Abstract
The concept of a ‘recommended dietary allowance’ (RDA) and similar terms describing the daily intake of essential nutrients recommended for healthy individuals is widely used by various health authorities around the world. For vitamin C, however, there remain significant discrepancies in the criteria used to establish dietary recommendations and consequently, global recommendations for daily vitamin C intake vary by more than five fold. While it appears that the scientific data underlying the recommendations are more or less the same, the interpretation differs considerably. Moreover, although a number of the assumptions used in e.g. the body pool estimates of the 1960s and 1970s have later been proven wrong and give rise to significant underestimations, these data are still used as the main support of several recommendations. Aspects that modify vitamin C requirements, such as gender, age, pregnancy, lactation, and smoking, have been taken into consideration by many but not all regulatory authorities, and are thus subject of debate. In contrast, body weight, a significant predictor of vitamin C status and requirement, has not been taken into consideration with respect to vitamin C recommendations, even in the face of the looming global obesity pandemic. The present review examines the discrepancies in vitamin C dietary recommendations of international authorities and critically discusses representative examples of criteria and the underlying health perspectives used to derive current recommended intakes of vitamin C. New biological signatures of vitamin C nutriture are also explored with regard to their potential use for future updates of dietary recommendations.
Introduction
The human need for vitamin C to prevent the ultimately fatal disease scurvy has been undisputed for almost a century. Nevertheless, the actual amount necessary for optimal health remains widely debated, and various arguments and approaches have been adopted by authorities around the world leading to very different recommended daily intakes depending on country, region and organization. This diversity of guidelines ranging from 40 to 220 mg vitamin C per day may be surprising at first, since the actual data underlying and presumably supporting the various recommendations are indeed more or less the same. However, one major explanation for the differences can be identified by looking more closely at the health perspectives forming the basis for the various recommendations. These can be summarized into four main categories: (i) preventing scurvy, (ii) partly saturating immune cells with vitamin C, whilst limiting its urinary excretion, (iii) replacing the daily turnover of vitamin C to maintain ‘adequate’ plasma vitamin C status, and finally (iv) attempting to optimize health of the individual by ingestion of the ‘optimal’ rather than a sufficient amount of vitamin C.
The Food and Nutrition Board of the United States National Academy of Sciences first established RDAs for selected nutrients, including vitamin C, in the early 1940s as a way to ensure superior nutrition for civilians and avoid nutrition problems in connection with National Defense (Food and Nutrition Board of the National Research Council Citation1943; National Academy of Sciences 1989). The RDAs established for vitamin C in 1943 were 70 mg/d for women and 75 mg/d for men (Food and Nutrition Board of the National Research Council Citation1943). The Food and Nutrition Board have subsequently revised the RDAs every five to ten years, with the RDA for vitamin C being decreased to 60 mg/d in 1968 (7th edition) (Sebrell Citation1968; Staff of National Academy of Science Citation1968), decreased again to 45 mg/d in 1974 (8th edition) (Committee on Dietary Allowances Citation1974; Pauling Citation1974), then increased back to 60 mg/d in 1980 and 1989 (9th and 10th editions) (Food and Nutrition Board of the National Research Council Citation1980, National Academy of Sciences Citation1989; Young Citation1996). This value was based on an intake level that would prevent the development of scurvy for about one month on a diet lacking vitamin C (National Academy of Sciences 1989). There are a number of authorities around the world that continue to base their RDA for vitamin C on the prevention of deficiency, including the Food and Agriculture Organization of the World Health Organization (FAO/WHO) (Food and Agriculture Organization Citation2001). As such, the RDA for vitamin C in these countries remains at 40–45 mg/d (National Health and Medical Research Council Citation2006; Dietary reference values for food energy and nutrients for the United Kingdom Citation1991; Indian Council of Medical Research 2010). However, lack of overt deficiency does not necessarily equate to adequacy of intake (Levine et al. Citation1995).
During the most recent revision of the US RDA for the antioxidant nutrients (2000, 11th edition) the Food and Nutrition Board sought to change the criteria for establishing the RDA from prevention of deficiency disease to evaluation of their potential for prevention of chronic disease. Although the RDA for vitamin C was subsequently increased to 75 mg/d for women and 90 mg/d for men (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000), the criteria used were still based on indicators of status rather than health effects. More recently, some health authorities, such as the European Food Safety Authority (EFSA) and Germany, Austria and Switzerland (DACH), modified their criteria for deriving the RDA for vitamin C from prevention of deficiency to maintaining ‘adequate’ plasma vitamin C status and, as such, increased their RDA from 45 mg/d in the 1990s (Scientific Committee for Food Citation1993) to 95 mg/d for women and 110 mg/d for men in 2013 (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013). Other countries have adopted two recommendations, one for prevention of deficiency and a higher recommendation for decreasing non-communicable and chronic disease risk (National Health and Medical Research Council Citation2006; Chinese Nutrition Society Citation2014).
This review outlines the various vitamin C dietary recommendations of international authorities and critically discusses representative examples of criteria and the underlying health perspectives used to derive current recommended intakes of vitamin C.
Vitamin C dietary reference intakes defined
The terminology for ‘recommended dietary allowance’ (RDA) (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000) varies by country and organization, e.g. ‘recommended dietary intake’ (RDI) (National Health and Medical Research Council Citation2006), ‘recommended nutrient intake’ or ‘reference nutrient intake’ (RNI) (Food and Agriculture Organization Citation2001; Dietary reference values for food energy and nutrients for the United Kingdom Citation1991), or ‘population reference intake’ (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013). This has resulted in calls for alignment and harmonization of recommendations (Van't Veer et al. Citation2013; King and Garza Citation2007; International Life Sciences Institute Southeast Asia Region Citation2005). For the purposes of this review, we will use the term RDA. The RDA is defined as the “average daily dietary intake level that is sufficient to meet the nutrient requirements of nearly all (97–98%) healthy individuals in a particular life stage and gender group” () (National Health and Medical Research Council Citation2006; Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). The RDA is derived from the estimated average requirement (EAR), which is defined as the “daily nutrient level estimated to meet the requirements of half the healthy individuals in a particular life stage and gender group” (). If the standard deviation (SD) of the EAR is available and the requirement for the nutrient is symmetrically distributed, the RDA is set at 2SD above the EAR (i.e. RDA = EAR + 2SDEAR). If data about variability in requirements are insufficient to calculate a SD, a coefficient of variation (CV) is used; twice that amount is added to the EAR to derive the RDA (i.e. RDA = EAR + 2CV). An average intake (AI) is used when an RDA cannot be determined, for example, in the case of infants (National Health and Medical Research Council Citation2006). Different criteria have been used by various authorities to determine the EARs for vitamin C, which has resulted in quite different RDAs globally.
Figure 1. EAR = estimated average requirement (meet requirement of 50% healthy individuals); RDA/A = recommended dietary intake (meet requirement of 97.5% healthy individuals); UL = tolerable upper intake level (highest intake likely to pose no adverse health effects). Figure is from (Zimmerman and Snow Citation2012) (CC BY-NC-SA 3.0).
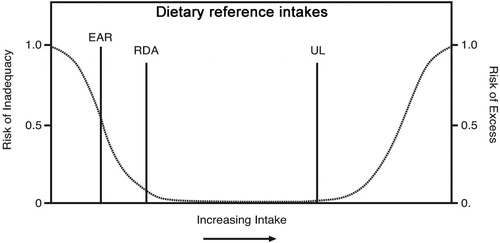
Table 1. Definitions of vitamin C dietary reference intake terminology.
The upper level of intake (UL) is defined as the “highest average daily nutrient intake level likely to pose no adverse health effects to almost all individuals in the general population. As intake increases above the UL, the potential risk of adverse effects increases.” (National Health and Medical Research Council Citation2006; Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Vitamin C has an excellent safety profile, primarily due to its high water solubility and rapid clearance of excess levels by the kidneys. As such, it is not possible to establish an UL for vitamin C. Nevertheless, values of 1000–2000 mg/day have been suggested as prudent limits by some countries, based on a potential risk of osmotic diarrhea and related gastrointestinal disturbances in some people at doses higher than these (National Health and Medical Research Council Citation2006; Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000; International Life Sciences Institute Southeast Asia Region Citation2005; Nutrition Information Centre of the University of Stellenbosch (NICUS) Citation2019; Expert Group on Vitamins and Minerals Citation2003; Scientific Committee on Food Scientific Panel on Dietetic Products Nutrition and Allergies Citation2006). Others have argued that the scientific evidence does not allow for defining an UL for vitamin C (Italian Society of Human Nutrition (SINU) Citation2014; Nordic Council of Ministers Citation2014).
