Abstract
Eight polycyclic aromatic hydrocarbons (PAHs) including benzo[a]pyrene were analyzed in sediment and benthos collected on the coast of Chiba Prefecture in Japan by gas chromatography-mass spectrometry (GC/MS). The total concentration of the PAHs ranged from 8 to 18 μ g/kg-dry in the sediments and from 36 to 59 μ g/kg-wet in the benthos, Mactra quadrangularis, Scoletoma nipponica, and Arenicola basiliensis. The PAH composition in the sediments and the benthos was similar at four sampling points and dominated by phenanthrene (Phe), fluoranthene (Flu), and pyrene (Pyr). The ratios of abundance of Flu to that of Pyr, Flu/Pyr, and of Phe/anthracene suggest that the PAHs in the benthos are derived from the combustion of fossil fuels. The ratio, R, of the PAH concentration in the benthos to that in the sediment is 2.0 for Scoletoma nipponica and 1.8 for Arenicola basiliensis in average, indicating that the bioaccumulation is not significant in the benthos. However, the R value for perylene in Scoletoma nipponica was 3.7 times as large as the average.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) exist widely in our environment and originate from a variety of source. There are two types of anthropogenic sources of PAHs: petrogenic and pyrogenic sources. Crude and refined petroleum contains PAHs (i.e., petrogenic PAHs). They are introduced to aquatic environments through accidental oil spills, discharge from routine tanker operations, municipal and urban runoff, and so on. The combustion of fossil fuel (coal and petroleum) and biomass also produces PAHs (i.e., pyrogenic PAHs), which are released into the environment in the form of exhaust and solid residues (Citation1). Some PAHs such as benzo[a]pyrene and benzo[a]anthracene are mutagenic and carcinogenic. In addition, a few of them such as benzo[a]pyrene have been listed as endocrine disruptors in the “environmental hormone” category (Citation2, Citation3). They tend to spread into sediments and soils due to their low solubilities in water (Citation4). Thus it is important to analyze the exact concentration and the source of PAHs in the environment, especially sediments, soils, and organisms living in them, in order to investigate their influence on the human body and the environment. In recent years, PAHs have been analyzed quantitatively in various areas in Japan. In 2002, the concentration of benzo[a]pyrene in water, sediment and aquatic organism samples were measured all over the country (Citation5). The results showed that the mean concentration of benzo[a]pyrene was 21 ng/g-dry in sediments. Although Chiba (located near Tokyo) has many people and factories, such analysis was performed only in one spot in Chiba.
In this study, we determined the concentration and composition of 8 PAHs including benzo[a]pyrene in sediment and benthos samples collected from 4 sampling points in Chiba including the Tokyo Bay. The analysis revealed that PAHs produced from combustion were detected in all sampling points and their concentrations were lower than the national average.
EXPERIMENTAL
Materials
Phenanthrene (Phe), anthracene (Anth), fluoranthene (Flu), pyrene (Pyr), chrysene (Chry), benzo[b]fluoranthene ([b]flu), benzo[a]pyrene ([a]pyr), and perylene (Pery) were selected as target compounds. Deuterium-labeled PAHs: phenanthrene-d 10 (Phe-d), anthracene-d 10 (Anth-d), fluoranthene-d 10 (Flu-d), pyrene-d 10 (Pyr-d), chrysene-d 12 (Chry-d), benzo[a]pyrene-d 12 ([a]pyr-d), and perylene-d 12 (Pery-d) were used as surrogate standards for determining the recovery rates of PAHs in the sample preparation procedure. p-Terphenyl-d 14 (p-ter) was used as an internal standard. These reagents were purchased from Wako Pure Chemicals Industries, Ltd. (Japan). Hexane, acetone, ethanol and sodium sulfate (Wako Pure Chemicals Industries, Ltd., Japan) were of residual agricultural chemicals grade. Water was purified by WT100 (Yamato Scientific Co., Ltd., Japan) and washed with hexane twice before using.
GC/MS Analysis
A GC/MS system (QP2010, SHIMADZU, Japan) equipped with 30 m Rtx-5MS fused silica capillary column (0.25 mm i.d. coated with a 0.25 μ m thickness 5% diphenyl-95% dimethyl polysiloxane film, Restek, USA) was used for the analysis of PAHs. High purity helium (99.999%) was used as a carrier gas. The temperature program was optimized to separate each PAH quickly as follows: initial temperature 50°C, held for 2 min, increased at 30°C min−1 to 170°C, then increased at 10°C min−1 to 320°C with a final held for 5 min. The mass spectrometer was operated in the electron impact ionization mode at 70 eV. The interface was kept at 250°C and the ionisation source 200°C. Measurements were performed in the SIM mode; the m/z values used for the detection of each PAH and an internal standard are shown in .
