ABSTRACT
Latest forecasts predict that half of the European population will be allergic within the coming 15 years, with food allergies contributing substantially to the total burden; preventive measures are urgently needed. Unfortunately, all attempted alimentary strategies for primary prevention of allergic diseases through allergen avoidance so far have failed. This also holds true for the prevention of food allergies in breastfed infants by the common practice of excluding certain foods with allergenic potential from the maternal diet. As a preventive measure, therefore, exclusion diets should be discouraged. They can exhaust nursing mothers and negatively impact both their nutritional status as well as their motivation to breastfeed. A prolonged exclusion diet may be indicated solely in cases of doctor-diagnosed food allergy following rigid medical tests (e.g. double-blind placebo-controlled food challenges). Indicated cases usually involve exclusion of only a few food items. Continued breastfeeding is generally important for many aspects of the infant's health, including the training of the infant's immune responses to foreign compounds and avoidance of overshooting inflammatory responses. Recent studies suggest that the presence of maternal dietary proteins in amniotic fluid, cord blood, and human milk might support the induction of tolerance towards solid foods in infants. These are exactly the same species of proteins or remnants thereof that, in comparatively few cases, trigger allergic responses. However, the insight that the proteins of maternal dietary origin in human milk are more likely to be cure (or, more precise, directing prevention) than curse has still largely evaded the attention of health care professionals consulted by worried breastfeeding mothers. In this paper, we summarize recent literature on the importance of exposure to dietary proteins in the establishment of immunological tolerance and hence prevention of allergic disease. Multiple organizations have used the scientific knowledge to build (local) guidelines (e.g. AAAAI, EAACI, BSACI) that can support health care professionals to provide the best strategy to prevent the onset of allergic diseases. We thus hope to clarify existing confusion about the allergenic propensities of dietary proteins during early life, which has contributed to exaggerated fears around the diet of pregnant and breastfeeding mothers.
Breastfeeding mothers fear food allergens in their diet for their infant's sake
Allergies, including food allergies, have increased dramatically to epidemic proportions worldwide (Berin and Sampson Citation2013). The European Academy of Allergy and Clinical Immunology predicts that in 15 years from now more than half of the European population will suffer from some type of allergy (Calderon et al. Citation2012). This forecast includes an expected further rise in the prevalence of food allergies from current 2–10% (Calderon et al. Citation2012; Praticò and Leonardi Citation2015). This forecast is substantiated by several data, including the US Center for Disease Control and Prevention that reports an increase in food allergies among children in the United States from 3.4% in 1997–1999 to 5.1% in 2009–2011 (Jackson et al. Citation2013), an increase which is also observed in Europe (Nwaru et al. Citation2014).
Proteins present in common foods can act as agents involved in sensitizing the skin, gut, and respiratory tract towards allergic reactions. That means that the body recognizes specific food proteins as harmful foreign material alike pathogenic microorganisms. The resultant immune response to expel these substances from the body typically involves mucus secretion, sneezing, itching, coughing, bronchoconstriction, tear production, inflammation, vomiting and diarrhea (http://www.worldallergy.org/professional/allergic_diseases_center/ige/). In contrast, a healthy immune state is characterized by directing protective responses exclusively to invading pathogenic microorganisms and avoiding excessive immune reactions to harmless proteins (Sakaguchi et al. Citation2008).
Breastfeeding mothers often learn through hearsay that some of the proteins they consume with their food can leak into their milk and may cause allergies in their infants. Women are also aware that allergen avoidance has been a common recommendation to safeguard welfare of both mother and infant (Vance et al. Citation2005). As a result, breastfeeding women who consider themselves normal eaters when they are not breastfeeding may decide to go on a strict elimination diet as soon as their infant shows gastrointestinal symptoms (e.g. colic, vomiting) or skin reactions (e.g. eczema). They hope that they can thus minimize their children's risk to develop serious allergic diseases. Many mothers are not aware, however, that the exclusion diet comes with its own risks.
This paper specifically aims to inform that a maternal exclusion diet does not help in the prevention of a misguided immune response towards food including serious allergic manifestations in breast-fed infants and young children (Kramer and Kakuma Citation2012). Breastfeeding should be encouraged for all infants, as the milk may contain dietary proteins with an immunological potential (Academy of Breastfeeding Medicine Citation2011). We back such notion by highlighting both recent epidemiological evidence as well as emerging knowledge about the mechanistic links between dietary protein exposure during pregnancy and breast feeding and the infant's immune responsiveness. Current insights into mechanistic aspects of immune training suggest that maternal elimination diets may result in insufficient induction of immune tolerance towards solid foods in children, so are counterproductive (Verhasselt Citation2010).
Both mothers and infants need proteins in their diet to prosper
A protein can be described as any of a group of complex organic macromolecules that contain carbon, hydrogen, oxygen, nitrogen, and usually sulfur and are composed of one or more chains of amino acids (Thefreedictionary Citation2017). Proteins are fundamental components of all living cells and include many substances, such as enzymes, hormones, and antibodies that are necessary for the proper functioning of an organism. They are essential in the diet of animals for the growth and repair of tissue and can be obtained from foods such as meat, fish, eggs, milk, and legumes”. Except for water, protein is the most abundant substance in the human body, both holding true for infants as well as adults (Snyder et al. Citation1975).
There should be no doubt that dietary protein per se is essential to build and resupply the body with this essential substance. According to the U.S. Institute of Medicine (Institute-of-Medicine Citation2017), breastfeeding women require about 1.05 g of dietary protein/kg body weight each day to maintain their own body and express protein in their breast milk for the infant. This is an average increment of 159 percent compared to non-lactating non-pregnant adult women, who are recommended to consume 0.66 g/kg body weight per day (Institute-of-Medicine Citation2017).
A maternal diet that is deficient in protein has severe consequences for both mother and child. Therefore, exclusion diets subscribed by medical specialists must never compromise on total protein intake. Protein under-nutrition of a pregnant woman may lead to intrauterine growth retardation of the fetus and low birth weight (Christian Citation2014), emphasizing why exclusion diets should always be put together by medical specialists. Even when a mother started a diet based on her own judgment, medical specialists can still protect a child from being born with a low birth weight. For example, it has been shown that protein-energy supplementation of the protein-malnourished pregnant mother increases the birth weight of newborns towards normal levels (Stevens et al. Citation2015). In addition, it has been shown that the average protein content of human milk from chronically protein deficient mothers in one study was 0.93 g/dL compared with 1.09 g/dL from healthy mothers; milk volume was also decreased by 22% (Hanafy et al. Citation1972). Lower milk volume together with reduced protein concentration may compromise infant's healthy growth. It is important to note that, whenever severe maternal under-nutrition is not an issue, the total protein need for growth of healthy infants born at term are covered through exclusive breastfeeding. These needs are estimated to range from 1.77 g/kg body weight per day in the first month after birth to 1.21 g/kg body weight per day in the fifth month (Panel Citation2014). The decision to exclude a broad range of food items from the diet of the expecting and nursing mother for longer periods can pose a problem for her physiological status (Vandenplas et al. Citation2007; Polloni et al. Citation2013). Moreover, nutrient deficiencies and energy imbalance can contribute to an emotional crisis that prompts mothers to abandon breastfeeding much earlier than originally intended (Forster Citation2010).
