ABSTRACT
This workshop, jointly presented by the ILSI North America Technical Committees on Food Microbiology and Sodium, aimed to provide greater knowledge and appreciation of the opportunities and challenges facing the food industry in answering the public health community's call to reduce sodium levels in the food supply. One major challenge is finding effective substitutes for the various antimicrobial and functional roles that sodium plays across different food categories. Sodium plays a critical role in retarding the growth of pathogens and food spoilage bacteria. Moreover, taste is an important factor for consumers when they choose food products, and the flavor changes that occur when salt is reduced or replaced must be considered and ingredients and processes adjusted accordingly. The workshop provided a platform for a multidisciplinary discussion among the microbiology, food science, nutrition, and public health communities to share progress and propose solutions, including the formation of public-private partnerships, to develop coordinated and comprehensive strategies. This paper provides an overview of the issues raised, rather than a specific summary of workshop discussions. The Food and Drug Administration's Draft Guidance for Industry on voluntary sodium reduction and the 2015 US Dietary Guidelines for Americans were released subsequent to the workshop and are also discussed.
Introduction
Salt, which is composed of 40% sodium and 60% chloride, is one of the most important food additives. The sodium component of salt is one of the most studied nutrients with respect to implementing dietary recommendations, particularly for lowering sodium levels in the food supply. Two recent Institute of Medicine (IOM) reports noted that sodium recommendations and most efforts to implement them have not been successful in the United States (IOM, Citation2010b, Citation2013). The IOM reports suggested that this is due, at least in part, to the failure to change the food environment. The US Department of Agriculture/US Department of Health and Human Services (via the 2010 and the 2015–2020 Dietary Guidelines for Americans; (USDA and DHHS, Citation2010, Citation2015)Footnote1 and Health Canada (Citation2010) both recommend that sodium be limited to less than 2,300 mg per day. Current sodium intake in the United States is about 3,400 mg per day (USDA, Citation2015), an estimated 75% of which comes from processed and commercially prepared (e.g., restaurant) foods (Mattes and Donnelly, Citation1991; CDC, Citation2012; Drewnowski and Rehm, Citation2013).
In 2015, the ILSI North America Technical Committees on Food Microbiology and Sodium jointly presented a workshop on “The Safety of Sodium Reduction in the Food Supply: A Cross-Discipline Balancing Act.” This workshop specifically addressed the technical challenges to reducing sodium in foods, rather than the relationship between sodium intake and health outcomes. The IOM (Citation2010b) report, Strategies to Reduce Sodium Intake in the United States, was the impetus for the workshop and focused on the following: the functions of sodium in food and how these functions relate to product development, consumer preferences, and health; the factors that could affect sodium reduction strategies; potential unanticipated consequences of sodium reduction; and the potential of food technology to develop alternatives to current sodium use in processed foods. The 2010 IOM report called on the US Food and Drug Administration (FDA) to consider issuing a range of sodium levels across product categories. The IOM report was the basis for an FDA initiative to reduce sodium in processed foods, which resulted in the June 2016 release of the FDA Draft Guidance for Industry for voluntary sodium reduction for 150 food categories (FDA, Citation2016).
Sodium is ubiquitous in the food supply, making it difficult for consumers to achieve the recommended intakes. Recommending that consumers choose lower-sodium foods simply is not enough to move the population toward meeting the dietary guidelines. When considering whether to reduce the sodium content of any food, there are concerns with microbial food safety, stability, flavor, texture, functionality, quality, and cost. In addition, a reduction in salt without other modifications has the potential to reduce shelf-life and thus increase spoilage and waste. In recent years, consumers have driven the trend in clean labeling, making the options for newer additives and salt replacements challenging. Food scientists have had to shift approaches for reducing sodium to meet this new consumer expectation. An ingredient that may have once been an acceptable salt substitute is no longer acceptable. In the case of salt, more than one substitute may be needed to replace all of salt's functional roles, which can further add to the cost of reformulation. Sodium reduction in foods is a balancing act for manufacturers, as they attempt to address every aspect from taste and functionality to safety and cost. It is a complex process that requires a different approach for each food category and even for each food within a category.
There are clear challenges ahead—for some foods more than others—and there is no simple solution. However, there are opportunities for food manufacturers, foodservice and restaurant industries, government agencies, nongovernment agencies, and nonprofit organizations to work together to discover new and innovative ways to ultimately reduce sodium in the food supply. This workshop was a major step in addressing the myriad of shifting challenges in sodium reduction and allowing for a genuine dialogue across disciplines.
The workshop agenda is provided in and videos of speaker presentations are available online (http://ilsina.org/event/the-safety-of-sodium-reduction-in-the-food-supply-a-cross-discipline-balancing-act/). The workshop focused on a range of technological and methodological considerations relative to sodium reduction in food and provided a number of case studies for food types ranging from breads and doughs to meats and cheeses. This paper builds on those presentations to provide an overview of the key considerations and challenges.
Table 1. Agenda for “The Safety of Sodium Reduction in the Food Supply: A Cross-Discipline Balancing Act”.
Background
Sodium in the food supply
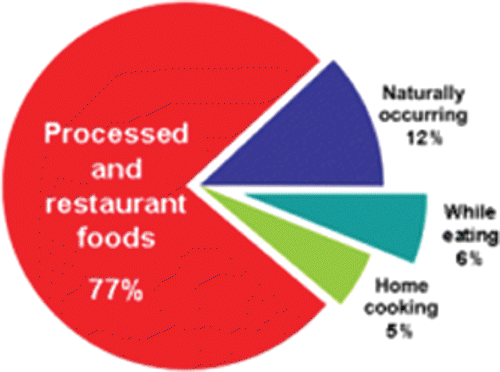
The majority of sodium in the American diet (77%) comes from processed foods () (Mattes and Donnelly, Citation1991; Anderson et al., Citation2010). Only 5% comes from salt added during cooking and 6% comes from salt added at the table.
