ABSTRACT
Anthocyanins are one of the most widespread families of natural pigments in the plant kingdom. Their health beneficial effects have been documented in many in vivo and in vitro studies. This review summarizes the most recent literature regarding the health benefits of anthocyanins and their molecular mechanisms. It appears that several signaling pathways, including mitogen-activated protein kinase, nuclear factor κB, AMP-activated protein kinase, and Wnt/β-catenin, as well as some crucial cellular processes, such as cell cycle, apoptosis, autophagy, and biochemical metabolism, are involved in these beneficial effects and may provide potential therapeutic targets and strategies for the improvement of a wide range of diseases in future. In addition, specific anthocyanin metabolites contributing to the observed in vivo biological activities, structure–activity relationships as well as additive and synergistic efficacy of anthocyanins are also discussed.
Abbreviations
| ABCA1 | = | ATP-binding cassette transporter A1 |
| ABCG1 | = | ATP-binding cassette transporter G1 |
| ACC | = | acetyl-CoA carboxylase |
| ACO | = | acyl-CoA oxidase |
| AD | = | Alzheimer's disease |
| AIF | = | apoptosis-inducing factor |
| AMPK | = | AMP-activated protein kinase |
| AP-1 | = | activator protein-1 |
| aP2 | = | adipocyte fatty acid binding protein |
| apo E−/− | = | apolipoprotein E-deficient |
| APP | = | amyloid precursor protein |
| Atg5 | = | autophagy-related gene 5 |
| ATGL | = | adipose triglyceride lipase |
| ATP | = | adenosine triphosphate |
| Aβ | = | amyloid-beta peptide |
| C/EBPδ | = | CCAAT/enhancer-binding protein |
| COX-2 | = | cyclooxygenase-2 |
| CPT1A | = | carnitine palmitoyltransferase-1A |
| CRP | = | C-reactive protein |
| eIF2α | = | eukaryotic initiation factor 2α-subunit |
| Endo G | = | endonuclease G |
| ERK | = | extracellular signal-regulated kinase |
| FFAs | = | free fatty acids |
| FoxO1 | = | forkhead box O1 |
| G6Pase | = | glucose-6-phosphatase |
| GFAT | = | glutamine:fructose 6-phosphate aminotransferase |
| Glut2 | = | glucose transporter 2 |
| Glut4 | = | glucose transporter 4 |
| GSK3β | = | glycogen synthase kinase 3β |
| HBP | = | hexosamine biosynthetic pathway |
| IL | = | interleukin |
| iNOS | = | inducible nitric oxide synthase |
| JNK | = | c-Jun N-terminal kinase |
| LPL | = | lipoprotein lipaseand |
| LPS | = | lipopolysaccharide |
| MAPK | = | mitogen-activated protein kinase |
| MMP | = | matrix metalloproteinase |
| mtGPAT1 | = | mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 |
| mTOR | = | mammalian target of rapamycin |
| NF-κB | = | nuclear factor κB |
| NO | = | nitric oxide |
| OGD | = | oxygen–glucose deprivation |
| oxLDL | = | oxidative modification of low-density lipoprotein |
| PARP | = | poly ADP-ribose polymerase |
| PCA | = | protocatechuic acid |
| PD | = | Parkinson's disease |
| PEPCK | = | phosphoenol pyruvate carboxykinase |
| PGE2 | = | prostaglandin E2 |
| PhIP | = | 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine |
| PKCζ | = | protein kinase C ζ |
| PPARγ | = | peroxisome proliferator-activated receptor γ |
| Pt | = | petunidin |
| RCT | = | reverse cholesterol transport |
| ROS | = | reactive oxygen species |
| RR-ARFs | = | anthocyanin-rich fractions from red raspberries |
| SCI | = | spinal cord injury |
| SGLT1 | = | sodium-dependent glucose transporter 1 |
| STAT3 | = | signal transducers and activators of transcription 3 |
| TG | = | triglycerides |
| TNF | = | tumor necrosis factor |
| TRAFs | = | tumor necrosis factor receptor-associated factors |
| UCP2 | = | uncoupling protein 2 |
| UDP-GlcNAc | = | UDP-N-acetylglucosamine production |
Introduction
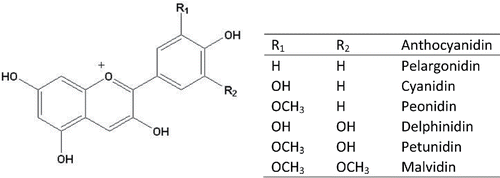
Anthocyanins are one of the most widespread families of natural pigments in the plant kingdom. They are responsible for the blue, purple, red, and orange colors of many fruits and vegetables. Anthocyanins belong to a large group of compounds, collectively known as flavonoids, which are a subgroup of an even larger group of compounds known as polyphenolics (Mazza and Miniati, Citation1993; Andersen, Citation2001; Andersen and Markham, Citation2006; McGhie and Walton, Citation2007). Anthocyanins are present in nature mainly in the form of heterosides. The aglycon forms of anthocyanins, also called anthocyanidin, are structurally based on the flavilium ion or 2-phenylbenzopyrilium, and comprise hydroxyl and methoxyl groups in different positions. According to the number and position of the hydroxyl and methoxyl moieties, more than 635 anthocyanins have been identified. Among them, the six most mentioned anthocyanidins in plants are pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin (de Pascual-Teresa and Sanchez-Ballesta, Citation2008; ). Moreover, they are widely present in many fruits and vegetables. shows the daily consumption of anthocyanins from fruits, vegetables, and beverages obtained from the National Health and Nutrition Examination Survey (NHANES, Citation2001; Wu et al., Citation2006).
Table 1. Estimation of daily consumption of anthocyanins from fruits, vegetables, and beverages. (Reproduced from the original source, Wu et al., Citation2006.)
During the last decade, several excellent reviews have illustrated these natural dietary phytochemicals in terms of their absorption (McGhie and Walton, Citation2007), metabolism (He and Giusti, Citation2010), bioavailability (Fernandes et al., Citation2014), and pharmacokinetics (Kay, Citation2006) as well as their various analytical techniques (Welch et al., Citation2008). The interest of knowing the health benefits of anthocyanins has also strongly increased in recent years. Ghosh and Konishi Citation(2007) summarized the antidiabetic and eye function properties of anthocyanins. de Pascual-Teresa Citation(2014) discussed the beneficial effects of anthocyanins on the prevention of cardiovascular diseases and neurological conditions. This review summarizes the most recent literature regarding the biological benefits of dietary anthocyanins, including anticancer activity, anti-inflammatory activity, neuroprotective activity, prevention of cardiovascular disease, anti-obesity, and antidiabetic activity, especially focusing on the molecular mechanisms of action. We believe that the present review will be helpful to better understand these dietary phytochemicals and apply them for the benefits of human health.
Anticancer activity
Epidemiological studies revealing the anticancer activity of anthocyanins on gastrointestinal tract cancer risk in humans have been well summarized (Kocic et al., Citation2011). However, convincing epidemiological evidence indicating a positive association between the intake of anthocyanins-rich food and other cancers is needed to be further determined (Wang and Stoner, Citation2008). We here discuss experimental findings related to the anticancer activity of anthocyanins ().
Table 2. Summary of the experimental findings on the anti-cancer activity of anthocyanin-rich fruits and vegetables.