Human milk protein is more than nourishment
Proteins are well represented in human milk: >2600 different protein species (van Herwijnen et al. Citation2016) each with amino acids bound in a different sequence. The major proteins in human milk, including caseins, α-lactalbumin, lactoferrin, secretory immunoglobulin IgA, lysozyme, and serum albumin are synthesized exclusively in the mammary tissue (Lonnerdal Citation2004). To support infant growth, milk proteins are broken down into constituent amino acid moieties in the digestive tract, which are in turn absorbed into the blood capillaries and carried on to the cells throughout the body to become reassembled into proteins. Overall, however, the amino acid composition of human milk protein is distinct from that of the synthesized tissue, which indicates that proteins may serve infant's healthy growth not only through providing adequate amounts of essential amino acids (Dupont Citation2003). Indeed, it has been found that several proteins are not purely nutritive, but exert a specific physiological function in the body before or independent of amino acid supply, which is dependent on a specific amino acid sequences. For example, immunoglobulins, kappa-casein, lysozyme, lactoferrin, haptocorrin, α-lactalbumin, and lactoperoxidase are relatively resistant against digestive break-down and can contribute in intact or partially digested form to the defense of breastfed infants against pathogenic bacteria and viruses (Lonnerdal Citation2004). Colostrum, the first milk produced in the breasts after childbirth, is particularly rich in maternal antibodies (Islam et al. Citation2006). When the child ingests these maternal antibodies through the milk, these antibodies can bind to pathogens present in the infant's gastro-intestinal tract, thereby inhibiting the entrance of these pathogens into the baby's tissue. These molecules thus provide antibody-mediated passive immunity at a time when the infant's own immune competence is still inadequate to deal with any serious pathogen assault (Newburg Citation2005; Field Citation2005). Another example is lactoferrin. Its primary role is to sequester free iron, thereby removing an essential substrate that is required for bacterial growth. Furthermore, lactoferrin binds to lipopolysaccharides present in bacterial cell walls where it can neutralize bacteria via the formation of peroxides. These peroxides can affect the membrane permeability of the bacteria and subsequently result in the cell lysis and bacterial death (Ellison et al. Citation1988).
As first highlighted 1974 by John W. Gerrard, a Canadian pediatrician, proteins in human milk with immunologically protective functions are also expected to provide protection against disease induction in humans (Gerrard Citation1974). In line with Gerrard's initial suggestion, exclusive breastfeeding for the first six months of life not only reduces the risk of infections, but also of cow's milk allergy and severe allergic symptoms in early childhood compared to infants fed with cow's milk (Harstad and Albers-Prock Citation2009; Chandra Citation1997; Saarinen and Kajosaari Citation1995). Recently, Tuokkola et al. showed that mothers with a high consumption of milk products during pregnancy may protect their children from developing CMA, especially in infants of non-allergic mothers (Tuokkola et al. Citation2016). Complete early weaning of an infant from maternal milk has several health disadvantages for the infant, both short- and long-term (Victora et al. Citation2016). Most disadvantages of completely weaning before 4 to 6 months or the advantages of continued breast- or specialized formula feeding, respectively, have been explained in recent seminal papers (Sakihara et al. Citation2016; Abeshu et al. Citation2016; Fewtrell et al. Citation2017; Lenja et al. Citation2016; Grimshaw et al. Citation2013; Levin et al. Citation2016). In short, they strongly emphasize immunological aspects of breastfeeding linked to the defense against actually harmful bacteria and viruses (Bode Citation2012), which is supported, for example, by compounds that transfer immunologic information from the mother to the offspring infant (Chirico et al. Citation2008; Goldman Citation1993; Goldman and Goldblum Citation1995; Admyre et al. Citation2007; M'Rabet et al. Citation2008). In fact, the United Nations Children's Fund (UNICEF), the World Health Organization (WHO) together with medical authorities like the American Academy of Pediatrics (Yu Citation2011) and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) all emphasize that breastfeeding passes anti-pathogenic benefits to the infant (Agostoni et al. Citation2009). Specific oligosaccharides, found in both human milk as well as specialized infant formula, also possess the potential to bind to specific pathogens, thereby preventing them to enter the infant's body (Koning et al. Citation2015).
Human milk protein as allergens
An allergen can be described as a substance, protein or non-protein, capable of inducing an allergy or specific hypersensitivity. Almost any substance in the external environment can act as an allergen. The list of known allergens includes plant pollens, spores of mold, animal dander, house dust, foods, feathers, dyes, soaps, detergents, cosmetics, plastics, and drugs” (Thefreedictionary Citation2017).
It is true that human milk contains proteins or their remnants, which fall within above description; however, the terms ‘proteins in human milk’ and ‘milk allergen’ are neither mutually exclusive nor synonymous as some worried mother may wrongly understand from reading the dictionary. It may be consoling mothers to inform them about a number of additional insights about the allergenic potential of milk proteins. For instance, that any allergic response towards a protein, including human milk proteins, is mainly due to the presence of very specific amino acids sequences (Bannon Citation2004). Proteins bearing such sequences with allergenic potential in human milk seem to be in minority amongst the >2600 different recorded protein species (van Herwijnen et al. Citation2016). They seem to be almost exclusively minor milk proteins that are not synthesized in the mammary gland or any other part of the human body, but do indeed directly originate from the maternal diet (van Herwijnen et al. Citation2016). Although the presence of maternal dietary proteins in human milk was already described many years ago (Shannon Citation1921; Cant et al. Citation1985), the low-abundant proteins or derived peptides might have been missed with the use of classical protein-detection techniques like ELISA. This might be due to the fact that proteins recognize a specific sequence of a protein, and when that specific sequence was not present, it would not be detected. With the new proteomics approaches, even low-abundant or cleaved proteins (peptides) are identifiable.