TABLE 1 SIM conditions for GC/MS analysis of PAHs and an internal standard
Sample Collection
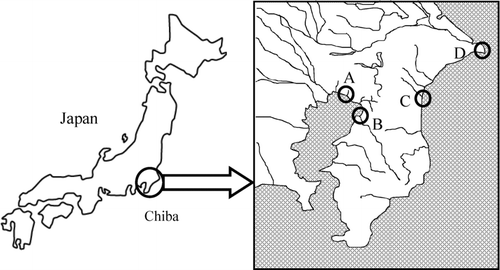
Sediment samples were collected in four sampling points which have different aspects in Chiba Prefecture in 2006 (). “Makuhari” is an artificial sandy shore surrounded by many office buildings and institutions. “Yoro” is a mud flat located in the factory district that has power plants using liquefied natural gas and many factories such as oil refining, petrochemical, and chemical. This tidal flat is in the large-scale factory district.
Moreover these two points face the Tokyo Bay. On the other hand, “Choshi” and “Naruto” are located in the east of Chiba and have smaller populations than “Makuhari” and “Yoro.” All implements and vessels used for sampling were washed by detergent and ion-exchanged water beforehand. The sediment samples were collected by digging a vinyl chloride pipe, a diameter of 8.5 cm and a length of 1 m, into the sediment. Then the samples were divided into 2 parts by the depth from the surface; a shallow part (0–20 cm) and a deep part (20–40 cm). The former part was oxidative, and the latter reductive. These samples were sealed in plastic vessels and stored at −20°C until analysis. The benthos samples, Mactra quadrangularis, Scoletoma nipponica, and Arenicola basiliensis, were gathered in Makuhari and Yoro. The samples were sealed in plastic vessels washed and stored at −20°C until analysis.
Sample Preparation
The collected sediment was thawed, mixed and sieved through 2-mm mesh. About 20 g of the sieved sample was placed in a centrifugation bottle with 50 ml acetone and 10 μ l of a surrogate standard mixture (10 μ g/ml). Then the sample was shaken for 10 min, sonicated for 10 min, and centrifuged 3000 rpm for 10 min. The supernatant acetone was transferred to an evaporation flask. This extraction process with acetone was repeated twice and the acetone extract (about 100 ml) was concentrated to about 20 ml with a rotary evaporator. The concentrated extract was alkaline decomposed by adding 50 ml of 1M KOH/EtOH solution and shaking (130 rpm) for 15 h at room temperature in the dark. After the decomposition, the solution was transferred to a separatory funnel and 20 ml of ethanol/hexane (1:1) solution, 50 ml of hexane, and 50 ml of refined water were poured into the funnel, which was shaken for 10 min. After removal of the hexane layer, the same procedure was repeated for the water layer remaining in the funnel. The extracted hexane solution (about 100 ml in total) was washed twice with refined water (50 and 25 ml), dried with Na2SO4, filtered, and concentrated to about 1 ml with a rotary evaporator and a gentle flow of N2. The concentrated solution was subjected to column chromatography with 5 g of 5% H2O-deactivated silica gel column and eluted with 17 ml of hexane followed by 100 ml of acetone: hexane (1:99, v/v). The second elute was concentrated by flowing N2 gas and diluted to 1 ml with hexane after the addition of 10 μ l of p-ter (10 μ g/ml) to the sample as an internal standard. The sample solution thus prepared was analyzed by GC/MS; the measurement was repeated twice.
On the other hand, benthos samples were prepared using a procedure almost the same as that used for the sediment samples. Bivalve samples were thawed out and microwaved for about 2 min. The internal organs and muscles were divided and individually homogenized. Annelid samples were homogenized after discharging the excrement from the body. About 2–10 g of the homogenized sample was placed in a brown plastic bottle and 50 ml of 1M KOH/EtOH solution and 10 μ l of surrogate standard mixture (10 μ g/ml) were added. These samples were decomposed for 15 h using a magnetic stirrer at room temperature. Then the samples were shaken for 2 h (170 rpm) to be perfectly decomposed of the sample adhered to the vessel, deposition, and adhered sample. The following procedure is the same as the sediment sample.
RESULTS AND DISCUSSION
Analytical Method
The PAHs were eluted within 10 to 20 min. To determine the instrument detection limit (IDL) and instrument quantification limit (IQL), we analyzed the standard solution (0.005 ppm) seven times repeatedly and obtained the relative standard deviation (σ) from the ratio of the peak area. IDL and IQL for each PAH were 0.41–1.08 μ g/l (3σ) and 1.24–3.23 μ g/l (9σ). Calibration plots were constructed in the range of 0.005 to 0.3 ppm and good correlation coefficients (≧ 0.999) were obtained for all PAHs and surrogates.
