Abstract
After the Erika oil spill in December 1999, several thousand tons of heavy fuel were released in marine environment and polluted the Atlantic French coast. DNA adducts were used as a biomarker of the Erika genotoxic impact on DNA of mussel digestive gland sampled at 15 locations from Finistère to Vendée county during a period of 11 months after the accident. Mussels collected in the impacted sites in December 1999 and January 2000 showed the highest total DNA adduct level (165 adducts/108 nucleotides). The level decreased dramatically in February 2000 to a stable level through the rest of the year. The site considered as reference zone was also impacted just after the Erika oil spill. The DNA adduct patterns are similar at all locations, indicating a common genotoxic impact along the coast. Two adducts persisted and were used to characterize and follow the Erika genotoxic impact. The analysis revealed that mussels from three sites (respectively Finistére, Loire Atlantique, and Vendée counties) have been highly impacted by the Erika oil spill.
On 12 December 1999, the tanker Erika broke in two parts at about 50 km from the French coast (Point of Penmarc'h, Sud Finistère, France). About 10,000 tons of fuel were released in the sea. The first signs of pollution were observed on the shore of Sud Finistère on 23 December, 11 days after the accident. The length of Atlantic coast impacted between 23 December 1999 and February 2000 was about 400 km from Morbihan to Vendée counties.
A number of analyses performed by independent institutes identified the spilled oil as fuel no. 2 (fuel no. 6 or Bunker C, CAS no. 68553-00-4) (Citation1). In addition to paraffinic, cycloparaffinic, and olefinic compounds fuel no. 2 included a mixture of polycyclic aromatic hydrocarbons (PAHs) and polycyclic heterocyclic hydrocarbons (HPHs) such as carbazoles and thiophenes (Citation1,Citation 2). This is a type of the so-called “heavy fuel oils” which constitute part of the “residual oils.” The chemical properties of heavy oil fuel no 6 have been reviewed by B. J. Baars (Citation3) who summarized the some data on heavy fuel no. 6 properties presented in ATSDR (Citation4,Citation 5), the International Agency for Research on Cancer (IARC) (Citation2), Irwin (Citation6), and Concawe reports (Citation7). Log Kow of the fuel no. 6 range from 2.7 to 7.1 according to Irwin and Concawe (Citation6,Citation 7) indicating a high bioaccumulation potential of at least part of this fuel. The evaporation and dissolution potencies are low implicating a long persistence of the contamination in the sediments, probably several years. The fuel density can be above 1 causing the sinking of the fuel, which can impact the benthic and sessile organisms. Oudot et al. (Citation8) showed experimentally that the biodegradation property of the Erika fuel is very slow due to the high proportion of refractory compounds such as asphalts, resins, and polycyclic saturated and aromatic hydrocarbons. It recently has been confirmed that the sediments (Citation9) and shellfish (Citation10) were contaminated by Erika petroleum product.
Birds were highly affected and many died (7,380 alive, 53,118 dead) from the direct physical toxic effect of the Erika fuel (Citation11). The reports from Institut National de l'Environnement Industriel et des Risques Industriels, ministere du territoire, France (INERIS) and the French Ministry of Environment pinpointed the potential risks of the Erika fuel on the aquatic ecosystem exposed to the soluble water fraction of the fuel. The impact of the Erika oil on macrofauna in the island of Groix has been studied during 1 year after the spill (Citation12). The damages observed are dependent on the type of habitat (bedrock, crevices, and boulders). The boulder habitat was the most impacted until 1 year after the spill. Consequences of an oil spill on fish are not clearly described. After the Exxon Valdez oil spill in Alaska in 1989, 25% to 32% of the embryos herring (Clupea pallasi) were damaged and authors hypothesized an indirect toxic effect of the oil on the adult herring population (Citation13).
When xenobiotics, such as PACs, enter a living organism, they are biotransformed into more hydrosoluble compounds that facilitate their elimination via excretion pathways. After oil spill contamination, the induction of xenobiotic metabolizing enzyme, such as cytochrome P-450 (CYP), has been shown in exposed fish (Citation14–17). During the biotransformation of a PAC, electrophilic metabolites of PAC might appear which can react with DNA bases and form DNA adducts. In ecotoxicological studies, the impact of a pollution might be assessed by the use of biomarkers reflecting different levels of ecological relevance (Citation18). DNA adducts represent a biological marker of high significance because they have been recognized to play a role in the initial step of environmental chemical carcinogenesis in general and notably in marine fish (Citation19). DNA adducts are recognized and widely used as a biomarker to detect genotoxic event in aquatic species and notably in fish (Citation20–27).
