Room-temperature ionic liquids are salts with a melting point close to or below room temperature. They form liquids composed in the majority of ions. This gives these materials the potential to behave very differently when they are used as solvents compared to conventional molecular liquids. The search for their application is growing in every area of analytical chemistry—electrochemistry, chromatography, electrophoresis, and even mass spectrometry. The literature on ionic liquids is growing almost exponentially. The basis for this activity is the easy preparation of salts with different ion constituents. This ability might best be described as the “chemical tunability” of ionic liquids, a class of solvents with members possessing similar physical properties but having different chemical behavior. Their good solvating properties, together with large spectral transparency, make ionic liquids suitable solvents for spectroscopic measurements. It has been demonstrated that task-specific ionic liquids have advantages compared to common solvents used as separation media in liquid-liquid extraction processes achieving high efficiencies and selectivities of separation. The main advantage for other applications of ionic liquids in analytical chemistry lies in their low volatility which makes them useful as solvents for working in both high-temperature (gas chromatography (GC) stationary phases) and high-vacuum (MALDI matrixes) environments. When using an ionic liquid as an electrolyte medium, it is possible to achieve a broader range of operational temperatures and conditions relative to other conventional electrolytic media, and this makes ionic liquids promising materials in various electrochemical devices (e.g., batteries, fuel cells, sensors, and electrochromic windows).
IONIC LIQUIDS AS ROOM-TEMPERATURE MOLTEN SALTS
One of the principal driving forces for the broad investigations in the area of new materials is the need to find replacements for environmentally hazardous volatile organic solvents in many different commercial chemical processes. Most of the familiar liquids are formed from neutral molecules and are non-ionic. There is a different class of room-temperature liquids that, unlike the molecular liquids, is constituted of ions. These liquids are called ionic liquids (ILs). For a substance to be considered a room-temperature ionic liquid (RTIL), its melting point should be below 100°C. Room-temperature molten salts are not new. The first representative of them, ethylammonium nitrate [C2H5NH3]+[NO3]− which has a melting point 12°C, has been known since 1914 [Citation1].
There are few classes of organic salts, such as the quaternary ammonium and imidazolium salts, which exhibit room-temperature liquid behavior. The major interest in ILs was first connected with their potential for the development of new electrolytes to be used in the electrical batteries and electroplating of aluminum. One such kind of room-temperature molten salt system was reported in the late 1940s by Hurley and Wier [Citation2, Citation3]. In their work, fused mixtures of ethylpyridinium bromide and metallic chlorides were studied.
It was the discovery of 1-ethyl-3-methylimidazolium [EMIm]-based chloroaluminate ionic liquids [Citation4] in 1982 that provided acceleration for an activity in the area of (RTILs). The named salt is one of the most widely studied room-temperature melt systems, which is liquid at room temperature for compositions between 33 and 67 mol% AlCl3. The exiting property of halogenoaluminate ILs is their ability for acid base chemistry, which can be varied by controlling the molar ratio of the two components. This kind of tuning makes these ILs attractive as nonaqueous reaction media [Citation5]. A considerable drawback of aluminum chloride-based ionic liquids concerns their moisture sensitivity and need to be protected rigorously from moisture. Air- and water-stable molten salts can be obtained using the weakly complexing anion in the imidazolium compound [Citation6]. Unlike [AlCl4]− anion, [CF3SO3]−, [BF4]−, [PF6]− anions do not form polynuclear anions, and these salts are neutral stoichiometric compounds. The inertness and facile handling capability of ILs has greatly enhanced the possibilities for their application. An excellent short history of ILs covering the most important moments of this area up to 1994 has been presented by eyewitness to and participant in crucial developments, Professor John S. Wilkes [Citation7].
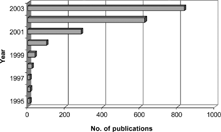
Figure 1. Publications containing the phrase “ionic liquid” in the title, abstract, or keywords, determined by SciFinder, as a function of time.
These new types of liquids can be classified as anhydrous, aprotic solvents, but the most characteristic property of ILs is their very low vapor pressure. Their physical and chemical properties can be finely tuned for a range of applications by varying the chemical constituents. For example, physical properties in class of alkylimidazolium ILs vary over wide ranges. They are more dense than water with density values from 1.0 to 1.5 g/cm3; viscous liquids similar to oils with viscosity values from 0.20 to 4.0 Poise at 25°C, refractive index 1.3–1.5, electrical conductivity in the range of 60 to 250 S/cm.
The same kind of variability is seen relation to water, for example, [BMImCl]-AlCl3 reacting with water; [BMIm][PF6] hydrophobic; [BMIm][CH3COO] water soluble.
One of the advantages of ILs is their thermal robustness. This means a large thermal operating range is possible (typically −40°C to 200°C) which enables a wide range of kinetic control for reactions that proceed in ILs. The few data on heat capacity show values similar on water and on thermal conductivity values closer to toluene [Citation8].
Ionic liquids not only can be applied in existing methods where improvement of sensitivity and selectivity of analysis is always needed, but their different behaviors and properties can offer original solutions in chemical analysis and the search for new applications of ILs is growing in every area of chemistry.
The use of ILs is a very rapidly developing area in chemistry according to the literature, and the number of publications on ILs is growing almost exponentially. In recent years several reviews have been published in different journals, where one can obtain data about properties and applications of ionic liquids [Citation9]. Chapters in the books and proceedings are also excellent sources for information about ILs [Citation10].
DEVELOPMENT OF METHODS, WHERE IL ARE USED AS NOVEL MEDIA
Electrochemistry
Electrochemists appear to prefer the term “room-temperature molten salt,” which must be kept in mind when doing literature searches. There have been some reviews [Citation11, Citation12] on electrochemical use of ILs examining the current state of IL-based electrochemistry, with particular focus on the work of the past decade. The widespread ILs are more or less be divided into three groups: (a) systems based on AlCl3 and organic salts such as 1-butylpyridinium chloride [BPCl], 1-alkyl-3-methylimidazolium chloride [CnMIm]Cl, and derivatives; (b) systems based on organic cations as in (a) and anions BF4, PF6 and SbF6; and (c) systems based on the aforementioned organic cations with anions of the type CF3SO3, (CF3SO2)2N and similar. Liquids made on the basis of the latter ions are stable under ambient conditions with only low water uptake.
Good electrolytes should have high conductivity, large electrochemical windows, excellent thermal and chemical stability, and negligible evaporation. When using IL as an electrolyte medium, it is possible to satisfy the conditions and achieve a broader range of operational temperatures and conditions relative to other conventional electrolytic media. This makes ILs promising materials in various electrochemical devices, such as, batteries, fuel cells, sensors, and electrochromic windows.
One report [Citation13] showed that a lithium ion cell with an IL electrolyte performed at a level of practical utility in terms of cell performance and cycle durability. Both the room-temperature IL [Citation14] and proton-conducting gelatinous electrolytes templated by RTILs [Citation15] have been studied as possible solvents in lithium batteries.
Metals that can be obtained from aqueous media in most cases can also be deposited from ILs, often with superior quality because hydrogen evolution does not occur. These features and their good ionic conductivities—between 10−3 and 10−2Ω−1cm−1—make ILs interesting solvents for low-temperature electrodeposition studies, especially with respect to elements that cannot be obtained from aqueous solutions (e.g., silicon, germanium, aluminum, titanium, and plutonium).
Most of the basic studies on metal and alloy deposition have been performed in AlCl3-based ILs. However, interesting systems that are made from organic salts and different metal salts also have been reported in the literature, and the new air-stable liquids are being intensively investigated for catalytic studies and green chemistry purposes. One example is Ref. [Citation16] where the report that silicon can be electrodeposited well on the nanoscale in the RTIL 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide saturated with SiCl4 is presented. This liquid exhibits on highly oriented pyrolytic graphite (HOPG) an electrochemical window of 4 V, which is limited in the anodic regime by the degradation of HOPG, in the cathodic regime by the irreversible reduction of the organic cation. A silicon layer with a thickness of 100 nm exhibits a band gap of 1.0 ± 0.2 eV, which is shown by in situ current/voltage tunneling spectroscopy, indicating that semiconducting silicon was electrodeposited.
However, although these systems have been known for a long time, industrial standard procedures have not yet been established. A shortcoming is that, for studies on less noble elements (e.g., germanium and aluminum), the liquids have to be free of water so that at least a final dry atmosphere is required. Nevertheless, this is not a principal problem and the wide electrochemical windows of at least 4 V are very attractive.
Microdroplet and thin film deposits of an IL, 1-methyl-3-(2,6-(S)-dimethylocten-2-yl)-imidazolium tetrafluoroborate [MDIM][BF4], on electrode surfaces have been studied in order to assess the ability of aqueous ions to partition into the IL [Citation17]. Two complementary methodologies were employed deposition of IL onto a 4.9-mm-diameter basal plane pyrolytic graphite electrode followed by voltammetric analysis and casting of an IL film onto a random array of 7-μm-diameter carbon microelectrodes (RAMTM electrode) followed by chronoamperometry. The ability of hydrophilic ions to partition from the water phase into the IL phase was unexpected and shown to depend strongly on (a) the type of ion, (b) the specific interaction of the ion with the IL, and (c) the concentration of the supporting electrolyte. Voltammetric responses obtained for the reduction of Fe(CN)6 3− partitioned into microdroplet and thin film deposits of MDIM+BF4 − indicate selective uptake of ferricyanide into the IL phase. Chronoamperometric experiments at RAMTM electrodes were used to quantify both the concentration of Fe(CN)6 3− in the IL and the diffusion coefficient.
In some cases, electrochemical properties of probes in ILs are not much different than in conventional organic solvents. This has been reported in a study [Citation18] on the voltammetric investigation of the reactivity of a cobalt Schiff base complex (namely, N, N′-bis(salicylidene)-ethylene diamino cobalt(II), so-called CoII–salen, in 1-butyl-3methylimidazolium hexafluorophosphate, [BMIm][PF6]) toward selected organohalogenated derivatives. The obtained results indicate that the electrochemical behavior of CoII–salen in the RTIL is similar to that previously reported in various organic solvents. Also, the nature of the involved mechanisms of the electroreductive activation of the organohalogenated derivatives was almost unchanged in [BMIm][PF6] compared with the usual conventional organic solvents. If when testing new methods this kind of result is disappointing, sometimes it would be useful to compare methods in parallel with ILs as was done in next study [Citation19] to obtain similar behavior. These results detail a novel methodology for the electrochemical detection of ammonia based on its interaction with hydroquinone in DMF. It was shown that ammonia reversibly removes protons from the hydroquinone molecules, thus facilitating the oxidative process with the emergence of a new wave at less positive potentials. The analytical utility of the proposed methodology was examined with a linear range from 10 to 95 ppm and corresponding limit-of-detection of 4.2 ppm achievable. Finally, the response of hydroquinone in the presence of ammonia was examined in the RTIL 1-ethyl-3-methylimidazolium bis(trifluormethylsulfonyl)imide, [EMIm][N(Tf)2]. Analogous voltammetric waveshapes to that observed in DMF were obtained, thereby confirming the viability of the method in either DMF or [EMIm][N(Tf)2] as solvent.
Electrolytes play an important role in determining the performance of conducting polymer electrochromic devices. The applications of ILs as electrolytes in electrochemical synthesis of conducting polymers, in electrochemical and electrochromic characterization of both electrochemical and chemically synthesized conducting polymers, and in fabrication of conducting polymer electrochromic devices have been explored [Citation20]. In ionic liquids, highly stable electroactivity was obtained for polyaniline in a wide potential range for greater than 1 million cycles. During the fabrication of electrochromic devices, electrochemically synthesized polymers were employed for displays, while chemically synthesized polymers (via spin-coating) were preferable for large-area electrochromic windows. Based on this, the prototypes of alphanumeric displays and large-area (5× 5 cm) electrochromic windows have been successfully fabricated. High device performance of low operation voltages (< 1.5 V), high coloration contrast (> 50%), fast coloration speed (< 100 ms), and high coulombic efficiency (> 98%) have been realized.