The anticancer activities of anthocyanins from many fruits and vegetables have been demonstrated to inhibit the initiation, promotion, and progression of several cancers, such as breast cancer (Singletary et al., Citation2007; Hui et al., Citation2010; Devi et al., Citation2011), prostate cancer (Reddivari et al., Citation2007), liver cancer (Bishayee et al., Citation2010, Citation2011), colorectal and intestinal cancers (Koide et al., Citation1996, Citation1997; Hagiwara et al., Citation2001; Bobe et al., Citation2006; Lala et al., Citation2006; Srivastava et al., Citation2007; Lim et al., Citation2013), blood cancer (Tsai et al., Citation2014), cervical cancer (Rugină et al., Citation2012), lung cancer (Aqil et al., Citation2012), fibrosarcoma (Filipiak et al., Citation2014), and metastatic melanoma (Bunea et al., Citation2013). In addition, some studies also demonstrated that anthocyanins from one fruit or vegetable might have chemoprotective activity against various cancers. For example, anti-proliferative effects of anthocyanins-rich extract from blackberry on colon cancer HT-29, breast cancer MCF-7, lung cancer A549, and leukemia HL-60 cells have been determined (Dai et al., Citation2009; Aqil et al., Citation2012). Barrios et al. Citation(2010) demonstrated that the anthocyanins-rich extract from Pourouma cecropiifolia fruits, a tropical plant, significantly reduced the viability of laryngeal cancer HEp-2, gastric cancer MKN-45, and breast cancer MCF-7 cells. Anthocyanin extract from blueberry significantly induced apoptosis in mouse melanoma B16-F10 and human colon cancer HT-29 cells (Srivastava et al., Citation2007; Bunea et al., Citation2013). However, there is little knowledge about synergistic or antagonastic effects of various anthocaynins on inhibiting the initiation, promotion, and progression of carcinogenesis, which should be highlighted in future. In addition, a recent study by Peiffer et al. Citation(2014) has demonstrated that protocatechuic acid (PCA), a major anthocyanin metabolite, effectively inhibited the development of esophageal cancer in rats. Similarly, Forester et al. Citation(2014) also found that three anthocyanin metabolites, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, could decrease cell viability and cause cell cycle arrest and apoptosis in colon cancer Caco-2 cells. Therefore, it is indispensable to investigate the anticancer activity of the individual metabolite of anthocyanins in future.
Several studies have shown that the chemical structures of anthocyanins do have a significant impact on their biological activities. Konczak-Islam et al. Citation(2003) demonstrated the anticancer property of the anthocyanins-rich extract from sweet potato in HL-60 cells. Composition analysis showed that the dominated non-acylated cyanidin 3-sophoroside-5-glucoside might be related to the observed anticancer activity. Further, Zhao et al. Citation(2004) demonstrated that the chokeberry extracts, containing high levels of monoglycosylated cyanidin derivatives, showed a stronger chemoprotective activity than grape and bilberry extracts (BBEs). Moreover, Jing et al. Citation(2008) used human colon cancer HT29 cell line to compare the anticancer properties of anthocyanins-rich extract from purple corn, chokeberry, bilberry, purple carrot, grape, radish, and elderberry. The degrees of growth inhibitory activity were as follows: purple corn > chokeberry and bilberry > purple carrot and grape > radish and elderberry. Statistical analyses suggested that non-acylated monoglycosylated anthocyanins had greater anticancer property than anthocyanins with pelargonidin, triglycoside, and/or acylation with cinnamic acid. Therefore, it appears that the type of aglycones, sugars, and acylated acids, and the position and degree of glycosylation and acylation are the main factors influencing the anticancer property. Nevertheless, the structure–activity relationship of anthocyanins as chemoprotective agents remains to be further elucidated.
Extensive investigations have been performed to determine the molecular mechanisms of the anticancer activity of anthocyanins, with results indicating that anthocyanins can inhibit several signaling pathways involved in tumor growth and apoptosis. In an early study, the anthocyanins-rich extract from Aronia meloncarpa induced G1/G0 and G2/M cell cycle arrest in HT-29 colon cancer cells by increasing the expression of p21WAF1 and p27KIP1 and decreasing the expression of cyclin A and cyclin B (Malik et al., Citation2003). Similarly, the anthocyanins-rich extract from potato also led to G1/G0 cell cycle arrest in prostate cancer LNCaP and PC-3 cells with the higher p27 protein levels. Moreover, the extract caused caspase-dependent apoptosis in LNCaP cells with the induction of poly ADP-ribose polymerase (PARP) cleavage and the activation of caspase 3 (cleavage), as well as caspase-independent cell death in PC-3 cells with mitochondrial release and nuclear uptake of the proapoptotic endonuclease G (Endo G) and apoptosis-inducing factor (AIF) proteins. Notably, both apoptotic pathways depended on upstream activation of mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways, indicating that MAPK signaling pathway might participate in the molecular mechanisms of anticancer activity (Reddivari et al., Citation2007). In addition, the activation of nuclear factor κB (NF-κB) pathways also might be shown to be involved in tumor growth and development. Afaq et al. Citation(2005) reported that the animals pretreated with anthocyanins-rich extract from pomegranate fruit resulted in substantially reduced tumor incidence and lower tumor body burden in 7,12-dimethylbenz(a)anthracene-initiated CD-1 mouse through the inhibition of phosphorylation of extracellular signal-regulated kinase (ERK)1/2, p38, and JNK1/2, as well as the activation of NF-κB and IκKα and phosphorylation and degradation of IκBα.
The Wnt pathway is crucial for cell proliferation, differentiation, and survival. β-catenin is a key component of the Wnt signaling pathway, and the over activation of β-catenin in cytosol is related to cancer metastasis. Under normal conditions, β-catenin is controlled by dephosphorylated glycogen synthase kinase 3β (GSK3β). When GSK3β is phosphorylated, GSK3β loses its activity and no longer controls β-catenin. Park et al. Citation(2014) demonstrated that anthocyanin extract from Korea wild berry Meoru effectively inhibited liver cancer Hep3B cell migration and invasion by decreasing the expression of phospho-GSK3β and β-catenin. Moreover, AMP-activated protein kinase (AMPK) activation induced by anthocyanins might be an upstream regulator of the GSK3β/β-catenin pathway. In addition, autophagic pathway has also been reported to be a novel therapeutic target for cancer treatment. Longo et al. Citation(2008) found that anthocyanin-induced autophagy was characterized by the upregulation of eukaryotic initiation factor 2α-subunit (eIF2α) and downregulation of mammalian target of rapamycin (mTOR) and Bcl-2. Inhibition of autophagy by either 3-methyladenine or autophagy-related gene 5 (Atg5) small interfering RNA enhanced anthocyanin-triggered apoptosis in liver cancer PLC/PRF/5 cells.
Anti-inflammatory activity
Many epidemiological and experimental studies have showed the anti-inflammatory activity of anthocyanins in foods for the amelioration of inflammation-associated diseases or disorders such as colitis (Akiyama et al., Citation2012), peyronie disease (Sohn et al., Citation2014), periodontal disease, laryngopharyngeal reflux (Samuels et al., Citation2013), postprandial inflammatory response (Edirisinghe et al. Citation2011), and pain behavior (Tall et al., Citation2004).
Inflammatory responses are a series of well-coordinated events that are controlled by several factors, including cytokines, enzymes, lipid mediators, and vasoactive mediators (Hassimotto et al., Citation2013). Cyclooxygenases (COXs) are the key pro-inflammatory enzymes that are involved in arachidonic acid metabolism. COX-2 is the main enzyme for the synthesis of lipid mediators such as prostaglandin E2 (PGE2), which is a potent vasodilator enhancing edema formation (Graf et al., Citation2013; Hassimotto et al., Citation2013). Hassimotto et al. Citation(2013) showed that dietary supplements of the anthocyanins-rich extract from wild mulberry and the cyanidin 3-glucoside are effective in suppressing carrageenan-induced edema and peritonitis through the downregulation of COX-2 expression and inhibition of PGE2 production. The inducible nitric oxide synthase (iNOS) is another key pro-inflammatory enzyme for the production of nitric oxide (NO). Excessive production of NO appears to be associated with the progression of many inflammatory diseases (Li et al., Citation2014). An early study by Tsuda et al. Citation(2002) demonstrated that cyanidin 3-O-β-glucoside suppressed the zymosan-induced inflammatory response in rats by reducing the level of iNOS, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in the peritoneal exudate cells. Poulose et al. Citation(2012) also showed that pretreatment of BV-2 microglial cells with the anthocyanins-rich extract from acai pulp was protective against lipopolysaccharide (LPS)-induced NO release, iNOS production, and COX-2 expression.
