Abstract
Pesticides are an important and diverse environmental and agricultural species. Their determination in formulations, in feed and food, and in complex environmental matrices (e.g., water, soil, sludge, sediments, etc.) often requires separation methods capable of high efficiency, unique selectivity, and high sensitivity. Because pesticides (organophosporus, organochlorine, carbamate, dithiocarbamate, etc.) are carcinogenic, they are problematic for humans in the course of the food chain. Residual analyses have been performed to find out the concentration and type of pesticides and their metabolites left in food at the time of consumption. This ultimately helps in production of better and human-friendly pesticides. A new and effective sampling technique, Solid-Phase microextraction (SPME), was developed in 1990 using a fused silica fiber coated on the outside with appropriate stationary phase in which analyte in the sample is directly extracted to the fibber coating. Two types of fiber techniques can be used to extract analytes: Headspace (HS-SPME), where the fiber is exposed to the vapor phase above a gas, and direct immersion (DI-SPME), where the fiber is directly immersed in the samples. More advanced in-tube SPME recently has been developed, which uses open tubular fused-silica capillary column instead of SPME fiber. SPME in combination with HPLC, GC, GC-MS, LC-MS is getting a wide acceptability as an analytical technique. These have great advantages over the classical sampling techniques, which are time consuming and require larger samples and solvents. This review discusses the various advantages and disadvantages of the SPME for the extraction of the pesticides and their analysis by HPLC.
INTRODUCTION
Most of the pesticides are organic compounds with varying functional groups, which cause geometrical and optical isomers. Most of the pesticides differ in their degree of ionization, polarity, and solubility in water. These compounds, when sprayed in the environment, undergo degradation and decomposition. Thus, the analysis of the pesticides and their metabolites in the environment is very important, and methods of high resolving power, high separation efficiency, high sensitivity and unique selectivity are required for their analysis and separation.
The need to provide food to the growing population has lead to tremendous growth in the use of the pesticides and fertilizers. While, pesticides such as triazines, phenylureas, phenoxy acids, carbamates, etc. have increased crop production, their presence in air, soil, and water has had a marked increase as well.
Within nature, most chemical substances tend to decompose to relatively harmless compounds. However, some pesticides are persistent enough to withstand the decomposition action of chemical, physical, and biological processes. The persistentence of pesticides is characterized by parameter T1/2, which denotes the time that the content of the relevant pesticide in a sample is halved in comparison to the original amount. The persistence of the pesticide in nature is governed by factors, such as the type of pesticide, soil type, moisture content, and temperature. Residue analysis of food predicts the persistence of pesticides. These undergo different processes after application such as voltalization, adsorption in the soil with high content of clay or organic matter, leaching to ground and surface water, and chemical, microbial thermal, and photolytic degradation.
The analytical methods must overcome the following basic problems:
| 1. | necessity of analyzing very low concentrations; | ||||
| 2. | need for analytical standards containing analyte of interest on the level comparable to concentration in real samples; | ||||
| 3. | sample preparation for the final analysis should not add to the environmental pollution. | ||||
The aim of this review is to provide an introduction to the hyphenation of solid phase microextraction to the high performance liquid chromatography (HPLC) and its application to the analysis of pesticides in various samples. While earlier reviews appeared on SPME, very little attention was given to the hyphenation of SPME with HPLC for the analysis of pesticides. Most of the work pertains to SPME-GC. This review presents a comprehensive study of SPME-HPLC () and its applications for the analysis of pesticides.
TABLE 1 SPME-HPLC characteristics of pesticides
SOLID PHASE MICROEXTRACTION TECHNIQUE
Traditional methods of residual analysis for pesticides involve their extraction by solvent before they are analyzed. This extraction is both time and solvent consuming. The need to develop a solvent free and sensitive technique arises because the use of organic solvents creates pollution-related problems; moreover organic solvents are costly and require time consuming procedures. Solid-phase microextraction is relatively a new approach invented and developed at the University of Waterloo (Ontario, Canada) by Pawliszyn and Associates (Belardi and Pawliszyn) and being sold by Supelco(Citation1, Citation2). The method involves the equilibrium sorption of analytes onto a small microfiber, which is made of a fused-silica optical fiber, coated with a hydrophobic polymer. Analytes, either in the air or in a water sample, come into equilibrium with the fiber according to their affinity for the solid phase. The microfiber is incorporated into HPLC interphase and the analytes are desorbed from the fiber and delivered to the column for separation.
Solid-phase microextraction coupled with chromatographic techniques is gaining a wide applicability as an analytical technique. The technique is based on the partition of the analyte between sample matrix and stationary phase (polyacrylate, polydimethyl siloxane, etc.) coated on fused silica fiber. Depending on the distribution coefficient, an equilibrium is reached between the concentration of the analyte in sample and the amount of analyte sorbed on the fiber.
Design of SPME
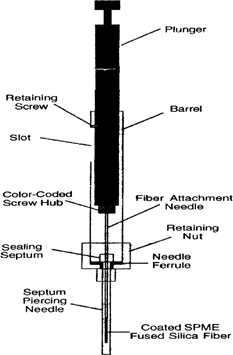
SPME is a modified syringe-like instrument (). The fused silica fiber, having a small size and cylindrical shape, is connected to a stainless-steel tubing that is used to provide additional mechanical strength to the fiber assembly for repeated sampling. This stainless-steel tubing is connected to a specially designed syringe-like instrument. The fused silica fiber is coated with a relatively thin film of several polymeric stationary phases. The fiber assembly is reusable and replaceable. Supelco (www.sigmaaldrich.com) provides seven different types of fibers. The small size and cylindrical geometry of fiber has some advantages such as easy placement of the sorbent fiber coating into a sample or headspace above the sample to extract the analytes; also it can be easily placed in desorption chamber of GC or interphase of the HPLC without any modification of GC or HPLC. Plunger movement and timing must be controlled carefully to perform adsorption and desorption correctly. It is very important for field sampling to prevent loss of analyte during transport. To do this, the needle opening of SPME devise must be sealed by using a septum and/or by cooling the needle.