Overview of global recommendations
Overall, the current global RDAs for vitamin C vary by almost three fold between different regions of the world, from 40 mg/d in the UK and India to 110 mg/d in a number of European countries (). Countries and authorities that base their RDAs on the prevention of deficiency, e.g. India, United Kingdom, Australia, New Zealand, and FAO/WHO, have RDAs of 40–45 mg/d. These RDAs are based on an EAR that is defined as “the intake at which body content is halfway between tissue saturation and the point at which clinical signs of scurvy appear” (National Health and Medical Research Council Citation2006; World Health Organization and Food and Agriculture Organization of the United Nations Citation2004). This was assumed to equate to a body content of 900 mg, i.e. halfway between tissue saturation (1500 mg) and the point at which clinical signs of scurvy appear (300–400 mg) (Food and Agriculture Organization Citation2001). However, the theoretical basis for the estimation of these body content values appears to be highly questionable as discussed later. Although the Netherlands also based their RDA on body pool, their recommended intake of 75 mg/d was to ensure maintenance of the total body pool (estimated to be 1500 mg) (Health Council of the Netherlands 2018).
Table 2. Examples of global RDAs for vitamin C.
In contrast, other countries, such as the USA and Canada, considered the potential health effects of vitamin C when deriving the RDAs. Their current RDA (90 mg/d for men and 75 mg/d for women) is based on “an amount of vitamin C that is thought to provide antioxidant protection as derived from correlation of such protection with neutrophil ascorbate concentrations” (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Specifically, the criterion chosen for the EAR is “the vitamin C intake that maintains near-maximal neutrophil concentrations with minimal urinary excretion of ascorbate”. These criteria will be discussed in more detail below. Japan and the Nordic countries have also used the antioxidant effects of vitamin C, as well cardiovascular disease prevention or decreased risk of cardiovascular or cancer mortality and morbidity, resulting in RDAs of 75–100 mg/d (Nordic Council of Ministers Citation2014; Ministry of Health Labour and Welfare Citation2018; Sasaki Citation2008). More recently, EFSA and DACH published new criteria based on an EAR determined from the “quantity of vitamin C that balances metabolic vitamin C losses and allows the maintenance of an adequate body pool characterised by fasting plasma ascorbate concentrations at around 50 μmol/L” (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013; German Nutrition Society Citation2015a). The resultant RDAs for these countries (110 mg/d for men and 95 mg/d for women) are presently among the highest in the world ().
Factors affecting RDAs: gender and age
Epidemiological studies have indicated that women tend to have higher vitamin C status than men, despite lower dietary intakes (Canoy et al. Citation2005; Galan et al. Citation2005; Hampl, Taylor, and Johnston Citation2004). This is thought to be partly due to the larger body and fat-free mass of men compared to women (Jungert and Neuhauser-Berthold Citation2015). The gender difference has been taken into account by some authorities, with lower recommendations for women based on their lower body weight (). The EAR values for children and adolescents are usually extrapolated from adult values and are based on their lower body weight (National Health and Medical Research Council Citation2006; Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000; European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013; German Nutrition Society, Citation2015a). Infants typically have AIs based on estimated vitamin C intakes from breast milk and food (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Some epidemiological research indicates that elderly people may have lower vitamin C status, suggesting higher requirements for the vitamin (Faure et al. Citation2006; Birlouez-Aragon et al. Citation2001; Ravindran et al. Citation2011; Nyyssonen et al. Citation1997; Wrieden et al. Citation2000). Only France (AFSSA) has a higher recommended intake for adults aged 75 years and older (120 mg/d) (Martin Citation2001).
Factors requiring increased intake: pregnancy and lactation
Due to the needs of the developing fetus and the growing infant, vitamin C requirements are enhanced for pregnant and lactating women. The vitamin C status of pregnant women is often lower than non-pregnant women, presumably due to hemodilution and active transfer of vitamin C to the fetus (Juhl et al., Citation2017a). Women with complications of pregnancy can have lower vitamin C status still (Kiondo et al. Citation2012). A significant correlation was recently reported between poor vitamin C status and increased risk of complications in pregnant women with type 1 diabetes (Juhl et al., Citation2017b). Supplementation of pregnant women with vitamin C can potentially decrease the risk of pregnancy-related complications such as premature rupture of membranes (Rumbold et al. Citation2015). It is noteworthy that the recently discovered epigenetic regulatory activities of vitamin C could also have important roles to play in fetal development (Camarena and Wang Citation2016). Most authorities have taken the enhanced needs of pregnant and lactating women into consideration, with recommendations of an additional 10–20 mg/d for pregnant women and an additional 20–60 mg/d for lactating women ().
Table 3. Increased RDAs for pregnancy and lactation.
Factors requiring increased intake: smoking
Smoking is known to increase oxidative stress and enhance the utilization of vitamin C (Lykkesfeldt et al. Citation2000; Marangon et al. Citation1998; Schectman, Byrd, and Gruchow Citation1989; Lykkesfeldt, Viscovich, and Poulsen Citation2004; Lykkesfeldt, Viscovich, and Poulsen Citation2003) and supplementation with vitamin C has been shown to decrease oxidative DNA damage in mononuclear blood cells of smokers (Moller et al. Citation2004). As such, smokers have higher requirements for the vitamin than nonsmokers (Schectman, Byrd, and Hoffmann Citation1991; Kallner, Hartmann, and Hornig Citation1981; Lykkesfeldt and Tveden-Nyborg Citation2019). Moreover, smokers generally have a lower dietary intake of vitamin C further contributing to a poor vitamin C status (Lykkesfeldt et al. Citation1996). Some authorities have considered these factors with higher recommendations for smokers (). For example, the United States and Canada have recommended an additional 35 mg/d vitamin C intake for smokers to compensate for increased oxidative stress and loss of vitamin C (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Several EU countries have recommended intakes of 120–155 mg/day for smokers, comprising additional intakes of 20 to 80 mg/d over the RDA for adults in these countries (Dietary reference values for food energy and nutrients for the United Kingdom Citation1991; Martin Citation2001; German Nutrition Society, Citation2015b). However, it is likely that smokers need substantially more vitamin C than these recommendations (see details below). Although there has been a general trend toward decreased prevalence of smoking globally, some countries continue to show an increased trend, which could potentially impact on their population vitamin C status and requirements (GBD 2015 Tobacco Collaborators Citation2017). Despite this, FAO/WHO has stated that there is no justification for an increased RDA for smokers. In addition, passive exposure to tobacco smoking has been shown repeatedly to lower plasma vitamin C status by about half of that of active smokers (reviewed in (Tveden-Nyborg and Lykkesfeldt Citation2013; Lykkesfeldt Citation2006)). However, so far no authorities have included increased recommendations for passive smokers.
Table 4. Increased RDAs for smokers.
Reexamining selected RDA criteria argumentation
Prevention of scurvy
Scurvy can be effectively prevented with relatively little vitamin C – some have estimated as little as 10 mg/day (Krebs Citation1953; Hodges et al. Citation1971). Although multiple potential health benefits of vitamin C have been suggested in the literature (reviewed in (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000; European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013)), scurvy remains the only globally accepted consequence of severe and prolonged vitamin C deficiency. As scurvy is an ultimately fatal condition, authorities using scurvy prevention as the health target of their recommendation have allowed for a relatively large safety margin with respect to scurvy risk (National Health and Medical Research Council Citation2006; Dietary reference values for food energy and nutrients for the United Kingdom Citation1991; World Health Organization and Food and Agriculture Organization of the United Nations Citation2004; Department of Health Panel on Dietary Reference Values Citation1991). Unfortunately, the specific determinations for the EARs are most likely flawed as they are based on body pool estimations from a time when the complexity of vitamin C pharmacokinetics was not fully understood (discussed below) (Lykkesfeldt and Tveden-Nyborg Citation2019). Using a more appropriate body pool target would in fact result in recommendations similar to that of EFSA and the comparable European countries (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013; German Nutrition Society Citation2015a; Martin Citation2001; German Nutrition Society Citation2015b; Blonk Environmental Consultants Citation2012).