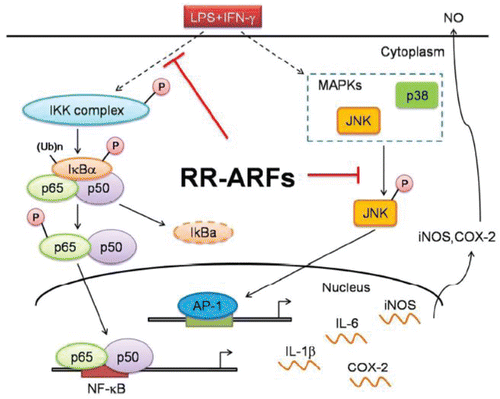
The NF-κB pathway plays an important role in triggering and regulating inflammatory processes. The NF-κB transcription factor exists in an inactive state in the cytosol by binding to IκB; upon its activation and translocation into the nucleus, genes involved in pro-inflammatory responses are induced, leading to the expression of cytokines and inflammatory enzymes, including iNOS and COX-2 (Taverniti et al., Citation2014). Taverniti and coworkers Citation(2014) showed that the anthocyanins-rich extract from wild blueberry displayed anti-inflammatory property by decreasing the activation of NF-κB in the presence of the pro-inflammatory stimulus IL-1β in human Caco-2 intestinal cells. In a retinal inflammatory mouse model, an anthocyanins-rich extract from bilberry showed anti-inflammatory activity by suppressing the expression of signal transducers and activators of transcription 3 (STAT3) and IL-6 through the reduction of NF-κB activation (Miyake et al., Citation2012). Moreover, delphinidin inhibited COX-2 expression in LPS-activated murine macrophage RAW264 cells by targeting the transcription factors NF-κB, CCAAT/enhancer-binding protein (C/EBPδ), and activator protein-1 (AP-1) (Hou et al., Citation2005). Xia et al. Citation(2007) demonstrated that intracellular tumor necrosis factor receptor-associated factors' (TRAFs) translocation to lipid rafts played pivotal role in CD40-mediated NF-κB activation, which induced inflammatory response in human endothelial cells. However, cyanidin 3-O-β-glucoside and peonidin 3-O-β-glucoside significantly inhibited CD-40-induced inflammatory signaling through the reduction of cholesterol distribution in lipid rafts, indicating that anthocyanins could act as lipid-dependent regulators of inflammatory response. In addition, MAPK signaling pathway also played a critical role in the regulation of inflammatory response. Hou et al. Citation(2005) reported that inhibition of COX-2 expression by blocking the activation of MAPK signaling pathway, including JNK, ERK, and p38 kinase contributed to the anti-inflammatory property of delphinidin. Xia et al. Citation(2009) demonstrated that cyanidin 3-O-β-glucoside and peonidin 3-O-β-glucoside also significantly prevented CD40-induced endothelial activation and apoptosis by inhibiting the production of pro-inflammatory cytokines and matrix metalloproteinases (MMP-1 and MMP-9). Notably, the anti-inflammatory activity might be due to the downregulation of JNK and p38 activation. The above findings suggested that the NF-κB and MAPK signaling pathways might provide key targets for the application of anthocyanins to improve the inflammation-associated diseases. A recent study by Li and coworkers Citation(2014) further confirmed this notion by determining the anti-inflammatory activity of anthocyanins-rich fraction from red raspberries (RR-ARFs) in RAW264.7 cells and an acute mouse colitis model. As shown in , RR-ARFs significantly inhibited p65 phosphorylation and its nuclear translocation as well as the activation of IKK, IκBα, and JNK, thereby suppressing the expression of pro-inflammatory genes such as iNOS, COX-2, IL-1β, and IL-6.
Figure 2. Schematic model showing the role of anthocyanin-rich fractions from red raspberries (RR-ARFs) in inflammatory signaling pathways. RR-ARFs attenuated LPS/IFN-γ-induced inflammatory responses through inhibition of NF-κB, MAPK/JNK activities, respectively, in LPS/IFN-γ-stimulated RAW264.7 cells. (Reproduced from the original source: Li et al., Citation2014).
Interestingly, Graf et al. Citation(2013) found that the anthocyanins-rich juice from grape–bilberry at physiological level did not showed any anti-inflammatory effects on the systemic immune system, gut-associated lymphoid tissue, and mesenteric adipose tissue in healthy rats, indicating that anti-inflammatory effects of anthocyanins might depend on various factors such as sources, doses, and categories of anthoycanins used in the experiments . Hou and colleagues Citation(2005) investigated the anti-inflammatory effects of five anthocyanins in LPS-activated murine macrophage RAW264 cells. It seemed that only delphinidin and cyanidin inhibited LPS-induced COX-2 expression, but pelargonidin, peonidin, and malvidin did not. Further analysis showed that the ortho-dihydroxyphenyl structure of anthocyanidins on the B-ring might be crucial for inhibitory activity. Furthermore, long-term supplementation with purified anthocyanins (cyanidin 3-O-glucoside or delphenidin 3-O-glucosides) derived from bilberries and blackcurrants inhibited inflammatory response in adults with hypercholesterolemia. Notably, this study showed that the mixture of the two purified anthocyanins caused a greater anti-inflammatory effect than single anthocyanin, which suggested that different anthocyanins might have additive or synergistic effects on mediating anti-inflammatory response (Zhu et al., Citation2013). In addition, the synergistic effect of anthocyanins and antimicrobials has been confirmed. Yoon et al. Citation(2013) reported that combination of anthocyanin extracts from black soybean and ciprofloxacin has more obvious anti-inflammatory and antimicrobial effects on treating chronic bacterial prostatitis than ciprofloxacin treatment alone. Therefore, the combined use of anthocyanins with other anti-inflammatory medicines may provide novel therapeutic strategies for the treatment of inflammation-associated diseases.
Neuroprotective activity
Significant evidence from epidemiological studies has suggested the neuroprotective activity of anthocyanins for their improvement on cognitive performance, memory performance, and motor performance, indicating their potential application for the prevention of many neurodegenerative diseases such as Parkinson's disease (PD) and Alzheimer's disease (AD) (Youdim et al., Citation2004).
Parkinson's disease is a neurodegenerative disorder that involves loss of dopaminergic neurons in the midbrain region. Dopaminergic cell death is suggested to be involved in the progression of PD. Strathearn et al. Citation(2014) demonstrated that anthocyanins-rich extract from blueberries, grape seed, hibiscus, blackcurrant, and Chinese mulberry significantly suppressed rotenone-induced dopaminergic cell death via interference with microglial activation and amelioration of mitochondrial dysfunction. Mitochondrial dysfunction caused by oxidative stress also leads to neuronal damage after ischemic stroke. Cyanidin 3-O-glucoside has been shown to exert its neuroprotective effect against ischemic stroke in mice by blocking AIF release from mitochondria (Min et al., Citation2011). One of the physiological roles of cell autophagy is to remove damaged organelles, including damaged mitochondria, thereby rescuing cells under stressed conditions. Kim and coworkers Citation(2012) demonstrated that anthocyanin extract from black soybean increased survival of U87 glioma cells exposed to oxidative stress induced by oxygen–glucose deprivation (OGD). Silencing Atg5 expression, an essential regulator of autophagy induction, reversed the cytoprotective effect of anthocyanin extract against OGD stress. However, treatment of U87 cells with rapamycin, an autophagy inducer, increased cell survival upon OGD stress, indicating that autophagy might be a neuroprotective mechanism for anthocyanins against oxidative stress-induced cytotoxicity in glial cells. Whether induction of autophagy is a common neuroprotective mechanism of various anthocyanins with different chemical structures, still needs to be further determined. Anyway, anthocyanins are still potential candidates to be the effective neuroprotective dietary complementation for the prevention or reduction of neuronal cell death. In addition, anthocyanin extract from bog bilberry exerted its neuroprotective effects by reducing glial scar formation, axonal loss, and inflammation and promoting remyelination and neuron survival in a rat model of spinal cord injury (SCI) (Wang et al., Citation2012a, Citation2012b). Similarly, treatment with cyanidin 3-O-β-glucoside could also reduce superoxide production, neuron cell damage, lesion volume, and neurological dysfunction in a traumatic SCI rat model (Kim et al., Citation2011). Therefore, an anthocyanins-rich dietary therapy may be used to improve the condition of SCI patients.