In this study we used mussels (Mytilus edulis) to follow DNA adduct evolution ERIKA oil spill. It has been shown that (a) mussels reflect chemical contamination in situ (Citation28), (b) mussels are able to biotransform compounds such as PAHs in DNA adducts (Citation29), (c) total PAHs concentration in whole tissus is correlated with DNA adducts level (Citation29,Citation 30). This allows the use of these animals in biomonitoring studies. The 32P-postlabeling method of analysis for the DNA adduct detection is a standardized method for the analysis of PAH-DNA adducts (Citation31,Citation 32). It is a useful and sensitive tool to follow the effect of exposure to environmental genotoxic pollutants in aquatics species (Citation33–38). This method can be also used for biomonitoring of low-level contaminations in environmental studies (Citation39). In this in situ study, we have analyzed DNA adducts in mussels (Mytilus edulis) a sentinel invertebrate and sessile species, living along the French coast exposed to Erika oil spill sampled at 15 different sites between Finistère to Vendée from December 1999 to November 2000. An in vitro study was also made to verify the ability of Erika mussels to form DNA adducts.
MATERIALS AND METHODS
Field Sampling
Mussels were collected from a total of 15 different sites along the French Brittany coast (). The majority of the sites were sampled every month from January 2000 to November 2000. At each site, three pools of 10 individual digestive gland were excised, frozen immediately, and then stored at −80°C until analysis.
32P-Postlabeling Method
Chemicals
Proteinase K, RNase A and T1, spleen phosphodiesterase micro-ccocal nuclease DNA from Salmon sperm were from Sigma (Saint Quentin Fallavier, Mexlan, France); nuclease P1 and T4 polynucleotide kinase was purchased from Roche Diagnostics (France) and [γ32P-ATP], 370 Tbq/mmol (5000 Ci/mmol) from Amersham (Les Ullis, France); rotiphenol from Rothsichel (Lauterbourg, France); cellulose MN 301 from Macherey Nagel (Düren, Germany); the Polyethyleneimine (PEI) was from Corcat (Virginia Chemicals, Portsmouth, Virginia, USA). The PEI/cellulose TLC plates were made in the laboratory.
Procedure
DNA adducts were detected by 32P-postlabeling. DNA was extracted and purified as described previously (Citation40). The method used for 32P-postlabeling was that previously described (Citation41) with modifications and all validation steps from the interlaboratory study described in Phillips and Castegnaro (Citation31) in which the authors participated. In brief, DNA (7 μg) was digested at 37°C for 4 h with micrococcal nuclease (183 mU) and spleen phosphodiesterase (12 mU) in a reaction mixture (total volume 10 μl) containing 20 mM sodium succinate and 10 mM CaCl2, pH 6. Subsequently, adducted nucleotides were enriched. Digested DNA was treated with nuclease P1 (6 μg) at 37°C for 45 min before 32P-postlabeling. Normal nucleotides, pyrophosphate, and excess ATP were removed by chromatography on polyethyleneimine cellulose plates in 2.3 M NaH2PO4, pH 5.7 (D1) overnight. Origin areas containing labeled adducted nucleotides were cut out and transferred onto another polyethyleneimine-cellulose plate, which was run in urea 8.5 M, lithium formate 5.3 M, pH = 3.5 (D2). Two further migrations (D3 and D4) were performed perpendicularly to D2. The solvant for D3 was lithium chloride 1 M, urea 8 M, Tris 0.5 M, pH = 8, and the buffer D4 was 1.7 M NaH2PO4, pH = 6. Autoradiography was carried out at −80°C for 24 or 48 h in the presence of an intensifying screen. The radioactivity of the spots is evaluated by a Bioimager and treated by Ambis software. A BaP modified DNA standard obtained during the European Union collaborative study on 32P-postlabeling validation method (Citation31) was used as a positive control. Purified DNA from salmon sperm which present no adduct was used as a negative control.
In Vitro Incubation
Chemicals
Nicotinamide adenine dinucleotide phosphate (NADPH), bovine serum albumin (BSA), aprotinine, phenylmethylsulfonylfluoride (PMSF), and arachidonic acid (AA) were obtained from Sigma (St. Quentin Fallavier, France).