Replacing daily turnover of vitamin C to maintain body Pool
Body pool estimations and turnover of ascorbate have been used as a basis for a number of international RDAs, particularly those based on the prevention of deficiency e.g. UK, AUS/NZ, FAO/WHO (National Health and Medical Research Council Citation2006; Dietary reference values for food energy and nutrients for the United Kingdom Citation1991; World Health Organization and Food and Agriculture Organization of the United Nations Citation2004; Department of Health Panel on Dietary Reference Values Citation1991). These values were determined in a series of studies in the 1960s and 1970s primarily using 14C-labelled ascorbate (Baker et al. Citation1969; Baker et al. Citation1971; Kallner, Hartmann, and Hornig Citation1977; Kallner, Hartmann, and Hornig Citation1979; Tolbert et al. Citation1967). Most of these studies arrived at a body pool of vitamin C of about 2 mg/100 g body weight corresponding to about 1500 mg for a 75 kg individual (Kallner, Hartmann, and Hornig Citation1979). According to the US RDA document, Kallner “reported that the body pool of vitamin C was saturated at an intake of 100 mg/d in healthy non-smoking men” (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). However, there are major methodological issues associated with the calculations because the true complexity of vitamin C pharmacokinetics were not known at the time (Padayatty et al. Citation2004). First, the calculations were based on compartment kinetics or non-compartment kinetics, both of which require that a terminal 1st order kinetics exists. For vitamin C, this is not the case within the physiological concentration range (Lykkesfeldt and Tveden-Nyborg Citation2019). Second, excretion was measured not as vitamin C but rather nonspecifically as radioactivity. This suggests that a variety of compounds including breakdown products may have contributed to the radioactivity. Thirdly, for ethical and safety reasons, the radioactive dose was limited to 20 µCi. As was rightfully admitted by Kallner et al, this greatly limited the period of time that vitamin C kinetics could be studied (Kallner, Hartmann, and Hornig Citation1980). Fourthly, the sodium dependent vitamin C transporters, that actively transport vitamin C from the extracellular to the intracellular space against a concentration gradient, were not known at the time. These transporters are high affinity transporters that are capable of maintaining a many fold higher intracellular concentration of vitamin C compared to plasma even during periods of reduced intake (reviewed in (Lindblad, Tveden-Nyborg, and Lykkesfeldt Citation2013)). As studied in detail in guinea pigs, that like humans do not have the ability to biosynthesize vitamin C, the brain is a particularly relevant example as it has a very high vitamin C content and a high preference in the systemic homeostasis of vitamin C (Hasselholt, Tveden-Nyborg, and Lykkesfeldt Citation2015; Frikke-Schmidt et al. Citation2011). Such deep compartments do not equilibrate rapidly with blood and other compartments and are therefore not assessed in short-term isotope dilution experiments such as those conducted by Kallner and others.
With our present knowledge, it is clear that the blood concentration of vitamin C cannot be used as a surrogate marker of the tissue or body concentration or content. The consequence of the methodological problems of these early studies is that the total body pool most likely has been significantly underestimated. Indeed, these calculations were immediately questioned by Ginter, who based his simple calculations on available data from human tissue samples and organ weights and arrived at a body pool of about 5000 mg (Ginter Citation1979; Ginter Citation1980), i.e. more than three times the number of Kallner et al (Kallner, Hartmann, and Hornig Citation1979). Very recently, modeling studies of the human body pool has resulted in similar estimates (Nygaard Citation2019).
Kallner’s body pool estimates have been used by several authorities as the basis for the calculation of the amount necessary to replace the daily turnover of vitamin C and consequently directly translated into the RDA. As mentioned earlier, the recommendation of e.g. WHO/FAO is based on the objective of preventing vitamin C deficiency (World Health Organization and Food and Agriculture Organization of the United Nations Citation2004). More specifically, the calculation has been based on a value half way between body pool saturation in men (1500 mg) and the point where deficiency occurs (about 300–400 mg), i.e. a value of 900 mg (9). Multiplying with the rate of turnover (2.9% per day) and correcting for absorption efficiency (85%) and twice the estimated coefficient of variation (estimated at 20%) results in 44 mg/day rounded up to the recommendation of 45 mg/day.
In contrast, EFSA’s calculation of the RDA for vitamin C is based on maintaining a near saturation of the body pool assuming that this is optimal for vitamin C functions (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013). EFSA used the following rationale: the body pool in men (1500 mg) is multiplied by the metabolic loss (2.9% per day) and compensated for the urinary loss (25% per day) and absorption efficiency (80%). Adding twice the estimated coefficient of variation (estimated at 10%) to include 97.5% of the population results in the recommendation of 110 mg/d for men (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013). This sought to provide a plasma vitamin C concentration of >50 µmol/L, an amount which is considered to be ‘adequate’ (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013). It is obvious from the RDA calculations based on body pool that a higher body pool estimate will result in correspondingly higher recommendations.
Near saturation of neutrophils whilst limiting urinary excretion of vitamin C
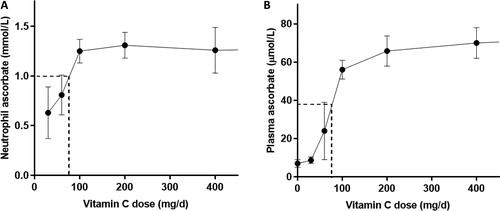
The criterion chosen for the US and Canadian EAR is “the vitamin C intake that maintains near-maximal neutrophil concentrations with minimal urinary excretion of ascorbate” (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Near-maximal neutrophil ascorbate concentration was selected to be 80% of the maximal concentration observed in seven healthy men () (Levine et al. Citation1996). This was not an actual data point, but rather an estimated midpoint between 60% maximal obtained from 60 mg/d (with little observed urinary excretion from ‘several volunteers’) and 100% maximal from 100 mg/d (with 25% urinary excretion). This equated to an intake of ∼75 mg/d, which provides ∼38 µmol/L plasma ascorbate (). However, it can be seen from the 60 mg/d intake data that there is large variability in plasma concentrations around these intakes (Levine et al. Citation1996). It was assumed that the extrapolated value “should potentially protect intracellular proteins from oxidative injury when these cells are activated during infectious and inflammatory processes”. Not only is it highly unusual to choose a point on an estimated graph that is not supported by direct data, but there is also no evidence that an intake of 75 mg/d protects neutrophil intracellular proteins from oxidation during activation of these cells.
Figure 2. Neutrophil and plasma ascorbate concentrations as a function of daily dose. Values were obtained from seven volunteers for neutrophils (A) and steady state plasma (B) concentrations (Levine et al. Citation1996). Dashed lines show values used for EAR determination (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000).
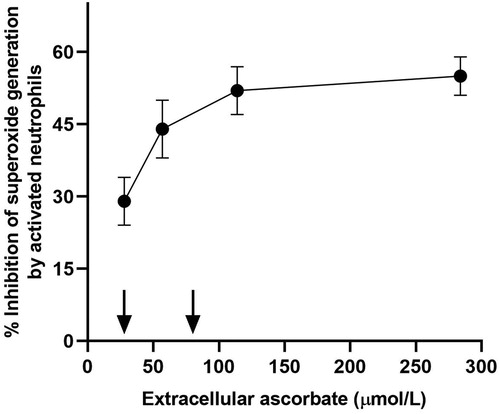
The data utilized for ‘antioxidant protection’ was from an in vitro study using neutrophils stimulated in the presence of varying concentrations of extracellular vitamin C (28–284 µmol/L) (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000; Anderson and Lukey Citation1987). The IOM stated that “antioxidant protection is increasingly provided as ascorbate concentrations increase with the greatest change in protection seen for ascorbate concentrations between 28 and 57 μmol/L.” However, the data shows maximal scavenging of superoxide generation by activated neutrophils at ascorbate concentrations between 57 and 114 µmol/L, which are saturating plasma concentrations (). Furthermore, it should be noted that the researchers used only 1 × 106 cells/ml, whereas plasma contains on average five times this many neutrophils per ml. Therefore, it is likely that these concentrations of vitamin C would be insufficient for maximal scavenging of the reactive oxygen species generated by 5 × 106 cells/ml. Thus, the amount of vitamin C required for neutrophil antioxidant protection has likely been underestimated by the IOM (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000).
Figure 3. Effect of varying extracellular ascorbate concentrations on scavenging of superoxide produced by activated neutrophils (1 × 106/mL). Arrows indicate the range of normal human plasma ascorbate concentrations (i.e. 22–85 µmol/L) (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Data is adapted from (Anderson and Lukey Citation1987).