According to the Centers for Disease Control and Prevention (CDC), the top five greatest contributors to sodium in the diet are breads and rolls, cold cuts and cured meats (e.g., deli or packaged ham or turkey), pizza, fresh and processed poultry, and soups (CDC, Citation2016). Some of these items (e.g., bread) do not contain excessive amounts of sodium but are consumed frequently and, as a result, significantly add to total sodium intake.
Current regulatory landscape
In June 2016, the FDA released a Draft Guidance for Industry entitled “Voluntary Sodium Reduction Goals: Target Mean and Upper Bound Concentrations for Sodium in Commercially Processed, Packaged, and Prepared Foods” (FDA, Citation2016). The agency established 150 food categories, similar to the approach that has been taken by Canada and the United Kingdom.
The FDA has committed to a sodium reduction effort that is based on the best available scientific evidence to obtain gradual, achievable, and sustainable reductions in sodium, while taking into account food microbial safety, the technical role that sodium plays in each food, and consumer acceptance. Sustained monitoring will be a part of this effort, to ensure consistent application of voluntary sodium goals over the entire food supply. The FDA established both short-term (2-year) and long-term (10-year) goals to better track progress. With the creation of different goals, the FDA acknowledges that some food categories can contribute more to the reduction in sodium in the food supply than others, which may require significant technical breakthroughs to further reduce sodium levels.
The extent of targeted reduction in each of the FDA's 150 food categories is influenced by the functions of sodium-containing ingredients, as well as the distribution of sodium concentrations in products within each category. Some goals aim for an eventual reduction of 50%, while others aim for only a 15% reduction (FDA, Citation2016). For example, the FDA's short-term guidance for canned, condensed soup is for a sodium reduction of 10%, with 26% over the long term. For frozen/refrigerated dough and batters, a 17% reduction is indicated over the short term compared with a 46% reduction over the long term. For deli ham products, a 13% reduction is indicated over the short term compared with a 31% over the long term.
The FDA's milestone date for its short-term goals for sodium reduction is the second year after final publication of the sodium guidance—the time frame required from the start of a project to market, as reported to the FDA by food manufacturers and ingredient suppliers. The FDA's milestone date for its long-term goals is the tenth year after final publication. This longer time frame considers the time, effort, and innovation required for voluntary large-scale reformulation of the food supply, should members of the food industry voluntarily pursue these goals (FDA, Citation2016).
As stated in the draft guidance, the voluntary goals are intended to provide a shared framework for describing and analyzing the success of voluntary reduction efforts by various industry stakeholders and to promote continued discussion on sodium reduction opportunities. This important FDA sodium-reduction initiative brings greater visibility to the issue and helps to highlight the industry's journey. It also provides the framework for the establishment of a research public-private partnership to accelerate sodium-reduction efforts. The agency will work to finalize the draft guidance following the public comment period.
Salt was included in the original 1959 GRAS (Generally Recognized as Safe) list along with ingredients such as pepper, vinegar, baking powder, and monosodium glutamate (MSG) (FDA, Citation1999). Labeling of the sodium content of foods has been mandatory since the Nutrition Labeling and Education Act of 1990 went into effect. However, the level of sodium on labels does not always reflect actual sodium content. The FDA allows a labeling tolerance of ±20% (Bender and Rader, Citation1998). Natural variations in the sodium content of products sometimes pose an obstacle to staying within the allotted tolerance, and products are often labeled with higher amounts of sodium than they actually contain to ensure compliance with the upper tolerance levels (Ahuja et al., Citation2014).
Salt preference
A salt appetite is present in many species of plant-eating animals, which innately recognize and respond to it when sodium is depleted. Humans do not possess this type of sodium appetite. However, humans and many animals will preferentially consume salt even when they have no need for sodium, and it appears that children are biologically disposed to like salt. One study found that the preferred level of salt in soup is greater for children than for adults (Coxson et al., Citation2013). In humans, in utero experiences modify salt preferences. Children of women who suffer from excessive vomiting during pregnancy (hyperemesis gravidarum) and experience sodium depletion desire more salt than children who were not exposed to this in utero experience (Malaga et al., Citation2005). Birth weight may also affect salt appetite. Measures related to salty-taste preference were found to be inversely related to birth weight over the first 4 years of life (Stein et al., Citation2006). However, this may be altered over time. A study in the 1980s found that a long-term reduction in sodium intake leads to a decrease in liking of high-salt foods (IOM, Citation2013). However, this was conducted in a controlled environment. Once these individuals were returned to their usual environment, they returned to their usual salt intakes. This finding suggests that reducing the sodium available in the usual food environment might be the best way to reduce sodium intake in the population.
Other contributors of sodium in processed foods
In addition to salt, several other compounds that contribute to sodium content are used in food processing, including sodium benzoate, sodium dehydroacetate, sodium diacetate, sodium citrate, sodium phosphate, sodium propionate, sodium acid pyrophosphate, and sodium sulfite. These sodium-containing compounds act as acidity regulators, preservatives, emulsifiers, and leavening agents and are important for providing a unique salty taste in foods that other ingredients cannot provide. These compounds are also used to effectively inhibit pathogen growth and preserve foods (meats, fish, dairy, and vegetables), to interact with gluten to optimize the volume and texture of baked products (breads, cakes, crackers, cookies, and pancakes), to enhance the color of baked goods, to effectively mask bitterness (processed foods, high-fiber foods like whole-wheat products, and foods fortified with nutraceutical ingredients), and to generally enhance flavor (all foods). In addition, sodium-containing compounds are used as buffering agents, anticaking agents, or neutralizing agents, and they are also used to thicken liquids, help retain moisture, modify texture, and help lighten the color of some foods. (Doyle and Glass., Citation2010; IOM, Citation2010a)
Salt functions in foods
No other single ingredient in food serves as many different functions as salt, making it valuable and difficult to replace. Removing salt can introduce serious microbial food safety concerns and dramatically alter flavor, which affect consumer acceptance. Salt also helps create texture, ranging from crispy to creamy, which is important for consumer acceptance (i.e., pickles, cheese, crackers). Lowering sodium in bread products, for example, may result in a gummy texture, which is unacceptable to consumers even though taste may not be affected.