Purification of Microsomes and Measurement of Proteins
Digestive glands of mussels were homogenized in a buffer solution (potassium chloride (KCl) 1.15%, 50 mM Na2KPO4, pH 7.4), with phenylmethylsulfonyl fluoride (10 μg/mL), and aprotinine (5 μg/mL). The buffer volume was three times the organ weight. After an initial centrifugation at 9000 g for 20 min, the supernatant was taken up and ultracentrifuged at 100,000 g for 1 h. The pellets were homogenized in 1 to 2 mL of pH 7.4 buffer containing NaH2PO4 50 mM, KCl 0.15 M, EDTA 1 mM, dithiothreitol (DTT) 1 mM, glycerol 20%. The mixture was centrifuged again at 100,000 g for 1 h. Finally, microsomes were resuspended in the potassium chloride (KCL) 1.15%, Na2KPO4 50 mM, pH 7.4 to obtain a final concentration of about 10 mg of protein and stored at −80°C prior to analysis. All steps of the isolation were carried out at 4°C.
Bradford method was used to measure the proteins level in microsomes as indicated by the instructions's manufacturor (Amresco, Solon, Ohio, USA). A sample of each microsomal preparation was diluted with a sodium chloride (NaCl, 0.15 N) solution. To 100 μL of this dilution, 1 mL of the reagent was added. The blue coloration of the mixture was measured with a spectrophotometer (595 nm). The absorbency was compared with a standard curve calculated with 25 μg/mL to 100 μg/mL solutions of bovine serum albumin (BSA).
In Vitro Incubation to Determine DNA Adduct Formation in Mussels
Microsomes containing 0.5 mg of protein were incubated in vitro in the presence of 70 μg DNA and Erika fuel extract (in DMSO) (10 μL) in 500 μL (final volume) of Tris-HCl 50 mM, EDTA 1 mM, pH 7.4. The mixture was incubated at 37°C for 3 min before addition of cosubstrate. For measurement of cytotochrome P450(CYP)-dependent DNA adduct formation, NADPH (10 μL, 10 mg/mL) was added. To determine DNA adduct formation induced by peroxydases (cyclooxygenase (COX) and lipoxygenase (LOX)) dependent activity, arachidonic acid (AA) (10 μL, 1 mg/mL) was added. The mixture was then incubated at 37°C for 45 min. Two controls were added: (Citation1) incubation of DNA without microsomes and (Citation2) incubation of microsomes alone. All incubations were performed in triplicate.
Statistical Analysis
Box Plots
The box plots were drawn with the SPSS.11 for windows. This representation allowed the distribution of the values (sites) to be followed for each variable (month). The box plot represents the median and the interquartile observations.
Centered and Reduced Value (CRV)
To compare the genotoxicity between sites each month, we used the centered and reduced values (CRV) (Citation42). Data were transformed in order to compare genotoxicity values (number of DNA adducts) at the same scale. CRV = (Avi − Avn)/en, where Avi = DNA adduct average at one site; Avn = DNA adduct average of all sites; en = standard deviation of the DNA adduct amount of all sites. A positive CRV (centered reduced value) indicates a higher DNA adduct level compared to the average of all DNA adduct levels measured in all sites.
RESULTS
Study of the Genotoxic Impact
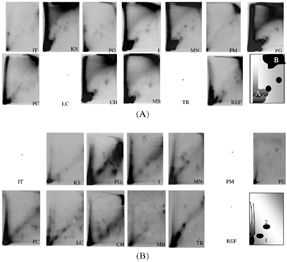
The typical DNA adduct patterns revealed at all sampled sites are presented in (period of December 1999 and January 2000) and 2B (February 2000). In January, except for IT (Ile Tudy, Finistère), PG (Poulgain, Finistère), and PM (pont Mahé, Sud Morbihan) the DNA adduct patterns are similar in all samples. We can distinguish two intense radioactive zones, one close to the origin (zone A) and the other on the top right of the autoradiogram (zone B). In addition, two to four other small adducts are observed in the center of the autoradiogram. In February, much less DNA adducts are observed. The DNA adduct pattern exhibited mainly a radioactive zone. Except for PB (Pen Bé, sud Morbihan), two adducts are detected numbered 1 and 2 in the scheme.
FIG. 2 (A) Typical DNA adduct patterns obtained in digestive gland from Mytilus edulis in January 2000 from the northeast sites (IT) to the southeast sites (REF). The scheme represents the DNA adduct pattern in December 1999 at Site PG. (B) Typical DNA adduct patterns of the digestive gland of Mytilus edulis in February 2000 from the northeast sites (IT) to the southeast sites (REF). The scheme represents the DNA adduct pattern in February.