Foreign allergenic proteins can be difficult to distinguish from endogenous human milk proteins. For example, Bertino et al. investigated the presence of the major cow's milk allergens ß-lactoglobulin and casein and found their detection method to be also sensitive to the human milk homologs (Bertino et al. Citation1996). However, the group managed to identify bovine αS1-casein in human colostrum (Coscia et al. Citation2012; Coscia et al. Citation2012). Other food proteins with allergenic potential that have been detected in human milk include hen's egg and peanut proteins (Coscia et al. Citation2012; Troncone et al. Citation1987; Jakobsson Citation1989; Cant et al. Citation1985; Vadas et al. Citation2001). It is, however, essential to realize that endogenous human milk proteins are present in the mg/ml range, whereas most maternal diet-derived food antigens are detected at concentrations a 1000-fold lower (Macchiaverni et al. Citation2014). It can be hypothesized that, like seen in immunotherapy approaches (Hussey Freeland et al. Citation2016), the initial exposure with low dosages that over time increase, will result in a tolerogenic response instead of the onset of an allergic response. Cases of allergic manifestations towards endogenous human milk proteins, purportedly through cross-reactivity with structurally similar cow's milk proteins, have been reported, but seem to be excessively rare (Jarvinen et al. Citation2012; Makinen-Kiljunen and Plosila Citation2004).
As a concrete example of maternal dietary protein transportation to milk, it has been shown that the consumption of one egg per day leads to higher concentrations of the chicken egg allergen ovalbumin (OVA) in human milk compared to egg-avoiding mothers (Palmer et al. Citation2008). However, up to 25% of the egg-consuming mothers had a delayed or even absent secretion of OVA to the human milk (Palmer et al. Citation2008; Palmer et al. Citation2005). It is still unclear whether this is a specific phenomenon for this particular allergen, or whether this is the case for more allergens in these ‘non-transporting’ and/or ‘non-processing’ mothers. Only a few research groups are working on the mechanism by which proteins are transported from the gastro-intestinal tract of the mother to her milk produced in the breast tissue. One of these groups showed that dephosphorylation of OVA reduces the passage through an intestinal epithelial Caco-2 cell monolayer (Matsubara et al. Citation2013). This means that specific enzymatic processes in the gastro-intestinal tract of the mother can change the fate of a protein: if the protein is not dephosphorylated, it will likely pass the gut-barrier and could head towards the milk, whereas a dephosphorylated protein will not be able to pass the barrier and will not be present in the milk. These differences in protein-processing might give a clue what determines the protein composition of the milk. In addition, these processes might differ between allergic and non-allergic mothers. Indeed, Hettinga et al. have shown that the milks of allergic and non-allergic mothers significantly differed in the composition of the protease inhibitors (Hettinga et al. Citation2015). It is to be expected that the contribution of protease inhibitors in human milk to the overall processing and absorption of proteins in the infant will be further investigated in the near future, as also described by Dallas et al. (Dallas et al. Citation2015).
Tolerance induction versus allergy development
In order to discuss the (mainly) preclinical data that is available on allergenic potential of proteins, the difference between the onset of an allergic type I response and the induction of oral tolerance needs to be given. The immunological process begins with an initial exposure. The food protein enters the body and is detected by immune cells. Antigen-presenting cells, such as dendritic cells, take up the protein antigen and degrade it. Even epithelial cells can act in processing and presenting of antigens as non-professional APC (e.g. in EoE (Mulder et al. Citation2011)). It then transports one of the fragments to the surface and presents it to a T-cell. There, two scenarios can take place: 1) Tolerance is the usual default response to an allergenic stimulus from the first days of life (Sansotta et al. Citation2013). The connection between dendritic cell and T cell initiates the secretion of anti-inflammatory cytokines as well as the transformation of the T cell into a regulatory T cell (Treg). Tolerance can be induced via several different mechanisms in direct interaction with both the T cell and the B cell. This way, the immune system has decided that this food protein can be tolerated which does not lead to allergic responses upon re-exposure and allergy is prevented. Oral tolerance can thus be defined as a lack of reactivity to an antigen/allergen or to a permanent immunologic state in which infrequent and repeated antigen exposures do not result in an allergic reaction; 2) In allergy development, the connection between a dendritic cell and a T cell within a non-tolerogenic environment initiates the secretion of pro-inflammatory mediators called cytokines as well as the transformation of the T cell into a T-helper 2 (Th2) cell. The Th2 cell then presents the protein fragment to a B cell. Together, these actions activate the B cell triggering a cascade resulting in the production of antibodies called antigen-specific immunoglobulins type E or G. Upon re-exposure with the food protein, IgE and IgG stimulate the mast cell to release predominantly histamine. This complicated cascade of events leads to allergic symptoms (See ).
Figure 1. Dependent on the composition of the maternal diet and the processing of the dietary proteins in the gastro-intestinal tract, specific proteins and/or peptides will be transported to the mother's milk. These proteins and/or peptides are combined with other immunomodulatory components like oligosaccharides in the milk. These factors together determine whether an allergic or tolerogenic response will develop within the infant.
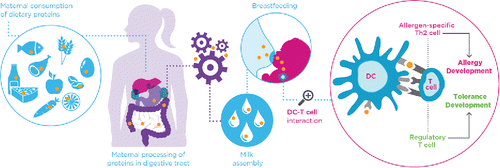
Effects of maternal dietary allergens through breastfeeding
The acquisition of antigen-specific tolerance of the offspring towards OVA-exposure via breast milk is described in mice (Verhasselt et al. Citation2008). This OVA-specific tolerance induction is attributed to the combined exposure to allergen and transforming growth factor-β (TGF-β) present in human milk, leading to the allergy-preventing development of Tregs in the offspring (Verhasselt Citation2010; Verhasselt Citation2010; Verhasselt Citation2010). Since these findings were done in healthy, non-allergic mice, the same group investigated whether similar results could be found in allergic murine mothers. They showed that breastfeeding by antigen-exposed sensitized mothers abolished asthma development in the offspring. However, in comparison to the allergy-preventing development of Tregs in non-allergic mice, protection conferred by sensitized mothers was more effective, and did not require the presence of TGF-β in the milk (Mosconi et al. Citation2010). When investigating the mechanism further, they discovered that antigen-IgG complexes present in milk were effectively transferred to the breastfed offspring through a specific receptor called FcRn which subsequently induced active tolerance (Mosconi et al. Citation2010). Furthermore, Fusaro et al. showed that prenatal murine exposure to allergens can both lead to tolerance and sensitization of the offspring, depending on the timing and amount of allergen administration (Fusaro et al. Citation2009). This means that the way a protein is processed by the mother and the way it is combined with other proteins present in milk determine whether a tolerogenic or allergic response will be promoted. A better understanding of which combinations of presented proteins and immunomodulating compounds in human milk are leading towards tolerance induction are required to better help health care professionals to judge whether an infant will be at risk of the development of allergy in the future.