Ionic liquids have the advantage of greater electrochemical stability than water, thus offering the possibility of higher actuation voltages for electrically controlled materials. An IL [EMIm][N(Tf)2] has been evaluated for use as electrolyte for electromechanical actuators based on polypyrrole [Citation21]. The actuator performance in IL electrolytes is significantly better than that in traditional organic and aqueous electrolytes.
In this work [Citation22], the use of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate IL was demonstrated as a viable solvent for Nafion polymer actuators and sensors. Experimental results indicate that Nafion transducers solvated with this IL have improved stability when operated in air as compared to the same materials solvated with water, although the magnitude of the response is decreased as compared to the water samples at high frequencies. The main drawback associated with the use of ILs is a reduction in the speed of the response as compared to water, although the initial results are promising and demonstrate the potential for this approach.
Solid-state amperometric O2 gas sensors were successfully fabricated by using supported 1-n-butyl-3-methylimidazolium hexafluorophosphate [BMIm][PF6] [Citation23] or 1-ethyl-3-methylimidazolium tetrafluoroborate [EMIm][BF4] [Citation24] porous polyethylene membrane as a solid-state electrolyte. The presented O2 gas sensor, which is capable of being operated at room temperature, shows a wide detection range and high stability, high sensitivity, and a excellent reproducibility.
An electrochemical sensor with organic ILs as an electrolyte can be used for analysis of air or as a fire detector [Citation25]. Imidazole or pyridine derivatives and, preferably, 1-ethyl-3-methyl-imidazolium tetrafluoroborate [EMIm][BF4] or 1-ethyl-3-methyl-imidazolium chloride [EMIm][Cl] are used as electrolytes which are applied onto a nonwoven material consisting of silicates or polymers. The detection can be carried out amperometrically or potentiometrically using a two- or three-electrode system.
An attempt has been made to use IL as an ethanol sensor [Citation26]. The detection limit is 0.13% (vol/vol) and its response time was 336 s with a linear relation between the response current and the concentration of ethanol in a relatively wide range.
Extraction
A method for using an IL as a solvent in headspace gas chromatography has been described in Ref. [Citation27]. Thus, ILs containing 39.5 μg ethanol, 45 μg ethylacetate, 39.0 μg cyclohexane, and 43.5 μg toluene were dissolved in 0.1 mL of 1-ethyl-3-methylimidazolium trifluormethanesulfonate [EMIm][CF3SO3] or 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)-imidate [EMIm][N(Tf)2] and gas chromatographic analytes were obtained by using FID detection at 280°C. The ILs may be used in place of conventional solvents in headspace gas chromatography.
Another direction for use of IL as a solvent is extraction, where nonvolatility could be an advantage in cases of large-scale processes, and RTILs thus may be suitable candidates for replacement of volatile organic solvents in liquid-liquid extraction processes. At the same time one can expect different partition coefficients for that kind of unusual liquid, which can make use of ILs preferable at least in chemical analysis. Considering that ILs can be easily prepared from relatively inexpensive materials and their characteristics adjusted by combination of different anions and cations, it is possible to prepare liquids for task-specific extraction of analytes from various solvent media and create new liquid-liquid extraction systems. The variability is shown in data provided in .
Table 1. 1-butyl-3-methyl imidazolium cation-based ionic liquids miscibility with other liquids (Ref. 9e)
There already have been some studies providing distribution coefficients between solvents or water and ILs, also discussing possible mechanisms of partition. The first data were published in Ref. [Citation28], where the partitioning of simple, benzene-substited derivatives between water and the RTIL [BMIm][PF6] was discussed on the basis of the solutes' charged state and relative hydrophobicity. These partition data show a close correlation with partition coefficients of the same solutes in a system of 1-octanol and water. Although the distribution coefficients for the IL-water system are, in general, an order of magnitude lower than those for the 1-octanol-water system, these values are suitable for practical purpose.
Since this selectivity, capacity, viscosity, and thermal stability of ILs can be customized, they appear superior to many conventional entrainers and extraction solvents. Ionic liquids have been found [Citation29] able to break a variety of azeotropic systems. For ethanol-water separation, the nonvolatile [EMIm][BF4] shows a remarkable entrainer performance and therefore can enable extractive distillation processes which require less energy than the conventional process using 1,2-ethanediol as an entrainer. In Ref. [Citation30], ternary vapor-liquid and liquid-liquid equilibriums of the azeotropic mixtures ethanol + water and THF + water containing different kinds of common available ILs were presented. Evaluation of a new THF-water separation process indicates the competitiveness of the suggested process and a considerable potential for using ILs as extraction solvents.
In many cases, the modification of cations and/or anions makes a substantial effect on extraction efficiency. An example of the utilization of RTILs in liquid-liquid partitioning from water has been provided in Ref. [Citation31], where ILs containing fused polycyclic N-alkylisoquinolinium cations ([Cnisoq]+) in combination with the bis(perfluoroethylsulfonyl)imide anion ([BETI]−) gave high distribution ratios for aromatic solutes, especially chlorobenzenes, between the RTIL and water.
For separation of chiral substances the search for new solvents is ongoing, and results on using chiral ILs for this are beginning to appear. It has been shown that the type of ILs based on isoquinoliniums I (R1–R8 = monovalent group; A− = anion) with bis(trifluoromethanesulfonyl)imide anion is useful for chiral resolution by extraction. An aqueous mixture of amino acid DL-Tyr was treated with II to remove 18% L-Tyr from the aqueous phase [Citation32].
Organic solvents are widely used in a range of multiphase bioprocess operations, including the liquid-liquid extraction of antibiotics and two-phase biotransformation reactions. There are, however, considerable problems associated with the safe handling of these solvents because of their toxic and flammable nature. In the work described in Ref. [Citation33], it was shown that RTILs, such as [BMIm][PF6], can be successfully used in place of conventional solvents for the liquid-liquid extraction of erythromycin-A and for the Rhodococcus R312–catalyzed biotransformation of 1,3-dicyanobenzene (1,3-DCB) in a liquid-liquid, two-phase system. Extraction of erythromycin with either butyl acetate or [BMIm][PF6] showed that values of the equilibrium partition coefficient, K, up to 20–25 could be obtained for both extractants. The variation of K with the extraction pH also was similar in the pH range of 5 to 9 though it differed significantly at higher pH values.
Biocompatible ILs can act as a substrate reservoir and an in situ extracting agent in biotransformations with whole cells [Citation34]. In the reduction of 4-chloroacetophenone to (R)-1-(4-chlorophenyl)ethanol, high product concentration (82 g/L−1) and purity (99.6% ee) were achieved without the addition of cofactors and without damaging the biocatalyst.
The extractions of organic acid using imidazolium-based ILs without any extractant are controlled by the hydrophobicity of the acids and, in all cases, the extractability is very low. The extraction behaviors of lactic acid with imidazolium-based ILs containing tri-n-butylphosphate extractant are similar to those of conventional organic solvents. The lactic acid–producing bacterium, Lactobacillus rhamnosus NBRC 3863, grew, consumed glucose, and produced lactate in the presence of imidazolium-based ILs. These findings suggest that ILs could be used in an in situ extractive fermentation process [Citation35].
Amino acids are not soluble in IL; however, it is possible to extract them partially by adding a crown ether to the IL phase. The positive form of amino acids is complexed by the crown ether and the complex is extracted in the IL phase working at low pH [Citation36]. Also, in Ref. [Citation37], amino acids Trp, Gly, Ala, Leu were extracted efficiently from aqueous solution at pH 1.5–4.0 (Lys and Arg at pH 1.5–5.5) into the RTIL [BMIm][PF6] with dicyclohexano-18-crown-6 (DC18C6). The most hydrophilic amino acids, such as Gly, were extracted as efficiently as the less hydrophilic ones (92–96%). The influence of pH, amino acid, and crown ether concentration; volume ratio of aqueous and organic phases; and presence of some cations on amino acid recovery were studied. The ratio of amino acid to crown ether in the extracted species is 1:1 for cationic Trp, Leu, Ala, and Gly and 1:2 for dicationic Arg and Lys. This IL extraction system was used successfully for the recovery of amino acids from pharmaceutical samples and fermentation broth, and was followed by fluorimetric detection.
Important parameters to assess efficiency of the liquid-liquid extraction process in the case of ILs are distribution coefficients, and the data are becoming available in the literature. The most popular ionic liquid [BMIm][PF6] was used as a solvent in Ref. [Citation36]. The ionic liquid/water (Pil/water) and ionic liquid/heptane (Pil/heptane) distribution coefficients of a set of 40 compounds with various functionalities (including organic acids, organic bases, amino acids, antioxidants, and neutral compounds) were measured using liquid chromatography. For ionizable compounds, the Pil/water values measured at pH 2, 5.1, and 10 were very different. These allowed the determination of both the molecular P(o)il(/water) values and the ion P(−)il(/water) value for each compound. These coefficients were compared to the corresponding Poct/water coefficients. Marked differences in the partitioning behavior of basic, acidic, and neutral compounds were observed. The relationship between Pil/water and Poct/water was different from that reported previously. By using the linear free energy solvation approach and the descriptors found for 12 solutes, the [BMIm][PF6] solvent parameters were calculated for the ionic liquid/water and ionic liquid/heptane biphasic systems. The regression parameters show a low basicity of the [BMIm][PF6] solvent compared to octanol. The high cohesion of the ions in the IL phase is also indicated by the regression equations obtained. Ionized phenols (phenoxide ions) associated more strongly with [BMIm][PF6] than most other ionized molecules.
J.-F. Liu et al. [Citation38] demonstrated that (ILs) such as 1-octyl-3-methylimidazolium hexafluorophosphate [C8MIm][PF6] can be used as extraction solvents in liquid-phase microextraction (LPME). The unique properties of nonvolatility and adequate viscosity allow IL to be conveniently adopted as extraction solvents in both direct-immersion and headspace LPME. Model compounds, polycyclic aromatic hydrocarbons (PAHs), are conveniently and rapidly enriched in a 3-μ L drop of [C8MIm][PF6] suspended on the tip of a microsyringe followed by liquid chromatography detection. Compared to 1-octanol, a larger volume drop of [C8MIm][PF6] can be formed and survive for a longer extraction time; therefore, a much higher enrichment factor for PAHs can be reached. For low-volatility PAHs, direct-immersion LPME provides higher enrichment factors than that of headspace LPME. However, the enrichment factor obtained by headspace LPME was almost three fold that by direct-immersion LPME in a 30-min extraction of the most volatile PAH, naphthalene. For 30-min direction-immersion LPME of U.S. Environmental Protection Agency (U.S. EPA) priority PAHs, the enrichment factor, correlation coefficient (R2), and reproducibility (relative standard deviation, n = 5) were at 42–166, 0.9169–0.9976, and 2.8–12.0%. Especially, this proposed method should have great potentiality in sample preparation. Also, the nonvolatility of IL makes it potentially useful for headspace LPME of volatile analytes. In another paper [Citation39], researchers demonstrated the use of 1-hexyl-3-methylimidazolium hexafluorophosphate [C6MIm][PF6] as extraction solvent, in LPME of 4-nonylphenol (4-NP) and 4-tert-octylphenol (4-t-OP). Although [C6MIm][PF6] can suspend a much larger volume of droplets on the needle of the microsyringe than the conventional solvents such as 1-octanol and carbon tetrachloride, the method sensitivity was analyte dependent because of the different partition coefficients and the relatively large viscosity of [C6MIm][PF6]. The proposed procedure has a detection limit and enrichment factor of 0.3 μg/L and 163 for 4-NP, and 0.7 μg/L and 130 for 4-t-OP, respectively. Aqueous samples including tap water, river water, and effluent from a sewage treatment plant were analyzed by the proposed method and the recoveries at the 10 μg/L spiked level were in the range of 90% to 113%.