Working with SPME
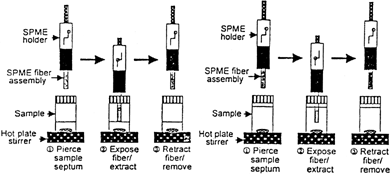
During the SPME operation, the fiber is first drawn into the syringe needle, then lowered into the vial (which is sealed with a septum type cap) by pressing the plunger. The fused silica fiber of suitable coating is used which is dependent upon the nature of the analyte. The fiber should be cleaned before analyzing any sample so as to remove contaminants that give a high background in the chromatogram. Cleaning can be performed in the desorption chamber of HPLC by running solvent. Now the cleaned fiber coating is exposed to a sample matrix for a predetermined, fixed period, which results in the adsorption of the analyte on the fiber coating (). This extraction can be performed in two ways (Citation3): 1) headspace SPME or HS-SPME where fiber is exposed in the vapor phase above a gaseous, liquid, or solid sample: 2) direct immersion DI-SPME, where the fiber is directly immersed in liquid samples.
Several methods to improve the extraction have evolved over time, including salting out, internal fiber cooling (Citation4), derivatization (Citation5, Citation6), Land vibration (Citation7) /rotation (Citation8) of the fiber.
| a. | Solubility of the analyte of interest can be decreased by addition of the salt in the liquid samples. This improves the HS extraction and thus the overall SPME/sample partition coefficient. | ||||
| b. | Increasing the temperature of the sample increases the vapor density of the analyte but increased temperature affects the coating of the fiber. Internal cooling of the fiber is done where the temperature of the sample is to be increased. This approach ensures increased sensitivity due to increase in partition coefficients of the HS/sample (due to increase in temperature of sample) and fiber/HS (due to decrease in temperature of the fiber). | ||||
| c. | Derivatization is based on analyte conversion to another compound by reaction with a specially selected reagent (called matrix derivatization) or by dipping the fiber coating in some suitable reagent so that the fiber becomes more selective for the analyte of interest. Derivatization makes the analyte more favorable for the SPME extraction (Citation5, Citation6) . | ||||
Although SPME has a maximum sensitivity at partition equilibrium, a proportional relationship is obtained between the amount of analyte adsorbed by the SPME fiber and the initial concentration in the sample matrix before reaching partition equilibrium (Citation9, Citation10), which implies that full equilibration is not necessary for quantitative analysis by SPME. Also adsorption of many chemical species like PCBs (Citation11) on the fiber is a slow process and sampling may take several days. A dynamic model has been proposed by Ai (Citation9). One conclusion of this model is that the amount of analyte adsorbed into the fiber is proportional to the initial concentration of the analyte in the sample matrix, once the agitation conditions and the sampling time are held constant, and hence, SPME quantization is feasible before adsorption equilibrium is reached. Once the sampling is complete, the fiber is directly transferred into the specially designed interface connected with HPLC for desorption of analyte, which is then analyzed.
Hyphenation of SPME with HPLC
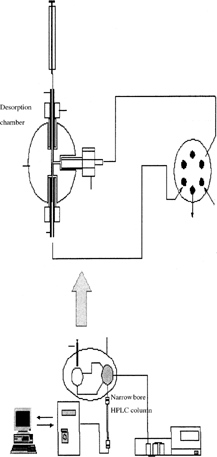
A special type of six-port interface (Citation1), which couples SPME with HPLC has been developed by Pawliszyn and sold by Supelco () (Citation12). This interface keeps intact the advantages of SPME as fast, solvent free, portable, and inexpensive sampling technique. In addition, it provides possibilities for the analysis of semi and nonvolatile organic compounds in water, an analysis that is difficult to achieve with GC. As some organophosphorous pesticides are less volatile, this hyphenation provides good alternative for their analysis.
SPME-HPLC interphase (Citation1) consists of the following three parts: 1) SPME device, 2) Interface, and 3) HPLC system. SPME assembly is similar to that discussed above. The type of fiber chosen is dependent on the polarity of the analyte and time required for the desorption. Time in turn depends on the thickness of the coating (Citation12) (time = L2/2D), where L is the thickness of coating and the D is the diffusion coefficient of an analyte.
A conventional SPME-HPLC interface (Citation13) reported by Pawliszyn et al. (Citation12), was modified so that the fiber in the injector extended into a piece of tubing outside the tee joint. This tubing was heated (either continually or with flash heating) during desorption using a heating wire coiled around the chamber with a d.c. power supply or capacitive discharge quick heating system.
Desorption of the Analyte
Two type of desorption techniques are used depending on the nature of interaction between analyte and fiber: static desorption (Citation14, Citation15) and dynamic desorption. When the analyte is not strongly adsorbed on the fiber, dynamic mode of desorption is sufficient; here the analyte can be removed by moving stream of mobile phase. But when the analytes are more strongly adsorbed on the fiber, the fiber is dipped in the mobile phase or other strong solvent for specified time. Desorption performed in this way is known as static. After desorption, fiber is injected in the HPLC column. Each type of desorption should be complete using minimal quantity of solvent. A portion of water containing the pesticides propham, siduron, linuron, chlorpropham, barban, and neburon were detected down to 1.5 ppb (Citation14) when subjected to SPME fiber coated with polydimethylsiloxane/ divinylbenzene.