In addition to the differences in the immunological path that is followed within the allergic compared to the non-allergic mothers, there are possibly also differences in the types of allergens the different individuals are exposed to. Besides cow's milk proteins, also hen's egg, wheat, soy, peanut, tree nut, fish and shell fish are major allergens reported by the European Academy of Allergy and Clinical Immunology (EAACI Citation2016). In contrast, the major allergens recently reported in China are shrimp, crab, mango, cow's milk and hen's egg (Zeng et al. Citation2015). Certain specific foods consumed mainly in the Asian region have resulted in allergies that are unique to their respective populations. Allergy to edible bird's nest from swiftlets has been described in the Chinese population in Asia (Lee et al. Citation2008). It is the most common cause of anaphylaxis in Singapore children. As peanuts are one of the most common and severe food allergens, much research has been performed on the allergenic properties of peanuts. From this work, it has become clear that not only the type of food, but also how it is prepared or manufactured plays a key role in its allergenicity. For example, Park et al. investigated whether the lower prevalence of peanut allergy in Korea compared to the USA could be explained by a difference in allergenic components in either raw or roasted peanuts originating from Korea or the USA. They concluded that, based on molecular mass and immunological assays, there were no differences between the allergenic components (Park et al. Citation2000). Mondoulet et al. later showed that there is no difference between the IgE-reactivity to whole peanut extract from raw or roasted peanuts, and the decreased allergenicity of boiled peanuts was mainly attributed to the loss of the low-molecular-weight allergens that diffused into the boiling water while cooking (Mondoulet et al. Citation2005). Other aspects that have been described to alter the allergenicity of allergens are glycation (Johnson et al. Citation2016), aggregation (Vissers et al. Citation2011) or dosing (Arps et al. Citation1998). Taken together, this implies that mothers from different geographical locations and/or different food cultures will have a different pattern of dietary proteins in their milk. In addition, different aspects of food-preparation can even interfere with each other, making the allergenic potential variable in different settings. This also suggests that we have to be cautious when extrapolating data regarding allergens from one setting to another, without cross-checking the allergenic potential of a protein or food source in each of the settings.
Exclusion diets are not an effective preventive measure
Generally, alimentary strategies for primary prevention of allergic diseases through allergen avoidance have not yielded the expected drop in allergy prevalence (Wahn Citation2013) and are therefore reconsidered in the past decade (Longo et al. Citation2013).
Recent reviews on the evidence for maternal allergen-avoidance during pregnancy, lactation or both to reduce atopic diseases in their infants conclude that allergen avoidance during pregnancy is unlikely to reduce atopic disease in the offspring, whereas avoidance during lactation might reduce eczema when the mother is classified as high-risk (Kramer and Kakuma Citation2012; Greer et al. Citation2008). Even when mothers are following a strict dietary avoidance regime for e.g. eggs, this is still not a guarantee that the infant will not be exposed to that allergen. This might be due to rare unintended ingestion of the protein by the mother, but might also be the result of environmental exposure through the mother's skin as was shown in severe eczema subjects (Vance et al. Citation2005). In addition, it is important to realize that the decision to exclude a broad range of food items from the diet of the expecting and nursing mother for longer periods can also pose a problem for the mother's physiological status (Vandenplas et al. Citation2007; Polloni et al. Citation2013). Moreover, nutrient deficiencies and energy imbalance can contribute to an emotional crisis that prompts mothers to abandon breastfeeding much earlier than originally intended.
This insight has been taken into account in the latest guidelines of the relevant medical societies in the United States, Australasia, and Europe. As a preventive measure, restrictions on consuming food with allergenic potential, including dairy products during pregnancy or lactation, are not recommended, also not regarding infants considered at risk, because of a family history of allergy (Greer et al. Citation2008; Boyce et al. Citation2010; Prescott et al. Citation2008; Host et al. Citation2008). Instead, the current recommendation for the long-term prevention of food allergy in a breastfed infant is to refrain from feeding complementary foods or drinks before 4 to 6 months of age and to continue breastfeeding while introducing complementary food like cow's milk after 4–6 months (Smith and Becker Citation2016). However, in cases of a proven food allergy in the breastfed infant the elimination of the identified food item(s) from the maternal diet is usually recommended for short-term relief of symptoms of the infant (Academy of Breastfeeding Medicine Citation2011; Eigenmann et al. Citation2013; Isolauri et al. Citation1999).
Taken together, there is no solid evidence that allergic disease can be prevented through the exclusion of dietary allergenic proteins during lactation. However, Martin-munoz et al. studied infants that developed allergic symptoms at the time they were breastfed, suggesting they were already sensitized in utero. They showed that peanut, chicken egg and/or cow's milk were demonstrated as the hidden responsible allergens. None of the infants returned to develop symptoms once mother started a diet free of the foods that evoked the allergic response in the child. Interestingly, three out of the five infants tolerated other food allergens identified in their human milk, suggesting that not all proteins with allergenic potential result in sensitization in these children (Martin-Munoz et al. Citation2016).
All these different aspects make it difficult to predict which allergen will be consumed by which mother, and which of the processing-induced alterations might in turn affect how the allergen is processed inside the mothers' body and eventually her milk. It therefore remains crucial to consider whether the presence of proteins of maternal dietary origin in human milk poses a risk for the breastfed infant or may instead be of positive or neutral value. For now, this consideration is dependent on the judgment of the medical specialist. Acquisition of immune competence whether regarding mounting an appropriate defense against threatening foreign compounds or tolerating harmless food substances at least requires prior exposure (Bannon Citation2004). Hopefully, in the future, more mechanistic approaches will give a better insight into the ways how allergenic proteins are recognized and/or processed differently by atopic compared to non-atopic individuals. In addition, there are at least two more possible routes for allergen exposure of a newborn: via ingestion of allergen-containing amniotic fluid into the gastrointestinal (GI) tract of the fetus, and via direct transfer of across the placenta (Tuokkola et al. Citation2016; Palmer et al. Citation2008; Verhasselt et al. Citation2008; Verhasselt Citation2010; Verhasselt Citation2010; Verhasselt Citation2010).