Liquid-liquid extraction is common method in certain metal processing, and the search for better solvents is continuing task. For example, 1-alkyl-3-methylimidazolium hexafluorophosphate [CnMIm][PF6] was employed as VOC replacement in liquid/liquid separations of metal ions from aqueous solutions [Citation40]. Here it was shown that the partitioning of metal ions in these novel biphasic systems is consistent with traditional liquid/liquid separations: The metal ion affinity for the hydrophobic phase necessitates the presence of an extractant. In this report the application of well-known extractants was explored, that is, 1-(2-pyridylazo)-2-naphthol (PAN) and 1-(2-thiazolylazo)-2-naphthol (TAN) and inorganic modifiers (i.e., CN−, OCN−, SCN−,) and halides for partitioning a variety of metal cations between [C4Mim][PF6] or [C6Mim][PF6] and an aqueous phase. PAN and TAN showed pH dependent extraction of Cd2 +, Co2 +, Ni2 +, and Fe3 + where their partitioning to the RTIL increases at least two orders of magnitude from pH 1 to 13. The effect of the halides on the partitioning of Hg2 + complexes increases F− < Cl− < Br− < I−. Pseudohalides, especially SCN−, had the greatest effect on enhancing the partitioning of Hg2 + to the RTIL, whereas CN− and OCN− provided little benefit for the extraction of any of the metal ions examined. The extraction temperature and pH of the aqueous phase are important parameters to optimize efficiency on the extraction of heavy metal ions (copper, zinc, and chromium) [Citation41].
Different additives have been used in studies on metal extraction. In Ref. [Citation42], dithizone was employed as a metal chelator to form neutral metal-dithizone complexes with heavy metal ions to extract metal ions from aqueous solution into [BMIm][PF6]. Since the distribution ratios of metal dithiozonates between [BMIm][PF6] and the aqueous phase are strongly pH dependent, the extraction efficiencies of metal complexes can be manipulated by tailoring the pH value of the extraction system. Hence the extraction, separation, and preconcentration of heavy metal ions with the biphasic system of [BMIm][PF6] and the aqueous phase can be achieved by controlling the pH value of the extraction system. Preliminary results have indicated that the use of [BMIm][PF6] as an alternate solvent to replace traditional organic solvents in liquid/liquid extraction of heavy metal ions is very promising.
Changing alkyl chain lengths in the 1-alkyl-3-methyl-imidazolium cation, in combination with PF6 or N(SO2CF3)2 anions, produces hydrophobic ILs with rheological properties suitable for their use in liquid/liquid separations of important materials. Actinides exhibit significant partitioning to these ILs from aqueous solutions with the addition of a modifier (e.g., octyl(phenyl)-N, N-diisobutylcarbamoylmethyl phosphine oxide) to the IL [Citation43]. Ionic liquids thus can be considered for actinide chemistry as a new class of materials with adjustable solvent characteristics.
The recovery of gold and silver from ore in an IL has been reported for the first time in [Citation44]. The 1-butyl-3-methyl-imidazolium hydrogen sulfate ionic liquid [BMIm][HSO4] was employed, with iron(iii) sulfate oxidant and thiourea added. Selective extraction of gold (> 85%) and silver (> 60%) from powder ore (of dominantly chalcopyrite/pyrite/pyrrhotite/sphalerite mineralogy) was achieved at room temperature in 50 h, with other lower-value metals present in the ore (Cu, Zn, Pb, Fe) extracted to only low percentages. Gold extraction was similar to that achieved in aqueous H2SO4/thiourea/Fe2(SO4)3, and silver extraction was significantly better. Moreover, the IL can be recycled following selective stripping of gold and silver on activated charcoal, with reuse in at least four successive treatments leading to neither IL degradation nor any loss in extraction efficiency.
Octyl(phenyl)-N,N-diisobutylcarbamoylmethyl phosphine oxide (CMPO) dissolved in an IL [BMIm][PF6] greatly enhances extractability and selectivity of lanthanide cations compared to that dissolved in conventional organic solvents; further, the recovery of lanthanides extracted into ILs can be accomplished using several stripping solutions containing complexing agents [Citation45].
Some additives can substantially change the distribution coefficient for certain substances. The preliminary results presented in [Citation46] showed that unprecedentedly large distribution coefficient (D) values can be achieved using ILs as extraction solvents for the separation of metal ions by crown ethers. This work highlighted the vast opportunities in separation applications for ILs with crown ethers. This was further systematically studied in [Citation47] where a series of RTILs 1-alkyl-3-methyl-imidazolium hexafluorophosphates, in which the 1-alkyl group is varied systematically from butyl to nonyl, were used as solvents in extraction. For solvent extraction of aqueous solutions of alkali metal chlorides with solutions of dicyclohexano-18-crown-6 (DC18C6) in these RTILs, the extraction efficiency generally diminished as the length of the 1-alkyl group was increased. Under the same conditions, extraction of alkali metal chlorides into solutions of DC18C6 in chloroform, nitrobenzene, and 1-octanol was undetectable. The extraction selectivity order for DC18C6 in the RTILs was K+ > Rb+ > Cs+ > Na+ > Li+. As the alkyl group in the RTIL was elongated, the K+/Rb+ and K+/Cs+ selectivities exhibited general increases with the larger enhancement for the latter. For DC18C6 in [C8MIm][PF6], the alkali metal cation extraction selectivity and efficiency were unaffected by variation of the aqueous-phase anion from chloride to nitrate to sulfate.
A series of N-alkyl aza-18-crown-6 ethers in ILs were investigated as recyclable extractants for separation of Sr2+ and Cs+ from aqueous solutions [Citation48]. The pH-sensitive complexation capability of these ligands allows for a facile stripping process to be developed so that both macrocyclic ligands and ILs can be reused. The strong dependence of selectivity on the type of IL indicates an important role played by solvation in solvent extraction processes based on ILs. The optimization of macrocyclic ligands and ILs led to an extraction system that is highly selective toward Sr2 +.
There are two modes of strontium ion transfer from acidic nitrate media into a series of 1-alkyl-3-methylimidazolium-based RTILs containing dicyclohexano-18-crown-6 (DCH18C6), which can be shifted from cation exchange [Citation49] to strontium nitrate-crown ether complex partitioning as the hydrophobicity of the IL cation is increased [Citation50].
Another interesting additive for the IL phase on extraction was found to be a calix[4]arene bearing pyridine, which has a molecular structure similar to the constitutional unit of 3-methylimidazolium hexafluorophosphate-based RTILs. It is soluble in ILs, and showed 230-fold extractability for silver ions in IL over that in chloroform [Citation51, Citation52]. In a competitive extraction test using five different metal ions (Ag+, Cu2 +, Zn2 +, Co2 +, Ni2 +), only silver ions were transferred by the calix[4]arene from the aqueous feed phase into the RTILs through a cation-exchange mechanism. The pyridinocalix[4]arene forms a stable 1:1 complex with silver ions, both by slope analysis and by Job's method. Since it is easy to strip silver ions from RTILs by controlling the aqueous-phase pH, the extraction performance of calix[4]arene in RTILs was maintained after five repeated uses.
Solvent extraction of cesium ions from aqueous solution to hydrophobic ILs without the introduction of an organophilic anion in the aqueous phase was demonstrated using calix[4]arene-bis(tert-octylbenzo-crown-6) (BOBCalixC6) as an extractant [Citation53].
A series of hydrophobic task-specific ionic liquids (TSILs) designed to extract Hg2 + and Cd2 + from water were prepared by appending urea-, thiourea-, and thioether-substituted alkyl groups to imidazoles and combining the resulting cationic species with [PF6] [Citation54]. These ILs were characterized and investigated for their metal ion extraction capabilities. When used in liquid/liquid extraction of Hg2+ and Cd2+ from aqueous solutions, the metal ion distribution ratios increased several orders of magnitude, regardless of whether the ILs were used as the sole extracting phase or doped into a series of [1-alkyl-3-methylimidazolium][PF6] (alkyl = n-C4-C8) ionic liquids to form a 1:1 solution. In the 1:1 mixtures, as the length of the alkyl chain increased from butyl to hexyl to octyl, the metal ion distribution ratios increased. Increasing the ratio TSIL/[BMIm][PF6] resulted in higher distribution ratios for both Hg2+ and Cd2+. Overall the thiourea- and urea-derivatized cations yielded the highest distribution ratios, and those for Hg2+ were higher than those for Cd2+; however, a change in aqueous-phase pH does not promote the stripping of metal ions from the extracting phase.
Task-specific ionic liquids containing a bis-imidazolium cation incorporating a short ethylene-glycol and longer decyl-glycol spacers, 1,1′-[1,2-ethanediylbis(oxy-1,2-ethanediyl)] bis[3-methyl-1H-imidazolium-1-yl] imide [N(Tf)2] has been prepared from the corresponding chloride salt [Citation55]. Introducing the ethylene-glycol functionality dramatically increases the distribution ratio of mercury ions, but not cesium, from aqueous solution to the hydrophobic IL. This is an example of pH-dependent partitioning and stripping of mercury from ionic liquid/aqueous two-phase systems. The crystal structure of the related mercury(II) carbene complex reveals the possibility of a carbene extraction mechanism.
The development of simple systems for cleaning of fuels has large practical importance [Citation56]. Extraction of S- and N-compounds from gasoline and diesel oil by ILs indicates that such a process could be an alternative to common hydrodesulfurization (HDS) for deep desulfurization down to values of 10 ppm S or even lower [Citation57]. The results showed the selective extraction properties of ILs, especially with regard to those S-compounds which are hard to remove by HDS (e.g., dibenzothiophene derivatives present in middle distillates like diesel oil). The application of mild process conditions (ambient pressure and temperature) and the fact that no hydrogen is needed are additional advantages compared to HDS. Very promising ILs are [BMIm][C8SO3] and [EMIm][C2SO3], as they are halogen-free and available from relatively inexpensive starting materials. Extraction with ILs is not limited to diesel oil, but probably is even more attractive for FCC-gasoline. Although HDS of S-species present in this gasoline constituent (mainly thiophenes) is relatively straightforward, a major drawback is the loss in octane number by olefin saturation, which favors extraction with ILs.
The most widespread [EMImBF4], [BMImPF6], [BMImBF4], and heavier trimethylamine hydrochloride (AlCl3-TMAC) showed high selectivity, particularly toward aromatic sulfur and nitrogen compounds, for extractive desulfurization and denitrogenation [Citation58]. The used ILs were readily regenerated either by distillation or by water displacement of absorbed molecules. The absorbed aromatic S-containing compounds were quantitatively recovered. Organic compounds with higher aromatic π-electron density were favorably absorbed. Alkyl substitution on the aromatic rings was found to significantly reduce the absorption capacity, as a result of a steric effect. The cation and anion structure and size in the ILs are important parameters affecting the absorption capacity for aromatic compounds. At low concentrations, the N- and S-containing compounds were extracted from fuels without mutual hindrance. AlCl3-TMAC ILs were found to have remarkably high absorption capacities for aromatics.
There are constant searches for effective systems, and one interesting proposal can be brought out where CuCl-based IL exhibited remarkable ability in the desulfurization of gasoline when used as an extraction absorbent [Citation59]. Also, it has been proposed an one-pot operation, where the sulfur-containing compounds in the light oils were extracted into RTILs and then S-oxidized (H2O2-acetic acid) to form the corresponding sulfones [Citation60]. The advantage of performing both extraction and oxidation of sulfur compounds from light oil simultaneously in RTILs is that this process increases the desulfurization yield by about an order of magnitude relative to that of merely extracting with RTILs. The room temperature ILs can be recycled after workup and reused without any loss of activity.