Salt can have a significantly different effect depending on whether it is an ingredient in a food or added to the surface of the food. In soup, salt brings out the brightness of carrots and the savory notes in chicken broth and it improves the taste of vegetables. While salt itself imparts flavor to food, it can also act as a blocker of bitter flavors, making the food taste sweeter and altering the overall taste profile.
Additional factors can affect the relationship between salt and shelf-life, including the quality of the raw material, sanitation practices in the operation, manufacturing procedures, the inclusion of other antimicrobial ingredient(s), and the survival of spoilage and pathogen microorganisms post processing. In many food processing formulations, however, the amount of salt is not sufficient by itself to adequately preserve foods. When salt is reduced, it may be necessary to add or modify an array of critical factors to ensure that there are adequate margins to protect the safety and quality of the product. (IOM, Citation2010a; Doyle and Glass, Citation2010)
Salt exerts selective action against microbes but does not inhibit the growth of all microbes. Microorganisms require an adequate amount of water in order to survive the food matrix. Salt is a small, highly charged molecule that causes cells to lose water (reduces water activity) and undergo osmotic shock, which then causes cell death or growth retardation. (IOM, Citation2010a)
Salt sometimes functions as a microbiological stabilizer for spoilage organisms, rather than for microbial food safety. Included in that group of food products are pickles, olives, butter, poultry cuts, and condiments (salad dressings, ketchup). Foods in the United States for which salt is added for functions other than preservation or microbial food safety include breads, dry snack foods (crackers, chips, popcorn), boxed/dried prepared foods (spice and cheese packets in rice meals, macaroni and cheese), cereals, beverages, flavorings, and frozen foods (raw meat, fruits and vegetables, pot pies, pizzas). Because different food products have different requirements for salt, uniform reductions across all product categories or even within a product line are not feasible. (IOM, 2010)
In addition, for salt to perform its intended function, timing of its addition is critical. In dough preparation, salt is added in the beginning of the process so that it dissolves, resulting in a more even distribution and uniform salty taste. If salt is added at the end of the dough mixing process and has a larger crystal size, it will be unevenly dispersed, will dissolve less, and will tend to be localized within the dough structure. This can help preserve more of the salty flavor due to the taste contrast between salted and unsalted sections within the bakery product (Noort et al., Citation2012). With meats, it is important to add salt and bacterial cultures such as Pediocccus acidilactici simultaneously. In cheese, the starter culture is added in the beginning of the process and salt is added at the end to create the desired pH, draw moisture from the curd, and prevent growth of spoilage and pathogenic organisms. If salt is added too early, the fermentation stops, resulting in a lower-quality and potentially less safe product (Guinee and Fox, Citation1993).
Salt functions are further highlighted in the three sections below, focusing on meats and meat products, bakery products, and cheese and cheese products.
Meats and meat products
Meat naturally contains sodium but in amounts less than 100 mg per 100 g. Most of the sodium in meat products comes from the salt that is added during processing (Coxson et al., Citation2013). Reducing sodium can compromise the safety of meat products by allowing growth and survival of pathogenic organisms, such as Listeria monocytogenes, Clostridium botulinum (proteolytic and nonproteolytic), Bacillus cereus, Yersinia enterocolitica, Clostridium perfringens, Aeromonas hydrophila, Campylobacter, Escherichia coli O157:H7, Salmonella, and Staphylococcus aureus (IOM, Citation2010a). Salt in meat allows for proper progression and growth of lactic acid bacteria, which helps to control pH and prevent the growth of both spoilage microbes and microbial pathogens that produce toxins. Other ingredients are sometimes added that act synergistically with sodium chloride. For example, adding sodium phosphates can enhance the effectiveness of sodium chloride, resulting in increased functionality and decreased levels of sodium. Sodium also acts synergistically with nitrites. Some studies have suggested that 50 ppm of sodium nitrite has the food safety preservation equivalence impact of 1% of salt (Sebranek and Fox, Citation1985).
Salt helps to bind meat proteins together and acts as a binding agent between meat components and fat. In addition, it improves texture and tenderness by gelatinization, as well as by the release of muscle proteins. Below a critical level of 1.5% to 1.6% salt, solubilization thresholds are reached, leading to binding issues, and texture and moisture management problems begin to occur.
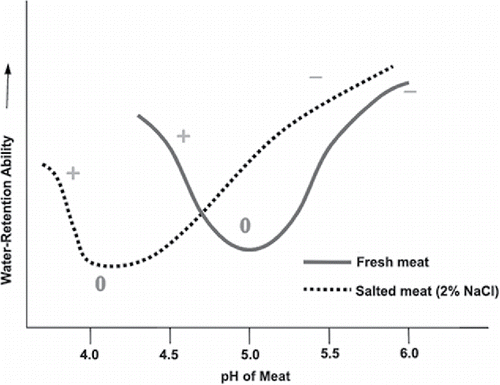
In meat products, reduced levels of sodium result in an increase in moisture loss, which affects texture. The meat and poultry industry is currently at or near the lowest levels of salt in a product that can be used without significantly affecting quality. As seen in , as sodium content decreases, moisture loss increases with cooking, leading to meat toughness and unacceptability to consumers. Moisture loss is also a pH-dependent phenomenon. The inverse bell curve shows that as the pH increases, there is an increase in water-holding capacity (i.e., an increase in better moisture management). This improves with the addition of salt to the meat system. The addition of salt increases the water-binding capacity of meat protein at above a pH of 5.2.
