citation1993). The limited response of algal production to fish stocking in the Uinta Mountains could be explained if fish stocking or survivorship were lower in the Uinta Mountains than at other locations. In the Canadian Rockies, a 10-fold increase in algal production was observed in lakes following stocking, while virtually no change occurred in a control, unstocked lake (Leavitt et al. Citation1994). Stocking rates in the Canadian Rockies (∼400 fish/ha/yr; Leavitt et al. Citation1994) were about twice that of the Uinta Mountains (200 fish/ha/yr). Although we do not have specific information on fish survivorship, Utah State Fish and Wildlife Department records indicate healthy fish populations in Jessen and Hidden lakes. Given the 10-fold increase in primary production due to fish stocking observed in the Canadian Rockies, it is surprising that there is not more evidence of a response to fish stocking in the Uinta Mountains.
Perhaps the effects of atmospheric deposition on algal production mask those due to fish stocking. Although earlier research showed that nutrient loading from fish stocking is greater or equivalent to atmospheric deposition, the relative importance of atmospheric inputs may have been underestimated (Schindler et al. Citation2001, Lyons et al. Citation2016, Milardi et al. Citation2016). For example, earlier comparisons of the relative contributions of these nutrient sources did not include P in dust, which has been shown to be significant (Psenner Citation1999, Sickman et al. Citation2003, Pulido-Villena et al. Citation2008, Brahney et al. Citation2015).
Another possibility to explain the muted response to fish stocking is differences in nutrient limitation. Linkages made between fish stocking and increased primary production typically focus on the effects of fish stocking on P, partly because N:P in fish excretions is low (Vanni Citation2002) and partly because lakes in previous studies were generally P-limited (Schindler et al. Citation2001). Although speculative, it is possible that because our study lakes are more often N-limited, there was less response to fish stocking (Lyons et al. Citation2016). This response could shift if nutrient limitation changed from N to P. Our calculations suggest that N deposition would have to increase by 0.05 to 2 kg/ha/year from today’s values to cause the lakes to be P-limited. The increase in N deposition in this region from 1980 is about ∼1 kg/ha/year (NADP 2020). Most of the N presently being deposited in the Uinta Mountains is from wet deposition of NH4+ that likely orginated from distant agricultural fields; this source of N is unlikely to decrease (Hundey et al. 2016). Oil and gas extraction, which occur south of the study sites and are important sources of several N constituents (e.g., NO2, N2O5, HONO, PANs, and alkyl nitrates), has rapidly expanded, providing a new source of atmospheric N (Edwards et al. Citation2014, Gorchov Negron et al. Citation2018, Coughlin et al. Citation2020). It is therefore possible that we eventually may observe increases in atmospheric deposition of N of the magnitude we suggest at these sites. If nutrient limitation changed from N to P, then small additions of P from fish stocking might become more important in driving primary production.
Differences in algal production between Hidden and Jessen Lakes
Owing to Jessen Lake’s smaller catchment to lake area ratio and greater depth, its Chl-a(s) is half the value of measurements at Hidden Lake. With most of the nutrient load to these lakes likely coming from atmospheric deposition, Jessen Lake’s larger water volume will likely dilute nutrient concentration and limit primary production more effectively than for Hidden Lake. Lake production for a deep and shallow lake is also regulated by thermal stability (Perin et al. Citation1996). The absence of thermal stratification allows for continuous mixing and effective nutrient cycling, increasing total nutrient availability in shallow lakes (Schallenberg and Burns Citation2004). In deep, oligotrophic lakes, thermal stratification can result in the loss of nutrients from the epilimnion to the hypolimnion through sedimentation, resulting in less primary production in the epilimnion (Lepori et al. Citation2018).
Warmer air temperatures will lead to increasing lake-water temperatures and longer and more stable stratification in deep lakes, such as Jessen Lake. More stable stratification will likely reduce nutrient cycling in Jessen Lake, and warmer lakewater temperatures will promote increased algal production, assuming sufficient nutrient availability (Lepori et al. Citation2018). These complex interactions point to the importance of considering climate warming and its effects on nutrient loading and primary production in shallow and deep lakes.