The distribution of DNA adduct values, detected in sites sampled each month during the year of 2000, are given in . The gray box contains 50% of the DNA adduct values detected in sites that have been sampled, the black line represents the median value, and the limits of the box represent the inferior and superior quartiles. The number of sites sampled monthly (N) is indicated on the x-axis and under the box. In the first 2 months (December and January 2000), a very high DNA adduct quantity were measured in the digestive gland of the sampled mussels (; ). Only one station has been sampled in December, just after the accident, and the DNA adduct level reached 164.71 adduct/108 nucleotides at PG (Poulguin, Finistère). In January, 10 stations were analyzed (N = 10). The DNA adduct level ranged between 1.2 adduct/108 nucleotides (PM: Pont Mahé in Loire Atlantique) and 138 adduct/108 nucleotides (MN: Merrien in Finistère). A decrease, ranging between 50% to 99% was observed between January and February at all sites, except for PG where the total DNA adduct level increased (14.6/108 nucleotide versus 2.45/108 nucleotide) (). For a better readability of the results, DNA adduct values in December 1999 and in January 2000 were excluded in . Between February and November, DNA adduct levels were never over 40/108 nucleotides. We can also observe an increase of the DNA adduct in October 2000 in all sites sampled.
FIG. 3 Box plots representing the evolution of the mean of DNA adducts quantified in mussel digestive glands from December 1999 to November 2000. N represents the number of sites analyzed each month.
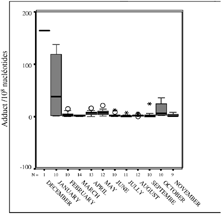
FIG. 4 Box plots representing the evolution of the mean of DNA adducts quantified in mussel digestive glands from February to November 2000. N represents the number of sites analyzed each month.
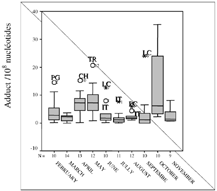
TABLE 1 Comparison of DNA Adduct/108 Nucleotides According to Site and Time of Sampling
The comparison between the different sites in January through the centered and reduced values (CRV) is depicted in in order to assess the genotoxic impact depending on site. In January, half of the sites analyzed exhibit a positive CRV (black) indicating a higher genotoxicity at these sites (superior to the average of all sites). The most impacted sites were KS (Kerist), I (Ile), MN (Merrien) localized in the Finistère, CH (pointe de Chemoulin, Loire Atlantique), and MB (Maison Blanche, Vendée).
TABLE 2 Scheme of the VCR Values According to Sampling Sites and Time (December 1999 to November 2000)
Globally, the most impacted counties are Sud Finistère, Loire Atlantique, and Vendée until May 2000. Two sites, IT (Finistère) and LC (Loire Atlantique) have been highly impacted by genotoxic compounds at least until October 2000.
Presence of Adducts 1 and 2 and Correlation with the CRV Values
Qualitative analysis of DNA adduct patterns demonstrated the presence and the persistence of two adducts (called 1 and 2). The occurrence of these adducts is reported in . Apart from two sites (Groix, GX, and Le Maresclé, MS) where they are never detected, adducts 1 and 2 are detected at all sampling sites from January to April–May 2000 (). These two adducts persisted at IT, LC, and TR sites during all the sampling period ( and ). For some Loire Atlantique sites (CH, PM-PB, PC-C), these adducts reappeared in September-October.
TABLE 3 Scheme of the Persistence of DNA Adducts 1 and 2 According to Sampling Sites and Time (December 1999 to November 2000)
FIG. 5 Typical DNA adduct pattern of digestive gland of Mytilus edulis at IT (Finistere), LC (Loire Atlantique), and TR (Vendée) sites from January to September 2000.
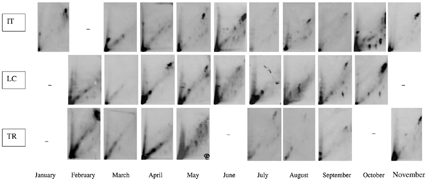
In the period June–August 2000, the DNA adduct pattern is slightly different at all sites except IT, LC, and TR (). Adducts 1 and 2 and the diagonal zone are absent.
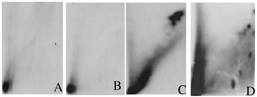
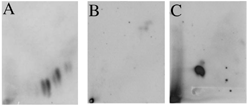
FIG. 6 (A) Typical DNA adduct pattern of the digestive gland of Mytilus edulis observed during the period June–August 2000 (except for IT, LC, and TR). (B) Negative control: purified DNA from salmon sperm. (C) Positive control: BAP-DNA adduct.