Figure 2. Sodium chloride levels versus cook loss. Cook loss is defined as the degree of shrinkage of meat during cooking due to loss of drippings and volatile losses (3.7% salt = 4% cook loss; 2.9% salt = 6% cook loss; 2.1% salt = 9% cook loss; 1.3% salt = 22% cook loss). Source: Data are from Youling Xiong, University of Kentucky.
A literature review conducted by the FDA identified studies of successful sodium reduction in a number of meat and poultry products, including cooked and cured hams, sausage, frankfurters, and deli meats. These studies identified sodium concentrations that were lower than what is commonly used but may be compatible with microbiological stability. However, many of the studies addressed sodium reduction only on a laboratory level. Scaling those findings up to a mass production level will require additional evaluation, ideally in public-private partnerships with food manufacturers.
Some standards of identify for meats, such that for prosciutto and ham, require as much as 4% salt by weight in the finished product. This amount cannot be reduced unless the standard of identity is changed through a regulatory process and research confirms equivalency in shelf-life stability and microbial safety (U.S. National Archives and Records Administration. Code of Federal Regulations [CFR], 1999).
Salt increases palatability, as volatile compounds are released from the food matrix during cooking, improving the aroma. Other sodium-containing ingredients are used in meat to improve color, texture, and flavor. Potassium acetate is an approved flavoring additive and the meat industry has begun to apply it in an effort to use less sodium. Most ready-to-eat (RTE) meat products use blends of sodium-containing ingredients that can include sodium lactate and sodium diacetate, for Listeria control.
In addition, there is a trend toward “clean” labels, fueled by consumers' dislike for chemical-sounding names (“ics,” “ates,” and “ites”) on the label. Substituting vinegar for more common preservatives is one approach being used to add acetic acid to products and provide the perception that the ingredient is clean and natural. A pilot study performed by Smithfield Foods determined that the addition of vinegar solids and lemon juice (acetic acid and citric acid) added in lieu of lactate and acetate suppressed the growth of L. monocytogenes well into the shelf-life of a deli meat product (unpublished data). In another pilot study, adding 0.6% dried vinegar controlled the growth of pathogens sufficiently to replace high-pressure processing (HPP) in a low-sodium ham, which is an expensive process used to kill and suppress growth of pathogens (unpublished data). Vinegar or HPP can be utilized in any RTE meat to control the growth of pathogens. Both vinegar and HPP are techniques to produce a “clean label” and lower the sodium of a product while maintaining food safety (unpublished data).
Bakery products
The rheological properties of dough are dependent upon the concentration of salt, as it aids in gluten development, which helps strengthen the dough and crumb of the finished bakery product. Sodium chloride is also critical in controlling the yeast fermentation rate and water activity. If too much salt is used, the yeast-leavened dough will rise too slowly; if too little is used, excess gas is produced and the dough tastes sour due to an accelerated rate of yeast formation. The lack of gluten development results in an uneven distribution of air cells in the finished product.
There are several sodium-containing ingredients used in baking, in addition to sodium chloride, that add to the sodium content. These include chemical leavening agents such as sodium bicarbonate, sodium acid pyrophosphate, sodium aluminum phosphate and sodium propionate. Quick breads, cakes, and cookies typically rely on chemical leavening agents, rather than yeast to create the desired texture. Some of the most commonly used leavening agents contain sodium, including baking soda (sodium bicarbonate) and baking powder (a combination of sodium bicarbonate and one of, or a combination of, potassium hydrogen tartrate, sodium aluminum sulfate, sodium acid pyrophosphate, and calcium acid phosphate) (IOM, Citation2010b).
A factor to be considered when lowering sodium in wheat-based bakery products is that wheat is a crop. Each year the protein composition (required for gluten development) changes and the wheat must be characterized for protein quality. Changes in the production process are required to adapt to the protein composition of the new crop. Flour manufacturers can blend flours or add gluten to achieve the desired protein level, illustrating that solutions for sodium reduction are not static but must be adjusted to accommodate other changing environmental factors.
General Mills has made efforts to reduce sodium in pressurized, canned, refrigerated dough. Through experimentation, it was determined that as sodium levels were reduced, can pressures were significantly reduced as well, resulting in a failure of the cans to seal properly (unpublished data). Sealing of the pressurized can is critical for performance and to prevent spoilage. When salt is added to the system, it drives the leavening reaction and increases can pressure. The addition of ≤ 0.5% sodium chloride is associated with failure to pressurize. If magnesium salts were added instead, the opposite occurred; the reactions were accelerated substantially and the dough could not be contained in the cans (unpublished data). Both the type and amount of salt are critical for controlling leavening reactions in dough. (Domingues et al., Citation2016) The rate of reaction is a function of the acid/soda concentration, acid type, temperature and dissolved ions.
Sodium affects the ionic strength of the aqueous medium of the dough, which in turn affects the dissociation of the acids.
Cheese and cheese products
Salt performs many technical functions in cheesemaking, and industry reformulations to reduce sodium are constrained by regulatory standards of identity for natural cheeses. Unlike in meat products, where it might be possible to replace sodium chloride with alternatives such as potassium chloride, standards of identity for cheese do not allow for that option.
Salt is important for the texture of cheese. Salt removes excess water, or whey, resulting in a firmer texture and, in some cases, a rind. Furthermore, salt allows for control of the activity of starter cultures in cheesemaking. A lower salt content results in a cheese that is soft, pasty, and adhesive. For example, removing salt in the production of mozzarella cheese results in a mushy texture, which is unacceptable to consumers. Salt also contributes to the meltability, shredding, and stretching of cheese.