Minimal urinary excretion of vitamin C was the other criteria used by the IOM (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). However, the rationale that emerging urinary excretion is indicative of saturation of body stores is not in agreement with data from Levine and coworkers, who showed that plasma steady state concentrations plateaued at much higher intakes than those necessary to produce urinary excretion and also concluded that the bioavailability of a 200 mg dose was complete (Levine et al. Citation1996). Moreover, as mentioned above, the equilibration with so-called deep compartments may not be particularly quick less prioritized tissues such as muscle and thus, although accounting for a large part of the volume of distribution, will likely not have achieved steady state saturation when urinary excretion commences.
Levine’s data used as the basis for the IOM recommendation were based on data obtained from seven young healthy males, i.e. probably the ‘ideal’ situation representing a minimum requirement rather than a population average (Levine et al. Citation1996). In 2001, one year after the most resent IOM revision, Levine and coworkers published a follow up study conducted in healthy women (Levine et al. Citation2001). These data are similar to those obtained in men but are tighter being obtained from 13 individuals. The use of data based selectively on healthy young individuals may result in the requirement of the general population to achieve similar protection to be underestimated by the IOM. Moreover, choosing a target point on the steepest part of the dose versus concentration curve also results in relatively higher variation and may result in cases of deficiency even among individuals meeting the RDA ().
Effect of smoking on requirements
Looking closer at attempts made to estimate the additional turnover of vitamin C due to smoking, different approaches have been used. Kallner et al. used 14C-labelled ascorbate to estimate the turnover of vitamin C in 17 male smokers aged 21–69 years and weighing between 55 and 110 kg (Kallner, Hartmann, and Hornig Citation1981). Urinary excretion of radioactivity was used to estimate the vitamin C pharmacokinetics using a three-compartment model. Based on these data, Kallner et al. concluded that smokers needed only about 35 mg more vitamin C than nonsmokers per day to compensate for their habit (Kallner, Hartmann, and Hornig Citation1981). This recommendation was later adopted by the IOM in their dietary reference intakes (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). As mentioned below, Lykkesfeldt et al. arrived at a higher turnover as estimated by steady state ascorbate oxidation (Lykkesfeldt et al. Citation1997). This was noted by the IOM but was not taken into account in their recommendation. However, as discussed earlier, several problems are associated with Kallner’s approach and these also apply to their estimations in smokers. Thus, it appears likely that the turnover in smokers was significantly underestimated by Kallner et al. (Kallner, Hartmann, and Hornig Citation1981).
In support of this premise, Schectman et al. analyzed the NHANES II data, comparing daily intake versus serum concentrations of ascorbate among 4182 smokers and 7020 nonsmokers. Using regression analysis, they were able to estimate that smokers would need an additional 130 mg vitamin C per day to overcome the effect of increased vitamin C turnover due to smoking per se (Schectman, Byrd, and Gruchow Citation1989). In a separate analysis, it was concluded that smokers need an intake >200 mg/d to lower the risk of vitamin C deficiency to that of nonsmokers (Schectman, Byrd, and Hoffmann Citation1991). These conclusions were later supported by measuring the steady state oxidation ratio of vitamin C among smokers and nonsmokers (Lykkesfeldt et al. Citation1997). It was found that smokers with poor vitamin C status had an increased steady state oxidation of their vitamin C pool compared to nonsmokers and it was estimated by regression that smokers need at least 200 mg vitamin C per day to compensate for the effect of smoking on the oxidation of vitamin C (Lykkesfeldt et al. Citation1997). This increased oxidation apparently occurs in spite of increased ascorbate recycling observed in smokers (Lykkesfeldt, Viscovich, and Poulsen Citation2003).
Effect of obesity on requirements
Numerous epidemiological studies have found correlations between vitamin C status and body weight, with higher weight associated with lower status (Canoy et al. Citation2005; Galan et al. Citation2005; Jungert and Neuhauser-Berthold Citation2015; Langlois, Cooper, and Colapinto Citation2016; Schleicher et al. Citation2009; Pearson et al. Citation2017). Higher body weight can also attenuate the response to supplementation indicating higher requirements for the vitamin in people with increased body weight (Block et al. Citation1999; Carr et al. Citation2016). Initial vitamin C status and body weight were found to account for 30% of the lack of response to low-dose supplementation, likely due to insufficient tissue status (Carr et al. Citation2016). The generally larger body weight of men relative to women has been taken into account with respect to RDAs in many countries ().
However, the overall increase in body weight globally is reaching pandemic proportions, with the prevalence of obesity exceeding 50% in some countries (Ng et al. Citation2014; Yatsuya et al. Citation2014). Therefore, it would be prudent to ensure that global RDAs meet this markedly changing demographic. In 1999, Block et al. (Block et al. Citation1999) proposed that recommended vitamin C intakes should be based on a dose per kg body weight or in terms of desirable plasma concentrations, the latter of which has been used by EFSA and DACH for their most recent RDA determinations (European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies Citation2013; German Nutrition Society Citation2015a; German Nutrition Society Citation2015b). Alternatively, recommendations specifically aimed at overweight or severely obese individuals are clearly needed, similar to those for smokers and pregnant women.
Optimizing health
A number of countries now base their RDAs for vitamin C on its potential health effects, including decreased risk of cardiovascular or cancer morbidity and mortality (Nordic Council of Ministers Citation2014; Ministry of Health Labour and Welfare Citation2018; Sasaki Citation2008). Although there is little evidence from randomized controlled intervention studies demonstrating additional benefits for healthy individuals of intakes of >200 mg/d, vitamin C is completely safe and the potential benefits appear to outweigh the potential negative consequences of inadequacy or suboptimal vitamin C status (Frei, Birlouez-Aragon, and Lykkesfeldt Citation2012). It should also be noted that most intervention studies have suffered from the fact that their study populations have been close to saturation and thus only small incremental improvements, if any, can be expected, albeit potentially important on a long-term basis (Lykkesfeldt and Poulsen Citation2010). Furthermore, several of the negative intervention studies actually allowed the placebo group to continue taking supplements up to the RDA further limiting the possibility of observing a short-term health benefit (Tveden-Nyborg and Lykkesfeldt Citation2013). In spite of these shortcomings, a meta-analysis of the effect of vitamin C on blood pressure did reveal a significant positive effect on both systolic and diastolic blood pressure with a median intake of 500 mg vitamin C/d (Juraschek et al. Citation2012).
In 2006, Australia and New Zealand developed an additional reference value, the suggested dietary target (SDT), to address the issue of chronic disease prevention (see definition in ) (Australian Government Department of Health, Citation2015-2017). The SDT relates to selected nutrients for which there was a reasonable body of evidence of a potential chronic disease preventive effect at levels substantially higher than the EAR and RDA or AI. The STD for vitamin C for healthy men and women was determined to be 220 mg/d and 190 mg/d, respectively (); this was equivalent to the 90th centile of intake in the Australian and New Zealand populations (National Health and Medical Research Council Citation2006). Similarly, China recently adopted a category known as a proposed intake (PI) (Chinese Nutrition Society Citation2014). The purpose for establishing a PI was to prevent or reduce the risk of non-communicable diseases. The PI target of 200 mg/d was to raise plasma vitamin C concentrations in healthy individuals to achieve 70 µmol/L, which is saturation. An intake of 200 mg/d can be obtained from a diet of five or more servings of fresh fruit and vegetables, provided that at least one serving is a high vitamin C food. Alternatively, it can be obtained through dietary supplements, which have comparable bioavailability to food sources of the vitamin (Carr and Vissers Citation2013). In smokers and other individuals with increased turnover and requirements, dietary supplements are probably necessary to achieve saturation.
Future directions
Although the antioxidant function of vitamin C is not disputed (Carr and Frei, Citation1999a; Carr and Frei, Citation1999b), and has been taken into consideration by a number of authorities during their development of RDAs (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000; Ministry of Health Labour and Welfare Citation2018; Sasaki Citation2008), the need for a concentration versus functional or clinical outcome measure remains. In 2000, the IOM pointed out that ‘despite the many known biochemical roles of ascorbic acid, no reliable biochemical or physiologically based functional measures of vitamin C nutriture have been established. Knowledge of vitamin C intakes needed to fulfill specific functional roles of ascorbate will allow more accurate and precise determinations of the individual and average population requirements of the vitamin’ (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000).