Comparison of the two Tables (2 and 3) shows a parallelism between the presence of these two adducts and the corresponding CRV values. When the CRV is positive (most impacted sites), the adducts 1 and 2 are present from December 1999 to April 2000, and also in October and November 2000. This is clear for the IT site (Finistère) and LC site (Loire Atlantique). For some sites a positive CRV is not directly correlated to these two adducts (TR in August and September, I in April, KS in June, and CH in September). This could be explained by the fact that, (a) the adducts 1 and 2 are present but at very low concentration (b) the total genotoxicity is not due exclusively to these two adducts. In the same way, some other sites (PG/PL in March and May, MS in March, PC/C and CH in August, and MB in September) with a positive CRV value do not exhibit adducts 1 and 2.
In Vitro Biotransformation of Erika Petroleum by Mussel Microsomes
To confirm that mussels have the ability to biotransform the Erika fuel and compare the DNA adduct pattern, we incubated microsomes from mussels' digestive gland, in the presence of Erika fuel extract and DNA. No DNA adducts are observed when Erika fuel is incubated in presence of microsomes alone (). In the same way, no DNA adducts are observed when microsomes are incubated alone (). A diagonal radioactive zone and a high radioactive zone in the top of the pattern are observed when Erika fuel was incubated in presence of microsomes, DNA, and NADPH (). Several individual DNA adducts are observed when Erika fuel is incubated in presence of microsomes, DNA, and AA ().
DISCUSSION
The aim of this study was to follow the genotoxic impact of the Erika oil spill, on digestive gland of mussels (Mytilus edulis), by detection of DNA adducts for a long period. Unfortunately, we have no basis for comparison with the situation prior to the accident because no sampling had been made before the Erika oil spill to detect DNA adducts in mussels. However, this field study demonstrates a genotoxic event on the digestive glands of the mussels living in the coast impacted by the Erika spill. Immediately after the Erika accident (December 1999 and January 2000), a very high amount of DNA adduct could be observed even in the reference site (REF). As the DNA adduct pattern at the REF site is similar in January to those in the other sites, essentially adducts 1 and 2, we can conclude that this site has also been impacted by a drifted Erika oil slick. However the total DNA adduct returned to very low amounts the rest of the year. From February to March, a dramatic decrease of DNA adduct in all the sites was observed and could be explained by the depuration of the water probably due to the weathering and the cleaning operations. The Erika oil contains a high amount of PAH (naphthalene, phenanthrene, chrysene) and PHH (dibenzothiophene) compounds (Citation9) able to react with DNA. To confirm that the DNA adduction could be related to the Erika fuel, we have incubated in vitro microsomes from digestive gland of mussels. Comparison of these patterns with those obtained from digestive glands of mussels living in field analyzed after the Erika oil spill, confirmed that the diagonal zone but also the radioactive zone in the top of the pattern (adducts observed in January 2000) are due to biotransformation of Erika fuel by cytochrome P 450 into genotoxic metabolites reactive with DNA. Biotransformation of Erika fuel in presence of AA by cyloxygenase and/or lipoxygenase also induces the formation of several DNA adducts which have chromatographic properties similar to DNA adducts observed in mussels. Interestingly, the adducts observed in the patterns from June to August () correspond to those formed via the AA pathway. These results demonstrated that Erika fuel is genotoxic after metabolic activation by cytochrome P-450, cyloxygenase, and lipoxygenase.
The monthly sampling time has allowed us to determine the presence and the persistence of two adducts until the end of the study in specific sites. The two papers reporting the use of DNA adduct detection in invertebrate species exposed to an oil spill have not shown the presence of any DNA adducts in the species studied (Citation33,Citation 34). Indeed, the sampling was performed very late after the accident and this corresponds, in our study, to a low quantity of DNA adduct in mussels. These data are not therefore contradictory to ours. When we compare presence of adducts with the relative genotoxic impact between sites (CRV values), it is possible to determine two periods of genotoxic events. A first period from January to April–May 2000 and then in October 2000. In the RNO 02 report (Citation10), the analysis of the chemical contamination (Σ 13 PAH/dws) in mussels exposed to the Erika oil spill along the Atlantic coast has revealed the two same periods of chemical contamination which correspond to the direct chemical contamination by the oil spill from January to April–May 2000 and then the remobilization of the pollutant in the environment in October 2000. The results of the genotoxicity depending on location in our study is in accordance with the spatial chemical contamination in mussels reported in the RNO 02 report (Citation10) leading to the same conclusion that the Finistère, Loire Atlantique, and Vendée counties were the most impacted sites. The persistence of the two adducts represented particularly at the IT (Finistère), LC (Loire Atlantique), and TR (Vendée) sites confirms that these sites where the most impacted.