In utero-exposure to maternal dietary allergens
The fetus is exposed to allergens in utero and can mount an antigen-specific IgE response. Intrauterine allergen exposure is probably very common, especially for dietary allergens, as a large proportion of neonates have positive proliferative responses to allergens such as cow's milk protein (Szepfalusi et al. Citation1997), peanut protein (DesRoches et al. Citation2010; Frank et al. Citation1999), ovalbumin (Vance et al. Citation2005; Edelbauer et al. Citation2004; Edelbauer et al. Citation2003; Szepfalusi et al. Citation2006) and apple protein (Abelius et al. Citation2014), indicating their immune system has already been activated by these proteins before. The most commonly postulated pathway for allergen exposure of the fetus is transplacental, probably by IgG/allergen immune complex transfer from the mother to the fetus. Whilst IgG antibodies are transferred from the mother to the child via the placenta, allergen-specific IgE antibodies cannot cross the placenta barrier (Thornton and Vance Citation2002; Flicker et al. Citation2009; Szepfalusi et al. Citation2000). Another suggested route is the transamniotic route (i.e. by ingesting the allergen – maybe bound to maternal IgE – with the amniotic fluid) (Holloway et al. Citation2000).
Braegger et al. published a review on the ontogenetic aspects of the human intestinal immune system, explaining that newborns already have a functioning immune system that becomes apparent around week 16 of gestation. In this period, clusters of sub-epithelial lymphoid cells (albeit without cellular zonation) are visible by light microscopy. At the same gestational age, predominantly CD4-positive T cells and naïve B cells (double positive for IgM and IgD) are present in the Peyer's patches (Braegger et al. Citation1992). M cells (microfold cells) can be identified in the fetus as early as 17 weeks' gestation (Braegger et al. Citation1992). By 19 weeks' gestation, well-defined Peyer's patches are present and detectable IgM-positive B cells appear in the circulation, indicating an already full functioning sensitization process (Vance et al. Citation2005). Furthermore, the lamina propria contains few lymphocytes at week 11 of gestation, increasing to scattered CD4+ T cells in week 14 and large numbers of T cells in week 20 of gestation. Both B cells and plasma cells are absent in the lamina propria up to 22 weeks' gestation (Braegger et al. Citation1992). In addition, at 22 weeks of gestation, allergen-specific immune responses are detected in cord blood mononuclear cells (Jones et al. Citation1996).
Although the summary above shows that the immune system of the infant is already capable of mounting an immune response at 22 weeks of gestation, it remains open whether this means that the infant can already become allergic during pregnancy. It could also hint at a crucial time window to acquire tolerance to maternal dietary proteins. Multiple studies have shown that manipulation of the maternal immune response by allergen immunization during pregnancy reduces the allergen-specific IgE responses in their offspring after immunization (Hansen et al. Citation2012). There are some indications that immunotherapy during pregnancy might be associated with a favorable immune modulation and some data suggest that changes in allergen-specific IgG antibodies occurring in the mother can influence the atopic status of offspring (Glovsky et al. Citation1991). It has been shown that the circulating maternal antibodies in the offspring may diminish allergen processing and presentation by antigen presenting cells to T cells, thereby preventing neonatal sensitization (Jenmalm and Bjorksten Citation2000; Siegrist Citation2003). Furthermore, maternal immunization up-regulates the inhibitory IgG receptor FcγRIIb on neonatal B cells in early life. If thereafter maternal antibodies and the specific allergen form a complex, these complexes will cross-link the FcγRIIb, leading to B cell inhibition (Victor et al. Citation2010).
Thus, maternal dietary allergens are detected in both maternal and infants' blood directly after birth and there are indications that the fetus may be exposed to dietary allergens from the second trimester of pregnancy onwards through both trans-amniotic and trans-placental routes (Vance et al. Citation2005). Although these studies do not exclude that the effects are solely described to the prenatal or the postnatal period, it is suggested that postnatal mucosal allergen exposure could induce allergen-specific tolerance as the newborns' immune system is still maturing. However, there is still much debate about this early life introduction of allergens to prevent allergy.
Breastfeeding while introducing complimentary foods
In a similar trend to that observed in the elimination of allergens from the maternal diet during pregnancy and lactation, discussions remain ongoing in literature about when to start to introduce solid foods, in which order and in which format. In 2013, Sansotta et al. make a stance for changing the weaning practices from after the 4th or 6th month towards starting at the 17th week with weaning, and introducing almost all foods before the 27th week of life. This proposal was already given in literature, as there was no evidence that the delayed introduction of solid products after more than 4–6 months prevented the risk of allergies. Early total weaning (so no maternal milk at all anymore) has several health disadvantages for the infant, both short- and long-term (Victora et al. Citation2016). Most disadvantages of completely weaning an infant from maternal milk before 4 to 6 months or the advantages of continued breastfeeding, respectively, have been explained in recent seminal papers (Grimshaw et al. Citation2013; Levin et al. Citation2016). These papers strongly emphasize that continuing breastfeeding while introducing small amounts of solid foods provides a favorable setting. In addition, complementary breast feeding provides immunological aspects of breastfeeding linked to the defense against actually harmful bacteria and viruses (Bode Citation2012), which is supported, for example, by compounds that transfer immunologic information from the mother to the offspring (Chirico et al. Citation2008; Goldman Citation1993; Goldman and Goldblum Citation1995; Admyre et al. Citation2007; M'Rabet et al. Citation2008).
In addition, the more recent LEAP study showed that there was a 70 to 86% relative reduction in the incidence of peanut allergy in high-risk infants when peanut was introduced between 4 and 11 months of age (Du Toit et al. Citation2015). These outcomes are in line with earlier statements that the development of immune tolerance is a critical process in early life and that both too early or too late introduction of allergens will lead to adverse effects (Sansotta et al. Citation2013). However, Rabinovitch et al. warrant caution with implementing this early exposure to children between 4 to 11 months, as serious and potentially life-threatening events have already been reported (Rabinovitch et al. Citation2015). Recently, Turati et al. found that early weaning, defined as the introduction of solid foods at 4 or 5 months of age, was inversely related to the risk of AD compared to those exclusively breastfed and confirms recent results indicating a beneficial role of early weaning on AD (Turati et al. Citation2016). In an additional study, it was shown that early introduction, before 6 months of age, of at least some amount of multiple allergenic foods appears achievable and did not affect the quality of breastfeeding (Perkin et al. Citation2016). This new insight in the potential of early introduction of specific dietary proteins, without endangering the breast feeding rate and duration, has important implications for the evaluation of food allergy prevention strategies. Furthermore, the group of Hayashi showed that early introduction of cow's milk formula is associated with lower incidence of IgE-CMA when comparing 51 patients with IgE-mediated CMA with 102 healthy controls and 32 unmatched patients with IgE-mediated hen's egg allergy (Onizawa et al. Citation2016).