Research has revealed the level of complexity that exists when reducing sodium in cheese while still maintaining safety (USDA and DHHS, Citation2010; Shrestha et al., Citation2011a, Citationb; Oh et al., Citation2014). Using a cheddar cheese extract, a study examined the effects of sodium chloride, pH, and lactic acid on the growth of pathogenic bacteria. Four pathogens (E. coli, L. monocytogenes, S. aureus, and Salmonella) were examined in a model system to determine the lowest levels of the three inhibitory factors that would be effective. The results revealed that lower pH and higher lactic acid levels from starter cultures had a greater impact on inactivation of Shiga toxin–producing E. coli than high salt concentrations alone, suggesting that the greatest antimicrobial factor may not be salt, but the pH and lactic acid levels. Shiga toxin–producing E. coli were of greatest concern in cheddar cheese varieties with altered compositions, and the pH was the microbial hurdle primarily responsible for controlling these bacteria.
Starter cultures and nonstarter cultures, such as lactic acid–producing bacteria, serve as competitive bacteria against pathogenic and spoilage microorganisms. Adding cultured dairy solids can provide additional organic acids, as well as help control bacterial activity. However, although the product is improved from a food safety perspective, the higher acidity typically results in a poorer-quality product throughout the shelf-life.
Because processed cheese products must undergo a heating step, the levels of the starter culture present that may be protective against pathogen growth are greatly reduced or eliminated. However, studies determined that these products could be rendered shelf-stable and safe from C. botulinum toxin formation by formulating them with specific combinations of pH, water activity, and antimicrobial ingredients (Tanaka et al., Citation1986). More specifically, processed cheese depends on salt and emulsifying salts, such as sodium phosphate, for safety, flavor, and functionality, but these also add to the sodium content.
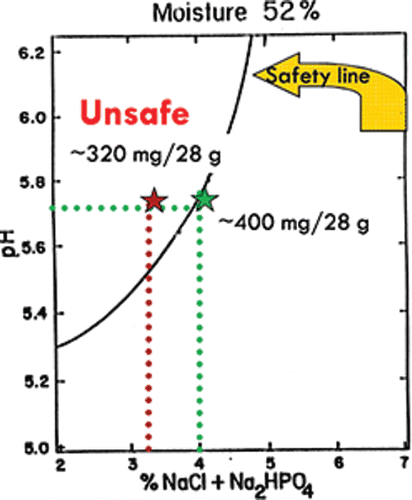
Processed cheese products used in the US school lunch program contain approximately 350–400 mg of sodium per 1-ounce serving, which provides microbial safety but is greater than the school lunch program target of 200–300 mg of sodium per 1-ounce serving (a 25%–43% reduction from current levels) (USDA, Citation2012). If sodium is simply reduced, the product falls into the unsafe zone unless validated alternative preservatives are added ().
Figure 3. Control of Clostridium botulinum in shelf-stable process cheese. Source: Adapted from Tanaka et al. (Citation1986).
Nisin is an effective antibotulinum agent (Somers and Taylor, Citation1987) but it can be cost prohibitive if added in the amounts necessary for antimicrobial activity. If sodium chloride in processed cheese is replaced with potassium chloride, there are limitations due to the metallic/bitter aftertaste, a possibly reduced water-holding capacity, and, if added on a percentage basis, reduced microbiological activity. However, sorbic acid/potassium sorbate is another potential alternative that is being studied for its ability to reduce sodium in processed cheese. Based on several studies, sodium reduction by substitution with potassium salts on a molar basis, rather than a percentage basis, could achieve a 50% reduction in sodium (Glass, Citation2016). Fat reduction is also being examined; the lower the fat content, the more stable the product, as antimicrobial activity could be sequestered in the fat phase if the antimicrobial is fat soluble. (Glass and Johnson, Citation2004a; Glass and Johnson, Citation2004b)
Strategies and challenges
The workshop participants discussed emerging strategies to reduce the sodium content of foods that are already beginning to show promising results. The challenges for the industry are many and will require monetary and time commitments.
The costs associated with sodium reduction efforts cannot be ignored and may include those involved with research and development, new ingredients, process validation, and labeling. Researchers may develop excellent alternatives to salt, but the cost-in-use of the alternative ingredient or processing conditions will come into play.
Leveraging collaborations
One example of collaborative leveraging comes from the National Dairy Council, which has created a 27-member Cheese and Sodium Best Practices Task Force, representing producers of approximately 80% of the volume of cheese produced nationally. The task force was created to review the science and conduct new research on the best ways to reduce sodium in cheese and cheese products, while maintaining quality and safety. The first task force–sponsored study analyzed 1,665 cheese samples and found a wide variation in sodium levels, not only across different types of cheese but also among brands, forms, and even from sample to sample (National Dairy Council, unpublished data). The study also determined that the amount of sodium in cheese is typically lower than the labeled amount. The task force has undertaken research to develop methods for rapid direct sodium testing of cheese to enable cheesemakers to make adjustments in real time and tighten the variability of sodium content. A second study of consumers' sensory perception of cheese determined that there is a fairly narrow range of sodium that results in the greatest consumer satisfaction (National Dairy Council, unpublished data). For mild cheddar, results of consumer sensory testing showed that a range of 630–670 mg of sodium per 100 g had the highest level of satisfaction. The range was 630–690 mg for mozzarella and 1,150–1,250 mg for processed cheese. It was concluded that, for cheese, the best approach for further reducing sodium is to reduce it in small increments and then to work toward a goal of reducing sodium variability within products.
In the United Kingdom, the food industry has funded a collaborative project through the Food and Drink Federation and the British Retail Consortium to investigate ways to reduce salt concentration in foods. Leatherhead Food Research in the United Kingdom jointly funded the project to identify new and emerging technical solutions to sodium reduction (Leatherhead Food Research, unpublished data). Eight product categories have been identified that present the most significant challenges: meat and meat products; bread, particularly specialty and morning goods; cheese, particularly cheddar and soft cheeses; extruded and pelleted snacks; cakes, pastries, and fruit pies; pesto and other thick sauces; all other puddings; and canned fish.