This work demonstrates the usefulness of DNA adduct detection as a biomarker to determine and follow the genotoxic impact of the Erika oil spill although the genotoxic chemical part of this PAH complex mixture is unknown (Citation37).
CONCLUSION
To our knowledge, this study is the first that uses analysis of DNA adducts on M. edulis, a sentinel organism, to follow the impact of a catastrophic pollution caused by an oil spill during 11 months. Although precise chemical data were not available to establish an indisputable correlation between contamination level in the environment and the biological effect such as DNA adducts, the qualitative pattern compared to the in vitro work with the same fuel provides an evidence of the impact of the oil spill. The persistence of two adducts for several months shows a recurrent genotoxic event on mussels and allow us to discern two periods of contamination and the three sites the most impacted. The genotoxic events seem to be correlate to the chemical contamination revealed in shellfish (Citation10). This study demonstrates that the use of the DNA adduct was suitable to follow the genotoxic impact of the Erika oil spill.
Acknowledgments
The authors thank Ifremer for collection of the material analyzed and financial support. This work was part of the Monerika program set up after the Erika oil spill.
REFERENCES
- Boudet , C. , Chemin , F. and Bois , F. 2000 . Evaluation du risque sanitaire de la marée noire consécutive au naufrage de l'ERKA rapport 6, Institut national de l'environnement industriel et des risques, Ministère de l'Aménagement du Territoire et de l' Environnement, Unité d'Evaluation des Risques Sanitaires, Direction des Risques Chroniques
- International Agency for Research on Cancer (IARC) . 1989 . Occupational Exposures in Petroleum Refining Crude Oil and Major Petroleum Fuels. Monographs on the Evaluation of Carcinogenic Risks to Humans , Lyon, , France : IARC .
- Baars , B. J. 2002 . The wreckage of the oil tanker “Erika” Human Health Risk Assessment of Beach Cleaning, Sunbathing and Swimming . Toxicology Letters , 128 : 55 – 68 .
- Agency for Toxic Substances and Disease Registry (ATSDR) . 1995 . Toxicological Profile for Fuel Oils , Atlanta : U.S. Department of Health and Human Services, Public Health Service .
- Agency for Toxic Substances and Disease Registry (ATSDR) . 1999 . Toxicological Profile for Total Petroleum Hydrocarbons , Atlanta : U.S. Department of Health and Human Services, Public Health Service .
- Irwin , R. J. , VanMourik , M. , Stevens , L. , Seese , M. D. and Basham , W. “ Encyclopaedia-Entry on Fuel Oil ” . In Environmental Contaminants , Vol. 6 , Fort Collins, Colo. : National Park Service, Water Resources Division .
- Concawe (the oil companies' organization for environmental and health protection) . 1998 . Heavy Fuel Oils, Product dossier no. 98/109 , Brussels : Concawe .
- Oudot , J. 2000 . Biodegradability of Erika Fuel Oil, Comptes Rendus De l'Académie des Sciences, Series III . Sciences De La Vie , 323 : 945 – 950 .
- Mazeas , L. and Budzinski , H. 2001 . Polycyclic Aromatic Hydrocarbon 13C/12C Ratio Measurement in Petroleum and Marine Sediments: Application to Standard Reference Materials and a Sediment Suspected of Contamination from the Erika Oil Spill . Journal of Chromatography , A923 : 165 – 176 .
- RNO . Surveillance du milieu marin. Travaux du réseau national d'observation de la qualité du milieu marin, Edition 2002 Ministère de l'Ecologie et du développement durable, ISSN 1620–1124
- Lacroix , G. Mars. Evaluation sanitaire des risques lors des soins apportés aux oiseaux mazoutés, rapport 2 Institut national de l'environnement industriel et des risques, Ministère de l'Aménagement du Territoire et de l'Environnement, Unité d'Evaluation des Risques Sanitaires, Direction des Risques Chroniques
- Le Hir , M. and Hily , C. 2002 . First Observations in a High Rocky-Shore Community after the Erika Oil Spill (December 1999, Brittany, France) . Marine Pollution Bulletin , 44 : 1243 – 1252 . http://dx.doi.org/10.1016/S0025-326X%2802%2900217-5
- Carls , M. G. , Babcock , M. M. , Harris , P. M. , Irvine , G. V. , Cusick , J. A. and Rice , S. D. 2001 . Persistence of Oiling in Mussel Beds after the Exxon Valdez Oil Spill . Marine Environmental Research , 51 : 167 – 190 .