In conclusion it can be said that, in many cases, ILs have advantages compared to common solvents used as separation media in liquid-liquid extraction processes. Their unique properties can be developed to allow achievement of high efficiencies and selectivities of separation. For analytical applications, more data to perform quantitative extractions are needed. Also problems with IL recycling must be solved, especially when industrial application is in question.
Stationary Phases for Gas Chromatography
Synthetic routes to over 200 RTILs are known, but for most ILs physicochemical data are generally lacking or incomplete. Chromatographic and spectroscopic methods provide suitable tools for the study of solvation properties under conditions that approximate infinite dilution. The best review of this area has been provided by Colin Poole in [Citation61], which covers and summarizes not only imidazolium-based ILs, but also other salts exhibiting properties similar to ILs. Gas-liquid chromatography (GLC) is suitable for the determination of gas-liquid partition coefficients and activity coefficients as well as thermodynamic constants derived from either of these parameters and their variation with temperature. Using inverse gas chromatography (GC), one can examine the nature of ILs via their interactions with a variety of compounds. The solvation parameter model can be used to define the contribution from individual intermolecular interactions to the gas-liquid partition coefficient.
The wetting ability and viscosity of RTILs allow them to be coated onto fused silica capillaries. Thus, 1-butyl-3-methylimidazolium hexafluorophosphate and the analogous chloride salt were used as stationary phases GC [Citation62]. The Rohrschneider-McReynolds constants were determined for both ILs and a popular, commercial polysiloxane stationary phase. Ionic liquid stationary phases seem to have a dual nature. They appear to act as a low-polarity stationary phase to nonpolar compounds. However, molecules with strong proton donor groups, in particular, are tenaciously retained. The nature of the anion can have a significant effect on both the solubilizing ability and the selectivity of IL stationary phases. It appears that the unusual properties of ILs could make them beneficial in many areas of separation science.
Results of systematic study of imidazolium-based ILs as GLC stationary phases was presented in [Citation63] by J. Anderson and D. Armstrong. Many of the ILs used in this study suffer from low thermal stability and possess unfavorable retention behavior for some classes of molecules. Two new ILs were engineered and synthesized to overcome these drawbacks. These new ILs, 1-benzyl-3-methylimidazolium trifluoromethanesulfonate and 1-(4-methoxyphenyl)-3-methylimidazolium trifluoromethanesulfonate are based on bulky imidazolium cations with trifluoromethanesulfonate anions. Their solvation characteristics were evaluated using the Abraham solvation parameter model and correlations made between the structure of the cation and the degree to which the ILs retain certain analytes. The new ILs have good thermal stability up to 260°C, provide symmetrical peak shapes, and, because of their broad range of solvation-type interactions, exhibit dual-nature selectivity behavior. The IL stationary phases provided different retention behavior for many analytes when compared to a common methylphenyl polysiloxane GLC stationary phase. This difference in selectivity is due to the unique solvation characteristics of the ILs and allow us to make an optimistic conclusion about them as useful dual-nature GLC stationary phases.
Using ILs for chiral separations can be accomplished via two ways: (1) a chiral selector can be dissolved in an achiral ionic liquid or (2) the ionic liquid itself can be chiral. The first precedent to separate chiral compounds with help of ILs was not very successful [Citation64]. There, [BMIm][PF6] was used as a solvent to dissolve permethylated-β-cyclodextrin (BPM) and dimethylated-β-cyclodextrin (BDM) to prepare stationary phases for capillary columns in GC for chiral separation. The RTIL-containing columns were compared to common columns containing the same chiral selectors. A set of 64 chiral compounds separated by the common BPM column was tested on the RTIL BPM column. Only 21 were enantio-resolved. Similarly, a set of 80 compounds separated by the common BDM column was passed on the RTIL BDM column with only 16 position separations. Probably the imidazolium ion pair could make an inclusion complex with the cyclodextrin cavity, blocking it for chiral recognition. All the chiral compounds recognized by the RTIL columns had their asymmetric carbon that was part of a ring structure. The retention factors of the derivatized solutes were lower on the RTIL columns than those obtained on the common equivivalent column. The peak efficiencies obtained with the RTIL capillary were significantly higher than that obtained with the common column.
Reference [Citation65] presented enantiomeric separations using chiral IL stationary phases in GC. Compounds that have been separated using these IL chiral selectors include alcohols, diols, sulfoxides, epoxides, and acetylated amines. Because of the synthetic nature of these chiral selectors, the configuration of the stereogenic center can be controlled and altered for mechanistic studies and reversing enantiomeric retention.
Some other groups are using inverse GC with ILs as stationary phases to measure the activity coefficients at infinite dilution, for both polar and nonpolar solutes in an IL. In Ref. [Citation66] the activity coefficients have been detected on [C8MIm][BF4], at T = (298.15 K and 323.15 K). The selectivity values have been calculated at 298.15 K, and the results indicate that the IL [C8MIM][BF4] should be a good solvent for separation of benzene and alkanes. Similar results were obtained in Ref. [Citation67] where the activity coefficients for hydrocarbon solutes at infinite dilution in 1-methyl-3-octyl-imidazolium chloride have been measured. Activity coefficients at infinite dilution γ iα of the linear and branched C1 to C6 alcohols, acetone, acetonitrile, ethylacetate, alkylethers, and chloromethanes in the IL 4-methyl-N-butyl-pyridinium tetrafluoroborate were determined in [Citation68], and in the ILs 1-methyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide and 1,2-dimethyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide [Citation69] by GC using the IL as the stationary phase. The measurements were carried out at different temperatures between 313.1°K and 363.1°K. From the temperature dependence of the limiting activity coefficients partial molar excess enthalpies at infinite dilution γ iα of these polar solutes in the IL have been derived.
Ionic liquid [BMIm][PF6] was employed as the stationary liquid in capillary column chromatography with supercritical carbon dioxide (scCO2) as the mobile phase [Citation70]. Open tubular capillary columns were used to measure the retention factors of anisole, azulene, benzil, α-ionone, napthalene, pyrene, and vertrole within 313–353 K and 8.1–23.2 MPa. The retention factors were converted to infinite-dilution solute partition coefficients in the [BMIm][PF6]-scCO2 system. Analysis of the relative retention data by regular solvation theory resulted in approximate values of the solubility parameter of CO2-expanded [BMIm][PF6]. It confirms viability of open tubular supercritical fluid chromatography to provide partitioning data on low-volatility solutes in biphasic RTIL-scCO2 systems.
The liquid state of ILs makes them possible candidates as solvents in countercurrent chromatography (CCC), which uses solvents as both the mobile and stationary phases. It was found that the viscosity of pure RTILs is too high for direct use as a liquid phase in CCC. The addition of a third solvent was needed to decrease viscosity. The 40:20:40% wt/wt water-acetonitrile-[BMIm][PF6] liquid system was found to be appropriate as a biphasic liquid system for CCC. The ternary phase diagrams of [BMIm][PF6]-water and acetonitrile, methanol, ethanol, 1-propanol, and 2-propanol are presented in mass and mole percentages. The organic solvent-RTIL-water systems form two liquid phases with a viscosity low enough to allow CCC operation. The partitioning of 38 aromatic derivatives with acid, base, or neutral functionalities was studied by CCC [Citation71]. The resulting Pil/w constants were compared with the corresponding literature on octanol-water partition coefficients, Po/w. [Citation72]. The important drawbacks in the use of RTILs in CCC were clearly pointed out: high viscosity producing pressure buildup, UV absorbance limiting the use of the convenient UV detector, and non volatility precluding the use of the evaporative light-scattering detector for continuous detection.
Solvents for Spectroscopy
It has been reported in [Citation73] that ILs are able to solvate a wide range of species including organic, inorganic, and organometallic compounds; notably, a variety of transition metal complexes, which are unstable in other media, may be studied in ambient temperature ILs. The use of RTILs as solvents for UV-visible and IR spectroscopy for highly charged complex ions with high- or low-oxidation states like [MXn]y-complexes (M = transition metal; X = Cl, Br) circumvents the problems of solvation and solvolysis and permits reliable solution spectra to be recorded for these species. A selection of spectra is presented which were obtained for M = Ir, Os, Ru using ILs based on 1-methyl-3-ethylimidazolium chloride: AlCl3 and 1-methyl-3-ethylimidazolium bromide: AlBr3 mixtures. Enhanced resolution and significant spectra shifts compared with the standard published solution spectra were observed. The results most closely resemble those recorded in solvents of low dielectric constants such as CH2Cl2. Several examples using room-temperature haloaluminate ILs, specifically N-butylpyridinium chloride-aluminum(III) chloride and 1-methyl-3-ethylimidazolium halide-aluminum(III) halide (halide = chloride or bromide), as solvents for studying the solution electronic absorption spectra of transition metal halide complexes have been described by K. R. Seddon in [Citation74]. Also, water- and air-stable ILs have good solvating properties together with large spectral transparency (UV cut-off wavelengths of alkylimidazolium-based ILs are in the range of 230 to 250 nm), which makes them suitable solvents for spectroscopic measurements especially in the visible region.
The importance of the purity of the solvent for spectroscopic studies has been highlighted in Ref. [Citation75]. Results from small-angle X-ray scattering indicate that the pure solvent exhibits a local organization. Eu(II), which appears to be unusually stable in [BMIm][PF6], was characterized by spectroscopic techniques (absorption, luminescence). Solvation of Eu(II) in [BMIm][PF6] and complexation effects in the presence of the crown ether DC15C5 solubilized in the IL were discussed.
The combination of ILs with the use of scCO2 as an extractant offers potential for chemical reaction and downstream separation. Spectroscopic studies can offer reliable data about the properties of media. The authors in [Citation76] studied the solvent properties of mixtures of [BMIm][PF6] and CO2 as functions of temperature (35°C to 50°C) and CO2 pressure (0–230 bar). The results are consistent with a picture of local enhancement of an RTIL compound around a chromophore, maintaining solvent strength even at fairly high loadings of CO2, whereas the microviscosity in the vicinity of the solute is dramatically reduced, leading to enhanced mass transport and facilitated separation. They can be used together with organic cosolvents which “solvate” the constituent ions of the IL, resulting in a decrease in the aggregation of these ions (lower viscosity and higher conductivity).
Spectroscopic measurements of solvatochromic and fluorescent probe molecules in RTILs have provided insights into solvent intermolecular interactions, although interpretation of the different and generally uncorrelated “polarity” scales has sometimes been ambiguous. The IL [BMIm][PF6] has been used as solvent for a series of representative photochemical reactions [Citation77]. The aim was to cover a wide range of photochemical reactions, including energy transfer, hydrogen abstraction, oxygen quenching, and electron transfer. The major features found in this study for this prototypical IL are: (a) remarkable low oxygen solubility ([O2] < 0.2 mmol/L) at atmospheric oxygen pressure; (b) slow molecular diffusion rendering diffusion-controlled processes about two orders of magnitude slower than in common organic solvents; (c) long lifetime of triplet excited states (one order of magnitude) and radical ions; (d) weaker CT interaction decreasing the association constant of the CT complexes and shifting the λmax longer wavelength.
In view of the presented data, ILs not only are suitable for electron transfer processes as reported up to present, but they also are useful for energy transfer and hydrogen transfer, although not for singlet oxygen generation. Finally, the slowdown of the rate constants could be useful for doing fast processes (sub ns) in more widely accessible timescales (ns systems).