Stealth reduction in salt
When salt is reduced in products, the flavor profile is usually altered. Despite the findings of surveys indicating that consumers want to reduce their sodium intakes, the experience of some food companies fails to support this (unpublished data). In consumer tests, the Campbell Soup Company found that 75% of those asked said they were more likely to purchase a tomato soup with lower sodium levels, and 80% said that the benefit of sodium reduction would be one of the reasons they would choose the product. However, when lower-sodium labels were tested without the consumers having tasted the product, intent to purchase was significantly reduced. (Attitude Management Corporation Survey of Buyers of Campbell's reduced-sodium soups, unpublished data) The decision was eventually made to reduce sodium without highlighting it on the label. Other manufacturers have taken the approach of a “stealth reduction in salt” as well, and some have positioned products as containing “a hint of salt” rather than being labeled as “reduced salt.”
Salt alternatives
Flavors, herbs, and spices are effective at rebuilding much of the flavor lost when salt is reduced or removed, and proteins and texturants (starches, soy protein, whey protein) return texture that is lost with salt reduction.
Potassium chloride is often used as a salt substitute, but not everyone perceives it as a salty taste. For some people, potassium chloride can impart a bitter, metallic taste, and adding sodium chloride may help but not entirely suppress the off-flavor. In addition, potassium does not completely substitute for sodium in many other critical functions, such as the rate of yeast fermentation (slightly faster than salt) or the Maillard reaction (which creates an appealing brown crust in breads and other baked products). (Doyle and Glass, Citation2010)
The amino acid l-arginine does not provide a salty taste by itself, but it has a bitterness-suppressive property that can partially mask the bitterness of potassium chloride. When l-arginine is added to salt at low levels (50 mM or 75 mM), the saltiness of a product is almost doubled (Health Canada, Citation2010). However, there are limitations to the amount of saltiness l-arginine can impart and to its ability to mask the bitterness of a potassium chloride salt substitute. A salt substitute containing l-arginine has not been commercially developed. However, if this option is pursued, the heat and cold stability of l-arginine may determine how well it substitutes for salt's other functions in food processing and, therefore, must be investigated (Coxson et al., Citation2013). While l-arginine is a GRAS substance, regulatory barriers exist for its use in products with standards of identity.
Consumer ratings of alternatives to salt, including disodium guanylate, disodium inosinate, hydrolyzed yeast protein, hydrolyzed wheat gluten, MSG, and autolyzed yeast extract, indicate low acceptability for all but natural flavor, yeast extract, and potassium chloride (unpublished data). In order to meet consumer demands, ingredients that were once standard items in the food manufacturer's toolbox for ensuring food safety and product quality are no longer used. Alternative ingredients that can carry the “clean” label and are just as effective must be developed for use on a large scale. New ingredient technologies, such as a proprietary novel MSG-free sodium replacement ingredient derived from vegetables and sea salt, are being studied. This replacement ingredient has been tested in canned tuna and was found to enable a 35% reduction in sodium from 350 mg to 250 mg, without affecting taste (unpublished data).
Changing the salt distribution in the product
If the size of the salt granule is reduced, it yields a more intense salty flavor. New technologies applying this concept are currently being developed to reduce the sodium content of bread without affecting flavor. One such technology employs “inhomogeneous spatial distribution” of salt in the food matrix, in which salt is intermittently dispersed through the product, achieving up to a 25% reduction of salt in bread without the loss of flavor intensity and without the addition of taste enhancers, aromas, or salt replacers (Noort et al., Citation2010; Coxson et al., Citation2013).
Focus on the whole food
While individual reductions may be small, aggregate reductions can be significant enough to have an impact on public health. Single ingredients in multi-ingredient foods, such as pizza, cannot carry the entire sodium-reduction load. However, when the dough, cheese, tomato sauce, and all or most of the other ingredients have reduced sodium, a significant reduction in the sodium of the whole product can be achieved (unpublished data). Leprino Foods, a major producer of mozzarella cheese in the United States, has taken a whole-food approach to lowering sodium. Rather than focusing on a single ingredient, the company is working with partners and suppliers of other ingredients, such as dough, sauce and meats, to make reductions in sodium to produce a greater sodium reduction in the final product.
The same analogy can be realized across the food supply. The basic approach to reducing sodium in mixed dishes is to address each component, as follows:
| • | Identify the sodium target. | ||||
| • | Deconstruct the food into components. | ||||
| • | Identify sources of sodium in each ingredient. | ||||
| • | Determine the functional role of the sodium-containing ingredients. | ||||
| • | Reformulate and conduct prototype testing. | ||||
| • | Conduct sensory testing. | ||||
| • | Conduct thermal/nonthermal processing validation testing for food safety. | ||||
| • | Conduct shelf-life testing. | ||||
The food manufacturer's challenge is that there are often different suppliers for each food component in a mixed dish like pizza, and the solution for one component may not complement another in terms of taste or texture.
provides examples of factors and, in turn, challenges that the food industry faces in reducing sodium. Overall, it should be noted that process validation studies (defined as the collection and evaluation of data, from the process design stage throughout production, which establishes scientific evidence that a process is capable of consistently delivering quality products) using food safety models with specific salt levels are needed to provide scientific support for any approach undertaken. These studies are expensive to conduct and, depending on the product, can require approximately 12–18 months to complete in order to verify microbial food safety and shelf-life stability. Even a minor adjustment of sodium level can require that a new series of process validation studies be conducted. This complicates the public health community's recommendation to implement small incremental reductions in sodium over time so that consumers adjust their taste preferences.
Table 2. Examples of factors and challenges the food industry faces in reducing sodium.