Management implications
Our research shows that fish stocking is having little effect on primary production in Uinta Mountain lakes, but this may be due to our study lakes being N-limited. We argue that primary production initially increased in the Uinta Mountains circa 1950 due to atmospheric deposition of nutrients, particularly N. The observed increase in primary production has been exacerbated by warming temperatures beginning circa 1980. Nutrient limitation in lakes can shift from N-limitation to P-limitation, resulting from atmospheric deposition of N (Elser et al. Citation2009). If this happens, increased P from fish stocking could maintain the increasing trajectory of primary production. Furthermore, as primary production continues to increase and lakes shift from oligotrophic to mesotropic or even eutrophic states, the direct effects of fish stocking may become more important (Elser et al. Citation1990).
Of the factors considered, fish stocking has the smallest impact on primary production in these lakes. Our research shows that atmospheric deposition of nutrients and climate warming, which are more challenging to manage, are the main drivers of increasing primary production. Before the challenge of N deposition can be addressed, more information must be generated through (1) effective monitoring of atmospheric deposition and (2) research into the processes influencing transport pathways from the source to the atmosphere, catchment, and lake, with particular attention to the effects of climate change. Such information will help to pinpoint specific sources of N and identify the lakes that are most sensitive. Globally, oligotrophic ecosystems are disappearing (Stoddard et al. Citation2016), and the Uinta Mountain lakes are no exception. Protecting these important mountain lake ecosystems poses a considerable challenge because the most damaging stressors are the result of distant human activities.
Supplemental Material
Download PDF (295.4 KB)Acknowledgments
We thank Shirley Ngai, Chris Plunkett, Melissa Hendrickson, and Nick Oprandy for their assistance in field sample collection. We thank Chris Plunkett for kindly providing accommodations during field work. Thanks to Dr. John Smol, Dr. Neal Michelutti, and Chris Grooms for providing the equipment necessary to conduct Chl-a(s) analysis. Karen VanKerkoerle provided maps and assisted with figure design. We thank 2 anonymous reviewers and Chris Plunkett for reviewing and providing suggestions that have improved our article.
Additional information
Funding
References
- Andersen T, Carstensen J, Hernández-García E, Duarte CM. 2009. Ecological thresholds and regime shifts: approaches to identification. Trends Ecol Evol. 24(1):49–57. doi:10.1016/j.tree.2008.07.014.
- Anderson KA, Downing JA. 2006. Dry and wet atmospheric deposition of nitrogen, phosphorus and silicon in an agricultural region. Water Air Soil Pollut. 176(1–4):351–374. doi:10.1007/s11270-006-9172-4.
- Appleby PG. 2001. Chronostratigraphic techniques in recent sediments In: Last WM, Smol JP, editors. Tracking environmental change using lake sediments: basin analysis, coring and chronological techniques. Dordrecht: Kluwer Academic Press; p. 171–203.
- Bahls P. 1992. The status of fish populations and management of high mountain lakes in the western United States. Northwest Sci. 66(3):183–193.
- Bai J, Perron P. 2003. Computation and analysis of multiple structural change models. J Appl Econ. 18(1):1–22. doi:10.1002/jae.659.
- Ballantyne AP, Brahney J, Fernandez D, Lawrence CL, Saros J, Neff JC. 2011. Biogeochemical response of alpine lakes to a recent increase in dust deposition in the southwestern US. Biogeosciences. 8(9):2689–2706. doi:10.5194/bg-8-2689-2011.
- Baron JS, Rueth HM, Wolfe AM, Nydick KR, Allstott EJ, Minear JT, Moraska B. 2000. Ecosystem responses to nitrogen deposition in the Colorado Front Range. Ecosystems. 3(4):352–368. doi:10.1007/s100210000032.
- Bayley SE, Schindler DW, Beaty KG, Parker BR, Stainton MP. 1992. Effects of multiple fires on nutrient yields from streams draining boreal forest and fen watersheds: nitrogen and phosphorus. Can J Fish Aquat Sci. 49(3):584–596. doi:10.1139/f92-068.