Mass Spectrometry
The low volatility of ILs makes them useful as solvents working in a high vacuum and, together with their more amorphous solid analogs, they merit further study as MALDI matrixes. Ionic liquids have excellent solubilizing properties and vacuum stability compared to other commonly used liquid and solid matrixes. Certain ionic matrices, however, produce homogeneous solutions of higher ion peak intensity and equivalent or lower detection limits than currently used solid matrixes. Also, they vary widely in their ability to produce analyte gas-phase ions.
These matrix systems allow a homogenous sample preparation with a thin IL layer having negligible vapor pressure. The vacuum-stable, liquid consistency of IL matrix sample preparations has considerably enhanced MALDI-MS analysis in terms of shot-to-shot reproducibility, and this has led to a facilitated qualitative and quantitative measurement of the analytes compared with classical solid matrixes.
There are some groups working on this area and searching the TSILs for MALDI-MS applications. Several different ionic matrixes have been synthesized and tested, using peptides, proteins, and poly(ethylene glycol) (PEG-2000) [Citation78]. The IL matrixes are composed of equimolar combinations of classical MALDI matrixes (sinapinic acid, α-cyano-4-hydroxycinnamic acid, or 2,5-dihydroxybenzoic acid) with organic bases. The use of ILs as matrixes for the qualitative and quantitative analysis of low-molecular-weight a compounds like amino acids, sugars, and vitamins was studied in [Citation79]. As a result of these experiments, it appears that IL matrices can successfully replace solid matrices wherever the latter are used for analysis of biologically interesting low-molecular-weight compounds.
The usefulness of using ionic matrices to determine the molecular weight of DNA oligomers by direct TOF mass spectrometric analysis was demonstrated in [Citation80]. Three oligonucleotides were tested, d(pT)(10), d(pC)(11), and d(pC)(12), with several ionic matrices synthesized from different bases associated with two acids (3-hydroxypicolinic acid and 2,5-dihydroxybenzoic acid). The results obtained show that the best ionic matrices enhance the ion peak intensity of the oligonucleotides with respect to conventional molecular matrices under our experimental conditions. In one case, an ionic matrix provided a signal-to-noise ratio 10 times higher than the corresponding molecular matrix.
The performance of the new IL MALDI-MS matrix 2,5-dihydroxybenzoic acid butylamine (DHBB) was assessed and compared to results obtained with the IL MALDI-MS matrixes α-cyano-4-hydroxycinnamic acid butylamine (CHCAB), 3,5-dimethoxy-4-hydroxycinnamic acid triethylamine (SinTri), and the frequently used solid MALDI matrixes 2,5-dihydroxybenzoic acid (DHB) and α-cyano-4-hydroxycinnamic acid (CHCA) [Citation81]. Consequently, relative standard deviations serving as a measure for reproducibility of intensity values acquired from 90 different spots on one MALDI-MS preparation were approximately one-half as high when solid DHB was replaced by the IL DHBB and eight times lower after exchange of solid CHCA by the IL CHCAB. Interestingly, the IL MALDI-matrix DHBB conserved the broad applicability of its solid analog DHB, reduced MALDI induced fragmentation of monosialylated glycans and gangliosides, and was the superior IL matrix for MALDI-MS analysis of oligosaccharides and polymers, such as poly(ethylene glycol). It also worked well with glycoconjugates, peptides, and proteins; however, the tendency of DHBB to form multiple alkali adduct ions with peptides and proteins made CHCAB the IL matrix of choice for peptides. SinTri was the best IL matrix for proteins of high molecular weight, such as IgG. Furthermore, it was demonstrated that solvent properties and MALDI matrix properties of ILs, such as DHBB, can be combined to enable fast, direct screening of an enzymic reaction. This was proven by the desialylation of sialylactose with sialidase from Clostridium perfringens in the presence of diluted aqueous DHBB and subsequent direct MALDI-MS analysis of the reaction mixture.
Electrospray ionization mass spectrometry is gaining larger attention in both a theoretical and practical sense. When a liquid meniscus is charged to a sufficiently high voltage with respect to neighboring grounded electrodes, the liquid surface deforms into a so-called Taylor cone, whose apex ejects a thin jet. Ionic liquids, being highly conducting liquids, of low volatility, can be used for generation this kind of jet. The modest spread of ion energies observed and considerable currents attained (0.6 μA) suggest the use of ILs as ion beam sources with a much wider range of mass/charge and chemical compounds than previously available [Citation82, Citation83].
Electrospray ionization mass spectrometry of neat ILs does not require continuous sample injection and the presence of a molecular solvent facilitates analysis of the IL itself and dissolved analytes [Citation84]. Ionic liquids were analyzed in undiluted form [Citation85], where results indicate that signal-to-noise ratios for minor constituents are comparable to those observed in conventional, diluted ES-MS and that this approach could be readily applied for mass spectrometric analysis of ILs and ionic impurities/additives dissolved therein, especially those that are solvent reactive. This was realized in a purity check of microwave synthesized ILs [Citation86]. On the other hand, the characterization of a ruthenium catalyst immobilized in an IL has been described [Citation87].
DEVELOPMENT OF METHODS, WHERE IL ARE USED AS NOVEL ADDITIVES
Liquid Chromatography
It seems that ILs are not very good media for separation and applications in liquid chromatography and capillary electrophoresis with use of them as specific additives. Silica-based stationary phases are commonly used in liquid chromatography, but their surface acidity causes known problems, especially when separating basic compounds. Deleterious effects of free silanols are not fully removed by standard prevention procedures consisting of adding alkylamines or other amino quenchers to the eluents. Researchers found in [Citation88] that ILs of the imidazolium tetrafluoroborate class, added to mobile phases at concentrations of 0.5–1.5% (v/v), blocked silanols and provided excellent thin-layer chromatographic separations of strongly basic drugs which were otherwise not eluted, even with neat acetonitrile as the mobile phase. The silanol-suppressing potency of imidazolium tetrafluoroborates was demonstrated to markedly exceed that of the standard mobile phase additives, such as triethylamine, dimethyloctylamine, and ammonia. The proposed new mobile phase additives were also demonstrated to provide reliable lipophilicity parameters of base drug analytes as determined by the gradient mode of high-performance liquid chromatography. By applying the readily available and environmentally friendly imidazolium tetrafluoroborate ILs, simple and efficient means of improvement of liquid chromatographic analysis of organic bases have been elaborated.
The positive effects of IL addition on the separation of ephedrines (norephedrine, ephedrine, pseudoephedrine, and methylephedrine) on a C18 column—decreasing band tailing, reducing band broadening, and improving resolution—were followed in [Citation89]. The retention times of the analytes increased at first and then decreased with increase in the concentration [BMIm][BF4] IL. This effect may be attributed to the competition between imidazolium cations and the polar groups of the analytes for the silanol group on the alkylsilica surface, and also to the formation of a weak bilayer electronic structure on the C18 column. Several ILs with different alkyl substituents on the imidazolium cations or with different counterions as the eluent were compared.
Some amines including benzidine, benzylamine, N-ethylaniline, and N,N-dimethylaniline have been separated using ILs as additives for the mobile phase in high-performance liquid chromatography. The differences between ILs and tetrabutylammonium bromide (TBA) on the separation of o-, m-, p-phthalic acids were compared. It was concluded that ILs are ion-pair reagents in essence, although their hydrophobicity and hydrogen bonding play important roles [Citation90].
Electrophoresis
There is one example on electrophoretic separation of highly hydrophobic compounds and hydrophobic proteins in a nonaqueous solvent. The nonaqueous solvent was an IL or a mixture of an organic solvent containing an IL in an amount to render the solvent electrically conductive and amenable for electrophoretic separation. The hydrophobic proteins were separated by electrophoresis using an electrophoresis gel that was compatible with the organic solvent and IL. The formation of a nonaqueous electrophoresis polyurethane gel containing the IL [EMIm][TfO] was demonstrated [Citation91]. Hypol G-50 prepolymer was used as a precursor for the polyurethane gel.
More studies have been performed in capillary electrophoresis (CE) on use of ILs. Acetonitrile is a well-suited medium for nonaqueous capillary electroseparations and enables extending the range of applications of CE techniques to more hydrophobic species. In many cases salts which are liquid at room temperature, show a better solubility in organic solvents, and can be used in nonaqueous capillary zone electrophoresis as ionic additives for adjustment of analyte mobility and separation. The separation of different analytes in organic solvents is achieved because they become charged in the presence of ILs in separation media. The first such kind of application of ILs was demonstrated in [Citation92]. In the next study [Citation93, Citation94], 1-alkyl-3-methylimidasolium-based ILs were used as additives in separation media to assess the interactions between the analytes and the ionic additive present, to find an influence of the type and concentration of the ionic additive, and also to find an influence of the nature of the nonaqueous medium employed. The anionic part as well as the concentration of an IL influenced the general electrophoretic mobility of the buffer system. Different organic solvents (acetonitrile and methanol) contributed differently to the conversion of analytes into a charged form. Complexes with either an anionic or a cationic part of the IL additive were formed. The separation of phenols and aromatic acids, as well as polyphenolic compounds was discussed.
Also in aqueous media, use of 1-alkyl-3-methylimidazolium-based ILs was successful [Citation95] for resolving phenolic compounds found in grape seed extracts. The method is simple and reproducible. The separation mechanism seems to involve the association between the imidazolium cations and the polyphenols. The same kind of buffer was found useful for the analysis of basic proteins including lysozyme, cytochrome c, trypsinogen, and α -chymotrypsinogen A [Citation96]. The method, in which 1-alkyl-3-methylimidazolium-based ILs were used as the running electrolytes, led to a surface charge reversal on the capillary wall. The effects of the alkyl group, imidazolium counterion, and the concentration of the ILs were discussed. The optimum buffer system was a 90 mM [EMIm][BF4] solution. Baseline separation, high efficiencies, and symmetrical peaks of four proteins were obtained. The RSD values of migration times and peak areas were < 0.68% and < 3.0%, respectively. The separation mechanism seems to involve the association between the imidazolium cations and the proteins.
Aqueous CE studies were performed to investigate interactions between halophenols and 1-ethyl-3-methylimidazolium tetrafluoroborate or tetraethylammonium tetrafluoroborate electrolytes [Citation97]. In both cases, increased halogen size correlated with increased affinity for the electrolyte cation. For isomers, the ortho-substituted isomer exhibited higher affinity than the para isomer. Irreproducible CE results for analyte pairs in the presence of the IL stimulated investigations of the interactions between halophenols as well as with the cations of the electrolyte. These interactions were explored by proton and fluorine one-dimensional nuclear magnetic resonance (NMR). The NMR results indicated differences in the interactions between tetraethylammonium/iodophenols and imidazolium/iodophenols. The NMR results also indicated hydrophobic stacking interactions between the iodophenols and possible similar interaction among phenols and imidazolium.
The electroosmotic flow of bare silica capillary was reversed by the covalently bonded RTIL coating [Citation98]. With run buffer of 5 mM [EMIm] with different anions (BF4, Cl, PF6, and TfO) at pH 8.5, NH4+ in human urine was separated from the K+ matrix and was detected to be 0.37 ± 0.012%. K+, Na+, Li+, Ca2+, Mg2+, and Ba2+ were baseline separated in RTIL-coated capillary with run buffer of 10 mM [EMIm][OH]–acetic acid at pH 5, and the concentrations of the above ions in a red wine were detected to be 907, 0, 27.9, 0, 71.0, 83.4, and 31.1 μg/mL, respectively. The same kind of coating by IL was used in [Citation99] to eliminate the adsorption of the analytes onto the bare capillary wall. Sildenafil (SL) and its metabolite UK-103,320 (UK) in human serum were detected by solid-phase extraction followed by capillary zone electrophoresis-mass spectrometry analysis. The drugs were baseline separated within 14 min with detection limits (S/N = 3) of 14 and 17 ng/mL for SL and UK, respectively. The method developed showed good intraday precision in terms of RSD with respect to migration time (RSD ≤ 0.76%, n = 5) and peak area (RSD ≤ 3.4%, n = 5). Also an IL-coated capillary was prepared and investigated for DNA separation [Citation100]. Below 900 base pairs, the larger DNA fragment suffered more retardation in the IL-coated capillary due to the increasing charge density of the fragment with size. In the presence of 4% hydroxyethylcellulose, the phiX174 DNA-Hae III digest fragments were baseline separated in both IL- and polyacrylamide-coated capillary except for the fragments of 271 and 281 base pairs; the analysis time was shorter in the IL-coated capillary. These experiments indicated that the IL-coated capillary could work stably in the run buffer for at least 96 h with no notable deterioration in performance.