Furthermore, the primary message for sodium reduction is that there is no one-size-fits-all solution. The composition, texture, and function of the food affect the approach and feasibility of successful sodium reduction efforts. In soups, for example, it is easier to reduce sodium in those products that have high glutamic acid and nucleotide contents, as these interact with umami receptors to bring out savory notes. An increased salt perception also occurs in high-acid foods, such as tomato products, which provide opportunities for sodium reduction (unpublished sensory data).
For example, the Campbell Soup Company was able to successfully reduce the sodium content in its classic tomato soup from about 700–800 mg per serving to about 520 mg per serving (a 26%–35% reduction) and maintain an acceptable level of liking by consumers (unpublished data). The findings were quite different for the company's classic chicken noodle soup product. Only a 100-mg reduction in sodium was achieved in the chicken noodle soup, while still retaining consumer acceptance. This provides additional evidence that sodium cannot be reduced across the board, even within a single food category. Because foods vary greatly, a “recipe” approach is needed to determine what is acceptable for each individual product.
Next steps
The FDA acknowledged in its 2016 draft guidance that while sodium reduction may technically be possible, it may be less feasible for industry to implement in some cases. It will take a concerted effort to identify the challenges ahead and to set priorities for research and product development. This workshop provided a much-needed forum for discussion and collaboration among federal partners, international government partners, academia, food industry, representatives, and other health professionals on approaches to sodium reduction. The participants agreed that there will be many new, innovative solutions coming into the marketplace to adhere to the FDA's guidance and reduce the overall level of sodium in the food supply.
Reducing sodium in the food supply will take true collaboration among federal partners, such as the FDA, CDC, USDA (Center for Nutrition Policy and Promotion, Food Safety and Inspection Service, and Agriculture Research Service), and National Institutes of Health (NIH), alongside organizations outside the government, including food manufacturers, retailers and wholesalers, restaurant and food service operators, public health organizations, and foreign governments. A comprehensive strategy of multiple public-private partnerships seems essential to the success of overall sodium intake reduction. At the national level, there are opportunities at agencies such as the NIH and USDA to support fundamental research needed to identify and understand sodium receptors behind the salty-taste mechanism. The newly established USDA Foundation for Food and Agriculture Research has the mission to establish public-private partnerships and may, along with the Reagan-Udall Foundation for the FDA, facilitate effective partnerships for developing practical, effective solutions for reducing sodium in the food supply. The FDA has requested specific technical information about reformulations to better understand what is working in a broad range of categories, and this can be accomplished by the food industry as well as academic research centers.
Since the release of the IOM 2010 report on Strategies to Reduce Sodium Intake in the United States, much progress has been made to reduce the levels of sodium in the food supply, much of which was highlighted at the workshop. This was reinforced by the release of the FDA's Draft Guidance for Sodium Reduction in June 2016. However, workshop participants agreed that much remains to be accomplished and that it will be a slow, stepwise process. There is no panacea for reducing sodium in the food supply, but there are many opportunities for research, new product development, and collaboration.
Disclosure statements
Michael Doyle is a member of the ILSI North America Board of Trustees. Christine Taylor is a government liaison to the ILSI North America Technical Committee on Sodium.
Funding details
Densie Webb received funds from ILSI North America for her work on this article. The workshop was sponsored by the ILSI North America Technical Committees on Food Microbiology and Sodium.
Acknowledgments
ILSI North America is grateful to the workshop speakers who reviewed the manuscript. ILSI North America is a public, nonprofit foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply by sponsoring research programs, educational seminars and workshops, and publications. ILSI North America receives support primarily from its industry membership. The views expressed in this article are those of the co-authors.
Notes
1 The 2015–2020 US Dietary Guidelines for Americans were released shortly after this workshop.
References
- Ahuja, J. K., Wasswa-Kintu, S., Daniel, M., Thomas, R., Nickle, M., Pehrsson, P. and Cogswell, M. (2014). Do the labels tell the truth about sodium? J. Acad. Nutr. Diet. 114:A48.
- Anderson, C. A., Appel, L. J., Okuda, N., Brown, I. J., Chan, Q., Zhao, L., Ueshima, H., Kesteloot, H., Miura, K., Curb, J. D., Yoshita, K., Elliott, P., Yamamoto, M. E., and Stamler, J. (2010). Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J. Am. Diet. Assoc. 110:736–745.
- Attitude Management Corporation Survey of Buyers of Campbell's reduced-sodium soups, unpublished data.
- Bender, M. M. and Rader, J. I. (1998). Guidance for industry: Nutrition labeling manual—a guide for developing and using databases. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm063113.htm [Accessed October 19, 2016].
- CDC. (2012). Vital signs: Food categories contributing the most to sodium consumption—United States, 2007–2008. MMWR Morb Mortal Wkly Rep. 61:92–98.
- CDC. (2016). Top 10 sources of sodium. Available from: http://www.cdc.gov/salt/sources.htm [Accessed October 19, 2016].
- U.S. National Archives and Records Administration. Code of Federal Regulations. (1999). Food Safety and Inspection Service. USDA. Pt. 319.106, Volume 64, No. 246.
- Coxson, P. G., Cook, N. R., Joffres, M., Hong, Y., Orenstein, D., Schmidt, S. M. and Bibbins-Domingo, K. (2013). Mortality benefits from US population-wide reduction in sodium consumption: Projections from 3 modeling approaches. Hypertension 61:564–570.
- Domingues, D., Dowd, C. and Atwell, W. (2016). Encyclopedia of Food Grains. Second Edition, Volume 3, Refrigerated Dough, pp. 359–366. Wrigley, Colin, Corke, Harold, Seetharaman, Kooshik and Faubion, Jon, Eds., Academic Press, editors. Oxford, UK.
- Doyle, M. E. and Glass, K.A. (2010). Sodium reduction and its effect on food safety, food quality, and human health. Compr. Rev. Food Sci. Food Saf. 9:44–56.