- Bergström A-K. 2010. The use of TN:TP and DIN:TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat Sci. 72(3):277–281. doi:10.1007/s00027-010-0132-0.
- Bergström A-K, Jansson J. 2006. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Change Biol. 12(4):635–643. doi:10.1111/j.1365-2486.2006.01129.x.
- Blaauw M, Christen JA. 2011. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 6(3):457–474.
- Blaauw M, Christen JA, Aquino MA. 2020. Rbacon: age-depth modelling using Baysian statitsics. R package 2.4.2.
- Brahney J, Ballantyne AP, Turner BL, Spaulding SA, Otu M, Neff JC. 2014. Separating the influences of diagenesis, productivity and anthropogenic nitrogen deposition on sedimentary δ15N variations. Org Geochem. 75:140–150. doi:10.1016/j.orggeochem.2014.07.003.
- Brahney J, Mahowald N, Ward DS, Ballantyne AP, Neff JC. 2015. Is atmospheric phosphorus pollution altering global alpine lake stoichiometry? Global Biogeochem Cycles. 29(9):1369–1383. doi:10.1002/2015GB005137.
- Cao P, Lu C, Yu Z. 2018. Historical nitrogen fertilizer use in agricultural ecosystems of the contiguous United States during 1850–2015: application rate, timing, and fertilizer types. Earth Syst Sci Data. 10(2):969–984. doi:10.5194/essd-10-969-2018.
- Carlisle DM, Hawkins CP. 1998. Relationships between invertebrate assemblage structure, 2 trout species and habitat structure in Utah mountain lakes. J North Am Benthol Soc. 17(3):286–300. doi:10.2307/1468332.
- Carpenter SR, Cottingham KL, Schindler DE. 1992. Biotic feedbacks in lake phosphorus cycles. Trends Ecol Evol (Amst). 7(10):332–336. doi:10.1016/0169-5347(92)90125-U.
- Carpenter SR, Kitchell JF, Hodgson JR. 1985. Cascading trophic interactions and lake productivity. Bioscience. 35(10):634–639. doi:10.2307/1309989.
- Carpenter SR. Kitchell JF. 1993. The trophic cascade in lakes. Cambridge: Cambridge University Press.
- Condie KC, Lee D, Lang Farmer G. 2001. Tectonic setting and provenance of the Neoproterozoic Uinta Mountain and Big Cottonwood groups, northern Utah: constraints from geochemistry, Nd-isotopes and detrital modes. Sediment Geol. 141–142:443–464. doi:10.1016/S0037-0738(01)00086-0.
- Cornett RJ, Chant L, Link D. 1984. Sedimentation of Pb-210 in Laurentian shield lakes. Water Pollut Res J Can. 19(2):97–109. doi:10.2166/wqrj.1984.018.
- Coughlin JG, Elliott EM, Rose LA, Pekney NJ, Reeder M. 2020. Quantifying atmospheric reactive nitrogen concentrations, dry deposition, and isotope dynamics surrounding a marcellus shale well pad. Atmos Environ. 223:117196. doi:10.1016/j.atmosenv.2019.117196.
- Coveney MF, Lowe EF, Battoe LE, Marzolf ER, Conrow R. 2005. Response of a eutrophic, shallow subtropical lake to reduced nutrient loading. Freshwater Biol. 50(10):1718–1730. doi:10.1111/j.1365-2427.2005.01435.x.
- Das B, Vinebrooke RC, Sanchez-Azofeifa A, Rivard B, Wolfe AP. 2005. Inferring sedimentary chlorophyll concentrations with reflectance spectroscopy: a novel approach to reconstructing historical changes in the trophic status of mountain lakes. Can J Fish Aquat Sci. 62(5):1067–1078. doi:10.1139/f05-016.
- Dean WE. 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss-on-ignition – comparisons with other methods. J Sediment Petrol. 44(1):242–248.
- Edwards PM, Brown SS, Roberts JM, Ahmadov R, Banta RM, deGouw JA, Dubé WP, Field RA, Flynn JH, Gilman JB, et al. 2014. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature. 514(7522):351–354. doi:10.1038/nature13767.