Alzheimer's disease is an irreversible degenerative brain disease caused by the hyperphosphorylation of Tau protein aggregation. Two kinds of anthocyanins, cyanidin 3-O-glucoside and malvidin 3-O-glucoside, have been shown to induce FK506 binding protein 52 (FKBP52) activation, leading to the reduction of hyper-phosphorylated Tau protein aggregation, and thereby improving the treatment of AD (Hung et al., Citation2014). It has also been reported that the altered amyloid precursor protein (APP) processing leading to increased amyloid-beta peptide (Aβ) accumulation is a key pathogenic feature of AD. Vepsäläinen et al. Citation(2013) observed that long-lasting supplementation with anthocyanins-rich extract from bilberry and blackcurrant exerted beneficial effects on APP and Aβ metabolism and alleviated behavioral abnormalities in a mouse model of AD. Aβ is known to induce redox imbalance, mitochondrial dysfunction, and caspase activation, resulting in neuronal cell death. Pretreatment with anthocyanins-rich extract from purple sweet potato reduced Aβ-induced apoptosis by inhibiting the reactive oxygen species (ROS) generation, lipid peroxidation, caspase-3 activity, and decreasing levels of intracellular Ca2+ and membrane potential loss in PC12 cells (Ye et al., Citation2010). In another study, anthocyanins-rich extract from mulberry significantly inhibited the accumulation of Aβ and improved learning and memory ability in a senescence-accelerated mice model (Shih et al., Citation2010).
Prevention of cardiovascular disease
The relationship between anthocyanins intake and the reduced risk of developing cardiovascular disease has been indicated in several epidemiological studies (PrRimm et al., Citation1991; Wallace, Citation2011). A recent study of young and middle-aged women showed a high intake of anthocyanins was associated with a 32% reduction in risk of myocardial infarction (Cassidy et al., Citation2013). In addition, an Iowa Women's Health Study with 34,489 women showed a significant reduction in cardiovascular disease mortality associated with anthocyanins-rich strawberry intake (Mink et al., Citation2007).
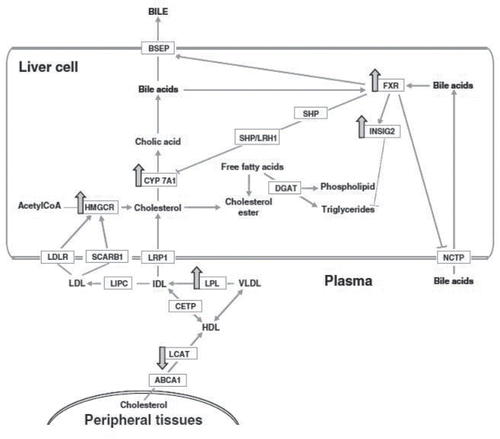
Atherosclerosis is characterized by vascular obstruction from the deposits of lipids, resulting in reduced blood flow (Mauray et al., Citation2012). The liver is the main organ regulating plasma lipids levels and lipoprotein metabolism. It plays a central role in atherosclerosis. Mauray et al. Citation(2010) showed that two-week supplementation with anthocyanins-rich extract from bilberry significantly reduced plasmatic total cholesterol and hepatic triglyceride levels in apolipoprotein E-deficient (apo E−/−) mice. Microarray analysis showed that numerous over-expressed genes (CYP7A1, HMGCR, LPL, NR1H4, and INSIG2) were involved in bile acid synthesis and excretion, which enhances cholesterol elimination in the plasma and the reduction of hepatic lipogenesis (). In addition, downregulation of many pro-inflammatory genes (ALOX5AP, CX3CL1, and TNFRSF14) was also observed, indicating that anti-inflammatory response might also participate in the protection against atherosclerosis (Mauray et al., Citation2010), and its mechanism of action was possibly inhibiting atherosclerotic plaque progression and increasing the stability of vulnerable plaque (Xia et al., Citation2006). Further study showed that anthocyanin-rich extract from bilberry also markedly modulated the expression of genes in the aortas of apo E−/− mice. Bioinformatic analysis revealed that the identified numerous aortic genes seemed to be related to increased inter-cellular adhesion (CDH4, CTNNB1, JAM-A, and VCAM1), decreased monocyte recruitment (RDX and ARPC5), cellular contractility (MYLC2B and PAK1), and vascular permeability (KDR, FAK, and VEGFR2), thereby decreasing the risk of endothelial dysfunction, which is an early marker of the development of atherosclerosis (Mauray et al., Citation2012). In addition, endothelial function has also been demonstrated to be improved by delphinidin 3-O-glucoside and cyanidin 3-O-glucoside through the activation of the NO-cGMP signaling pathway in hypercholesterolemic individuals (Zhu et al., Citation2011), inhibition of mitochondria-mediated apoptotic signaling pathway in human umbilical vein endothelial cells and bovine aortic endothelial cells (Zapolska-Downar et al., Citation2008; Paixão et al., Citation2011) as well as the activation of cAMP-PKA-eNOS signaling pathways in human aortic endothelial cells (Liu et al., Citation2014).
Figure 3. Schematic model showing changes in the expression of genes involved in cholesterol metabolism by an anthocyanin-rich bilberry extract (BBE) in apoE−/− mice. Large arrows indicate up- or down-regulation of genes involved in bile acid synthesis and excretion, which enhances cholesterol elimination in the plasma and the reduction of hepatic lipogenesis. (Reproduced from the original source: Mauray et al., Citation2010.)
Oxidative modification of low-density lipoprotein (oxLDL) is involved in the pathogenesis of atherosclerosis through the formation of macrophage-derived foam cells. Therefore, the inhibition of oxLDL formation might effectively lower the risk of atherosclerosis. Anthocyanin extract from Hibiscus was demonstrated to inhibit oxLDL and foam cell formation by downregulating the expression of CD36 and increasing nuclear peroxisome proliferator-activated receptor γ (PPARγ) protein levels in mouse macrophage J774A.1 cells (Kao et al., Citation2009). Similarly, anthocyanin extract from mulberry also decreased macrophage death and foam cell formation in Cu2+-mediated oxLDL (Liu et al. Citation2008).
Reverse cholesterol transport (RCT) is a process that entails the efflux of excess cholesterol from macrophages into the liver. The promotion of macrophage RCT by upregulating the expression of adenosine triphosphate (ATP)-binding cassette transporter A1 (ABCA1) and G1 (ABCG1) in macrophages may be a potential novel approach for prevention and treatment of atherosclerosis. PCA is determined as the main gut microbiota metabolite of cyanidin 3-O-β-glucoside. Wang et al. Citation(2012a), Citation(2012b) demonstrated that PCA, but not its precursor, induced ABCA1 and ABCG1 expression in macrophages by decreasing the expression of miR-10b, which contributed to the accelerated macrophage RCT. Importantly, the intestinal microbiota ecosystem might be a potential target for the prevention and treatment of chronic diseases such as atherosclerosis because of its strong metabolic ability.
In summary, anthocyanins-rich extract from fruits and vegetables could effectively reduce the risk of atherosclerosis by improving endothelial dysfunction, inhibiting oxLDL formation, and promoting macrophage RCT.
Anti-obesity and antidiabetic activity
Many epidemiological studies have confirmed inverse association between anthocyanin intake and the risk of obesity and diabetes. In a study of 1997 females from the United Kingdom, higher intake of anthocyanins was associated with significantly lower concentrations of high-sensitivity C-reactive protein (CRP), a marker of obesity and diabetes (Wang et al., Citation2013). Similarly, a large cohort study of 200,994 health professionals from the United States revealed that consumption of anthocyanins-rich foods was inversely associated with the risk of diabetes (Muraki et al., Citation2013). In line with these findings, a cross-sectional study suggested consumption of high amounts of anthocyanins might have beneficial effect on improving lipid profile in Chinese women, indicating the efficacy of anthocynins for the treatment of obesity (Li et al., Citation2013).