A capillary zone electrophoresis-potential gradient detection (PGD) method coupled with field-amplified sample injection was developed to determine alkali metal, alkali-earth metal, nickel, lead, and ammonium ions [Citation101]. The capillary surface was coated with dialkylimidazolium-based IL and thus the electroosmotic flow of the capillary was reversed. The buffer was composed of 7.5 mM lactic acid, 0.6 mM 18-crown-6, 12 mM α-cyclodextrin (α-CD); it was adjusted to pH 4.0 by 1-hexyl-3-methylimidazolium hydroxide. The 11 cations were baseline separated within 14 min with 5.1–18.9 * 104 plates (for 40-cm-long capillary) in separation efficiency, and the detection limits were at 0.27–7.3 ng/mL. The method showed good reproducibility in terms of migration time with RSD ± 0.90% for run-to-run and RSD ± 1.65% for day-to-day assessment.
The use of ILs as modifiers in the separation of achiral and chiral analytes in micellar electrokinetic chromatography has been reported [Citation102]. Polymeric surfactants and ILs were added to a low-conducting buffer solution. The polymeric surfactants used in this study were poly(sodium N-undecylelinic sulfate) and poly(sodium oleyl-L-leucylvalinate). It was expected that these ILs would have the ability to assist in the separation of hydrophobic mixtures while maintaining adequate background current. Three analyte mixtures were separated using various buffer combinations of polymeric surfactant and ILs. The ILs improved the resolution and peak efficiency of the analytes while maintaining adequate background current.
Application of ILs as useful additives is also possible in atomic spectroscopy. The analysis of gold by inductively coupled plasma atomic emission spectrometry (ICP-AES) in aqueous solution in the presence of up to 50% w/v of IL has been reported [Citation103]. The ILs investigated contain [BMIm] cation with the anions Cl−, BF4−, HSO4−, or N(CN)2−. A facile route to the HSO4− salt was also described. The presence of ILs altered the nebulization efficiency and sample transport properties, and the AES signal intensity and apparent concentration of gold in solution usually was suppressed principally as a result of increased viscosity of solutions containing an IL. However, the counterplay between a lower surface tension and a higher viscosity was illustrated by the results for the [BMIm][BF4] IL. The presence of this liquid at low concentrations caused an enhanced apparent concentration of gold, whereas at higher concentrations the apparent concentration was diminished as the viscosity of the solution increased. Comparative data with simple sodium salts was also reported. Use of the standard addition method to compensate for matrix effects in the presence of ILs is effective.
CONCLUSIONS
All evidence points to ILs as a unique class of polar solvents suitable for technical development. Ionic liquid studies indicate their unique solvent properties: low cohesion for ILs with weakly associated ions compared with molecular liquids of similar polarity, greater hydrogen-bond basicity than typical polar molecular solvents, and a range of dipolarity/polarizability that encompasses the same range as occupied by the most polar molecular liquids [Citation104]. In terms of designer solvents, however, further work is needed to fill the gaps in our knowledge of the relationship between ion structures and physicochemical properties to develop a comprehensive approach for design of ILs. There are several proofs of principle for an advantageous use of some ILs, but the road to designing and optimization of TSILs has a long way to go.
REFERENCES
- Walden , P. 1914 . Molecular weights and electrical conductivity of several fused salts . Bull. Acad. Imper. Sci. (St. Petersburg) , : 405 – 422 .
- Hurley , F. H. Electrodeposition of aluminum . U.S. Patent No. 2 445 331 . 1948 .
- Wier , T. P. and Hurley , F. H. U.S. Patent No. 2 446 349 . 1948 .
- Wier , T. P. U.S. Patent No. 2 446 350 . 1948 .
- Hurley , F. H. and Wier , T. P. 1951 . The electrodeposition of aluminium from nonaqueous solutions at room temperature . J. Electrochem. Soc. , 98 : 207 – 212 . [CSA]
- Wilkes , J. S. , Levisky , J. A. , Wilson , R. A. and Hussey , C. L. 1982 . Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy, and synthesis . Inorg. Chem. , 21 : 1263 – 1264 . [CSA] [CROSSREF]
- Tait , S. and Osteryoung , R. A. 1984 . Infrared study of ambient-temperature chloroaluminates as a function of melt acidity . Inorg. Chem. , 23 : 4352 – 4360 . [CSA] [CROSSREF]
- Wilkes , J. S. and Zaworodtko , M. J. 1992 . Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids . J. Chem. Soc., Chem. Commun. , 13 : 965 – 967 . [CSA] [CROSSREF]
- Wilkes , J. S. 2002 . A short history of ionic liquids—From molten salts to neoteric solvents . Green Chem , 4 : 73 – 80 . [CSA] [CROSSREF]
- Wilkes , J. S. 2004 . Propeties of ionic liquid solvents for catalysis . J. Mol. Cat. A. , 214 : 11 – 17 . [CSA] [CROSSREF]
- Welton , T. 1999 . Room-temperature ionic liquids: Solvents for synthesis and catalysis . Chem. Rev , 99 : 2071 – 2084 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Olivier , H. 1999 . Recent developments in the use of non-aqueous ionic liquids for two-phase catalysis . J. Mol. Catal. A: Chem , 146 ( 1–2 ) : 285 – 289 . [CSA] [CROSSREF]
- Earle , M. J. and Seddon , K. R. 2000 . Ionic liquids: Green solvents for the future . Pure Appl. Chem , 72 ( 7 ) : 1391 – 1398 . [CSA]
- Wasserscheid , P. and Keim , W. 2000 . Ionic liquids—New “solutions” for transition metal catalysis . Angew. Chem. Int. Ed. , 39 : 3772 – 3789 . [CSA] [CROSSREF]
- Koel , M. 2000 . Physical and chemical properties of ionic liquids based on the dialkylimidazolium cation . Proc. Est. Acad. Sci. Chem. , 49 : 145 – 155 . [CSA]
- Brennecke , J. F. and Maginn , E. J. 2001 . Ionic liquids: Innovative fluids for chemical processing . AIChE Jour. , 47 : 2384 – 2389 . [CSA] [CROSSREF]
- Sheldon , R. A. , Lau , R. M. , Sorgedrager , M. J. , van Rantwijk , F. and Seddon , K. R. 2002 . Biocatalysis in ionic liquids . Green Chem , 4 : 147 – 151 . [CSA] [CROSSREF]
- Marsh , K. N. , Deev , A. , Wu , A. C. T. , Tran , E. and Klamt , A. 2002 . Room temperature ionic liquids as replacements for conventional solvents—A review . Korean J. Chem. Eng , 19 : 357 – 362 . [CSA]
- Olivier-Bourbigou , H. and Magna , L. 2002 . Ionic liquids: Perspectives for organic and catalytic reactions . J. Mol. Catal. A: Chem. , 182–183 : 419 – 437 . [CSA] [CROSSREF]
- Zhao , D. , Wu , M. , Kou , Y. and Min , E. 2002 . Ionic liquids: Applications in catalysis . Catal. Today , 74 : 157 – 189 . [CSA] [CROSSREF]
- Dupont , J. , de Souza , R. F. and Suarez , P. A. Z. 2002 . Ionic liquid (molten salt) phase organometallic catalysis . Chem. Rev , 102 : 3667 – 3692 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- van Rantwijk , F. , Lau , R. M. and Sheldon , R. A. 2003 . Biocatalytic transformations in ionic liquids . Trends in Biotechnology , 21 : 131 – 138 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Wilkes , J. S. 2004 . Properties of ionic liquid solvents for catalysis . J. Mol. Catal. A: Chem. , 214 : 11 – 17 . [CSA] [CROSSREF]
- Marsh , K. N. , Boxall , J. A. and Lichtenthaler , R. 2004 . Room temperature ionic liquids and their mixtures—A review . Fluid Phase Equil , 219 : 93 – 98 . [CSA] [CROSSREF]
- Seddon , K. R. Ionic liquids for clean technology: An update . Molten Salt Forum: Proceedings of 5th International Conference on Molten Salt Chemistry and Technology . Edited by: Wendt , H. Vol. 5–6 , pp. 53 – 62 . Switzerland : Trans Tech Publications .
- Rooney , D. W. and Seddon , K. R. 2001 . “ Ionic liquids ” . In Handbook of Solvents , Edited by: Wypych , G. 1459 – 1484 . Toronto : ChemTech Publishing .
- Earle , M. J. and Seddon , K. R. 2002 . “ Ionic liquids: Green solvents for the future ” . In Clean Solvents: Alternative Media for Chemical Reactions and Processing , ACS Symposium Series Edited by: Abraham , M. and Moens , L. Vol. 819 , 10 – 25 . Washington, D.C. : American Chemical Society .
- Ohno , H. , ed. 2003 . Ionic Liquids: The Front and Future of Material Development , Tokyo : CMC Press .
- Rogers , R. D. and Seddon , K. R. , eds. 2002 . Ionic Liquids: Industrial Applications for Green Chemistry , ACS Symposium Series Vol. 818 , Washington, D.C. : American Chemical Society .
- Rogers , R. D. and Seddon , K. R. , eds. 2003 . Ionic Liquids as Green Solvents: Progress and Prospects , ACS Symposium Series Vol. 856 , Washington, D.C. : American Chemical Society .
- Rogers , R. D. , Seddon , K. R. and Volkov , S. , eds. 2002 . Green Industrial Applications of Ionic Liquids , NATO Science Series II: Math., Phys. and Chem. Vol. 92 , Dordrecht : Kluwer .
- Wasserscheid , P. and Welton , T. , eds. 2003 . Ionic Liquids in Synthesis , Weinheim : Wiley-VCH .