- Elser JJ, Carney HJ, Goldman CR. 1990. The zooplankton-phytoplankton interface in lakes of contrasting trophic status – an experimental comparison. Hydrobiologia. 200–201(1):69–82. doi:10.1007/BF02530330.
- Elser JJ, Andersen T, Baron JS, Bergström A-K, Jansson M, Kyle M, Nydick KR, Steger L, Hessen DO. 2009. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science. 326(5954):835–837. doi:10.1126/science.1176199.
- Enache M, Prairie YT. 2000. Paleolimnological reconstruction of forest fire induced changes in lake biogeochemsitry (Lac Francis, Abitibi, Quebec, Canada). Can J Fish Aquat Sci. 57(S2):146–154. doi:10.1139/f00-114.
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 320(5878):889–893. doi:10.1126/science.1136674.
- Gąsiorowski M, Sienjiewicz E. 2013. The sources of carbon and nitrogen in mountain lakes and the role of human activity in their modification determined by tracking stable isotope composition. Water Air Soil Poll. 224:1–9.
- Glew JR, Smol JP, Last WM. 2001. Sediment core collection and extrusion In: Last WM, Smol JP, editors. Tracking environmental change using lake sediments: basin analysis, coring and chronological techniques. Dordrecht: Kluwer Academic Press; p. 73–105.
- Gorchov Negron AM, McDonald BC, McKeen SA, Peischl J, Ahmadov R, de Gouw JA, Frost GJ, Hastings MG, Pollack IB, Ryerson TB, et al. 2018. Development of a fuel-based oil and gas inventory of nitrogen oxides emissions. Environ Sci Technol. 52(17):10175–10185. doi:10.1021/acs.est.8b02245.
- Heiri O, Lotter AF, Lemcke G. 2001. Loss on ignition as a method for estimating organics and carbonate in sediments: reproducibility and comparability of results. J Paleolimnol. 25(1):101–110. doi:10.1023/A:1008119611481.
- Holtgrieve GW, Schindler DE, Hobbs WO, Leavitt PR, Ward EJ, Bunting L, Chen G, Finney BP, Gregory-Eaves I, Holmgren S, Lisac MJ, Lisi PJ, Nydick KR, Rogers LA, Saros JE, Selbie DE, Shapley MD, Walsh PB, Wolfe AP. 2011. A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science. 334:1545–1548.
- Hundey, EJ, Moser KA, Longstaffe, FJ, Michelutti, N, Hladyniuk R. 2014. Recent changes in production in oligotrophic Uinta Mountain lakes, Utah, identified using paleolimnology. Limnol Oceanogr. 59(6): 1987–2001.
- Jeppesen E, Jensen JP, Søndergaard M, Lauridsen T, Landkildehus F. 2000. Trophic structure, species richness and biodiversity along a phosphorus gradient. Freshwater Biol. 45(2):201–213. doi:10.1046/j.1365-2427.2000.00675.x.
- Jiménez L, Conde-Porcuna JM, García-Alix A, Toney JL, Anderson RS, Heiri O, Pérez-Martínez C. 2019. Ecosystem responses to climate-related changes in a Mediterranean alpine environment over the last 180 years. Ecosystems. 22(3):563–577. doi:10.1007/s10021-018-0286-5.
- Kim SL, Shuman BN, Minckley TA, Marsicek JP. 2016. Biogeochemical change during climate-driven afforestation: a paleoecological perspective from the Rocky Mountains. 2016. Ecosystems. 19(4):615–624. doi:10.1007/s10021-015-9955-9.
- LaMotte AE. 2015. Selected items from the Census of Agriculture at the county level for the conterminous United States, 1950–2012. U.S. Geological Survey Data Release. 10.5066/F7H13016.
- Leavitt PR, Schindler DE, Paul AJ, Hardie AK, Schindler DW. 1994. Fossil pigment records of phytoplankton in trout-stocked alpine lakes. Can J Fish Aquat Sci. 51(11):2411–2423. doi:10.1139/f94-241.