Obesity is the result of accumulated excessive adipose tissue caused by the imbalance of energy intake and expenditure. It is usually associated with various metabolic disorders. Jurgoński et al. Citation(2013) reported that the anthocyanins-rich extract from Kamchatka honeysuckle berry was able to ameliorate disturbances in lipid and glucose metabolism in rats. In line with this result, Wu and coworkers Citation(2013) observed that anthocyanin extract from honeysuckle suppressed body weight gain, reduced serum and liver lipid profiles, ameliorated impaired hepatic function, and significantly increased serum adiponectin concentration while decreasing serum insulin and leptin levels in a high fat diet-induced mouse model. Continuous hyperglycemia could enhance lipolysis of triglycerides (TG) in the adipocytes, resulting in elevated levels of plasma-free fatty acids (FFAs), which have been demonstrated to potentially link obesity (Guo et al., Citation2012). Therefore, decreasing glucose uptake of the adipocytes may be a potential approach for the prevention of obesity. Alzaid et al. Citation(2013) found that acute exposure to the anthocyanins-rich extract from berry significantly decreased both Na+-dependent and Na+-independent glucose uptake in Caco-2 cells. Further, longer-term exposure with berry extract markedly reduced the expression levels of glucose transporter 2 (Glut2) and sodium-dependent glucose transporter 1 (SGLT1), and resulted in significant inhibition of glucose uptake (Alzaid et al., Citation2013).
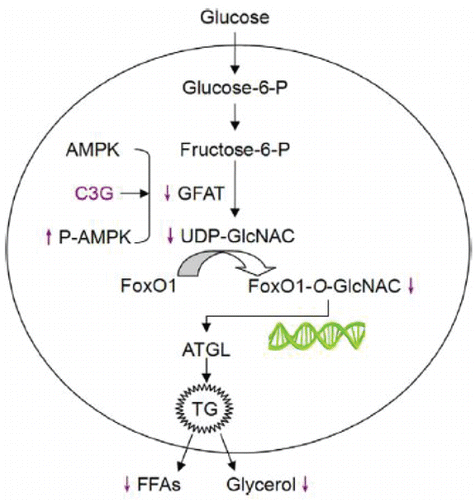
In addition, the adipose tissue secretes several adipocytokines (e.g. adiponectin, leptin, and resistin), which have important regulatory functions in the development of metabolic diseases such as obesity. Therefore, the regulation of adipocytokine secretion or the adipocyte-specific gene expression is one of the most important strategies for the prevention of obesity. Graf et al. Citation(2013) observed that rats fed with anthocyanins-rich grape–bilberry juice showed reduced levels of serum cholesterol, TG, leptin, and resistin. In addition, increased proportion of polyunsaturated fatty acids and decreased amount of saturated fatty acids in plasma were also observed. Similarly, Tsuda et al. Citation(2004) demonstrated that anthocyanins enhanced adipocytokine (adiponectin and leptin) secretion and the expression of PPARγ, lipoprotein lipase (LPL), adipocyte fatty acid binding protein (aP2), and uncoupling protein 2 (UCP2) in isolated rat adipocytes. Notably, AMPK activation might be one of the possible mechanisms for the regulation of adipocyte-specific gene expression. Guo et al. Citation(2012) revealed a novel mechanism by which anthocyanin cyanidin 3-O-β-glucoside effectively eliminated the impacts of high glucose on the induction of adipocyte lipolysis (). In that study, cyanidin 3-O-β-glucoside increased the activity of AMPK, decreased the activity of glutamine:fructose 6-phosphate aminotransferase (GFAT), and reduced cellular UDP-N-acetylglucosamine production (UDP-GlcNAc), thereby suppressing the hexosamine biosynthetic pathway (HBP). In addition, it also attenuated high-glucose-promoted forkhead box O1 (FoxO1), resulting in decreased expression of adipose triglyceride lipase (ATGL), thus inhibited the lipolysis of TG and decreased the levels of FFAs in the plasma (Guo et al., Citation2012).
Figure 4. Schematic model showing the antilipolytic role of anthocyanin in high glucose-incubated adipocytes through regulating FoxO1-mediated transcription of ATGL. Cyanidin 3-O-β-glucoside treatment decreases the cellular GFAT activity resulting in diminished formation UDP-N-acetylglucosamine. This disrupts high-glucose-induced O-GlcNAc modification of FoxO1, inhibits ATGL transcription, and limits lipolysis. (Reproduced from the original source: Guo et al., Citation2012.)
Insulin secreted from the β-cells of the pancreas is responsible for stimulation of blood glucose transport into skeletal muscle and adipose tissue as well as suppression of hepatic glucose production. Type 2 diabetes is a metabolic disorder associated in part with insulin resistance (Ghosh and Konishi, Citation2007). Guo et al. Citation(2007) showed that dietary anthocyanins-rich extract from black rice was capable of preventing the development of insulin resistance in fructose-fed rats, and the underlying mechanism might be related mainly to inhibiting oxidative stress and improving the plasma lipid profile. Further, Takikawa et al. Citation(2010) showed that the dietary anthocyanins-rich extract from bilberry reduced the blood glucose level and improved insulin sensitivity via the activation of AMPK in type 2 diabetic mice. As shown in , dietary BBE-activated AMPK in the white adipose tissue, skeletal muscle, and the liver. In the white adipose tissue and skeletal muscle, activation of AMPK increased the expression of glucose transporter 4 (Glut4), which resulted in the enhancing of glucose uptake and utilization in these tissues. In the liver, activated AMPK decreased the expression of phosphoenol pyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), leading to a decrease in glucose output into the blood. In addition, AMPK activation in the liver also resulted in significantly decreased liver and serum lipid content via the phosphorylation of acetyl-CoA carboxylase (ACC) and upregulation of PPARα, acyl-CoA oxidase (ACO), and carnitine palmitoyltransferase-1A (CPT1A) gene expression (Takikawa et al., Citation2010). Mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 (mtGPAT1) controls the rate-limiting step of TG synthesis, which plays important roles in the development of metabolic diseases such as obesity and type 2 diabetes. Guo and coworkers Citation(2011) demonstrated that protein kinase Cζ (PKCζ) might be a potential target for the prevention and treatment of the steatotic liver associated with obesity and type 2 diabetes. In that study, anthocyanin cyanidin 3-O-β-glucoside stimulated PKCζ activation in HepG2 cells by increasing PKCζ phosphorylation and membrane translocation from endoplasmic reticulum to outer mitochondrial membrane to phosphorylate the mtF0F1-ATPase β-subunit. As mentioned above, AMPK is an important energy metabolic regulator, whether AMPK activation was associated with the regulatory process of PKCζ activation remains to be clarified.
Figure 5. Proposed mechanisms for amelioration of hyperglycemia and insulin sensitivity by dietary BBE. BBE activates AMPK in the white adipose tissue and skeletal muscle. This activation induces upregulation of Glut4 and enhancement of glucose uptake and utilization in these tissues. In the liver, dietary BBE reduces glucose production via AMPK activation, which ameliorates hyperglycemia in type 2 diabetic mice. The AMPK activation induces phosphorylation of acetylCoA carboxylase (ACC) and upregulation of PPARα, acylCoA oxidase (ACO), and carnitine palmitoyltransferase-1A (CPT1A) gene expression in the liver. These changes lead to reductions in lipid content and increase in insulin sensitivity via reduction of lipotoxicity. (Reproduced from the original source, Takikawa et al., Citation2010.)