Selection of Fiber
SPME has advantages of simplicity, low cost, ease of use, rapid preconcentration, and rapid extraction compared to other sample preparation techniques. The technique has found limited use in HPLC applications because of the unavailability of fibers that are stable and durable in strong organic solvents. Proper fiber selection is important for the efficient extraction of the analyte from the sample, and should be based on the nature of the analyte. There are seven different types of fibers available with Supelco: Polydimethylsiloxane (PDMS) (Citation15, Citation16), Polydimethylsiloxane/Divenylbenzene (PDMS/DVB) (Citation17, Citation14), stableflex Polydimethylsiloxane/ Divenylbenzene (PDMS/DVB), Polyacrylate(PA) (Citation18, Citation19, Citation20, Citation21), Carboxen/Polydimethyl-siloxane(CAR/PDMS), Carbowax/ Divinylbenzene (CW/TPR) (Citation22, Citation23, Citation24, Citation25), stableflex Divenylbenzene/Carboxen/ Polydimethylsiloxane (DVB/CAR/PDMS).
Low molecular weight or volatile compounds usually requires a 100 μ m PDMS coated fiber. Larger molecular weight or semivolatile compounds are more effectively extracted with 30 μ m PDMS fiber or 7 μ m PDMS fiber. To extract very polar analyte from general polar samples, use an 85 μ m PA coated fiber. More volatile polar analyte, such as alcohols or amines, are adsorbed more effectively and released fast with a 65 μ m PDMS/DVB coated fiber. A 60 μ m PDMS/DVB fiber is general purpose fiber for HPLC. For trace level volatile analysis, use of 75 μ m PDMS/Carboxan fiber is recommended. For an expanded range of analytes (C3–C20), use a 50/30 DVB/Carboxan on PDMS fiber. Most of these fibers are compactable with HPLC solvents, but the 100 μ m, 30 μ m, and 7 μ m PDMS coated fibers cannot be used with hexane.
Some modification of the fiber report using sol gel process (Citation26), these fibers are stable in strong organic solvents (xylene and methylene chloride) as well as acidic and basic solutions. The hydrolytic stability of these sol-gel prepared SPME fibers toward organic solvents and high and low pH solutions can be attributed to the fact that the coating is chemically bonded to the surface of the fused silica substrate. These fibers have been subsequently used to extract organo-arsenic, organo-mercury, and organo-tin compounds from aqueous solutions followed by separation using HPLC with UV absorbance detection.
Negligible depletion SPME (nd-SPME) (Citation27) is an application of SPME in which a negligible part of the freely dissolved concentration is extracted. Hence, it can be applied for measuring freely dissolved concentration (Citation28) and partition coefficients (Citation29).
An alternative technique has been developed by Aranda et al. (Citation30) using polycrystalline graphites (pencil lead and vitreous carbon) as sorbents for SPME of a nonionic alkylphenol ethoxylate surfactant (Triton X-100). Analyses were performed by reversed-phase HPLC with fluorimetric detection. The presence of the benzene ring in the congeners of Triton X-100 also allowed their direct detection at 310 nm (excitation at 230 nm). Results were compared to those obtained using polymeric fibres such as PDMS/DVB and Carbowax/TPR. The chemical resistance and low cost of the polycrystalline graphites are advantageous over commercially available fibres.
Liao et al (Citation31) reported a method in which fused-silica fiber after being activated in ethacryloxypropyltrimethoxysilane and coated with polyacrylic acid by immersing in 0.05M-sodium phosphate buffer of pH 7 containing 50 percent acrylic acid, ammonium persulfate, andNNN'N'-tetramethylethylenediamine for 12 h.
PAH in water were determined using a 100 μm polydimethylsiloxane fiber (Citation16) as well poly (dimethylsilane) (PDMS) (Citation1, Citation14).
PDMS/DVB
An acetonitrile-conditioned 60 μ m polydimethylsiloxane/ divinylbenzene fiber PDMS/DVB was used for the determination of diethyl phthalate (Citation17) and other pesticidespropham, siduron, linuron, chlorpropham, barban, and neburon.
Polyacrylate (PA)
Simazine, thiram, chlorothalonil, bensulide, thiobencarb, and EPN in water were determined (Citation18) by using PA fiber. The recovery and selectivity of very polar pesticides, for example, simazine and carbamates, (Citation21) in SPME was low.
Phenothiazines in human bodily fluids (Citation19), polar pesticides in soil (Citation21) and other pesticides (Citation20) were analyzed with a polyacrylate-coated fiber. Detection limits were 0.5–8 ppb with the 4.6 mm i.d. column and 0.1–5 ppb with the 1.5 mm i.d. column (Citation20).
Carbowax
Quinolones in humic acid/buffer samples were extracted on to Carbowax/templated polymer resin SPME fibers, then desorbed and analyzed on a 5 μ m C-18 column with gradient elution with H2O/methanol/2 mM-H3PO4 and detection at 260 nm (Citation27).
CAR/TPR Carbowax Template Resin
SPME, using a Carbowax/Templated Resin fiber, was optimized for the determination of mycotoxin cyclopiazonic acid (CPA) (Citation22) and pollutant phenolic compounds in water samples (Citation23). Detection limits of 1–10 μ g/l were achieved under the optimized conditions.