- Lepori F, Roberts JJ, Schmidt TS. 2018. A paradox of warming in a deep peri-alpine lake (Lake Lugano, Switzerland and Italy. Hydrobiologia. 824(1):215–228. doi:10.1007/s10750-018-3649-1.
- Lyons RA, Johnson LK, McIntyre BM. 2016. Phosphorous loading rates in lakes with development and stocked fish in the Sierra Nevada Mountains, California, USA. Ecosphere. 7(11):e01554. doi:10.1002/ecs2.1554.
- MacDonald GM, Tingstad A. 2007. Recent and multicentennial precipitation variability and drought occurrence in the Uinta Mountains region, Utah. Arct Antarct Alp Res. 39(4):549–555. doi:10.1657/1523-0430(06-070)[MACDONALD]2.0.CO;2.
- Mahowald N, Jickells TD, Baker AR, Artaxo P, Benitez-Nelson CR, Bergametti G, Bond TC, Chen Y, Cohen DD, Herut B. 2008. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem Cy. 22(4):GB4026.
- Mahowald NM, Scanza E, Brahney J, Goodale CL, Hess PG, Moore JK, Neff J. 2017. Aerosol deposition impacts on land and ocean carbon cycles. Curr Clim Change Rep. 3(1):16–31. doi:10.1007/s40641-017-0056-z.
- McColl JG, Grigal DF. 1977. Nutrient changes following a wildfire in Minnesota: effects in watersheds with differing soils. Oikos. 28(1):105–112. doi:10.2307/3543329.
- McKnight D, Smith RL, Bradbury JP, Baron JS, Spaulding S. 1990. Phytoplankton dynamics in three Rocky Mountain lakes, Colorado, USA. Arct Antarct Alp Res. 22(3):264–274. doi:10.2307/1551589.
- Michelutti N, Smol JP. 2016. Visible spectroscopy reliably tracks trends in paleo-production. J Paleolimnol. 56(4):253–265. doi:10.1007/s10933-016-9921-3.
- Michelutti N, Blais JM, Cumming BF, Paterson AM, Rühland K, Wolfe AP, Smol JP. 2010. Do spectrally inferred determinations of chlorophyll a reflect trends in lake trophic status? J Paleolimnol. 43(2):205–217. doi:10.1007/s10933-009-9325-8.
- Milardi M, Lappalainen J, McGowan S, Weckström J. 2016. Can fish introductions alter nutrient cycles in previously fishless high-altitude lakes? J Limnol. 76(1):1–14. doi:10.4081/jlimnol.2016.1364.
- Moser KA, Baron JS, Brahney J, Oleksy IA, Saros JE, Hundey EJ, Sadro SA, Kopáček J, Sommaruga R, Kainz MJ, et al. 2019. Mountain lakes: eyes on global environmental change. Global Planet Change. 178:77–95. doi:10.1016/j.gloplacha.2019.04.001.
- Munroe JS. 2007. Exploring relationships between watershed properties and Holocene loss-on-ignition records in high-elevation lakes, southern Uinta Mountains, Utah, U.S.A. Arct Antarct Alp Res. 39(4):556–565. doi:10.1657/1523-0430(06-096)[MUNROE]2.0.CO;2.
- Munroe JS, Laabs BJC, Moser KA, Gurrieri JT. 2007. UINTAS 2006: The Uinta Mountain Interdisciplinary Assessment Symposium, Snowbird, Utah, May 2006- Introduction. Arct Antarct Alp Res. 39(4):517–520. doi:10.1657/1523-0430(07-500)[MUNROE]2.0.CO;2.
- Nanus L, Clow DW, Saros JE, Stephens VC, Campbell DH. 2012. Mapping critical loads of nitrogen deposition for aquatic ecosystems in the Rocky Mountains, USA. Environ Pollut. 166:125–135. doi:10.1016/j.envpol.2012.03.019.
- Nanus L, Williams MW, Campbell DH, Elliott EM, Kendall C. 2008. Evaluating regional patterns in nitrate sources to watersheds in national parks of the Rocky Mountains using nitrate isotopes. Environ Sci Technol. 42(17):6487–6493. doi:10.1021/es800739e.