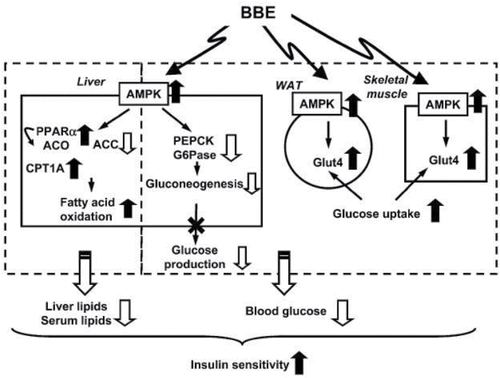
In summary, anthocyanins are able to ameliorate disturbances in lipid and glucose metabolism, which are fundamental risk factors for obesity and diabetes. AMPK signaling pathway is one of the crucial factors for cellular energy homeostasis, which can be recognized as a key target in the prevention and treatment of obesity and diabetes.
Other health benefits
Gout is a clinical syndrome in which tissue damage is induced by a chronic metabolic disorder associated with increased concentrations of uric acid in the blood. In a sodium oxonate-induced hyperuricemia mouse model, Hwa et al. Citation(2011) reported the hypouricemic effects of anthocyanins-rich extract from the purple-fleshed sweet potato. In addition, the ameliorative effect of anthocyanin extract from black rice against d-galactose-induced senescence has been demonstrated in a mice model (Lu et al., Citation2014). Jang and coworkers Citation(2010) proposed that anthocyanins might be effective in treating prostatic hyperplasia. On the other hand, the protective roles of anthocyanin extracts from Justicia secunda Vahl against sickle cell disease was indicated by both stabilizing the red blood cell membrane and inhibiting polymerization of hemoglobin (Mpiana et al., Citation2010). Moreover, anthocyanins could even improve the developmental competence by stimulating nuclear reprogramming through the increased transcription factor expression (You et al., Citation2010). However, detailed mechanisms of action of anthocyanins for these beneficial effects remain to be clearly studied.
Conclusions and future perspectives
Interests in anthocyanins have increased substantially during the past two decades. In this review, diverse health benefits of anthocyanins and molecular mechanisms have been described in detail. Several signaling pathways, including MAPK, NF-κB, AMPK, and Wnt/β-catenin, as well as some crucial cellular processes, such as cell cycle, apoptosis, autophagy, and biochemical metabolism, are involved in these beneficial effects and may provide potential therapeutic targets and strategies for the improvement of a wide range of diseases in future. Nevertheless, much remains to be elucidated before better applying them for the health benefits of humans.
First, more studies on the biological activity of the certain anthocyanin metabolites are desired, since it has been suggested that anthocyanins are absorbed and transported in human serum and urine primarily as metabolites, and these metabolites may totally or partially contribute to the above-mentioned biological activities of anthocyanins.
In addition, health benefits of dietary anthocyanins have been demonstrated in many in vivo and in vitro studies as well as epidemiological and clinic research of human volunteers. However, the low bioavailability of anthocyanins appears to be an obvious obstacle in achieving the desired beneficial effects (Xiao and Högger, Citation2015). Novel approaches for enhancing the bioavailability of these beneficial molecules in human bodies are desired to be developed. Fortunately, some new technologies, such as nanotechnology, may provide promising tools to solve this problem.
Moreover, purified anthocyanin individuals might exert different biological activities due to their specific chemical structures. Future studies should focus on careful and accurate characterization of different anthocyanins in order to better elucidating the molecular mechanism of their health benefits.
Funding
This work was supported by Program for New Century Excellent Talents in University (NCET) No. 12–0520, the National Basic Research Program of China (973 Program) No. 2012CB720805, and the National Natural Science Foundation of China (NSFC) No. 81102152, and the National Science & Technology Support Plan of the Chinese Ministry of Education No. 2011BAD39B00.
References
- Afaq, F., Saleem, M., Krueger, C. G., Reed, J. D. and Mukhtar, H. (2005). Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer. 113:423–433.
- Akiyama, S., Nesumi, A., Maeda-Yamamoto, M., Uehara, M. and Murakami, A. (2012). Effects of anthocyanin-rich tea “Sunrouge” on dextran sodium sulfate-induced colitis in mice. Biofactors. 38:226–233.
- Alzaid, F., Cheung, H. M., Preedy, V. R. and Sharp, P. A. (2013). Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS One. 8:e78932.
- Andersen, Ø. M. (2001). Anthocyanins. John Wiley, Chichester, UK.
- Andersen, Ø. M. and Markham, K. R. (2006). Flavonoids: Chemistry, Biochemistry, and Applications. CRC, Taylor & Francis, Boca Raton FL.
- Aqil, F., Gupta, A., Munagala, R., Jeyabalan, J., Kausar, H., Sharma, R. J., Singh, I. P. and Gupta, R. C. (2012). Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer. 64:428–438.
- Barrios, J., Cordero, C. P., Aristizabal, F., Heredia, F. J., Morales, A. L. and Osorio, C. (2010). Chemical analysis and screening as anticancer agent of anthocyanin-rich extract from Uva Caimarona (Pourouma cecropiifolia Mart.) fruit. J. Agric. Food Chem. 58:2100–2110.
- Bishayee, A., Háznagy-Radnai, E., Mbimba, T., Sipos, P., Morazzoni, P., Darvesh, A. S., Bhatia, D. and Hohmann, J. (2010). Anthocyanin-rich black currant extract suppresses the growth of human hepatocellular carcinoma cells. Nat. Prod. Commun. 5:1613–1618.
- Bishayee, A., Mbimba, T., Thoppil, R. J., Háznagy-Radnai, E., Sipos, P., Darvesh, A. S., Folkesson, H. G. and Hohmann, J. (2011). Anthocyanin-rich black currant (Ribes nigrum L.) extract affords chemoprevention against diethylnitrosamine-induced hepatocellular carcinogenesis in rats. J. Nutr. Biochem. 22:1035–1046.
- Bobe, G., Wang, B., Seeram, N. P., Nair, M. G. and Bourquin, L. D. (2006). Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC (Min) mice fed suboptimal levels of sulindac. J. Agric. Food Chem. 54:9322–9328.
- Bunea, A., Rugină, D., Sconţa, Z., Pop, R. M., Pintea, A., Socaciu, C., Tăbăran, F., Grootaert, C., Struijs, K. and VanCamp, J. (2013). Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry. 95:436–444.
- Cassidy, A., Mukamal, K. J., Liu, L., Franz, M., Eliassen, A. H. and Rimm, E. B. (2013). A high anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 127:188–196.
- Dai, J., Gupte, A., Gates, L. and Mumper, R. J. (2009). A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food Chem. Toxicol. 47:837–847.
- de Pascual-Teresa, S. (2014). Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Arch. Biochem. Biophys. 559:68–74.
- de Pascual-Teresa, S. and Sanchez-Ballesta, M. T. (2008). Anthocyanins: From plant to health. Phytochem. Rev. 7:281–299.
- Devi, P. S., Kumar, M. S. and Das, S. M. (2011). Evaluation of antiproliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line (mcf-7). Int. J. Breast Cancer. 891481.
- Edirisinghe, I., Banaszewski, K., Cappozzo, J., Sandhya, K., Ellis, C. L., Tadapaneni, R., Kappagoda, C. T. and Burton-Freeman, B. M. (2011). Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 106:913–922.
- Fernandes, I., Faria, A., Calhau, C., de Freitas, V. and Mateus, N. (2014). Bioavailability of anthocyanins and derivatives. J. Funct. Foods. 7:54–66.
- Filipiak, K., Hidalgo, M., Silvan, J. M., Fabre, B., Carbajo, R. J., Pineda-Lucena, A., Ramos, A., de Pascual-Teresa, B. and de Pascual-Teresa, S. (2014). Dietary gallic acid and anthocyanin cytotoxicity on human fibrosarcoma HT1080 cells. A study on the mode of action. Food Funct. 5:381–389.
- Forester, S. C., Choy, Y. Y., Waterhouse, A. L. and Oteiza, P. I. (2014). The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 53:432–439.
- Ghosh, D. and Konishi, T. (2007). Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia. Pac. J. Clin. Nutr. 16:200–208.