Applications of SPME-HPLC to Different Pesticides
Carbamates
Carbamates are group of insecticides having anticholinesterase activity. There are three major sub groups: sub group A comprises of carbaryl, propoxur, metalkamate, methiocarb; sub group B of Carbofuron, Pirimicarb; and subgroup-C of Aldicarb, Oxamyl, Methomyl. Carbamate insecticides are particularly useful for dealing with aphids and other pests that have developed resistence to organophosphorus compounds or that are difficult to control for the other reasons Whiteflies, leaf miners, ants, mealy bugs, scale insects, cockroaches, earwings, and wasps are among the pests controlled by various carbamates.
Comparison of two commercially available autosamplers were made (Citation32) for online coupling of in-tube SPME to HPLC for analysis of carbamates in water samples. In-tube SPME is a new online sample preparation technique that is easily installed on a conventional HPLC autosampler, and has been used previously with the LC Packings fully automated online sampler (FAMOS) autosampler without modification of the autosampler itself. The extraction and desorption processes with this system were studied thoroughly for a group of carbamate pesticides in water samples. The similarities and differences of the in-tube SPME technique with the two different autosamplers, FAMOS and Hewlett Packard, were compared during method development (Citation32).
In-tube SPME is an automated version of SPME that can be easily coupled to a conventional HPLC autosampler for online sample preparation, separation and quantitation. It has been termed “in-tube” SPME because the extraction phase is coated inside a section of fused-silica tubing rather than coated on the surface of a fused-silica rod as in the conventional syringe-like SPME device. The new in-tube SPME technique has been demonstrated as a very efficient extraction method for the analysis of polar and thermally labile analytes. The in-tube SPME-HPLC method, used with the FAMOS autosampler from LC Packings, was developed for detecting polar carbamate pesticides in clean water samples. The main parameters relating to the extraction and desorption processes of in-tube SPME (selection of coatings, aspirate/dispense steps, selection of the desorption solvents, and the efficiency of desorption solvent, etc.) were investigated. The method was evaluated according to the reproducibility, linear range and limit of detection. This method is simple, effective, reproducible, and sensitive. The relative standard deviation for all the carbamates investigated was between 1.7 percent and 5.3 percent. For the carbamates studied, the limits of detection observed are lower than or similar to that of U.S. Environmental Protection Agency or National Pesticide Survey methods. Detection of carbaryl present in clean water samples at 1 ng/l was possible (Citation33).
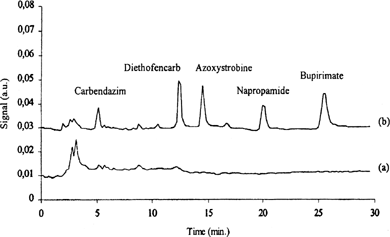
Focused microwave-assisted extraction (FMAE) (Citation22) and SPME, coupled HPLC, was applied for the determination of pesticides in strawberries () for the five compounds (carbendazim, diethofencarb, azoxystrobine, napropamide, and bupirimate) which can not be be analyzed easily by GC.
FIG. 4A SPME/HPLC chromatograms from strawberries extract detected at 205 nm: a) strawberry blank; b) strawberry spiked at 0.3 mg/kg. Reproduced with permission from Zambonin et al. (Citation22).
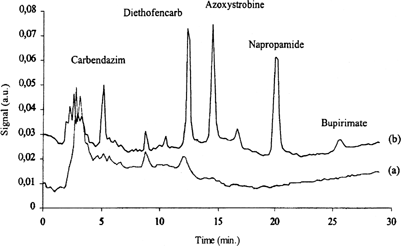
FIG. 4B SPME/HPLC chromatograms from strawberries extract detected at 240 nm: a) strawberry blank; b) strawberry spiked at 0.3 mg/kg. Reproduced with permission from Zambonin et al. (Citation22).
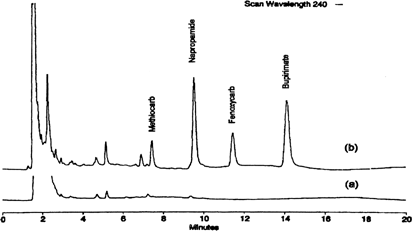
Methiocarb, napropamide, fenoxycarb, and bupirimate were analyzed (Citation45) () from spiked strawberry samples using polydimethylsiloxane/divinylbenzene-coated silica fiber and photodiode-array detection at 205 nm and 240 nm. Detection limits for methiocarb, napropamide, fenoxycarb, and bupirimate were 15, 10, 25, and 50 mg/kg, respectively, at 205 nm and 50, 12, 25, and 12 mg/kg, respectively, at 240 nm.
FIG. 5A SPME/HPLC chromatograms from strawberries extract detected at 205 nm: a) strawberry blank; b) strawberry spiked at 0.5 mg/kg. Reproduced with permission from Wang et al. (Citation45).
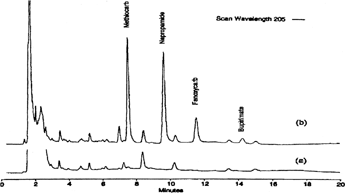
FIG. 5B SPME/HPLC chromatograms from strawberries extract detected at 240 nm: a) strawberry blank; b) strawberry spiked at 0.5 mg/kg. Reproduced with permission from Wang et al. (Citation45).
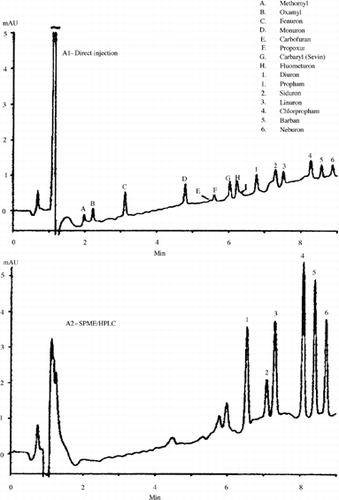
SPME also was applied for the extraction and analysis of the carbamate and urea pesticides using PDMS/DVB fiber. clearly demonstrates the extraction of the pesticides after preconcentration at low levels.