Summary
The dietary protein intake during pregnancy and lactation does not only fulfill the need for nourishment, but also provides functional proteins that help the developing infant to train its body to flourish in a world full of immunological challenges. The avoidance strategies have not resulted in a decrease in allergic manifestations, and insights into the mechanisms involved in tolerance induction even warrant controlled exposure to potential allergens in order to train the immune system. With this paper, we hope to have shown that breast feeding provides much more than only the potential allergenic proteins, providing a better setting to have the proper immune training in infants. Furthermore, the introduction of solid foods might also benefit from continued breast feeding while introducing, as again the immunological context provided by breast milk might potentiate the tolerogenic outcome and attenuate the potential allergenicity of the combined exposure.
References
- Academy of Breastfeeding Medicine. 2011. ABM Clinical Protocol #24: Allergic Proctocolitis in the Exclusively Breastfed Infant. Breastfeed Medicine 6 (6):435–40.
- Abelius, M. S., et al. 2014. Placental immune response to apple allergen in allergic mothers. Journal of Reproductive Immunology.
- Abeshu, M. A., A. Lelisa, and B. Geleta. 2016. Complementary Feeding: Review of Recommendations, Feeding Practices, and Adequacy of Homemade Complementary Food Preparations in Developing Countries – Lessons from Ethiopia. Frontiers in Nutrition 3:41.
- Admyre, C., et al. 2007. Exosomes with immune modulatory features are present in human breast milk. Journal of Immunology 179 (3):1969–78.
- Agostoni, C., et al. 2009. Breast-feeding: A commentary by the ESPGHAN Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 49 (1):112–25.
- Arps, V., S. Sudowe, and E. Kolsch. 1998. Antigen dose-dependent differences in IgE antibody production are not due to polarization towards Th1 and Th2 cell subsets. European Journal of Immunology 28 (2):681–6.
- Bannon, G. A. 2004. What makes a food protein an allergen? Current Allergy and Asthma Reports 4 (1):43–6.
- Berin, M. C., and H. A. Sampson. 2013. Food allergy: an enigmatic epidemic. Trends in Immunology 34 (8):390–397.
- Bertino, E., et al. 1996. Human milk proteins may interfere in ELISA measurements of bovine beta-lactoglobulin in human milk. Acta Paediatrica 85 (5):543–9.
- Bode, L. 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22 (9):1147–62.
- Boyce, J. A., et al. 2010. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. Journal of Allergy and Clinical Immunology 126 (6 Suppl):S1–58.
- Braegger, C. P., J. Spencer, and T. T. MacDonald. 1992. Ontogenetic aspects of the intestinal immune system in man. International Journal of Clinical and Laboratory Research 22 (1):1–4.
- Calderon, M. A., et al. 2012. EAACI: A European Declaration on Immunotherapy. Designing the future of allergen specific immunotherapy. Clinical and Translational Allergy 2:20–20.
- Cant, A. J., J. A. Bailes, and R. A. Marsden. 1985. Cow's milk, soya milk and goat's milk in a mother's diet causing eczema and diarrhoea in her breast fed infant. Acta Paediatrica Scandinavica 74 (3):467–8.
- Cant, A., R. A. Marsden, and P. J. Kilshaw. 1985. Egg and cows' milk hypersensitivity in exclusively breast fed infants with eczema, and detection of egg protein in breast milk. BMJ 291 (6500):932–935.
- Chandra, R. K. 1997. Five-year follow-up of high-risk infants with family history of allergy who were exclusively breast-fed or fed partial whey hydrolysate, soy, and conventional cow's milk formulas. Journal of Pediatric Gastroenterology and Nutrition 24 (4):380–8.
- Chirico, G., et al. 2008. Antiinfective properties of human milk. Journal of Nutrition 138 (9):1801S–1806S.
- Christian, P. 2014. Fetal growth restriction and preterm as determinants of child growth in the first two years and potential interventions. Nestlé Nutrition Institute Workshop Series 78:81–91.
- Coscia, A., et al. 2012. Cow's milk proteins in human milk. Journal of Biological Regulators and Homeostatic Agents 26 (3 Suppl):39–42.
- Coscia, A., et al. 2012. Detection of cow's milk proteins and minor components in human milk using proteomics techniques. Journal of Maternal-Fetal and Neonatal Medicine 25 (Suppl 4):54–6.
- Dallas, D. C., N. M. Murray, and J. Gan. 2015. Proteolytic Systems in Milk: Perspectives on the Evolutionary Function within the Mammary Gland and the Infant. Journal of Mammary Gland Biology and Neoplasia 20 (3–4):133–47.
- DesRoches, A., et al. 2010. Peanut allergy: is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? Journal of Investigational Allergology and Clinical Immunology 20 (4):289–94.
- Du Toit, G., et al. 2015. Randomized trial of peanut consumption in infants at risk for peanut allergy. New England Journal of Medicine 372 (9):803–13.
- Dupont, C. 2003. Protein requirements during the first year of life. The American Journal of Clinical Nutrition 77 (6):1544S–1549S.
- EFSA NDA Panel. 2014. Scientific opinion on the essential composition of infant and follow-on formulae. EFSA Journal 12 (7):106.
- EAACI. 2016. Food Allergy and Anaphylaxis guidelines. Available from: http://www.eaaci.org/resources/guidelines/faa-guidelines.html.
- Edelbauer, M., et al. 2004. Maternally delivered nutritive allergens in cord blood and in placental tissue of term and preterm neonates. Clinical and Experimental Allergy 34 (2):189–93.
- Edelbauer, M., et al. 2003. Dose-dependent and preterm- accentuated diaplacental transport of nutritive allergens in vitro. International Archives of Allergy and Immunology 130 (1):25–32.
- Eigenmann, P. A., et al. 2013. Testing children for allergies: why, how, who and when: an updated statement of the European Academy of Allergy and Clinical Immunology (EAACI) Section on Pediatrics and the EAACI-Clemens von Pirquet Foundation. Pediatric Allergy and Immunology 24 (2):195–209.
- Ellison, R. T., T. J. Giehl 3rd, and F. M. LaForce. 1988. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infection and Immunity 56 (11):2774–81.