- Graf, D., Seifert, S., Jaudszus, A., Bub, A. and Watzl, B. (2013). Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in Fischer rats. PLoS One. 8:e66690.
- Guo, H., Guo, J., Jiang, X., Li, Z. and Ling, W. (2012). Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 50:3040–3047.
- Guo, H., Li, D., Ling, W., Feng, X. and Xia, M. (2011). Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ. J. Lipid Res. 52:908–922.
- Guo, H., Ling, W., Wang, Q., Liu, C., Hu, Y., Xia, M., Feng, X. and Xia, X. (2007). Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 62:1–6.
- Hagiwara, A., Miyashita, K., Nakanishi, T., Sano, M., Tamano, S., Kadota, T., Koda, T., Nakamura, M., Imaida, K., Ito, N. and Shirai, T. (2001). Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 171:17–25.
- Hassimotto, N. M., Moreira, V., do Nascimento, N. G., Souto, P. C., Teixeira, C. and Lajolo, F. M. (2013). Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. Biomed. Res. Int. 146716.
- He, J. and Giusti, M. M. (2010). Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food. Sci. Technol. 1:163–187.
- Hou, D. X., Yanagita, T., Uto, T., Masuzaki, S. and Fujii, M. (2005). Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 70:417–425.
- Hui, C., Bin, Y., Xiaoping, Y., Long, Y., Chunye, C., Mantian, M. and Wenhua, L. (2010). Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer. 62:1128–1136.
- Hung, T. C., Chang, T. T., Fan, M. J., Lee, C. C. and Chen, C. Y. (2014). In Silico insight into potent of anthocyanin regulation of FKBP52 to prevent Alzheimer's disease. Evid. Based Complement. Alternat. Med. 450592.
- Hwa, K. S., Chung, D. M., Chung, Y. C. and Chun, H. K. (2011). Hypouricemic effects of anthocyanin extracts of purple sweet potato on potassium oxonate-induced hyperuricemia in mice. Phytother. Res. 25:1415–1417.
- Jang, H., Ha, U. S., Kim, S. J., Yoon, B. I., Han, D. S., Yuk, S. M. and Kim, S. W. (2010). Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 58:12686–12691.
- Jing, P., Bomser, J. A., Schwartz, S. J., He, J., Magnuson, B. A. and Giusti, M. M. (2008). Structure–function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 56:9391–9398.
- Jurgoński, A., Juśkiewicz, J. and Zduńczyk, Z. (2013). An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition. 29:898–902.
- Kao, E. S., Tseng, T. H., Lee, H. J., Chan, K. C. and Wang, C. J. (2009). Anthocyanin extracted from Hibiscus attenuate-oxidized, LDL-mediated foam cell formation involving regulation of CD36 gene. Chem. Biol. Interact. 179:212–218.
- Kay, C. D. (2006). Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 19:137–46.
- Kim, K. T., Nam, T. K., Park, Y. S., Kim, Y. B. and Park, S. W. (2011). Neuroprotective effect of anthocyanin on experimental traumatic spinal cord injury. J. Korean. Neurosurg. Soc. 49:205–211.
- Kim, Y. K., Yoon, H. H., Lee, Y. D., Youn, D. Y., Ha, T. J., Kim, H. S. and Lee, J. H. (2012). Anthocyanin extracts from black soybean (Glycine max L.) protect human glial cells against oxygen--glucose deprivation by promoting autophagy. Biomol. Ther. 20:68–74.
- Kocic, B., Filipovic, S., Nikolic, M. and Petrovic, B. (2011). Effects of anthocyanins and anthocyanin-rich extracts on the risk for cancers of the gastrointestinal tract. J. BUON. 16:602–608.
- Koide, T., Hashimoto, Y., Kamei, H., Kojima, T., Hasegawa, M. and Terabe, K. (1997). Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother. Radiopharm. 12:277–280.
- Koide, T., Kamei, H., Hashimoto, Y., Kojima, T. and Hasegawa, M. (1996). Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 11:273–277.
- Konczak-Islam, I., Yoshimoto, M., Hou, D. X., Terahara, N. and Yamakawa, O. (2003). Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L.). J. Agric. Food Chem. 51:5916–5922.
- Lala, G., Malik, M., Zhao, C., He, J., Kwon, Y., Giusti, M. M. and Magnuson, B. A. (2006). Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer. 54:84–93.
- Li, L., Wang, L., Wu, Z., Yao, L., Wu, Y., Huang, L., Liu, K., Zhou, X. and Gou, D. (2014). Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 4:6234.
- Li, G., Zhu, Y., Zhang, Y., Lang, J., Chen, Y. and Ling, W. (2013). Estimated daily flavonoid and stilbene intake from fruits, vegetables, and nuts and associations with lipid profiles in Chinese adults. J. Acad. Nutr. Diet. 113:786–794.
- Lim, S., Xu, J., Kim, J., Chen, T. Y., Su, X., Standard, J., Carey, E., Griffin, J., Herndon, B., Katz, B., Tomich, J. and Wang, W. (2013). Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 57:1908–1917.
- Liu, L. K., Lee, H. J., Shih, Y. W., Chyau, C. C. and Wang, C. J. (2008). Mulberry anthocyanin extracts inhibit LDL oxidation and macrophage-derived foam cell formation induced by oxidative LDL. J. Food Sci. 73:H113–H121.
- Liu, Y., Li, D., Zhang, Y., Sun, R. and Xia, M. (2014). Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 306:E975–E988.
- Longo, L., Platini, F., Scardino, A., Alabiso, O., Vasapollo, G. and Tessitore, L. (2008). Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 7:2476–2485.
- Lu, X., Zhou, Y., Wu, T. and Hao, L. (2014). Ameliorative effect of black rice anthocyanin on senescent mice induced by d-galactose. Food Funct. 5:2892–2897.
- Malik, M., Zhao, C., Schoene, N., Guisti, M. M., Moyer, M. P. and Magnuson, B. A. (2003). Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer. 46:186–196.
- Mauray, A., Felgines, C., Morand, C., Mazur, A., Scalbert, A. and Milenkovic, D. (2010). Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 5:343–353.
- Mauray, A., Felgines, C., Morand, C., Mazur, A., Scalbert, A. and Milenkovic, D. (2012). Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 22:72–80.
- Mazza, G. and Miniati, E. (1993). Anthocyanins in Fruits, Vegetables, and Grains. CRC Press, Boca Raton, FL.
- McGhie, T. K. and Walton, M. C. (2007). The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 51:702–713.
- Min, J., Yu, S. W., Baek, S. H., Nair, K. M., Bae, O. N., Bhatt, A., Kassab, M., Nair, M. G. and Majid, A. (2011). Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci. Lett. 500:157–161.
- Mink, P. J., Srafford, C. G., Barraj, L. M., Harnack, L., Hong, C. P., Nettleton, J. A. and Jacobs, D. R. (2007). Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 85:895–909.
- Miyake, S., Takahashi, N., Sasaki, M., Kobayashi, S., Tsubota, K. and Ozawa, Y. (2012). Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Invest. 92:102–109.
- Mpiana, P. T., Ngbolua, K. N., Bokota, M. T., Kasonga, T. K., Atibu, E. K., Tshibangu, D. S. and Mudogo, V. (2010). In vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of haemoglobin S and membrane stability of sickle erythrocytes. Blood Transfus. 8:248–254.
- Muraki, I., Imamura, F., Manson, J. E., Hu, F. B., Willett, W. C., van Dam, R. M. and Sun, Q. (2013). Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 347:f5001.
- NHANES. (2001). National Health and Nutrition Examination Survey Data. NHANES, Hyattsville, MD.
- Paixão, J., Dinis, T. C. and Almeida, L. M. (2011). Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: An in vitro approach. Apoptosis. 16:976–989.
- Park, S. Y., Lee, Y. K., Lee, W. S., Park, O. J. and Kim, Y. M. (2014). The involvement of AMPK/GSK3-beta signals in the control of metastasis and proliferation in hepato-carcinoma cells treated with anthocyanins extracted from Korea wild berry Meoru. BMC Complement Altern. Med. 14:109.