FIG. 6 Separation of carbamate and urea pesticides from water sample. Reproduced with permission from Gora-Maslak and Mani (Citation49).
Endocrine Disruptors
Endocrine system in humans and other animals produces the hormones that regulate the body's various processes, such as metabolism and reproduction, that are vital to the chemistry of life (e.g., insulin breaks down sugar, adrenalin pumps us up so we can deal with stress or danger). Research has shown that very high doses of some phthalates impeded the sexual development of male rats.
An automated online method for the analysis of endocrine (Citation34) disruptors was developed by Kataoka et al. Suspected endocrine disrupting compounds, bisphenol, alkylphenols, and phthalate esters tested in this study were well separated with a Hypersil ODS column with diode array detection using acetonitrile/water as the mobile phase.
Trace determination of diethyl phthalate (Citation17) in aqueous media by SPME—liquid chromatography was performed using an acetonitrile-conditioned 60 μm polydimethylsiloxane/divinylbenzene fiber. After extraction, the fiber was withdrawn and was placed in the interface between valves in a column switching system. The fiber was swept directly onto the HPLC column. Diethyl phthalate was analyzed on a 5 μm Nucleosil C-18 column.
Organometallic Pesticides
Organometallic compounds mostly are used as fungicides. There is always a probability of growth of fungus from few hypal strands. Therefore, the fungicides should possess some degree of persistence.
An analytical method has been developed for the determination of the trimethyllead (TML) and triethyllead (TEL) species in aqueous samples (Citation35). In-tube SPME and HPLC are coupled to a quadrupole mass spectrometer using an electrospray as an ionization interface. Elemental lead-208 (Pb+) and molecular forms of TML and TEL (m/z) were monitored simultaneously to provide complete speciation information. Results from the in-tube SPME-HPLC-ESMS experiment indicated that complete separation and detection of TML and TEL can be achieved in under 5 min. Precision was greater than 5 percent and estimated limits of detection were 11.3 and 12.6 ng/mL, respectively, for TML and TEL.
A simple and sensitive method has been developed (Citation36) for the determination of tributyltin (TBT) in aqueous samples and environmental samples. Automated in-tube SPME and HPLC were coupled to a quadrupole mass spectrometer (MS) using electrospray (ES) as an ionization source. To achieve optimum performance, the conditions for both in-tube SPME and ESMS detection were optimized for the analysis of TBT. A commercially available capillary, Supel-Q PLOT, showed the best extraction efficiency for TBT and, therefore, it was selected for in-tube SPME. The in-tube SPME-HPLC-ESMS method provided a linear relationship between the analyte concentration and signal intensity in the range of 0.5–200 ng/mL TBT, with a detection limit of 0.05 ng/mL. The method performance was evaluated by determining the content of TBT in a sediment reference material.
The application of SPME-HPLC-electrospray mass spectrometry (SPME-HPLC-ESMS) to the analysis of organolead compounds in the aqueous environment is described by Mester et al. (Citation37). Stock organolead solutions were obtained by dissolving trimethyllead chloride and triethyllead chloride methanol. Working standards were prepared from these stock standards.
Sol-gel fibers prepared by Gbatu et al. (Citation26) have been subsequently used to extract organo-arsenic, organo-mercury, and organo-tin compounds from aqueous solutions followed by separation using HPLC with UV absorbance detection.
Organophosphorus Pesticides
The primary site of action of organophosphorus compounds is the enzyme cholinestrase which is present in the nervous system. Main chemical groups of organophosphorus insecticides are orthophosphates, thionphosphates, thiolphosphate, dithiophosphates, phosphonates, pyrophosphoramides. These are less persistence as compared to the organochlorine pesticides.
Organophosphorus pesticides spiked in water sample solutions were extracted with SPME technique and were desorbed by supercritical fluid carbon dioxide (SFCO2) before online introduction into HPLC system. All of the SFCO2 injected into the HPLC system was dissolved into the CH3OH/H2O mobile phase without affecting the baseline of the UV detector signal (Citation37).
Herbicides
Degradation of chlortoluron (Citation38) in water disinfection processes were performed. The kinetics of the reaction between chlortoluron, a phenylurea herbicide [N′-(2-hydroxy-4-methyl-5-chlorophenyl)-NN-dimethylurea], and hypochlorite, the active species in water disinfection processes involving chlorine, were investigated by HPLC-UV and HPLC-electrospray ionization (ESI) MS. In particular, the concentrations of the main chlortoluron byproducts were monitored as a function of time by HPLC-ESI-MS and a kinetic model was developed to fit the relevant curves. The results showed that chlortoluron degradation starts with two parallel pathways, namely, chlorination and hydroxylation of the aromatic ring, which are then followed by consecutive chlorination reactions, and after almost two weeks by ring opening and partial mineralization, as confirmed by headspace SPME-GC-MS and total organic carbon measurements. Kinetic constants for the first reactions of the overall process, under pseudo-first-order conditions (hypochlorite excess) are reported.