- National Atmospheric Deposition Program (NADP). 2020. NRSP-3. NADP Program Office, Wisconsin State Laboratory of Hygiene, Madison, WI. http://nadp.slh.wisc.edu/data/ntn/.
- National Oceanic and Atmospheric Administration (NOAA). 2020. National Centers for Environmental Information. Climate at a glance: county time series. https://www.ncdc.noaa.gov/cag/.
- Neill WE, Peacock A. 1980. Breaking the bottleneck: interactions of invertebrate predators and nutrients in oligotrophic lakes In Kerfoot WC. Editor. Evolution and ecology zooplankton communities. Hanover (NH): University Press of New England; p.715–725.
- Ngai S. 2014. Potential effects of changing climate on the physical, chemical and biological characteristics of Alpine Lakes, Uinta Mounatins, Utah, USA [M.S. thesis]. London (ON): The Universtiy of Western Ontario.
- Parker BR, Vinebrooke RD, Schindler DW. 2008. Recent climate extremes alter alpine lake ecosystems. Proc Natl Acad Sci USA. 105(35):12927–12931. doi:10.1073/pnas.0806481105.
- Perin S, Pick FR, Lean DRS, Mazumder A. 1996. Effects of planktivorous fish and nutrient additions on primary production of shallow versus deep (stratified) lake enclosures. Can J Fish Aquat Sci. 53(5):1125–1132. doi:10.1139/f96-024.
- Psenner R. 1999. Living in a dusty world: airborne dust as a key factor for alpine lakes. Water Air Soil Poll. 112(3/4):217–227. doi:10.1023/A:1005082832499.
- Pulido-Villena E, Reche I, Morales-Baquero R. 2008. Evidence of an atmospheric forcing on bacterioplankton and phytoplankton dynamics in a high mountain lake. Aquat Sci. 70(1):1–9. doi:10.1007/s00027-007-0944-8.
- R Core Team. 2020. R: a language and environment for statitsical computing. Vienna, Austria: R Foundation for Statitsical Computing. http://R-project.org/.
- Reynolds RL, Mordecai JS, Rosenbaum JG, Ketterer ME, Walsh MK, Moser KA. 2010. Compositional changes in sediments of subalpine lakes, Uinta Mountains (Utah): evidence for the effects of human activity on atmospheric dust inputs. J Paleolimnol. 44(1):161–175. doi:10.1007/s10933-009-9394-8.
- Sadro S, Sickman JO, Melack JM, Skeen K. 2018. Effects of climate variability on snowmelt and implications for organic matter in a high-elevation lake. Water Resour Res. 54(7):4563–4578. doi:10.1029/2017WR022163.
- Schabetsberger R, Luger MS, Drozdowski G, Jagsch A. 2009. Only the small survive: monitoring long-term changes in the zooplankton community of an alpine lake after fish introduction. Biol Invasions. 11(6):1335–1345. doi:10.1007/s10530-008-9341-z.
- Schallenberg M, Burns CW. 2004. Effects of nutrient resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal entrainment. Freshwater Biol. 49(2):143–159. doi:10.1046/j.1365-2426.2003.01172.x.
- Schaus MH, Vanni MJ, Wissing TE, Bremigan MT, Garvey JE, Stein RA. 1997. Nitrogen and phosphorus excretion by detritivorous gizzard shad in a reservoir ecosystem. Limnol Oceanogr. 42(6):1386–1397. doi:10.4319/lo.1997.42.6.1386.
- Schindler DW, Curtis PJ, Parker BR, Stainton MP. 1996. Consequences of climate warming and lake acidification for UV-B penetration in North American boreal lakes. Nature. 379(6567):705–708. doi:10.1038/379705a0.
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM. 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci USA. 105(32):11254–11258. doi:10.1073/pnas.0805108105.
- Schindler DE, Kitchell JF, He X, Carpenter SR, Hodgson JR, Cottingham KL. 1993. Food web structure and phosphorus cycling in lakes. Trans Ame Fish Soc. 122(5):756–772. doi:10.1577/1548-8659(1993)122<0756:FWSAPC>2.3.CO;2.