Vitamin C has long been known to be a cofactor for a family of metalloenzymes with various biosynthetic roles, including the synthesis of collagen, carnitine, catecholamines and peptide hormones (Englard and Seifter Citation1986). The IOM suggested that ‘some current candidates that could be used as functional measures include pathways related to collagen and carnitine metabolism’, although the methods by which these would be assessed are not clear (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). In the early 1990s, Levine and coworkers proposed determining vitamin C requirements via assessing the in vitro conversion of tyrosine to norepinephrine, which includes vitamin C-dependent steps (Levine et al. Citation1991). The experiments comprised incubation of adrenal medulla chromaffin cells in culture with increasing concentrations of vitamin C, which resulted in a dose-dependent increase in norepinephrine generation. However, it is not easy to translate the data generated from these in vitro experiments into whole body requirements. Other potential biomarkers include measurement of amidated peptide hormones or their non-amidated precursors as the enzyme peptidylglycine α-amidating monooxygenase is the only known enzyme in the body to generate carboxy terminal amidated hormones and vitamin C is a cofactor for the enzyme reaction (reviewed in (Carr et al. Citation2015)). However, hormones can be difficult analytes to measure as they are often transient and are usually only released under specific circumstances.
The IOM also highlighted that research on new functions of vitamin C is needed (Institute of Medicine Panel on Dietary Antioxidants and Related Compounds Citation2000). Since that statement was made, a plethora of new discoveries have been made with respect to the cofactor functions of vitamin C (Du, Cullen, and Buettner Citation2012). These include its pleiotropic gene regulatory roles, first discovered in the early 2000s, when vitamin C was found to downregulate the transcription factor hypoxia inducible factor (HIF) (Hirsila et al. Citation2003; Koivunen et al. Citation2004; Hirota and Semenza Citation2005). Vitamin C acts as a cofactor for the dioxygenase enzymes which hydroxylate specific proline and asparagine residues in HIF resulting in decreased DNA and coactivator binding and enhanced proteosomal degradation of the transcription factor (Hirsila et al. Citation2003; Koivunen et al. Citation2004; Hirota and Semenza Citation2005). HIF regulates numerous pro-survival genes and has been implicated in cancer cell survival and tumor growth (Du, Cullen, and Buettner Citation2012). More recent research has uncovered a role for vitamin C in epigenetic regulation via acting as a cofactor for DNA and histone hydroxylases, which belong to the same family of metalloenzymes as the collagen and HIF dioxygenases (Camarena and Wang Citation2016; Gillberg et al. Citation2017). The ten-eleven translocation (TET) dioxygenases hydroxylate methylated cytosine residues in DNA, generating hydroxymethylcytosine residues, whilst Jumonji C domain-containing histone demethylases (JHDM) hydroxylate methylated lysine and arginine residues in histones resulting in demethylation and subsequent alterations in gene transcription (Camarena and Wang Citation2016; Blaschke et al. Citation2013; Minor et al. Citation2013; Yin et al. Citation2013; Tsukada et al. Citation2006). Thus, vitamin C has the potential to up- and down-regulate many thousands of genes.
Regulation of the epigenome by vitamin C may comprise a major role in its pleiotropic health promoting and disease modifying effects. For example, reduced TET activity and hydroxylation of DNA results in the over-proliferation of various leukocyte precursors in bone marrow and the development of acute myelocytic leukemia and other myeloproliferative neoplasms (Magotra et al. Citation2016). Interestingly, researchers have found that vitamin C is present at very high concentrations in hematopoietic stem cells and this prevents their progression to a leukemic phenotype in a TET-dependent manner (Agathocleous et al. Citation2017; Cimmino et al. Citation2017). Pre-clinical models indicated that vitamin C administration was able to increase hydroxymethylation of peripheral blood DNA, suppress leukemia progression and improve animal survival (Agathocleous et al. Citation2017; Cimmino et al. Citation2017). Preliminary human intervention studies have indicated that intravenous vitamin C can improve clinical remission and survival in acute myeloid leukemia patients, particularly those with TET and related mutations (Zhao et al. Citation2018; Das et al. Citation2019). Also, normalization of vitamin C status using oral supplementation was recently shown to restore TET activity in myeloid cancer patients (Gillberg et al. Citation2019). Continuing research in this field will no doubt reveal new exciting insights and treatment options, particularly for non-communicable diseases such as myelodysplastic syndrome and other haematological cancers.
However, the utility of these more recently discovered gene regulatory functions of vitamin C for the assessment of dietary recommendations has not yet been elucidated. The concentrations of ascorbate that permit the HIF dioxygenases to achieve half maximal rates of reaction (i.e. Km) have been determined and are of the same order of magnitude as that required for collagen prolyl dioxygenase (180 µmol/L and 300 µmol/L, respectively) (Hirsila et al. Citation2003; Myllyharju and Kivirikko Citation1997). The Km of ascorbate with the TET dioxygenases has not yet been reported, however, this is likely to be of a comparable concentration to the other dioxygenases. Therefore, measurement of hydroxymethylcytosine marks in DNA may comprise a new physiological biomarker for the assessment of vitamin C requirements. Although methodologies exist for measuring global DNA hydroxymethylation, such as mass spectrometry and immunological methods, epigenetic modification of specific DNA regions may prove to be a more sensitive marker of vitamin C requirements than assessment of global hydroxymethylcytosine status. Next generation sequencing methodologies have proved useful for assessing specific epigenetic alterations (Meaburn and Schulz Citation2012). As such, it may be possible to assess differential hydroxylation of defined DNA regions in participants who have been supplemented with various doses of vitamin C. These studies would ideally be carried out in metabolic units in order to closely control the participants’ dietary intakes and to assure low baseline vitamin status.
Peripheral blood leukocytes could be utilized as surrogates to study vitamin C-dependent alterations to the epigenome (Hohos et al. Citation2016). Preliminary in vivo evidence for the involvement of vitamin C in the regulation of epigenetic DNA marks in leukocytes has come from an observational study carried out in healthy controls and patients with inflammatory bowel disease, adenomatous polyps and colorectal cancer (Starczak et al. Citation2018). In this study, the researchers observed significant positive correlations between vitamin C status and hydroxymethylcytosine and hydroxymethyluracil marks in leukocyte DNA, with participants with vitamin C concentrations greater than 40 µmol/L exhibiting significantly higher concentrations of these epigenetic marks than those with plasma concentrations less than the hypovitaminosis C concentration of 20 µmol/L (Starczak et al. Citation2018). Recently, a vitamin C dose of 500 mg/d was shown to induce epigenetic changes in patients with myeloid cancer (Gillberg et al. Citation2019). Further research may indicate the minimum dose at which epigenetic changes can be observed, and the impact on functional or clinical outcomes, which would help to inform vitamin C intake requirements for the prevention and treatment of non-communicable diseases.
Concluding remarks
Overall, there are large variations in global RDAs for vitamin C, despite comparable data from the literature being utilized for their determinations. This is primarily due to the underlying premises for the RDA criteria varying from prevention of scurvy (∼45 mg/d) to optimization of health (∼200 mg/d). A number of authorities recommend the minimum amount of vitamin C, however, this is likely not meeting the health needs of various subpopulations. For example, smokers and people with obesity have higher requirements than the general population. Smoking rates continue to increase in some countries and evidence suggests that smokers may benefit from daily intakes of at least 200 mg of vitamin C. With increasing worldwide rates of obesity, and obesity-related comorbidities such as metabolic syndrome, diabetes and cardiovascular disease, more consideration needs to be given to appropriate recommendations to optimize vitamin C status in this rapidly increasing subpopulation.
Furthermore, we have ascertained that many current recommendations are based on rationales that likely underestimate the RDA of vitamin C, including those derived from body pool estimations. As such, it would be prudent for these authorities to reassess their dietary recommendations in accordance with correct assumptions in order for their RDAs to align appropriately with their stated health goals. To aid in this endeavor, specific biological signatures of physiologically sufficient versus insufficient vitamin C status and its potential relationship to specific disease conditions are needed. The recently identified epigenetic mark hydroxymethylcytosine may show promise as such a signature, and it is likely that other biomarkers of vitamin C function may yet be discovered.
Disclosure statement
The authors declare no conflicts of interest in relation to the present work.
Additional information
Funding
References
- Agathocleous, M., C. E. Meacham, R. J. Burgess, E. Piskounova, Z. Zhao, G. M. Crane, B. L. Cowin, E. Bruner, M. M. Murphy, W. Chen, et al. 2017. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549 (7673):476–481.