The application of a manually operated SPME-HPLC interface is discussed for the analysis of thermally labile analytes in flooded rice field to demonstrate its potential for real time extraction of the herbicide profoxydim (Citation39). The fibers were shipped back to the laboratory by express delivery where the target analyte was desorbed from the fiber and determined by HPLC-UV analysis. The SPME method was characterized by significant ruggedness where conventional techniques such as liquid–liquid extraction and SPE require additional shipping and handling costs and time-consuming multiple sample preparation steps. In general, any delay in shipping the aqueous samples to the laboratory has the potential for sample degradation and a loss in accuracy when using non-onsite extraction techniques. There were 50 μm Carbowax-templated resin coatings that were most suitable for coupling SPME to HPLC in order to achieve a high sensitivity for polar analytes. The SPME technique was characterized by a good sensitivity and a precision of < 10 percent RSD. The SPME-LC-UV method was linear over three orders of magnitude while achieving a limit of detection in the lower μg/l range. The onsite SPME method has shown significantly increased accuracy. Profoxydim was determined at concentrations of similar180 μ g/l on a flooded bare soil field.
Organochlorine Pesticides
The organochlorine insecticides are usually divided into three main families comprising respectively compounds related to DDT, γ-BHC and compounds related to aldrin. These are highly persistent insecticides leading to ban on some members of their group.The toxic effects of the three families of organochlorine compounds differ but all are neurotoxic substances and attack at the central nervous system.
A method for the determination of chlorinated phenoxy acid herbicides (Citation40) in river water has been developed using in-tube SPME followed by LC/ESI-MS. Simple mass spectra with strong signals corresponding to [M-H]− and [M-RCOOH]− were observed for all herbicides tested in this study. The best separation of these compounds was obtained with a C18 column using linear gradient elution with a mobile phase of acetonitrile-water containing 5 mmol/L dibutylamine acetate (DBA).
Optimization of SPME for widely used herbicide alachlor extraction from water samples have been performed by Gonzalez-Barreiro et al. (Citation41). Extraction time and sample volume were the only statistically significant factors from those studied. In the final optimized conditions the procedure was applied to the SPME-HPLC analysis of alachlor in spiked water samples with excellent figures of merit.
CONCLUSIONS
Analytes with low volatality are difficult to analyze with GC but can be easily estimated using hyphenation with HPLC. This hyphenation is relatively new and very limited literature is available. Initial work, reported by Pawliszyn et al. (Citation1) pertains to separation and identification of the PAHs. The extraction step is same for both the hypenations that is with GC and HPLC. But the difference between them lies in the method of desorption of the analyte from the fiber; in GC, an injector provides a mean for thermal desorption of the analyte, whereas in HPLC the analyte is desorbed from the fiber using the mobile phase. For this purpose a specialy designed interphase is used hyphenation is very simple and quick but fails to provide good results for the analytes which are thermally unstable.
The potential for the real time extraction can be visualized from the fact that it has been used for the onsite extraction of profoxydim in flooded rice fields and than analysis by HPLC after desorption from the fiber (Citation39).
Futher modifications in the SPME, that is, the in—tube SPME are highly promising. Thus, SPME can be considered as an extraction technique for the future.
REFERENCES
- Arthur , C. L. and Pawliszyn , J. 1990 . Solid phase microextraction with thermal desorption using fused silica optical fibers . Anal. Chem. , 62 : 2145 – 2148 .
- Zhang , Z. , Yang , M. J. and Pawliszyn , J. 1994 . Solid-phase microextraction: A new solvent-free alternative for sample preparation . Anal. Chem. , 66 : 844A – 853A .
- Kataoka , H. , Lord , H. L. and Pawliszyn , J. 2000 . Application of solid-phase microextraction in food analysis . J. of Chromatography A , 880 : 35 – 62 . [CROSSREF]
- Zhang , Z. and Pawliszyn , J. 1995 . Quantitative extraction using an internally cooled solid phase microextraction device . Anal. Chem. , 67 : 34 – 43 . [CROSSREF]
- Pan , L in and Pawliszyn , J. 1997 . Derivatization/Solid-phase microextraction: new approach to polar analytes . Anal. Chem. , 69 : 196 – 205 . [CROSSREF]
- Martos , P. A. and Pawliszyn , J. 1998 . Sampling and determination of formaldehyde using solid-phase microextraction with on–fiber derivatization . Anal. Chem. , 70 : 2311 – 2320 . [CROSSREF]
- Penton , Z. , Geppert , H. and Betz , V. 1997 . GIT spez. Chromatr , 776 : 293 – 303 .
- Geppert , H. 1998 . Solid-phase microextraction with rotation of the microfiber . Anal. Chem. , 70 ( 18 ) : 3981 – 3982 . [CROSSREF]
- Ai , J iu . 1997 . Solid phase microextraction for quantitative analysis in nonequilibrium situations . Anal. Chem. , 69 : 1230 – 1236 . [CROSSREF]
- Ai , J iu . 1997 . Headspace solid phase microextraction-dynamics and quantitative analysis before reaching a partition equilibrium . Anal. Chem. , 69 : 3260 – 3266 . [CROSSREF]
- Llompart , M. , Ken , Li and Fingas , M. 1998 . Solid-phase microextraction and headspace solid-phase microexraction for the determination of polychlorinated biphenyls in water samples . Anal. Chem. , 70 : 2510 – 2515 . [CROSSREF]
- Chen , J. and Pawliszyn , J. 1995 . Solid phase coupled to high-performance liquid Chromatography . Anal. Chem. , 67 : 2530 – 2533 . [CROSSREF]
- Louch , D. S. , Motlagh , S. and Pawliszyn , J. 1992 . Dynamics of organic compound extraction from water using liquid-coated fused silica fibers . Anal. Chem. , 64 : 1187 – 1199 . [CROSSREF]
- Daimon , H. and Pawliszyn , J. 1997 . Effect of heating the interface on chromatographic performance of solid-phase microextraction coupled to high-performance liquid chromatography . Analytical Communications , 34 ( 11 ) : 365 – 369 . [CROSSREF]
- Wang , C. Y. , Tao , J. Q. , Li , B. F. , Ma , Z. L. and Li , G. K. 2002 . Determination of trace chrysene in environmental water by solid-phase microextraction coupled with high-performance liquid chromatography . Sepu , 20 ( 1 ) : 59 – 62 .