- Fewtrell, M., et al. 2017. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 64 (1):119–132.
- Field, C. J. 2005. The immunological components of human milk and their effect on immune development in infants. Journal of Nutrition 135 (1):1–4.
- Flicker, S., et al. 2009. Placental transfer of allergen-specific IgG but not IgE from a specific immunotherapy-treated mother. Journal of Allergy and Clinical Immunology 124 (6):1358–60.e1.
- Forster, D. A., and M.H. L. 2010. Women's views and experiences of breast feeding: positive, negative or just good for the baby? Midwifery 26 (1):116–125.
- Frank, L., et al. 1999. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatric Allergy and Immunology 10 (1):27–32.
- Fusaro, A. E., et al. 2009. Balance between early life tolerance and sensitization in allergy: dependence on the timing and intensity of prenatal and postnatal allergen exposure of the mother. Immunology 128 (1 Suppl):e541–50.
- Gerrard, J. W. 1974. Breast-Feeding: Second Thoughts. Pediatrics 54 (6):757–764.
- Glovsky, M. M., L. Ghekiere, and E. Rejzek. 1991. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Annals of Allergy 67 (1):21–4.
- Goldman, A. S. 1993. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J 12 (8):664–71.
- Goldman, A. S., and R. M. Goldblum. 1995. Defence agents in human milk, in Handbook of milk composition, R. Jensen. Editor. San Diego (CA), USA: Academic Press. p. 727–45.
- Greer, F. R., S. H. Sicherer, and A. W. Burks. 2008. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 121 (1):183–91.
- Grimshaw, K. E., et al. 2013. Introduction of complementary foods and the relationship to food allergy. Pediatrics 132 (6):e1529–38.
- Hanafy, M. M., et al. 1972. Maternal nutrition and lactation performance: a study in urban Alexandria. J Trop Pediatr Environ Child Health 18 (3):187–91.
- Hansen, J. S., et al. 2012. Early life interventions to prevent allergy in the offspring: the role of maternal immunization and postnatal mucosal allergen exposure. International Archives of Allergy and Immunology 158 (3):261–75.
- Harstad, E., and L. Albers-Prock. 2009. Caring for your baby and Young child: Birth to Age 5, ed. A.A.o. Pediatrics. : Bantam Books. 892.
- Hettinga, K. A., et al. 2015. Difference in the breast milk proteome between allergic and non-allergic mothers. PLoS One 10 (3):e0122234.
- Holloway, J. A., et al. 2000. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet 356 (9245):1900–2.
- Host, A., et al. 2008. Dietary prevention of allergic diseases in infants and small children. Pediatric Allergy and Immunology 19 (1):1–4.
- Hussey Freeland, D. M., et al. 2016. Advances in food allergy oral immunotherapy: toward tolerance. Curr Opin Immunol 42:119–123.
- Institute-of-Medicine. 2017. Dietary Reference Intakes: Recommended Dietary Allowances and Adequate Intakes. Available from: www.nap.edu.
- Islam, S. N., et al. 2006. Immune components (IgA, IgM, IgG, immune cells) of colostrum of Bangladeshi mothers. Pediatrics International 48 (6):543–548.
- Isolauri, E., et al. 1999. Breast-feeding of allergic infants. J Pediatr 134 (1):27–32.
- Jackson, K., L. Howie, and L. Akinbami. 2013. Trends in allergic conditions among children: United States, 1997–2011. NCHS data brief, no 121. 2013. Hyattsville (MD, U.S.A.): National Center for Health Statistics.
- Jakobsson, I. 1989. Food Antigens in Human Milk, in Food Allergy in Infancy and Childhood, H. K. Harms and U. Wahn. Editors. Berlin Heidelberg: Springer. 47–52.
- Jarvinen, K. M., et al. 2012. Presence of functional, autoreactive human milk-specific IgE in infants with cow's milk allergy. Clinical and Experimental Allergy 42 (2):238–47.
- Jenmalm, M. C., and B. Bjorksten. 2000. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clinical and Experimental Allergy 30 (1):34–40.
- Johnson, K. L., et al. 2016. Enhanced Approaches for Identifying Amadori Products: Application to Peanut Allergens. J Agric Food Chem 64 (6):1406–13.
- Jones, A. C., et al. 1996. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatric Allergy and Immunology 7 (3):109–16.
- Koning, N., et al. 2015. Human Milk Blocks DC-SIGN-Pathogen Interaction via MUC1. Front Immunol 6:112.
- Kramer, M. S., and R. Kakuma. 2012. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev 9:CD000133.
- Lee, B. W., et al. 2008. Food allergy-lessons from Asia. World Allergy Organ J 1 (7):129–33.
- Lenja, A., et al. 2016. Determinants of exclusive breastfeeding practice to infants aged less than six months in Offa district, Southern Ethiopia: a cross-sectional study. Int Breastfeed J 11:32.
- Levin, M., et al. 2016. Allergy and infant feeding guidelines in the context of resource-constrained settings. Journal of Allergy and Clinical Immunology
- Longo, G., et al. 2013. IgE-mediated food allergy in children. Lancet 382 (9905):1656–64.
- Lonnerdal, B. 2004. Human milk proteins: key components for the biological activity of human milk. Adv Exp Med Biol 554:11–25.
- M'Rabet, L., et al. 2008. Breast-Feeding and Its Role in Early Development of the Immune System in Infants: Consequences for Health Later in Life. The Journal of Nutrition 138 (9):1782S–1790S.
- Macchiaverni, P., et al. 2014. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 69 (3):395–8.
- Makinen-Kiljunen, S., and M. Plosila. 2004. A father's IgE-mediated contact urticaria from mother's milk. Journal of Allergy and Clinical Immunology 113 (2):353–4.
- Martin-Munoz, M. F., et al. 2016. Food allergy in breastfeeding babies. Hidden allergens in human milk. European Annals of Allergy Clinical Immunology 48 (4):123–8.
- Matsubara, T., et al. 2013. Dephosphorylation reduces passage of ovalbumin antigen through intestinal epithelial Caco-2 cell monolayers. Journal of Biochemistry 153 (4):347–54.
- Mondoulet, L., et al. 2005. Influence of thermal processing on the allergenicity of peanut proteins. Journal of Agricultural and Food Chemistry 53 (11):4547–53.
- Mosconi, E., et al. 2010. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunology 3 (5):461–74.
- Mulder, D. J., et al. 2011. Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis. American Journal of Pathology 178 (2):744–53.