- Anderson, R., and P. T. Lukey. 1987. A biological role for ascorbate in the selective neutralization of extracellular phagocyte-derived oxidants. Annals of the New York Academy of Sciences 498 (1 Third Conference):229–47.
- Australian Government Department of Health. 2015-2017. Methodological framework for the review of Nutrient Reference Values. Canberra, Australia: Australian Government Department of Health.
- Baker, E. M., R. E. Hodges, J. Hood, H. E. Sauberlich, and S. C. March. 1969. Metabolism of ascorbic-1-14C acid in experimental human scurvy. The American Journal of Clinical Nutrition 22 (5):549–58.
- Baker, E. M., R. E. Hodges, J. Hood, H. E. Sauberlich, S. C. March, and J. E. Canham. 1971. Metabolism of 14C- and 3H-labeled L-ascorbic acid in human scurvy. The American Journal of Clinical Nutrition 24 (4):444–54. doi: 10.1093/ajcn/24.4.444.
- Birlouez-Aragon, I., C. Delcourt, F. Tessier, and L. Papoz. 2001. Associations of age, smoking habits and diabetes with plasma vitamin C of elderly of the POLA study. International Journal for Vitamin and Nutrition Research 71 (1):53–9.
- Blaschke, K., K. T. Ebata, M. M. Karimi, J. A. Zepeda-Martínez, P. Goyal, S. Mahapatra, A. Tam, D. J. Laird, M. Hirst, A. Rao, et al. 2013. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500 (7461):222–6.
- Block, G., A. R. Mangels, B. H. Patterson, O. A. Levander, E. P. Norkus, and P. R. Taylor. 1999. Body weight and prior depletion affect plasma ascorbate levels attained on identical vitamin C intake: A controlled-diet study. Journal of the American College of Nutrition 18 (6):628–37.
- Blonk Environmental Consultants. 2012. Food patterns and dietary recommendations in Spain. France and Sweden. Gouda;
- Camarena, V., and G. Wang. 2016. The epigenetic role of vitamin C in health and disease. Cellular and Molecular Life Sciences 73 (8):1645–58.
- Canoy, D., N. Wareham, A. Welch, S. Bingham, R. Luben, N. Day, and K.-T. Khaw. 2005. Plasma ascorbic acid concentrations and fat distribution in 19,068 British men and women in the European Prospective Investigation into Cancer and Nutrition Norfolk cohort study. The American Journal of Clinical Nutrition 82 (6):1203–9.
- Carr, A. C., and B. Frei. 1999a. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. The American Journal of Clinical Nutrition 69 (6):1086–107.
- Carr, A. C., and B. Frei. 1999b. Does vitamin C act as a pro-oxidant under physiological conditions? The Faseb Journal 13 (9):1007–24.
- Carr, A. C., and M. C. Vissers. 2013. Synthetic or food-derived vitamin C–are they equally bioavailable? Nutrients 5 (11):4284–304.
- Carr, A. C., G. M. Shaw, A. A. Fowler, and R. Natarajan. 2015. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock?. Critical Care 19 (1):e418.
- Carr, A. C., J. M. Pullar, S. M. Bozonet, and M. C. Vissers. 2016. Marginal ascorbate status (hypovitaminosis C) results in an attenuated response to vitamin C supplementation. Nutrients 8 (6):341.
- Chinese Nutrition Society. 2014. Chinese dietary reference intakes. Beijing: China Science Publishing House.
- Cimmino, L., I. Dolgalev, Y. Wang, A. Yoshimi, G. H. Martin, J. Wang, V. Ng, B. Xia, M. T. Witkowski, M. Mitchell-Flack, et al. 2017. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170 (6):1079–95. e20.
- Committee on Dietary Allowances. 1974. Recommended dietary allowances, 8th ed. Washington DC: National Academy of Sciences.
- Das, A. B., P. M. Kakadia, D. Wojcik, L. Pemberton, P. J. Browett, S. K. Bohlander, and M. C. M. Vissers. 2019. Clinical remission following ascorbate treatment in a case of acute myeloid leukemia with mutations in TET2 and WT1. Blood Cancer Journal 9 (10):82.
- Department of Health Panel on Dietary Reference Values. 1991. Dietary reference values for food energy and nutrients for the United Kingdom: report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. London: Her Majesty's Stationery Office (HMSO).
- Dietary reference values for food energy and nutrients for the United Kingdom. 1991. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Report on Health and Social Subjects (London) 41: 1–210.
- Du, J., J. J. Cullen, and G. R. Buettner. 2012. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochimica et Biophysica Acta 1826 (2):443–57. doi: 10.1016/j.bbcan.2012.06.003.
- Englard, S., and S. Seifter. 1986. The biochemical functions of ascorbic acid. Annual Review of Nutrition 6 (1):365–406.
- European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies. 2013. Scientific opinion on dietary reference values for vitamin C. EFSA Journal. 11 (11):3418–68.
- Expert Group on Vitamins and Minerals. 2003. Safe upper levels for vitamins and minerals. UK: Food Standards Agency.
- Faure, H., P. Preziosi, A.-M. Roussel, S. Bertrais, P. Galan, S. Hercberg, and A. Favier. 2006. Factors influencing blood concentration of retinol, alpha-tocopherol, vitamin C, and beta-carotene in the French participants of the SU.VI.MAX trial. European Journal of Clinical Nutrition 60 (6):706–17.
- Food and Agriculture Organization. 2001. Human vitamin and mineral requirements, 303. Rome: Food and Agriculture Organization.
- Food and Nutrition Board of the National Research Council. 1943. Recommended dietary allowances. Nutrition Reviews 1 (6):164–8.
- Food and Nutrition Board of the National Research Council. 1980. Recommended dietary allowances, 9th ed. Washington, D.C.: National Academy of Sciences.
- Food and Nutrition Research Institute. 2015a. Philippine dietary reference intake 2015: Summary of recommendations.
- Food and Nutrition Research Institute. 2015b. Philippine Dietary Reference Intakes 2015: Summary Tables: Food and Nutrition Research Institute. https://www.fnri.dost.gov.ph/images/images/news/PDRI-2018.pdf.
- Frei, B., I. Birlouez-Aragon, and J. Lykkesfeldt. 2012. What is the optimum intake of vitamin C in humans? Critical Reviews in Food Science and Nutrition 52 (9):815–29.
- Frikke-Schmidt, H., P. Tveden-Nyborg, M. M. Birck, and J. Lykkesfeldt. 2011. High dietary fat and cholesterol exacerbates chronic vitamin C deficiency in guinea pigs. British Journal of Nutrition 105 (1):54–61.
- Galan, P., F. E. Viteri, S. Bertrais, S. Czernichow, H. Faure, J. Arnaud, D. Ruffieux, S. Chenal, N. Arnault, A. Favier, et al. 2005. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. European Journal of Clinical Nutrition 59 (10):1181–90.,
- GBD 2015 Tobacco Collaborators. 2017. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 389 (10082):1885–906.
- German Nutrition Society (DGE). 2015a. New reference values for vitamin C intake. Annals of Nutrition and Metabolism 67 (1):13–20.
- German Nutrition Society (DGE). 2015b. AÖaSS. D-A-CH reference levels for nutrient intake. Bonn.
- Gillberg, L., A. D. Ørskov, A. Nasif, H. Ohtani, Z. Madaj, J. W. Hansen, N. Rapin, J. B. Mogensen, M. Liu, I. H. Dufva, et al. 2019. Oral vitamin C supplementation to patients with myeloid cancer on azacitidine treatment: Normalization of plasma vitamin C induces epigenetic changes. Clinical Epigenetics 11 (1):143.
- Gillberg, L., A. D. Orskov, M. Liu, L. B. S. Harslof, P. A. Jones, and K. Gronbaek. 2017. Vitamin C - A new player in regulation of the cancer epigenome. Seminars in Cancer Biology.
- Ginter, E. 1979. Chronic marginal vitamin C deficiency: Biochemistry and pathophysiology. World Review of Nutrition and Dietetics 33:104–41. doi: 10.1159/000402551.
- Ginter, E. 1980. What is truly the maximum body pool size of ascorbic acid in man? The American Journal of Clinical Nutrition 33 (3):538–9.
- Hampl, J. S., C. A. Taylor, and C. S. Johnston. 2004. Vitamin C deficiency and depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. American Journal of Public Health 94 (5):870–5.
- Hasselholt, S., P. Tveden-Nyborg, and J. Lykkesfeldt. 2015. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. British Journal of Nutrition 113 (10):1539–49.