- Schindler DE, Knapp RA, Leavitt PR. 2001. Alteration of nutrient cycles and algal production resulting from fish introductions into mountain lakes. Ecosystems. 4(4):308–321. doi:10.1007/s10021-001-0013-4.
- Schindler DW, Newbury RW, Beaty KG, Prokopowich J, Ruszczynski T, Dalton JA. 1980. Effects of a windstorm and forest fire on chemical losses from forested watersheds and on the quality of receiving streams. Can J Fish Aquat Sci. 37(3):328–334. doi:10.1139/f80-046.
- Schwarz G. 1978. Estimating the dimension of a model. Ann Statist. 6(2):461–464. doi:10.1214/aos/1176344136.
- Sickman JO, Melack JM, Clow DW. 2003. Evidence for nutrient enrichment of high-elevation lakes in the Sierra-Nevada. Limnol Oceanogr. 48(5):1885–1892. doi:10.4319/lo.2003.48.5.1885.
- Smol JP. 2008. Pollution of lakes and rivers: a paleoecological perspective. 2nd ed. Malden (MA): Blackwell Publishing.
- Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A, Pienitz R, Rühland K, Sorvari S, Antoniades D, Brooks SJ, et al. 2005. Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA. 102(12):4397–4402. doi:10.1073/pnas.0500245102.
- Søndergaard ME, Jeppesen E, Lauridsen TL, Skov C, Van Nes EH, Roijackers R, Lammens E, Portielje R. 2007. Lake restoration: successes, failures and long-term effects. J Appl Ecol. 44(6):1095–1105. doi:10.1111/j.1365-2664.2007.01363.x.
- Spaulding SA, Otu MK, Wolfe AP, Baron JS. 2015. Paleolimnological records of nitrogen deposition in shallow, high-elevation lakes of Grand Teton National Park, Wyoming, U.S.A. Arct Antarct Alp Res. 47(4):703–717. doi:10.1657/AAAR0015-008.
- Stoddard JL, Van Sickle J, Herlihy AT, Brahney J, Paulsen S, Peck DV, Mitchell R, Pollard AI. 2016. Continental-scale increase in lake and stream phosphorus: are oligotrophic systems disappearing in the United States? Environ Sci Technol. 50(7):3409–3415. doi:10.1021/acs.est.5b05950.
- Toms JD, Lesperance ML. 2003. Piecewise regression: a tool for identifying ecological thresholds. Ecology. 84(8):2034–2041. doi:10.1890/02-0472.
- Vanni MJ. 1996. Nutrient transport and recycling by consumers in lake food webs: impociations for algal communities In: Polis GA, Winemiller KO, editors. Food webs: integration of patterns and dynamics. New York (NY): Chapman & Hall; p. 81–95.
- Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst. 33(1):341–370. doi:10.1146/annurev.ecolsys.33.010802.150519.
- Vicars WC, Sickman JO, Ziemann PJ. 2010. Atmospheric phosphorus deposition at a montane site: size distribution, effects of wildfire, and ecological implications. Atmos Environ. 44(24):2813–2821. doi:10.1016/j.atmosenv.2010.04.055.
- Wetzel RG. 2001. Limnology: lake and river ecosystems. 3rd ed. San Diego (CA): Academic Press.
- Wolfe AP, Vinebrooke RD, Rivard B, Michelutti N, Das B. 2006. Experimental calibration of lake sediment spectral reflectance to chlorphyll a concentrations: methodology and paleolimnological validation. J Paleolimnol. 36(1):91–100. doi:10.1007/s10933-006-0006-6.
- Wright HE. Jr. 1976. The impact of forest fire on nutrient influxes to small lakes in northeastern Minnesota. Ecology. 57(4):649–662. doi:10.2307/1936180.
- Zeileis A, Kleiber C, Walter K, Hornik K. 2003. Testing and dating of structural changes in practise. Comput Stat Data Anal. 44(1–2):109–123. doi:10.1016/S0167-9473(03)00030-6.
- Zeileis A, Leisch F, Hornik K, Kleiber C. 2019. Strucchange: testing, monitoring and dating structural changes. R package 1.5-2.