- Endres , F. 2002 . Ionic liquids: Solvents for the electrodeposition of metals and semiconductors . ChemPhysChem , 3 : 144 – 154 . [CSA] [CROSSREF]
- Buzzeo , M. C. , Evans , R. G. and Compton , R. G. 2004 . Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review . ChemPhysChem , 5 ( 8 ) : 1106 – 1120 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Sato , T. , Maruo , T. , Marukane , S. and Takagi , K. 2004 . Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells . J. Power Sources , 138 ( 1–2 ) : 253 – 261 . [CSA] [CROSSREF]
- Garcia , B. , Lavallee , S. , Perron , G. , Michot , C. and Armand , M. 2004 . Room temperature molten salts as lithium battery electrolyte . Electrochim. Acta , 49 : 4583 – 4588 . [CSA] [CROSSREF]
- Li , Z. , Liu , H. , Liu , Y. , He , P. and Li , J. 2004 . A room-temperature ionic-liquid-templated proton-conducting gelatinous electrolyte . J. Phys. Chem. B , 108 : 17512 – 17518 . [CSA] [CROSSREF]
- Zein El Abedin , S. , Borissenko , N. and Endres , F. 2004 . Electrodeposition of nanoscale silicon in a room temperature ionic liquid . Electrochem. Comm , 6 : 510 – 514 . [CSA] [CROSSREF]
- Wadhawan , J. D. , Schröder , U. , Neudeck , A. , Wilkins , S. J. , Compton , R. G. , Marken , F. , Consorti , C. S. , de Souza , R. F. and Dupont , J. 2000 . Ionic liquid modified electrodes: Unusual partitioning and diffusion effects of Fe(CN)64-/3- in droplet and thin layer deposits of 1-methyl-3-(2,6-(S)-dimethylocten-2-yl)-imidazolium tetrafluoroborate . J. Electroanal. Chem. , 493 ( 1–2 ) : 75 – 83 . [CSA] [CROSSREF]
- Gaillon , L. and Bedioui , F. 2004 . Voltammetric analysis of the catalytic reactivity of electrogenerated CoI-salen with organohalogenated derivatives in an ionic liquid at room temperature . J. Mol. Cat. A: Chem. , 214 ( 1 ) : 91 – 94 . [CSA] [CROSSREF]
- Giovanelli , D. , Buzzeo , M. C. , Lawrence , N. S. , Hardacre , C. , Seddon , K. R. and Compton , R. G. 2004 . Determination of ammonia based on the electro-oxidation of hydroquinone in dimethylformamide or in the room temperature ionic liquid, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide . Talanta , 62 : 904 – 911 . [CSA] [CROSSREF]
- Lu , W. , Fadeev , A. G. , Qi , B. and Mattes , B. R. 2004 . Fabricating conducting polymer electrochromic devices using ionic liquids . J. Electrochem. Soc , 151 : H33 – H39 . [CSA] [CROSSREF]
- Ding , J. , Zhou , D. , Spinks , G. , Wallace , G. , Forsyth , S. , Forsyth , M. and MacFarlane , D. 2003 . Use of ionic liquids as electrolytes in electromechanical actuator systems based on inherently conducting polymers . Chem. Materials , 15 : 2392 – 2398 . [CSA] [CROSSREF]
- Bennett , M. D. and Leo , D. J. 2004 . Ionic liquids as stable solvents for ionic polymer transducers . Sensors and Actuators, A: Phys. , 115 : 79 – 90 . [CSA] [CROSSREF]
- Wang , R. , Hoyano , S. and Ohsaka , T. 2004 . O2 gas sensor using supported hydrophobic room-temperature IL membrane-coated electrode . Chem. Lett , 33 : 6 – 7 . [CSA] [CROSSREF]
- Wang , R. , Okajima , T. , Kitamura , F. and Ohsaka , T. 2004 . A novel amperometric O2 gas sensor based on supported room-temperature IL porous polyethylene membrane-coated electrodes . Electroanalysis , 16 ( 1–2 ) : 66 – 72 . [CSA] [CROSSREF]
- Brinz , T. and Simon , U. Electrochemical gas sensor with ionic liquids as electrolyte . German Patent DE 2002-10245337 . 2004 .
- Lee , Y. G. and Chou , T.-C. 2004 . Ionic liquid ethanol sensor . Biosensors and Bioelectronics , 20 : 33 – 40 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Koch , P. and Kuesters , E. PCT Int. Appl . World Patent WO 2004013612 . 2004 . Ionic liquids as solvents in headspace gas chromatography
- Huddleston , J. G. , Willauer , H. D. , Swatloski , R. P. , Visser , A. E. and Rogers , R. D. 1998 . Room temperature ionic liquids as novel media for “clean” liquid-liquid extraction . Chem. Commun , 16 : 1765 – 1766 . [CSA] [CROSSREF]
- Seiler , M. , Jork , C. , Kavarnou , A. , Arlt , W. and Hirsch , R. 2004 . Separation of azeotropic mixtures using hyperbranched polymers or ionic liquids . AIChE Journal , 50 : 2439 – 2454 . [CSA] [CROSSREF]
- Jork , C. , Seiler , M. , Beste , Y. -A. and Arlt , W. 2004 . Influence of ionic liquids on the phase behavior of aqueous azeotropic systems . J. Chem. Eng. Data , 49 : 852 – 857 . [CSA] [CROSSREF]
- Visser , A. E. , Holbrey , J. D. and Rogers , R. D. 2001 . Hydrophobic ionic liquids incorporating N-alkylisoquinolinium cations and their utilization in liquid-liquid separations . Chem. Commun , 23 : 2484 – 2485 . [CSA] [CROSSREF]
- Nobuoka , K. , Korenaga , W. and Ishikawa , Y. Environmentally safe ionic liquids for chiral resolution . Japan Patent JP 2004277351 . 2004 .
- Cull , S. G. , Holbrey , J. D. , Vargas-Mora , V. , Seddon , K. R. and Lye , G. J. 2000 . Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations . Biotech. and Bioeng. , 69 : 227 – 233 . [CSA] [CROSSREF]
- Pfruender , H. , Midjojo , M. , Kragl , U. and Weuster-Botz , D. 2004 . Efficient whole-cell biotransformation in a biphasic ionic liquid/water system . Angew. Chem., Int. Ed , 43 : 4529 – 4531 . [CSA] [CROSSREF]
- Matsumoto , M. , Mochiduki , K. , Fukunishi , K. and Kondo , K. 2004 . Extraction of organic acids using imidazolium-based ionic liquids and their toxicity to Lactobacillus rhamnosus . Sep. Pur. Tech. , 40 : 97 – 101 . [CSA] [CROSSREF]
- Carda-Broch , S. , Berthod , A. and Armstrong , D. W. 2003 . Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid . Anal. and Bioanal. Chem. , 375 : 191 – 199 . [CSA]
- Smirnova , S. V. , Torocheshnikova , I. I. , Formanovsky , A. A. and Pletnev , I. V. 2004 . Solvent extraction of amino acids into a room temperature ionic liquid with dicyclohexano-18-crown-6 . Anal. and Bioanal. Chem. , 378 : 1369 – 1375 . [CSA] [CROSSREF]
- Liu , J.-F. , Jiang , G.-B. , Chi , Y.-G. , Cai , Y.-Q. , Zhou , Q.-X. and Hu , J.-T. 2003 . Use of ionic liquids for liquid-phase microextraction of polycyclic aromatic hydrocarbons . Anal. Chem. , 75 : 5870 – 5876 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Liu , J.-F. , Chi , Y.-G. , Jiang , G.-B. , Tai , C. , Peng , J.-F. and Hu , J.-T. 2004 . Ionic liquid-based liquid-phase microextraction, A new sample enrichment procedure for liquid chromatography . J. Chromatogr. A , 1026 ( 1–2 ) : 143 – 147 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Visser , A. E. , Swatloski , R. P. , Griffin , S. T. , Hartman , D. H. and Rogers , R. D. 2001 . Liquid/liquid extraction of metal ions in room temperature ionic liquids . Sep. Sci. and Tech. , 36 ( 5 to 6 ) : 785 – 804 . [CSA] [CROSSREF]
- Vidal , S. , Neiva Joana , C. M. , Marques , M. M. , Ismael , M. R. and Angelino Reis , M. T. 2004 . Studies on the use of ionic liquids as potential extractants of phenolic compounds and metal ions . Sep. Sci. and Tech , 39 : 2155 – 2169 . [CSA] [CROSSREF]
- Wei , G.-T. , Yang , Z. and Chen , C.-J. 2003 . Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions . Anal. Chim. Acta , 488 : 183 – 192 . [CSA] [CROSSREF]
- Visser , A. E. and Rogers , R. D. 2003 . Room-temperature ionic liquids : New solvents for f-element separations and associated solution chemistry . J. Solid State Chem. , 171 ( 1–2 ) : 109 – 113 . [CSA]
- Whitehead , J. A. , Lawrance , G. A. and McCluskey , A. 2004 . “Green” leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid . Green Chem , 6 : 313 – 315 . [CSA]
- Nakashima , K. , Kubota , F. , Maruyama , T. and Goto , M. 2003 . Ionic liquids as a novel solvent for lanthanide extraction . Anal. Sci. , 19 : 1097 – 1098 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Dai , S. , Ju , Y. H. and Barnes , C. E. 1999 . Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids . J. Chem. Soc. Dalton Trans.: Inorg. Chem. , 8 : 1201 – 1202 . [CSA] [CROSSREF]
- Chun , S. , Dzyuba , S. V. and Bartsch , R. A. 2001 . Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether . Anal. Chem. , 73 : 3737 – 3741 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Luo , H. , Dai , S. and Bonnesen , P. V. 2004 . Solvent extraction of Sr2+ and Cs+ based on room-temperature ionic liquids containing monoaza-substituted crown ethers . Anal. Chem. , 76 : 2773 – 2779 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Dietz , M. L. and Dzielawa , J. A. 2001 . Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: Implications for the “greenness” of ionic liquids as diluents in liquid-liquid extraction . Chem. Commun. , 20 : 2124 – 2125 . [CSA] [CROSSREF]
- Dietz , M. l. , Dzielawa , J. A. , Laszak , I. , Young , B. A. and Jensen , M. P. 2003 . Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids . Green Chem. , 5 : 682 – 685 . [CSA] [CROSSREF]
- Shimojo , K. and Goto , M. 2004 . First application of calixarenes as extractants in room-temperature ionic liquids . Chem. Lett. , 33 : 320 – 321 . [CSA] [CROSSREF]
- Shimojo , K. and Goto , M. 2004 . Solvent extraction and stripping of silver ions in room-temperature ionic liquids containing calixarenes . Anal. Chem. , 76 : 5039 – 5044 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Luo , H. , Dai , S. , Bonnesen , P. V. , Buchanan , A. C. , Holbrey , J. D. , Bridges , N. J. and Rogers , R. D. 2004 . Extraction of cesium ions from aqueous solutions using calix[4]arene-bis(tert-octylbenzo-crown-6) in ionic liquids . Anal. Chem. , 76 : 3078 – 3083 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Visser , A. E. , Swatloski , R. P. , Reichert , W. M. , Mayton , R. , Sheff , S. , Wierzbicki , A. , Davis , J. H. and Rogers , R. D. 2002 . Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: Synthesis, characterization, and extraction studies . Envir. Sci. and Tech. , 36 : 2523 – 2529 . [CSA] [CROSSREF]
- Holbrey , J. D. , Visser , A. E. , Spear , S. K. , Reichert , W. M. , Swatloski , R. P. , Broker , G. A. and Rogers , R. D. 2003 . Mercury(II) partitioning from aqueous solutions with a new, hydrophobic ethylene-glycol functionalized bis-imidazolium IL . Green Chem. , 5 : 129 – 135 . [CSA] [CROSSREF]
- Bösmann , A. , Datsevich , L. , Jess , A. , Lauter , A. , Schmitz , C. and Wasserscheid , P. 2001 . Deep desulfurization of diesel fuel by extraction with ionic liquids . Chem. Commun. , 23 : 2494 – 2495 . [CSA]
- Eber , J. , Wasserscheid , P. and Jess , A. 2004 . Deep desulfurization of oil refinery streams by extraction with ionic liquids . Green Chem. , 6 : 316 – 322 . [CSA] [CROSSREF]
- Zhang , S. , Zhang , Q. and Conrad Zhang , Z. 2004 . Extractive desulfurization and denitrogenation of fuels using ionic liquids . Ind. and Eng. Chem. Res. , 43 : 614 – 622 . [CSA] [CROSSREF]
- Huang , C. , Chen , B. , Zhang , J. , Liu , Z. and Li , Y. 2004 . Desulfurization of gasoline by extraction with new ionic liquids . Energy and Fuels , 18 ( 6 ) : 1862 – 1864 . [CSA] [CROSSREF]