- Health Council of the Netherlands. 2018. Dietary reference values for vitamins and minerals for adults.
- Health Promotion Board. 2019. Recommended dietary allowances. Singapore: Ministry of Health. https://www.healthhub.sg/live-healthy/192/recommended_dietary_allowances.
- Hirota, K., and G. L. Semenza. 2005. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochemical and Biophysical Research Communications 338 (1):610–6.
- Hirsila, M., P. Koivunen, V. Gunzler, K. I. Kivirikko, and J. Myllyharju. 2003. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. Journal of Biological Chemistry 278 (33):30772–80.
- Hodges, R. E., J. Hood, J. E. Canham, H. E. Sauberlich, and E. M. Baker. 1971. Clinical manifestations of ascorbic acid deficiency in man. The American Journal of Clinical Nutrition 24 (4):432–43.
- Hohos, N. M., K. Lee, L. Ji, M. Yu, M. M. Kandasamy, B. G. Phillips, C. A. Baile, C. He, R. J. Schmitz, and R. B. Meagher. 2016. DNA cytosine hydroxymethylation levels are distinct among non-overlapping classes of peripheral blood leukocytes. Journal of Immunological Methods 436:1–15.
- Indian Council of Medical Research. 2010. Nutrient requirement and recommended dietary allowances for Indians. A report of the Expert Group of the Indian Council of Medical Research.
- Institute of Medicine Panel on Dietary Antioxidants and Related Compounds. 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids, 529. Washington: National Academies Press.
- International Life Sciences Institute Southeast Asia Region. 2005. Recommended dietary allowances: Harmonization in Southeast Asia. Singapore.
- Italian Society of Human Nutrition (SINU). 2014. LARN Levels of reference intake for nutrients and energy for the Italian population. Rome: Italian Society of Human Nutrition (SINU).
- Ivanovitch, K., J. Klaewkla, R. Chongsuwat, C. Viwatwongkasem, and W. Kitvorapat. 2014. The intake of energy and selected nutrients by Thai urban sedentary workers: An evaluation of adherence to dietary recommendations. Journal of Nutrition and Metabolism 2014 (145182):1–17.
- Juhl, B., F. F. Lauszus, and J. Lykkesfeldt. 2017a. Is diabetes associated with lower vitamin C status in pregnant women? A prospective study. International Journal for Vitamin and Nutrition Research 86 (3–4):184–9.
- Juhl, B., F. F. Lauszus, and J. Lykkesfeldt. 2017b. Poor vitamin C status late in pregnancy is associated with increased risk of complications in type 1 diabetic women: A cross-sectional study. Nutrients 9 (3):186.
- Jungert, A., and M. Neuhauser-Berthold. 2015. The lower vitamin C plasma concentrations in elderly men compared with elderly women can partly be attributed to a volumetric dilution effect due to differences in fat-free mass. British Journal of Nutrition 113 (5):859–64.
- Juraschek, S. P., E. Guallar, L. J. Appel, and E. R. Miller. 2012. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition 95 (5):1079–88.
- Kallner, A. B., D. Hartmann, and D. H. Hornig. 1981. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. The American Journal of Clinical Nutrition 34 (7):1347–55.
- Kallner, A., D. Hartmann, and D. Hornig. 1977. Determination of bodypool size and turnover rate of ascorbic acid in man. Annals of Nutrition and Metabolism 21 (1):31–5.
- Kallner, A., D. Hartmann, and D. Hornig. 1979. Steady-state turnover and body pool of ascorbic acid in man. The American Journal of Clinical Nutrition 32 (3):530–9.
- Kallner, A., D. Hartmann, and D. Hornig. 1980. Reply to letter by Ginter. American Journal of Clinical Nutrition 33 (3):359.
- Khan, N. C., and P. V. Hoan. 2008. Vietnam recommended dietary allowances 2007. Asia Pacific Journal of Clinical Nutrition 17 (Suppl 2):409–15.
- King, J. C., and C. Garza. 2007. Harmonization of nutrient intake values. Food and Nutrition Bulletin 28 (1_suppl1):S3–S12.
- Kiondo, P., N. M. Tumwesigye, J. Wandabwa, G. Wamuyu-Maina, G. S. Bimenya, and P. Okong. 2012. Plasma vitamin C assay in women of reproductive age in Kampala, Uganda, using a colorimetric method. Tropical Medicine & International Health 17 (2):191–6.
- Koivunen, P., M. Hirsila, V. Gunzler, K. I. Kivirikko, and J. Myllyharju. 2004. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. Journal of Biological Chemistry 279 (11):9899–904.
- Krebs, H. A. 1953. The Sheffield experiment on the vitamin C requirement of human adults. Proceedings of the Nutrition Society 12 (3):237–46.
- Langlois, K., M. Cooper, and C. K. Colapinto. 2016. Vitamin C status of Canadian adults: Findings from the 2012/2013 Canadian Health Measures Survey. Health Reports 27 (5):3–10.
- Levine, M., C. Conry-Cantilena, Y. Wang, R. W. Welch, P. W. Washko, K. R. Dhariwal, J. B. Park, A. Lazarev, J. F. Graumlich, J. King, et al. 1996. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proceedings of the National Academy of Sciences 93 (8):3704–9.
- Levine, M., K. R. Dhariwal, P. W. Washko, J. D. Butler, R. W. Welch, Y. H. Wang, and P. Bergsten. 1991. Ascorbic acid and in situ kinetics: A new approach to vitamin requirements. The American Journal of Clinical Nutrition 54 (6):1157S–62S.
- Levine, M., K. R. Dhariwal, R. W. Welch, Y. Wang, and J. B. Park. 1995. Determination of optimal vitamin C requirements in humans. The American Journal of Clinical Nutrition 62 (6):1347S–56S.
- Levine, M., Y. Wang, S. J. Padayatty, and J. Morrow. 2001. A new recommended dietary allowance of vitamin C for healthy young women. Proceedings of the National Academy of Sciences 98 (17):9842–6.
- Lindblad, M., P. Tveden-Nyborg, and J. Lykkesfeldt. 2013. Regulation of vitamin C homeostasis during deficiency. Nutrients 5 (8):2860–79.
- Lykkesfeldt, J. 2006. Smoking depletes vitamin C: Should smokers be recommended to take supplements? In Cigarette smoke & oxidative stress, eds. B. Halliwell and H. E. Poulsen, 237–60. Berlin, Heidelberg: Springer Verlag.
- Lykkesfeldt, J., and H. E. Poulsen. 2010. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. British Journal of Nutrition 103 (9):1251–9.
- Lykkesfeldt, J., and P. Tveden-Nyborg. 2019. The pharmacokinetics of vitamin C. Nutrients 11 (10):2412.
- Lykkesfeldt, J., H. Prieme, S. Loft, and H. E. Poulsen. 1996. Effect of smoking cessation on plasma ascorbic acid concentration. BMJ 313 (7049):91.
- Lykkesfeldt, J., M. Viscovich, and H. E. Poulsen. 2003. Ascorbic acid recycling in human erythrocytes is induced by smoking in vivo. Free Radical Biology and Medicine 35 (11):1439–47.
- Lykkesfeldt, J., M. Viscovich, and H. E. Poulsen. 2004. Plasma malondialdehyde is induced by smoking: A study with balanced antioxidant profiles. British Journal of Nutrition 92 (2):203–6.
- Lykkesfeldt, J., S. Christen, L. M. Wallock, H. H. Chang, R. A. Jacob, and B. N. Ames. 2000. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. The American Journal of Clinical Nutrition 71 (2):530–6.
- Lykkesfeldt, J., S. Loft, J. B. Nielsen, and H. E. Poulsen. 1997. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. The American Journal of Clinical Nutrition 65 (4):959–63.
- Magotra, M., A. Sakhdari, P. J. Lee, K. Tomaszewicz, K. Dresser, L. M. Hutchinson, B. A. Woda, and B. J. Chen. 2016. Immunohistochemical loss of 5-hydroxymethylcytosine expression in acute myeloid leukaemia: Relationship to somatic gene mutations affecting epigenetic pathways. Histopathology 69 (6):1055–65.
- Marangon, K., B. Herbeth, E. Lecomte, A. Paul-Dauphin, P. Grolier, Y. Chancerelle, Y. Artur, and G. Siest. 1998. Diet, antioxidant status, and smoking habits in French men. The American Journal of Clinical Nutrition 67 (2):231–9.