Concentrations of PAHs in Sediment Samples
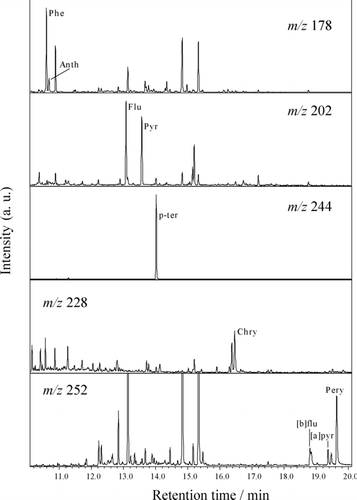
shows the SIM chromatograms of PAHs from the Yoro sediment sample. The peak of [b]flu at m/z = 252 was overlapped by other peaks; such overlap appeared in chromatograms for all sediment and benthos samples. We assigned the peak at a retention time (r) of 18.78 min to [b]flu by the standard addition method; the slower peak at r = 18.82 min was supposed to be due to benzo[k]fluoranthene, an isomer of [b]flu (Citation6). In this study, the shallow (0–20 cm) and deep (20–40 cm) parts were analyzed separately for all samples, but no difference was found between them. The mean (± σ) PAH concentrations in the sediment samples (0–40 cm) collected in each sampling point are shown in . The recovery rates ranged from 55 to 125% and there were no significant differences in the rates among the sampling points. The rates tend to increase with an increase in the number of benzene rings, or in the hydrophobicity of PAHs. This tendency may arise from the loss of PAHs with low molecular weights during the hexane extraction process.
TABLE 2 Concentrations of PAHs in sediment samples (μ g/ kg-dry)
The total PAH concentration of the four sampling points was 18 μ g/kg-dry in Yoro, 11 μ g/kg-dry in Makuhari, 9 μ g/kg-dry in Choshi, and 8 μ g/kg-dry in Naruto. The difference in the PAH concentrations can be ascribed to the difference in the density of population and the number of factories near the sampling point: there are power plants and chemical factories in the neighborhood of the Yoro river and as a result PAHs produced by combustion are discharged and deposited. On the other hand, there are far fewer houses and factories in Choshi and Naruto. It was reported by the Ministry of the Environment that the concentration of [a]pyr is 21 μ g/kg-dry on average, though it ranges from n.d. to 1200 μ g/kg-dry, [a]pyr concentration is 126 μ g/kg-dry in sediments on the coast of Tokyo Bay (Citation5). The present study revealed that the PAH concentration of our samples is somewhat lower than average. The finding is unexpected one. To confirm this, we are going to perform the analysis at the other points in Chiba.
The PAH composition in the sediments of the four sampling points was similar and dominated by Phe, Flu, and Pyr, except that Pery was detected at markedly high concentration (3.3 μ g/kg-dry) in the Yoro sediment. Such a high concentration of Pery in sediment was sometimes reported and discussed (Citation1, Citation7).
The ratios of the concentrations of the PAHs contained in sediment samples may correlate to the source of the PAH generation (Citation8, Citation9). When the ratio of the concentration of Flu to that of Pyr, Flu/Pyr, is greater than 1.0, it is most likely that the PAHs originate from pyrolytic and combustion related sources, and when the ratio less than 1.0, petrogenic sources are implied (Citation8). In addition, a ratio of Phe/Anth of less than 10 indicates the potential origin of PAHs from combustion processes (Citation9). In the case of Yoro, the ratios of Flu/Pyr and Phe/Anth were 1.3 and 6.5, respectively. Similar results were obtained in Makuhari, Choshi, and Naruto. These findings suggest that the PAHs in Yoro and other sediments are derived from the combustion of fossil fuels.
Concentrations of PAHs in Benthos Samples
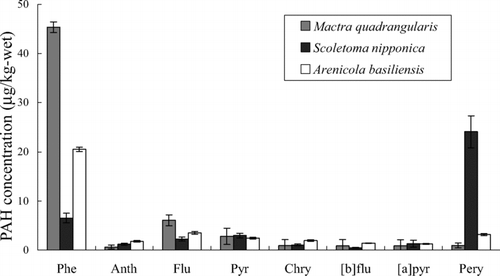
shows the PAHs concentrations in bentic organisms in the tidal flat of Yoro. We analyzed the internal organs and muscles separately for bivalve (Mactra quadrangularis), but there was no difference between them. Then the mean concentrations were shown in . Phenanthrene was detected in large quantities in all benthos living in Yoro, while Anth and penta-cyclic PAHs were scarcely detected (< 1 μ g/kg-wet) except Pery of Scoletoma nipponica. Phe was also detected in Mactra quadrangularis and Neverta ddidyma collected in Makuhari and Corbicula japonica in Tama River. The PAH composition of the benthos samples in Makuhari was almost the same as that in Yoro (data not shown). The high concentration of Phe especially in Mactra quadrangularis is ascribed to the nature of bivalves in that they are filter feeders and ingest pollutants adsorbed on particles and dissolved into water (Citation7). The main assimilation route for lower-molecular-weight and water-soluble PAHs is considered to be the direct absorption of dissolved compounds through interstitial water (Citation9). Thus it is suggested that Phe in Mactra quadrangularis were accumulated in bivalves through water.