- Lo , W.-H. , Yang , H.-Y. and Wei , G.-T. 2003 . One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids . Green Chem. , 5 : 639 – 642 . [CSA] [CROSSREF]
- Poole , C. F. 2004 . Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids . J. Chromatogr. A. , 1037 ( 1–2 ) : 49 – 82 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Armstrong , D. W. , He , L. and Liu , Y. S. 1999 . Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography . Anal. Chem. , 71 : 3873 – 3876 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Anderson , J. L. and Armstrong , D. W. 2003 . High-stability ionic liquids: A new class of stationary phases for gas chromatography . Anal. Chem. , 75 : 4851 – 4858 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Berthod , A. , He , L. and Armstrong , D. W. 2001 . Ionic liquids as stationary phase solvents for methylated cyclodextrins in gas chromatography . Chromatographia , 53 ( 1–2 ) : 63 – 68 . [CSA]
- Ding , J. , Welton , T. and Armstrong , D. W. 2004 . Chiral ionic liquids as stationary phases in gas chromatography . Anal. Chem. , 76 : 6819 – 6822 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Letcher , T. M. , Soko , B. , Reddy , P. and Deenadayalu , N. 2003 . Determination of activity coefficients at infinite dilution of solutes in the ionic liquid 1-hexyl-3-methylimidazolium tetrafluoroborate using gas-liquid chromatography at the temperatures 298.15 K and 323.15 K . J. Chem. and Eng. Data , 48 : 1587 – 1590 . [CSA] [CROSSREF]
- Warren , D. , Trevor , L. M. , Deresh , R. and Raal , D. J. 2003 . Activity coefficients of hydrocarbon solutes at infinite dilution in the IL, 1-methyl-3-octyl-imidazolium chloride from gas-liquid chromatography . J. Chem. Thermodyn. , 35 : 1335 – 1341 . [CSA] [CROSSREF]
- Heintz , A. , Kulikov , D. V. and Verevkin , S. P. 2002 . Thermodynamic properties of mixtures containing ionic liquids: Activity coefficients at infinite dilution of polar solutes in 4-methyl-N-butyl-pyridinium tetrafluoroborate using gas-liquid chromatography . J. Chem. Thermodyn. , 34 : 1341 – 1347 . [CSA] [CROSSREF]
- Heintz , A. , Kulikov , D. V. and Verevkin , S. P. 2002 . Thermodynamic properties of mixtures ccontaining ionic liquids: II. Activity coefficients at infinite dilution of hydrocarbons and polar solutes in 1-methyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide and in 1,2-dimethyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide using gas-liquid chromatography . J. Chem. and Eng. Data , 47 : 894 – 899 . [CSA] [CROSSREF]
- Planeta , J. and Roth , M. 2004 . Partition coefficients of low-volatility solutes in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate-supercritical CO2 system from chromatographic retention measurements . J. Phys. Chem. , 108 : 11244 – 11249 . [CSA]
- Berthod , A. and Carda-Broch , S. 2003 . A new class of solvents for CCC: The room temperature ionic liquids . J. Liq. Chrom. and Rel. Technol. , 26 ( 9–10 ) : 1493 – 1508 . [CSA] [CROSSREF]
- Berthod , A. and Carda-Broch , S. 2004 . Use of the IL 1-butyl-3-methylimidazolium hexafluorophosphate in countercurrent chromatography . Anal. and Bioanal. Chem. , 380 : 168 – 177 . [CSA]
- Appleby , D. , Hussey , C. L. , Seddon , K. R. and Turp , J. E. 1986 . Room-temperature ionic liquids as solvents for electronic absorption spectroscopy of halide complexes . Nature. , 323 : 614 – 615 . [CSA] [CROSSREF]
- Seddon , K. R. 1987 . Electronic Absorption Spectroscopy in Room-Temperature Ionic Liquids , NATO ASI Series. Ser. C: Math. Phys. Sci., 202 (Molten Salt Chemistry) 365 – 381 . Dordrecht : Reidel Publishing Co. .
- Billard , I. , Moutiers , G. , Labet , A. , El Azzi , A. , Gaillard , C. , Mariet , C. and Luetzenkirchen , K. 2003 . Stability of divalent europium in an ionic liquid: Spectroscopic investigations in 1-methyl-3-butylimidazolium hexafluorophosphate . Inorg. Chem. , 42 : 1726 – 1733 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Lu , J. , Liotta , C. L. and Eckert , C. A. 2003 . Spectroscopically probing microscopic solvent properties of room-temperature ionic liquids with the addition of carbon dioxide . J. Phys. Chem. A , 107 : 3995 – 4000 . [CSA] [CROSSREF]
- Álvaro , M. , Ferrer , B. , García , H. and Narayana , M. 2002 . Screening of an ionic liquid as medium for photochemical reactions . Chem. Phys. Lett. , 362 ( 5–6 ) : 435 – 440 . [CSA]
- Armstrong , D. W. , Zhang , L.-K. , He , L. and Gross , M. L. 2001 . Ionic liquids as matrixes for matrix-assisted laser desorption/ionization mass spectrometry . Anal. Chem. , 73 : 3679 – 3686 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Zabet-Moghaddam , M. , Heinzle , E. and Tholey , A. 2004 . Qualitative and quantitative analysis of low molecular weight compounds by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry using ionic liquid matrices . Rapid Comm. Mass Spec. , 18 : 141 – 148 . [CSA] [CROSSREF]
- Carda-Broch , S. , Berthod , A. and Armstrong , D. W. 2003 . Ionic matrices for matrix-assisted laser desorption/ionization time-of-flight detection of DNA oligomers . Rapid Comm. Mass Spec. , 17 : 553 – 560 . [CSA] [CROSSREF]
- Mank , M. , Stahl , B. and Boehm , G. 2004 . 2,5-Dihydroxybenzoic acid butylamine and other ionic liquid matrixes for enhanced MALDI-MS analysis of biomolecules . Anal. Chem. , 76 : 2938 – 2950 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Romero-Sanz , I. , Bocanegra , R. , Fernandez de la Mora , J. and Gamero-Castano , M. 2003 . Source of heavy molecular ions based on Taylor cones of ionic liquids operating in the pure ion evaporation regime . J. Appl. Phys. , 94 : 3599 – 3605 . [CSA] [CROSSREF]
- Romero-Sanz , I. and Fernandez de la Mora , J. 2004 . Energy distribution and spatial structure of electrosprays of ionic liquids in vacuo . J. Appl. Phys. , 95 : 2123 – 2129 . [CSA] [CROSSREF]
- Dyson , P. J. , Khalaila , I. , Luettgen , S. , McIndoe , J. S. and Zhao , D. 2004 . Direct probe electrospray (and nanospray) ionization mass spectrometry of neat ionic liquids . Chem. Commun. , 19 : 2204 – 2205 . [CSA] [CROSSREF]
- Jackson , G. P. and Duckworth , D. C. 2004 . Electrospray mass spectrometry of undiluted ionic liquids . Chem. Commun. , 5 : 522 – 523 . [CSA] [CROSSREF]
- Kärkkäinen , J. , Asikkala , J. , Laitinen , R. S. and Lajunen , M. 2004 . Effect of temperature on the purity of product in the preparation of 1-butyl-3-methylimidazolium-based ionic liquids . Zeitsch. Naturforsch. B: Chem. Sci. , 59 : 763 – 770 . [CSA]
- Zhao , D. 2004 . Analysis of ionic liquids and dissolved species by electrospray ionization MS . Australian J. Chem. , 57 : 509 – 510 . [CSA] [CROSSREF]
- Kaliszan , R. , Marszall , M. P. , Markuszewski , M. J. , Baczek , T. and Pernak , J. 2004 . Suppression of deleterious effects of free silanols in liquid chromatography by imidazolium tetrafluoroborate ionic liquids . J. Chromatogr. , 1030 ( 1–2 ) : 263 – 271 . [CSA]
- He , L. , Zhang , W. , Zhao , L. , Liu , X. and Jiang , S. 2003 . Effect of 1-alkyl-3-methylimidazolium-based ionic liquids as the eluent on the separation of ephedrines by liquid chromatography . J. Chromatogr. A , 1007 ( 1–2 ) : 39 – 45 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Xiao , X. , Liang , Z. , Xia , L. and Jiang , S. 2004 . Ionic liquids as additives in high performance liquid chromatography: Analysis of amines and the interaction mechanism of ionic liquids . Anal. Chim. Acta , 519 : 207 – 211 . [CSA] [CROSSREF]
- Anderson , N. G. and Braatz , J. A. Gel electrophoresis process using ionic liquids for solubilizing highly hydrophobic proteins . U.S. Patent Appl. US 2004031685 . 2004 .
- Vaher , M. , Koel , M. and Kaljurand , M. 2001 . Non-aqueous capillary electriphoresis in acetonitrile using ionic liquid buffer electrolytes . Chromatographia , 53 : 302 – 306 . [CSA]
- Vaher , M. , Koel , M. and Kaljurand , M. 2002 . Application of 1-alkyl-3-methylimidazolium-based ionic liquids in non-aqueous capillary electrophoresis . J. Chromatogr. A , 979 ( 1–2 ) : 27 – 32 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Vaher , M. , Koel , M. and Kaljurand , M. 2002 . Ionic liquids as electrolytes for nonaqueous capillary electrophoresis . Electrophoresis , 23 : 426 – 430 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Yanes , E. G. , Gratz , S. R. , Baldwin , M. J. , Robison , S. E. and Stalcup , A. M. 2001 . Capillary electrophoretic application of 1-alkyl-3-methylimidazolium-based ionic liquids . Anal. Chem. , 73 : 3838 – 3844 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Jiang , T.-F. , Gu , Y.-L. , Liang , B. , Li , J.-B. , Shi , Y.-P. and Ou , Q.-Y. 2003 . Dynamically coating the capillary with 1-alkyl-3-methylimidazolium-based ionic liquids for separation of basic proteins by capillary electrophoresis . Anal. Chim. Acta , 479 : 249 – 254 . [CSA]
- Cabovska , B. , Kreishman , G. P. , Wassell , D. F. and Stalcup , A. M. 2003 . Capillary electrophoretic and nuclear magnetic resonance studies of interactions between halophenols and ionic liquid or tetraalkylammonium cations . J. Chromatogr. A , 1007 ( 1–2 ) : 179 – 187 . [PUBMED] [INFOTRIEVE] [CSA]
- Qin , W. , Wei , H. and Li , S. F. Y. 2003 . 1,3-Dialkylimidazolium-based room-temperature ionic liquids as background electrolyte and coating material in aqueous capillary electrophoresis . J. Chromatogr. A , 985 ( 1–2 ) : 447 – 454 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Qin , W. and Li , S. F.Y. 2002 . An ionic liquid coating for determination of sildenafil and UK-103,320 in human serum by capillary zone electrophoresis-ion trap mass spectrometry . Electrophoresis , 23 : 4110 – 4116 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Qin , W. and Li , S. F. Y. 2003 . Electrophoresis of DNA in IL coated capillary . Analyst , 128 : 37 – 41 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Qin , W. and Li , S. F. Y. 2004 . Determination of ammonium and metal ions by capillary electrophoresis-potential gradient detection using ionic liquid as background electrolyte and covalent coating reagent . J. Chromatogr. A , 1048 : 253 – 256 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Mwongela , S. M. , Numan , A. , Gill , N. L. , Agbaria , R. A. and Warner , I. M. 2003 . Separation of achiral and chiral analytes using polymeric surfactants with ionic liquids as modifiers in micellar electrokinetic chromatography . Anal. Chem. , 75 : 6089 – 6096 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Whitehead , J. A. , Lawrance , G. A. and McCluskey , A. 2004 . Analysis of gold in solutions containing ionic liquids by inductively coupled plasma atomic emission spectrometry . Australian J. Chem. , 57 : 151 – 155 . [CSA] [CROSSREF]
- Anderson , J. L. , Ding , J. , Welton , T. and Armstrong , D. W. 2002 . Characterizing ionic liquids on the basis of multiple solvation interactions . J. Am. Chem. Soc. , 124 : 14247 – 14254 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]