- Haag , I. 1996 . Potential applications of coupled solid-phase microextraction-HPLC . LaborPraxis , 20 ( 11 ) : 66 – 68 . 71
- Kelly , M. T. and Larroque , M. 1999 . Trace determination of diethyl phthalate in aqueous media by solid-phase microextraction-liquid chromatography . J of Chromatography A , 841 ( 2 ) : 177 – 185 . [CROSSREF]
- Selleh , S. H. , Saito , Y. and Jinno , K. 1999 . Study on solventless sample preparation of pesticides with SPME-SFE technique . Chromatography , 20 ( 2 ) : 126 – 127 .
- Kumazawa , T. , Seno , H. , Watanabe-Suzuki , K. , Hattori , H. , Ishii , A. , Sato , K. and Suzuki , O. 2000 . Determination of phenothiazines in human body fluids by solid-phase microextraction and liquid chromatography-tandem mass spectrometry . Journal of Mass Spectrometry , 35 ( 9 ) : 1091 – 1099 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Jinno , K. , Muramatsu , T. , Saito , Y. , Kiso , Y. , Magdic , S. and Pawliszyn , J. 1996 . Analysis of pesticides in environmental water samples by solid-phase micro-extraction high-performance liquid chromatography . J of Chromatography A , 754 ( 1–2 ) : 137 – 144 . [CROSSREF]
- Moder , M. , Popp , P. , Eisert , R. and Pawliszyn , J. 1999 . Determination of polar pesticides in soil by solid-phase microextraction coupled to high-performance liquid chromatography-electrospray mass spectrometry . Fresenius' J of Anall. Chem. , 363 ( 7 ) : 680 – 685 . [CROSSREF]
- Zambonin , C. G. , Monaci , L. and Aresta , A. 2001 . Determination of cyclopiazonic acid in cheese samples using solid-phase microextraction with high performace liquid chromatography . Food Chemistry , 75 ( 2 ) : 249 – 254 . [CROSSREF] [CSA]
- Gonzalez-Toledo , E. , Prat , M. D. and Alpendurada , M. F. 2001 . Solid-phase microextraction coupled to liquid chromatography for the analysis of phenolic compounds in water . J of Chromatography A , 923 ( 1–2 ) : 45 – 52 . [CROSSREF]
- Ceglarek , U. , Efer , J. , Schreiber , A. , Zwanziger , E. and Engewald , W. 1999 . Determination of linear alkylbenzenesulfonates in communal wastewater by means of solid phase microextraction coupled with API-MS and HPLC-FLD . Fresenius' J of Anal Chem , 365 ( 8 ) : 674 – 681 . [CROSSREF] [CSA]
- Wu , L. , Almirall , J. R. and Furton , K. G. 1999 . An improved interface for coupling solid-phase microextraction (SPME) to high performance liquid chromatography (HPLC) applied to the analysis of explosives . J of High Resolution Chromatography , 22 ( 5 ) : 279 – 282 . [CROSSREF]
- Gbatu , T. P. , Sutton , K. L. and Caruso , J. A. 1999 . Development of new SPME fibers by sol-gel technology for SPME-HPLC determination of organometals . Analytica Chimica Acta , 402 ( 1–2 ) : 67 – 79 . [CROSSREF]
- Lutzhoft , H. C. H. , Vaes , W. H. J. , Freidig , A. P. , Halling-Sorenson , B. and Hermens , J. L. M. 2000 . Influence of pH and other modifying factors on the distribution behaviour of 4-quinolones to solid phases and humic acids studied by “negligible-depletion” SPME-HPLC . Environmental Science and Technology , 34 ( 23 ) : 4989 – 4994 . [CROSSREF] [CSA]
- Vaes , W. H. J. , Urrestarazu Ramos , E. , Verhaar , H. J. M. , Seinen , W. and Hermens , J. L. M. 1996 . Measurement of the free concentration using solid-phase microextraction: Binding to protein . Anal. Chem. , 68 : 4463 – 4467 . [CROSSREF]
- Vaes , W. H. J. , Urrestarazu Ramos , E. , Hamwijk , C. , Holsteijn , I. V. , Blaauboer , B. J. , Seinen , W. , Verhaar , H. J. M. and Hermens , J. L. M. 1997 . Solid phase microextraction as a tool to determine embrane/water partition coefficients and bioavailable concentrations in vitro systems . Chem. Res. Toxicol. , 10 : 1067 – 1072 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Aranda , R. , Kruus , P. and Burk , R. C. 2000 . Assessment of polycrystalline graphites as sorbents for solid-phase microextraction of nonionic surfactants . J of Chromatography A , 888 ( 1–2 ) : 35 – 41 . [CROSSREF]
- Liao , J. L. , Zeng , C. M. , Hjerten , S. and Pawliszyn , J. 1996 . Solid-phase microextraction of biopolymers, exemplified with adsorption of basic proteins onto a fiber coated with polyacrylic acid . Journal of Microcolumn Separations , 8 ( 1 ) : 1 – 4 . [CROSSREF]
- Gou , Y. , Tragas , C. , Lord , H. and Pawliszyn , J. 2000 . Online coupling of in-tube solid phase microextraction (SPME) to HPLC for analysis of carbamates in water samples: Comparison of two commercially available autosamplers . Journal of Microcolumn Separations , 12 ( 3 ) : 125 – 134 . [CROSSREF]
- Gou , Y. , Eisert , R. and Pawliszyn , J. 2000 . Automated in-tube solid-phase microextraction-high-performance liquid chromatography for carbamate pesticide analysis . Journal of Chromatography, A , 873 ( 1 ) : 137 – 147 . [CROSSREF]