- Newburg, D. S. 2005. Innate immunity and human milk. Journal of Nutrition 135 (5):1308–12.
- Nwaru, B. I., et al. 2014. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy 69 (1):62–75.
- Onizawa, Y., et al. 2016. The Association of the Delayed Introduction of Cow's Milk with IgE-Mediated Cow's Milk Allergies. Journal of Allergy and Clinical Immunology Pract 4 (3):481–488 e2.
- Palmer, D. J., M. S. Gold, and M. Makrides. 2008. Effect of maternal egg consumption on breast milk ovalbumin concentration. Clinical and Experimental Allergy 38 (7):1186–91.
- Palmer, D. J., M. S. Gold, and M. Makrides. 2005. Effect of cooked and raw egg consumption on ovalbumin content of human milk: a randomized, double-blind, cross-over trial. Clinical and Experimental Allergy 35 (2):173–8.
- Park, C. W., G. I. Kim, and C. H. Lee. 2000. A comparison study on allergen components between Korean (Arachis fastigiata Shinpung) and American peanut (Arachis hypogaea Runner). Journal of Korean Medical Science 15 (4):387–92.
- Perkin, M. R., et al. 2016. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. Journal of Allergy and Clinical Immunology 137 (5):1477–1486 e8.
- Polloni, L., et al. 2013. Nutritional behavior and attitudes in food allergic children and their mothers. Clinical and Translational Allergy 3 (1):41.
- Praticò, A. D., and S. Leonardi. 2015. Immunotherapy for food allergies: A myth or a reality? Immunotherapy 7 (2):147–161.
- Prescott, S. L., et al. 2008. The importance of early complementary feeding in the development of oral tolerance: concerns and controversies. Pediatric Allergy and Immunology 19 (5):375–80.
- Rabinovitch, N., D. Shah, and B. J. Lanser. 2015. Look before you LEAP: Risk of anaphylaxis in high-risk infants with early introduction of peanut. Journal of Allergy and Clinical Immunology 136 (3):822.
- Saarinen, U. M., and M. Kajosaari. 1995. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet 346 (8982):1065–9.
- Sakaguchi, S., et al. 2008. Regulatory T cells and immune tolerance. Cell 133 (5):775–87.
- Sakihara, T., S. Sugiura, and K. Ito. 2016. The ingestion of cow's milk formula in the first 3 months of life prevents the development of cow's milk allergy. Asia Pacific Allergy 6 (4):207–212.
- Sansotta, N., et al. 2013. Timing of introduction of solid food and risk of allergic disease development: understanding the evidence. Allergologia Et Immunopathologia (Madr) 41 (5):337–45.
- Shannon, W. 1921. Demonstration of food proteins in human breast milk by anaphylactic experiments on guinea-pigs: Their probable relationship to certain diseases of the nursing infant a preliminary report. American Journal of Diseases of Children 22 (3):223–231.
- Siegrist, C. A. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21 (24):3406–12.
- Smith, H. A., and G. E. Becker. 2016. Early additional food and fluids for healthy breastfed full-term infants. Cochrane Database Systematic Reviews (8):CD006462.
- Snyder, W., et al. 1975. Report of the Task Group on Reference Man. Oxford (United Kingdom): Pergamon Press, 1975.
- Stevens, B., et al. 2015. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: a systematic review and meta-analysis. Maternal & Child Nutrition 11 (4):415–432.
- Szepfalusi, Z., et al. 1997. Prenatal allergen contact with milk proteins. Clinical and Experimental Allergy 27 (1):28–35.
- Szepfalusi, Z., et al. 2006. Most of diaplacentally transferred allergen is retained in the placenta. Clinical and Experimental Allergy 36 (9):1130–7.
- Szepfalusi, Z., et al. 2000. Direct evidence for transplacental allergen transfer. Pediatric Research 48 (3):404–7.
- Thefreedictionary. 2017. Available from: www.thefreedictionary.com.
- Thornton, C. A., and G. H. Vance. 2002. The placenta: a portal of fetal allergen exposure. Clinical and Experimental Allergy 32 (11):1537–9.
- Troncone, R., et al. 1987. Passage of gliadin into human breast milk. Acta Paediatrica Scandinavica 76 (3):453–6.
- Tuokkola, J., et al. 2016. Maternal diet during pregnancy and lactation and cow's milk allergy in offspring. European Journal of Clinical Nutrition 70 (5):554–9.
- Turati, F., et al. 2016. Early weaning is beneficial to prevent atopic dermatitis occurrence in young children. Allergy 71 (6):878–88.
- Vadas, P., et al. 2001. DEtection of peanut allergens in breast milk of lactating women. JAMA 285 (13):1746–1748.
- van Herwijnen, M. J. C., et al. 2016. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Molecular & Cellular Proteomics 15 (11):3412–3423.
- Vance, G. H., et al. 2005. Exposure of the fetus and infant to hens' egg ovalbumin via the placenta and breast milk in relation to maternal intake of dietary egg. Clinical and Experimental Allergy 35 (10):1318–26.
- Vandenplas, Y., et al. 2007. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Archives of Disease in Childhood 92 (10):902–908.
- Verhasselt, V. 2010. Neonatal tolerance under breastfeeding influence. Current Opinion in Immunology 22 (5):623–630.
- Verhasselt, V., et al. 2008. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Naturalium Et Medicinalium 14 (2):170–5.
- Verhasselt, V. 2010. Neonatal tolerance under breastfeeding influence. Current Opinion in Immunology 22 (5):623–30.
- Verhasselt, V. 2010. Oral tolerance in neonates: from basics to potential prevention of allergic disease. Mucosal Immunology 3 (4):326–33.
- Verhasselt, V. 2010. Neonatal tolerance under breastfeeding influence: the presence of allergen and transforming growth factor-beta in breast milk protects the progeny from allergic asthma. Journal of Pediatrics 156 (2 Suppl):S16–20.
- Victor, J. R., et al. 2010. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunology 11:11.
- Victora, C. G., et al. 2016. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387 (10017):475–90.
- Vissers, Y. M., et al. 2011. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS One 6 (8):e23998.
- Wahn, U. 2013. Considering 25 years of research on allergy prevention–have we let ourselves down? Pediatric Allergy and Immunology 24 (4):308–10.
- Yu, W. 2011. New Mother's Guide to Breastfeeding, ed. J. Y. Meek. 272.
- Zeng, G. Q., et al. 2015. Food allergy and related risk factors in 2540 preschool children: an epidemiological survey in Guangdong Province, southern China. World Journal of Pediatrics 11 (3):219–25.