- Drewnowski, A. and Rehm, C. D. (2013). Sodium intakes of US children and adults from foods and beverages by location of origin and by specific food source. Nutrients 5:1840–1855.
- FDA. (1999). Substances generally recognized as safe (21CFR182.1). Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.1 [Accessed October 19, 2016].
- FDA. (2016). Draft guidance for industry: Voluntary sodium reduction goals: Target mean and upper bound concentrations for sodium in commericially processed, packaged, and prepared foods. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm494732.htm [Accessed October 19, 2016].
- Glass, K. A. and Johnson, E. A. (2004). Factors that contribute to the botunlinal safety of reduced-fat and fat-free process cheese products. J. Food Prot. 67:1687–1693.
- Glass, K. A., Mu, M., Rossi, F., LeVine, B. and McCoy, D. (2016). Modeling the inhibition of Closotridium botulinum in reduced-sodium pasterurized cheese products. J. Food Prot. 79(Sup A):79.
- Glass, K. A. and Johnson, E. A. (2004). Antagonistic effect of fat on the antibotuninal activity of food preservatives and fatty acids. Food Microbiol. 21:675–1693.
- Guinee, T. P. and Fox, P.F. (1993). Salt functionality and impact during and after cheesemaking is well documented. In: Cheese-Chemistry, Physics and Microbiology, Vol. 1, pp 257–268, Chapman & Hall, London, UK. pp 257–268.
- Health Canada. (2010). Sodium reduction strategy for Canada, recommendations of the sodium working group. Available from: http://www.hc-sc.gc.ca/fn-an/nutrition/sodium/related-info-connexe/strateg/reduct-strat-eng.php [Accessed October 19, 2016].
- International Dairy Federation. (2014). The importance of salt in the manufacture and ripening of cheese. Special Issue 1401. International Dairy Association, Brussels, Belgium.
- IOM. (2010a). Preservation and physical property roles of sodium in foods. In: Strategies to Reduce Sodium Intake in the United States, pp. 91–118. National Academies Press, Washington, DC.
- IOM. (2010b). Strategies to Reduce Sodium Intake in the United States. National Academies Press, Washington, DC.
- IOM. (2013). Sodium Intake in Populations: Assessment of Evidence. National Academies Press, Washington, DC.
- Malaga, I., Arguelles, J., Diaz, J. J., Perillan, C., Vijande, M. and Malaga, S. (2005). Maternal pregnancy vomiting and offspring salt taste sensitivity and blood pressure. Pediatr Nephrol. 20:956–960.
- Mattes, R. D. and Donnelly, D. (1991). Relative contributions of dietary sodium sources. J. Am. Coll. Nutr. 10:383–393.
- Noort, M. W., Bult, J. H. and Stieger, M. (2012). Saltiness enhancement by taste contrast in bread prepared with encapsulated salt. J. Cereal. Sci. 55:218–225.
- Noort, M. W., Bult, J. H., Stieger, M. and Hamer, R. J. (2010). Saltiness enhancement in bread by inhomogeneous spatial distribution of sodium chloride. J. Cereal. Sci. 52:378–386.
- NYC Health. (2015). High sodium warning label: Why it matters. Available from: https://www1.nyc.gov/assets/doh/downloads/pdf/cardio/high-sodium-warning-label.pdf [Accessed October 19, 2016].
- Oh, J. H., Vinay-Lara, E., McMinn, R., Jr., Glass, K. A., Johnson, M. E. and Steele, J. L. (2014). Evaluation of NaCl, pH, and lactic acid on the growth of Shiga toxin-producing Escherichia coli in a liquid Cheddar cheese extract. J. Dairy Sci. 97:6671–6679.
- Sebranek, J. G. and Fox, J. B. (1985). A review of nitrite and chloride chemistry: Interactions and implications for cured meats. J. Sci. Food Agric. 36:1169–1182.
- Shrestha, S., Grieder, J. A., McMahon, D. J. and Nummer, B. A. (2011a). Survival of Listeria monocytogenes introduced as a post-aging contaminant during storage of low-salt Cheddar cheese at 4, 10, and 21 degrees C. J. Dairy Sci. 94:4329–4335.
- Shrestha, S., Grieder, J. A., McMahon, D. J. and Nummer, B. A. (2011b). Survival of Salmonella serovars introduced as a post-aging contaminant during storage of low-salt Cheddar cheese at 4, 10, and 21 degrees C. J. Food Sci. 76:M616–M621.
- Somers, E. B. and Taylor, S. L. (1987). Antibotulinal effectiveness of nisin in pasteurized process cheese spreads. J. Food Prot. 50:842–848.
- Stein, L., Cowart, B. and Beauchamp, G. (2006). Salty taste acceptance by infants and young children is related to birth weight: Longitudinal analysis of infants within the normal birth weight range. Eur. J. Clin. Nutr. 60:272–279.
- Tanaka, N., Traisman, E., Plantinga, P., Finn, L., Flom, W., Meske, L. and Guggisberg, J. (1986). Evaluation of factors involved in antibotulinal properties of pasteurized process cheese spreads. J. Food Prot. 49:526–531.
- USDA. (2012). Nutrition standards in the National School Lunch and School Breakfast Programs. Final rule. Fed Regist. 77:4088–4167.
- USDA. (2015). Healthy People 2020. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/nutrition-and-weight-status/objectives [Accessed October 19, 2016].
- USDA and DHHS. (2010). Dietary Guidelines for Americans 2010. 7th ed. US Government Printing Office, Washington, DC.
- USDA and DHHS. (2015). 2015–2020 Dietary Guidelines for Americans. 8th ed. US Government Printing Office, Washington, DC.
- Xiong, Y. L. (2004). Encyclopedia of Meat Sciences, pp. 218–225. Jensen, W. K., Devine, C. and Kideman, M., Eds., Elsevier, Oxford, U.K.