- Martin, A. 2001. Apports nutritionnels conseilles pour la population francaise (Recommended dietary intakes for the French population).
- Meaburn, E., and R. Schulz. 2012. Next generation sequencing in epigenetics: Insights and challenges. Seminars in Cell & Developmental Biology 23 (2):192–9.
- Ministry of Health Labour and Welfare. 2018. Dietary Reference Intakes for Japanese (2015). Tokyo: Health Service Bureau, Ministry of Health, Labour and Welfare.
- Minor, E. A., B. L. Court, J. I. Young, and G. Wang. 2013. Ascorbate induces Ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. Journal of Biological Chemistry 288 (19):13669–74.
- Moller, P., M. Viscovich, J. Lykkesfeldt, S. Loft, A. Jensen, and H. E. Poulsen. 2004. Vitamin C supplementation decreases oxidative DNA damage in mononuclear blood cells of smokers. European Journal of Nutrition 43 (5):267–74.
- Moreiras, O., A. Carbajal, L. Cabrera, and C. Cuadrado. 2016. Ingestas diarias recomendadas de energía y nutrientes para la población española (Recommended daily energy and nutrient intakes for the Spanish population): Pyramid editions, 18th ed. https://www.ucm.es/data/cont/docs/458-2016-07-02-IR-tablas-Moreiras-col-2016-web.pdf.
- Myllyharju, J., and K. I. Kivirikko. 1997. Characterization of the iron- and 2-oxoglutarate-binding sites of human prolyl 4-hydroxylase. The EMBO Journal 16 (6):1173–80.
- National Academy of Sciences. 1989. National Research Council Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed. Washington (DC): National Academy of Sciences.
- National Health and Medical Research Council. 2006. Nutrient reference values for Australia and New Zealand: Executive summary. In Department of Health and Ageing, editor, 89. Canberra: National Health and Medical Research Council.
- Ng, M., T. Fleming, M. Robinson, B. Thomson, N. Graetz, C. Margono, E. C. Mullany, S. Biryukov, C. Abbafati, S. F. Abera, et al. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384 (9945):766–81.
- Nordic Council of Ministers. 2014. Nordic nutrition recommendations 2012. 5th ed., 627. Copenhagen: Narayana Press.
- Nutrition Information Centre of the University of Stellenbosch (NICUS). 2019. Vitamins: Vitamin C: Nutrition Information Centre of the University of Stellenbosch (NICUS). http://www.sun.ac.za/english/faculty/healthsciences/nicus/Pages/Vitamin-C.aspx.
- Nygaard, G. 2019. On a novel, simplified model framework describing ascorbic acid concentration dynamics. 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2880–6.
- Nyyssonen, K., M. T. Parviainen, R. Salonen, J. Tuomilehto, and J. T. Salonen. 1997. Vitamin C deficiency and risk of myocardial infarction: Prospective population study of men from eastern Finland. BMJ 314 (7081):634–8.
- Padayatty, S. J., H. Sun, Y. Wang, H. D. Riordan, S. M. Hewitt, A. Katz, R. A. Wesley, and M. Levine. 2004. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Annals of Internal Medicine 140 (7):533–7.
- Pauling, L. 1974. Are recommended daily allowances for vitamin C adequate? Proceedings of the National Academy of Sciences 71 (11):4442–6.
- Pearson, J., J. Pullar, R. Wilson, J. Spittlehouse, M. Vissers, P. Skidmore, J. Willis, V. Cameron, and A. Carr. 2017. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: Findings of the CHALICE cohort study. Nutrients 9 (8):831.
- Ravindran, R. D., P. Vashist, S. K. Gupta, I. S. Young, G. Maraini, M. Camparini, R. Jayanthi, N. John, K. E. Fitzpatrick, U. Chakravarthy, et al. 2011. Prevalence and risk factors for vitamin C deficiency in North and South India: A two centre population based study in people aged 60 years and over. PLoS One. 6 (12):e28588.
- Rumbold, A., E. Ota, C. Nagata, S. Shahrook, and C. A. Crowther. 2015. Vitamin C supplementation in pregnancy. The Cochrane Database of Systematic Reviews (9):Cd004072.
- Sasaki, S. 2008. Dietary Reference Intakes (DRIs) in Japan. Asia Pacific Journal of Clinical Nutrition 17 (Suppl 2):420–44.
- Schectman, G., J. C. Byrd, and H. W. Gruchow. 1989. The influence of smoking on vitamin C status in adults. American Journal of Public Health 79 (2):158–62.
- Schectman, G., J. C. Byrd, and R. Hoffmann. 1991. Ascorbic acid requirements for smokers: Analysis of a population survey. The American Journal of Clinical Nutrition 53 (6):1466–70.
- Schleicher, R. L., M. D. Carroll, E. S. Ford, and D. A. Lacher. 2009. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). The American Journal of Clinical Nutrition 90 (5):1252–63.
- Scientific Committee for Food. 1993. Nutrient and energy intakes for the European Community. Luxembourg: European Commission.
- Scientific Committee on Food Scientific Panel on Dietetic Products Nutrition and Allergies. 2006. Tolerable upper intake levels for vitamins and minerals.
- Sebrell, W. H. Jr. 1968. The new recommended dietary allowances. Nutrition Reviews 26 (12):355–7. doi:10.1111/j.1753-4887.1968.tb00849.x.
- Staff of National Academy of Science. 1968. Recommended dietary allowance, 7th ed. Washington, DC: National Academy of Science.
- Starczak, M., E. Zarakowska, M. Modrzejewska, T. Dziaman, A. Szpila, K. Linowiecka, J. Guz, J. Szpotan, M. Gawronski, A. Labejszo, et al. 2018. In vivo evidence of ascorbate involvement in the generation of epigenetic DNA modifications in leukocytes from patients with colorectal carcinoma, benign adenoma and inflammatory bowel disease. Journal of Translational Medicine 16 (1):204.
- Tee, E. S. 2009. Current status of recommended dietary allowances in Southeast Asia: A regional overview. Nutrition Reviews 56 (4):10–8.
- Tolbert, B. M., A. W. Chen, E. M. Bell, and E. M. Baker. 1967. Metabolism of l-ascorbic-4-3H acid in man. The American Journal of Clinical Nutrition 20 (3):250–2.
- Tsukada, Y-i, J. Fang, H. Erdjument-Bromage, M. E. Warren, C. H. Borchers, P. Tempst, and Y. Zhang. 2006. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439 (7078):811–6.
- Tveden-Nyborg, P., and J. Lykkesfeldt. 2013. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxidants & Redox Signaling 19 (17):2084–104.
- Van't Veer, P., E. Grammatikaki, C. Matthys, M. M. Raats, and L. Contor. 2013. EURRECA-framework for aligning micronutrient recommendations. Critical Reviews in Food Science and Nutrition. 53 (10):988–98.
- World Health Organization and Food and Agriculture Organization of the United Nations. 2004. Vitamin and mineral requirements in human nutrition: Report of a joint FAO/WHO expert consultation, Bangkok, Thailand, 21–30 September 1998. Rome: World Health Organization and Food and Agriculture Organization of the United Nations.
- Wrieden, W. L., M. K. Hannah, C. Bolton-Smith, R. Tavendale, C. Morrison, and H. Tunstall-Pedoe. 2000. Plasma vitamin C and food choice in the third Glasgow MONICA population survey. Journal of Epidemiology & Community Health 54 (5):355–60.
- Yatsuya, H., Y. Li, E. H. Hilawe, A. Ota, C. Wang, C. Chiang, Y. Zhang, M. Uemura, A. Osako, Y. Ozaki, et al. 2014. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circulation Journal 78 (12):2807–18.
- Yin, R., S. Q. Mao, B. Zhao, Z. Chong, Y. Yang, C. Zhao, D. Zhang, H. Huang, J. Gao, Z. Li, et al. 2013. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. Journal of the American Chemical Society.
- Young, V. R. 1996. Evidence for a recommended dietary allowance for vitamin C from pharmacokinetics: A comment and analysis. Proceedings of the National Academy of Sciences 93 (25):14344–8.
- Zhao, H., H. Zhu, J. Huang, Y. Zhu, M. Hong, H. Zhu, J. Zhang, S. Li, L. Yang, Y. Lian, et al. 2018. The synergy of vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leukemia Research 66:1–7.
- Zimmerman, M., and B. Snow. 2012. Understanding daily reference intakes. An Introduction to Nutrition 1.0: Unnamed Publisher.