- Kataoka , H. , Ise , M. and Narimatsu , S. 2002 . Automated online in-tube solid-phase microextraction coupled with high performance liquid chromatography for the analysis of bisphenol A, alkylphenols, and phthalate esters in foods contacted with plastics . Journal of Sep-aration Science , 25 ( 1-2 ) : 77 – 85 . [CROSSREF]
- Mester , Z. , Lord , H. and Pawliszyn , J. 2000 . Speciation of trimethyllead and triethyllead by in-tube solid-phase microextraction high-performance liquid chromatography electrospray ionization mass spectrometry . Journal of Analytical Atomic Spectrometry , 15 ( 6 ) : 595 – 600 . [CROSSREF]
- Wu , J. C. , Mester , Z. and Pawliszyn , J. 2001 . Determination of tributyltin by automated in-tube solid-phase microextraction coupled with HPLC-electrospray MS . Journal of Analytical Atomic Spectrometry , 16 ( 2 ) : 159 – 165 . [CROSSREF]
- Salleh , S. H. , Saito , Y. , Kiso , Y. and Jinno , K. 2001 . Solventless sample preparation procedure for organophosphorus pesticides analysis using solid phase microextraction and on-line supercritical fluid extraction/high-performance liquid chromatography technique . Analytica Chimica Acta , 433 ( 2 ) : 207 – 215 . [CROSSREF]
- Losito , H. , Zambonin , C. G. and Palmisano , F. 2000 . Degradation of chlortoluron in water disinfection processes: A kinetic study . Journal of Environmental Monitoring , 2 ( 6 ) : 582 – 586 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Eisert , R. , Jackson , S. and Krotzky , A. 2001 . Application of on-site microextraction in aquatic dissipation studies of profoxydim in rice . Journal of Chromatography, A , 909 ( 1 ) : 29 – 36 . [CROSSREF] [CSA]
- Takino , M. , Daishima , S. and Nakahara , T. 2001 . Automated on-line in-tube solid-phase microextraction followed by liquid chromatography/electrospray ionization-mass spectrometry for the determination of chlorinated phenoxy acid herbicides in environmental waters . Analyst , 126 ( 5 ) : 602 – 608 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Gonzalez-Barreiro , C. , Lores , M. , Casais , M. C. and Cela , R. 2000 . Optimization of alachlor solid-phase micro-extraction from water samples using experimental design . Journal of Chromatography, A , 896 ( 1–2 ) : 373 – 379 . [CROSSREF]
- Jinno , K. , Taniguchi , M. and Hayashida , M. 1998 . Solid-phase micro extraction coupled with semi-microcolumn high-performance liquid chromatography for the analysis of benzodiazepines in human urine . Journal of Pharmaceutical and Biomedical Analysis , 17 ( 6–7 ) : 1081 – 1091 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Jinno , K. , Muramatsu , T. , Saito , Y. , Kiso , Y. , Magdic , S. and Pawliszyn , J. 1996 . Analysis of pesticides in environmental water samples by solid-phase micro-extraction high-performance liquid chromatography . Journal of Chromatography, A , 754 ( 1–2 ) : 137 – 144 . [CROSSREF]
- Salleh , S. H. , Saito , Y. and Jinno , K. 2000 . An approach to solventless sample preparation procedure for pesticides analysis using solid-phase microextraction/supercritical-fluid extraction technique . Analytica Chimica Acta , 418 ( 1 ) : 69 – 77 . [CROSSREF]
- Wang , Z. , Hennion , B. , Urruty , L. and Montury , M. 2000 . Solid-phase microextraction coupled with high-performance liquid chromatography: a complementary technique to solid-phase microextraction-gas chromatography for the analysis of pesticide residues in strawberries . Food Additives and Contaminants , 17 ( 11 ) : 915 – 923 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Katayama , M. , Matsuda , Y. , Shimokawa , K. , Tanabe , S. , Hara , I. , Sato , T. , Kaneko , S. and Daimon , H. 2001 . Determination of -blockers by high-performance liquid chromatography coupled with solid-phase microextraction from urine and plasma samples . Analytical Letters , 34 ( 1 ) : 91 – 101 . [CROSSREF]
- Reyzer , M. L. and Brodbelt , J. S. 2001 . Analysis of fire ant pesticides in water by solid-phase microextraction and gas chromatography/ mass spectrometry or high-performance liquid chromatography/ mass spectrometry . Analytica Chimica Acta , 436 ( 1 ) : 11 – 20 . [CROSSREF] [CSA]
- Falqui-Cao , C. , Wang , Z. , Urruty , L. , Pommier , J. J. and Montury , M. 2001 . Focused microwave assistance for extracting some pesticide residues from strawberries into water before their determination by SPME/HPLC/DAD . Journal of Agricultural and Food Chemistry , 49 ( 11 ) : 5092 – 5097 . [PUBMED] [INFOTRIEVE] [CROSSREF] [CSA]
- Gora-Maslak , G. and Mani , V. 1997 . Solid-phase microextraction for HPLC analysis of pesticides . Supelco Reporter , 16 ( 3 ) : 5 [CSA]