- Peiffer, D. S., Zimmerman, N. P., Wang, L. S., Ransom, B. W., Carmella, S. G., Kuo, C. T., Siddiqui, J., Chen, J. H., Oshima, K., Huang, Y. W., Hecht, S. S. and Stoner, G. D. (2014). Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 6:574–584.
- Poulose, S. M., Fisher, D. R., Larson, J., Bielinski, D. F., Rimando, A. M., Carey, A. N., Schauss, A. G. and Shukitt-Hale, B. (2012). Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 60:1084–1093.
- PrRimm, E. B., Giovannucci, E. L., Willett, W. C., Colditz, G. A., Ascherio, A., Rosner, B. and Stampfer, M. J. (1991). Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 338:464–468.
- Reddivari, L., Vanamala, J., Chintharlapalli, S., Safe, S. H. and Miller, J. C. Jr. (2007). Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 28:2227–2235.
- Rugină, D., Sconţa, Z., Leopold, L., Pintea, A., Bunea, A. and Socaciu, C. (2012). Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on HeLa human cervical tumor cells. J. Med. Food. 15:700–706.
- Samuels, T. L., Pearson, A. C., Wells, C. W., Stoner, G. D. and Johnston, N. (2013). Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. Ann. Otol. Rhinol. Laryngol. 122:632–641.
- Shih, P. H., Chan, Y. C., Liao, J. W., Wang, M. F. and Yen, G. C. (2010). Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J. Nutr. Biochem. 21:598–605.
- Singletary, K. W., Jung, K. J. and Giusti, M. (2007). Anthocyanin-rich grape extract blocks breast cell DNA damage. J. Med. Food. 10:244–251.
- Sohn, D. W., Bae, W. J., Kim, H. S., Kim, S. W. and Kim, S. W. (2014). The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a peyronie disease rat model. Urology. 84:1112–1116.
- Srivastava, A., Akoh, C. C., Fischer, J. and Krewer, G. (2007). Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J. Agric. Food Chem. 55:3180–3185.
- Strathearn, K. E., Yousef, G. G., Grace, M. H., Roy, S. L., Tambe, M. A., Ferruzzi, M. G., Wu, Q. L., Simon, J. E., Lila, M. A. and Rochet, J. C. (2014). Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson's disease. Brain Res. 1555:60–77.
- Takikawa, M., Inoue, S., Horio, F. and Tsuda, T. (2010). Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 140:527–533.
- Tall, J. M., Seeram, N. P., Zhao, C., Nair, M. G., Meyer, R. A. and Raja, S. N. (2004). Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 153:181–188.
- Taverniti, V., Fracassetti, D., Del Bo’, C., Lanti, C., Minuzzo, M., Klimis-Zacas, D., Riso, P. and Guglielmetti, S. (2014). Immunomodulatory effect of a wild blueberry anthocyanin-rich extract in human Caco-2 intestinal cells. J. Agric. Food Chem. 62:8346–8351.
- Tsai, T. C., Huang, H. P., Chang, Y. C. and Wang, C. J. (2014). An anthocyanin-rich extract from Hibiscus sabdariffa linnaeus inhibits N-nitrosomethylurea-induced leukemia in rats. J. Agric. Food Chem. 62:1572–1580.
- Tsuda, T., Horio, F. and Osawa, T. (2002). Cyanidin 3-O-beta-Dglucoside suppresses nitric oxide production during a zymosan treatment in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 48:305–310.
- Tsuda, T., Ueno, Y., Aoki, H., Koda, T., Horio, F., Takahashi, N., Kawada, T. and Osawa, T. (2004). Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 316:149–157.
- Vepsäläinen, S., Koivisto, H., Pekkarinen, E., Mäkinen, P., Dobson, G., McDougall, G. J., Stewart, D., Haapasalo, A., Karjalainen, R. O., Tanila, H. and Hiltunen, M. (2013). Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer's disease. J. Nutr. Biochem. 24:360–370.
- Wallace, T. C. (2011). Anthocyanins in cardiovascular disease. Adv. Nutr. 2:1–7.
- Wang, J., Ma, C., Rong, W., Jing, H., Hu, X., Liu, X., Jiang, L., Wei, F. and Liu, Z. (2012a). Bog bilberry anthocyanin extract improves motor functional recovery by multifaceted effects in spinal cord injury. Neurochem. Res. 37:2814–2825.
- Wang, L. S. and Stoner, G. D. (2008). Anthocyanins and their role in cancer prevention. Cancer Lett. 269:281–290.
- Wang, D., Xia, M., Yan, X., Li, D., Wang, L., Xu, Y., Jin, T. and Ling, W. (2012b). Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 111:967–981.
- Wang, C., Yatsuya, H., Tamakoshi, K., Uemura, M., Li, Y., Wada, K., Yamashita, K., Kawaguchi, L., Toyoshima, H. and Aoyama, A. (2013). Positive association between high-sensitivity C-reactive protein and incidence of type 2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes. Metab. Res. Rev. 29:398–405.
- Welch, C. R., Wu, Q. and Simon, J. E. (2008). Recent advances in anthocyanin analysis and characterization. Curr. Anal. Chem. 4:75–101.
- Wu, X., Beecher, G. R., Holden, J. M., Haytowitz, D. B., Gebhardt, S. E. and Prior, R. L. (2006). Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 54:4069–4075.
- Wu, T., Yu, Z., Tang, Q., Song, H., Gao, Z., Chen, W. and Zheng, X. (2013). Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 4:1654–1661.
- Xia, X., Ling, W., Ma, J., Xia, M., Hou, M., Wang, Q., Zhu, H. and Tang, Z. (2006). An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J. Nutr. 136:2220–2225.
- Xia, M., Ling, W., Zhu, H., Ma, J., Wang, Q., Hou, M., Tang, Z., Guo, H., Liu, C. and Ye, Q. (2009). Anthocyanin attenuates CD40-mediated endothelial cell activation and apoptosis by inhibiting CD40-induced MAPK activation. Atherosclerosis. 202:41–47.
- Xia, M., Ling, W., Zhu, H., Wang, Q., Ma, J., Hou, M., Tang, Z., Li, L. and Ye, Q. (2007). Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler. Thromb. Vasc. Biol. 27:519–524.
- Xiao, J. B. and Högger, P. (2015). Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 22:23–38.
- Ye, J., Meng, X., Yan, C. and Wang, C. (2010). Effect of purple sweet potato anthocyanins on beta-amyloid-mediated PC-12 cells death by inhibition of oxidative stress. Neurochem. Res. 35:357–365.
- Yoon, B. I., Bae, W. J., Choi, Y. S., Kim, S. J., Ha, U. S., Hong, S. H., Sohn, D. W. and Kim, S. W. (2013). The anti-inflammatory and antimicrobial effects of anthocyanin extracted from black soybean on chronic bacterial prostatitis rat model. Chin. J. Integr. Med. 14.
- You, J., Kim, J., Lim, J. and Lee, E. (2010). Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology. 74:777–785.
- Youdim, K. A., Shukitt-Hale, B. and Joseph, J. A. (2004). Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 37:1683–1693.
- Zapolska-Downar, D., Nowicka, G., Sygitowicz, G. and Jarosz, M. (2008). Anthocyanin-rich aronox extract from Aronia melanocarpa E protects against 7 beta-hydroxycholesterol-induced apoptosis of endothelial cells. Ann. Nutr. Metab. 53:283–294.
- Zhao, C., Giusti, M. M., Malik, M., Moyer, M. P. and Magnuson, B. A. (2004). Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 52:6122–6128.
- Zhu, Y., Ling, W., Guo, H., Song, F., Ye, Q., Zou, T., Li, D., Zhang, Y., Li, G., Xiao, Y., Liu, F., Li, Z., Shi, Z. and Yang, Y. (2013). Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 23:843–849.
- Zhu, Y., Xia, M., Yang, Y., Liu, F., Li, Z., Hao, Y., Mi, M., Jin, T. and Ling, W. (2011). Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 57:1524–1533.