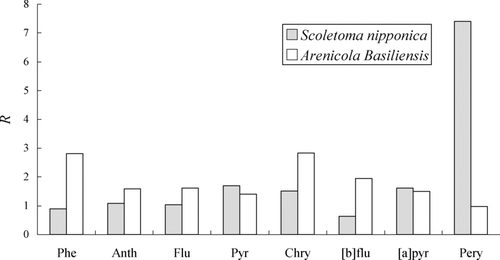
shows the ratios, R, of the PAH concentration in the body of Scoletoma nipponica and Arenicola basiliensis to that in the sediment; the R value is considered as a degree of the bioaccumulation for PAHs. An average of the R value is 2.0 for Scoletoma nipponica and 1.8 for Arenicola basiliensis. Therefore the degree of bioaccumulation is not very marked in the benthos. The concentrations of Phe in Arenicola basiliensis is significantly high in , but the corresponding R value is 2.8 in , only slightly larger than the average. Contrary to this, the data of Pery of Scoletoma nipponica is remarkable in both and . The findings indicate that Scoletoma nipponica seems to store Pery selectively. If this is the case, the metabolic pathway of Pery in the benthos is of much interest. To clarify the fate of PAHs in the sediment and benthos, analysis on the PAHs in the excrement of the benthos is needed. It may also provide useful information on the bioaccumulation.
CONCLUSION
The concentrations of 8 PAHs were measured in sediment and benthos samples collected in four sampling points in Chiba Prefecture. The total PAH concentration ranged from 8 to 18 μ g/kg-dry in sediments, which is lower than the national average. The PAH composition in the sediments of the four sampling points was similar and dominated by Phe, Flu, and Pyr which derived from the combustion of fossil fuels. The high concentration of Phe in Mactra quadrangularis suggested that the bivalve takes feed through water. The accumulation of the PAHs in the benthos was not significant, but that of Pery in Scoletoma nipponica was 3.7 times as large as the average.
REFERENCES
- Zakaria , M. P. , Takada , H. , Tsutsumi , S. , Ohno , K. , Yamada , J. , Kouno , E. and Kumata , H. 2002 . Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs . Environ. Sci. Technol. , 36 : 1907 – 1918 .
- Liu , X. and Korenaga , T. 2001 . Dynamics analysis for the distribution of polycyclic aromatic hydrocarbons in rice . J. Health Sci. , 47 : 446 – 451 .
- Webster , L. , Russel , M. , Packer , G. and Moffat , C. F. 2006 . Long term monitoring of polycyclic aromatic hydrocarbons (PAHs) in blue mussels (Mytilus edulis) from a remote Scottish location . Polycycl. Aromat. Comp. , 26 : 283 – 298 .
- Irwin , R. J. and National Park Service . 1997 . Environmental Contaminants Encyclopedia, s.v. “PAHS.” , Colorado : National Park Service .
- Ministry of the Environment, Government of Japan . Report on environmental survey and monitoring of chemicals, FY2003
- Li Liu , K. H. L. , Hashi , Y. , Maeda , T. and Lin , J. 2007 . Solid-phase extraction with C30 bounded silica for analysis of polycyclic aromatic hydrocarbons in airborne particulate matters by gas chromatography-mass spectrometry . J. Chromatogr. A , 1154 : 74 – 80 .
- Nakata , H. , Sakai , Y. , Miyawaki , T. and Takemura , A. 2003 . Bioaccumulation and toxic potencies of polychlorinated biphenyls and polycyclic aromatic hydrocarbons in tidal flat and coastal ecosystems of the Ariake Sea, Japan . Environ. Sci. Technol. , 37 : 3513 – 3521 .
- Sicre , M. A. , Marty , J. C. and Saliot , A. 1987 . Aliphatic and aromatic hydrocarbons in different sized aerosols over the Mediterranean Sea: Occurrence and origin . Atmos. Environ. , 21 : 2247 – 2259 .
- Pascale , B. , Helene , B. and Philippe , G. 1998 . Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean Sea . Environ. Toxicol. Chem. , 17 : 765 – 776 .