- Stagg , R. M. , Robinson , C. , McIntosh , A. M. , Moffat , C. F. and Bruno , D. W. 1998 . The Effects of the Braer Oil Spill, Shetland Isles, Scotland, on P4501A in Farmed Atlantic Salmon (Salmo salar) and the Common Dab (Limanda limanda) . Marine Environmental Research , 46 : 301 – 306 .
- Kirby , M. F. , Neall , P. and Tylor , T. 1999 . EROD Activity Measured in Flatfish From the Area of the Sea empress Oil Spill . Chemosphere , 38 : 2929 – 2949 .
- Jewett , S. C. , Dean , T. A. , Woodin , B. R. , Hoberg , M. K. and Stegeman , J. J. 2002 . Exposure to Hydrocarbons 10 Years after the Exxon Valdez Oil Spill: Evidence from Cytochrome P4501A Expression and Biliary FACs in Nearshore Demersal Fishes . Marine Environmental Research , 54 : 21 – 48 .
- Thomas , R. E. , Brodersen , C. , Carls , M. G. , Babcock , M. and Rice , S. D. 1999 . Lack of Physiological Responses to Hydrocarbon Accumulation by Mytilus trossulus after 3–4 Years Chronic Exposure to Spilled Exxon Valdez Crude Oil in Prince William Sound . Comparative Biochemistry and Physiology , 122 : 153 – 163 . http://dx.doi.org/10.1016/S0742-8413%2898%2910099-3
- Adams , S. M. , Shepard , K. L. , Greeley , J. , Jimenez , B. D. , Ryon , M. G. , Shugart , L. R. , McCarthy , J. F. and Hinton , D. E. 1989 . The Use of Bioindicators for Assessing the Effects of Pollutant Stress on Fish . Marine Environmental Research , 28 : 459 – 464 .
- van der Oost , R. , Goksoyr , A. , Celander , M. , Heida , H. and Vermeulen , N. P. E. 1996 . Biomonitoring of Aquatic Pollution with Feral Eel (Anguilla anguilla): II. Biomarkers: Pollution-Induced Biochemical Responses . Aquatic Toxicology , 36 : 189 – 222 .
- Dunn , B. P. , Black , J. J. and Maccubbin , A. 1987 . 32P-postlabeling Analysis of Aromatic DNA Adducts in Fish from Polluted Areas . Cancer Research , 47 : 6543 – 6548 .
- Kurelec , B. , Garg , A. , Krca , S. , Chacko , M. and Gupta , R. C. 1989 . Natural Environment Surpasses Polluted, Environment in Inducing DNA Damage in Fish . Carcinogenesis , 10 : 1337 – 1339 .
- Varanasi , U. , Reichert , W. L. and Stein , J. E. 1989 . 32P-postlabeling Analysis of DNA Adducts in Liver of Wild English Sole (Parophrys vetulus) and Winter Flounder (Pseudopleuronectes americanus) . Cancer Research , 49 : 1171 – 1177 .
- Stein , J. E. , Reichert , W. L. , French , B. and Varanasi , U. 1993 . 32P-postlabeling Analysis of DNA Adduct Formation and Persistence in English Sole (Pleuronectes vetulus) Exposed to Benzo[a]pyrene and 7H-dibenzo[c,g]carbazole . Chemico-Biological Interactions , 88 : 55 – 69 .
- French , B. L. , Reichert , W. L. , Hom , T. , Nishimoto , M. , Sanborn , H. R. and Stein , J. E. 1996 . Accumulation and Dose-Response of Hepatic DNA Adducts in English Sole (Pleuronectes vetulus) Exposed to a Gradient of Contaminated Sediments . Aquatic Toxicology , 36 : 1 – 16 .
- Burgeot , T. , Bocquéné , G. , Porte , C. , Dimeet , J. , Santella , R. M. , Garcia de la Parra , L. M. , Pfohl-Leszkowicz , A. , Raoux , C. and Galgani , F. 1996 . Bioindicators of Pollutant Exposure in the Northwestern Mediterranean Sea . Mar. Ecol. Prog. Ser. , 131 : 125 – 141 .
- Harvey , J. S. , Lyons , B. P. , Waldock , M. and Parry , J. M. 1997 . The Application of the 32P-postlabelling Assay to Aquatic Biomonitoring . Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis , 378 : 77 – 88 .
- Stephensen , E. , Svavarsson , J. , Sturve , J. , Ericson , G. , Adolfsson-Erici , M. and Forlin , L. 2000 . Biochemical Indicators of Pollution Exposure in Shorthorn Sculpin (Myoxocephalus scorpius), Caught in Four Harbours on the Southwest Coast of Iceland . Aquatic Toxicology , 48 : 431 – 442 .
- Baumard , P. , Budzinski , H. , Garrigues , P. , Sorbe , J. C. , Burgeot , T. and Bellocq , J. 1998 . Concentrations of PAHs (Polycyclic Aromatic Hydrocarbons) in Various Marine Organisms in Relation to Those in Sediments and to Trophic Level . Marine Pollution Bulletin , 36 : 951 – 960 .
- Akcha , F. , Izuel , C. , Venier , P. , Budzinski , H. , Burgeot , T. and Narbonne , J. F. 2000 . Enzymatic Biomarker Measurement and Study of DNA Adduct Formation in Benzo[a]pyrene-Contaminated Mussels, Mytilus galloprovincialis . Aquatic Toxicology , 49 : 269 – 287 .
- Xu , L. , Zheng , G. J. , Lam , P. K. S. and Richardson , B. J. 1999 . Relationship Between Tissues Concentrations of Polycyclic Aromatic Hydrocarbons and DNA Adducts in Green Lipped Mussels (Perna viridis) . Ecotoxicology , 8 : 73 – 82 .
- Phillips , D. H. and Castegnaro , M. 1999 . Standardization and Validation of DNA Adduct Postlabelling Methods: Report of Interlaboratory Trials and Production of Recommended Protocols . Mutagenesis , 14 : 301 – 315 .
- Reddy , M. V. 2000 . Methods for Testing Compounds for DNA Adduct Formation . Regulatory Toxicology and Pharmacology , 32 : 256 – 263 .
- Sole , M. , Porte , C. , Biosca , X. , Mitchelmore , C. L. , Chipman , J. K. , Livingstone , D. R. and Albaiges , J. 1996 . Effects of the Aegean Sea Oil Spill on Biotransformation Enzymes, Oxidative Stress and DNA-Adducts in Digestive Gland of the Mussel (Mytilus edulus L.) . Comparative Biochemistry and Physiology , 113 : 257 – 265 .
- Harvey , J. S. , Lyons , B. P. , Page , T. S. , Stewart , C. and Parry , J. M. 1999 . An Assessment of the Genotoxic Impact of the Sea Empress Oil Spill by the Measurement of DNA Adduct Levels in Selected Invertebrate and Vertebrate Species . Mutation Research/Genetic Toxicology and Environmental Mutagenesis , 441 : 103 – 114 .
- Livingstone , D. R. 1991 . Organic Xenobiotic Metabolism in Marine Invertebrates, in Advances in Comparative and Environmental Physiology , 45 – 185 . Berlin : Springer-Verlag .
- Dolcetti , L. , Zuanna , L. Dalla. and Venier , P. 2002 . DNA Adducts in Mussels and Fish Exposed to Bulky Genotoxic Compounds . Marine Environmental Research , 54 : 481 – 486 .
- Kurelec , B. and Gupta , R. C. 1993 . Biomonitoring of Aquatic Systems , 365 – 372 . Lyon : IARC . IARC Scientific Publications
- de Kok , T. M. C. M. , Moonen , H. J. J. , van Delft , J. and van Schooten , F. J. 2002 . Methodologies for Bulky DNA Adduct Analysis and Biomonitoring of Environmental and Occupational Exposures . Journal of Chromatography , B778 : 345 – 355 .
- Aas , E. , Baussant , T. , Balk , L. , Liewenborg , B. and Andersen , O. K. 2000 . PAH Metabolites in Bile, Cytochrome P4501A and DNA Adducts as Environmental Risk Parameters for Chronic Oil Exposure: A Laboratory Experiment with Atlantic Cod . Aquatic Toxicology , 51 : 241 – 258 .
- Pfohl-Leszkowicz , A. , Chakor , K. , Creppy , E. E. and Dirheimer , G. 1991 . “ DNA-Adducts Formation and Variation of DNA-Methylation after Treatment of Mice with Ochratoxine A ” . In Mycotoxins, Endemic Nephropathy and Urinary Tracts Tumours , 245 – 253 . Lyon : IARC Scientific Public . in
- Reddy , M. V. and Randerath , K. 1986 . Nuclease P1-Mediated Enhancement of Sensitivity of 32P-postlabeling Test for Structurally Diverse DNA Adducts . Carcinogenesis , 7 : 1543 – 1551 .
- Reyjol , Y. Variabilité spatio-temporelle de la transition SalmoniformesCypriniformes dans la Garonne Thèse de Doctorat, INP Toulouse