Abstract
Polar lipids including glycerophospholipids and sphingophospholipids are important nutrients and milk is a major source, particularly for infants. This systematic review describes the human and bovine milk polar lipid composition, structural organization, sources for formulation, and physiological functionality. A total of 2840 records were retrieved through Scopus, 378 were included. Bovine milk is a good source of polar lipids, where yield and composition are highly dependent on the choice of dairy streams and processing. In milk, polar lipids are organized in the milk fat globule membrane as a tri-layer encapsulating triglyceride. The overall polar lipid concentration in human milk is dependent on many factors including lactational stage and maternal diet. Here, reasonable ranges were determined where possible. Similar for bovine milk, where differences in milk lipid concentration proved the largest factor determining variation. The role of milk polar lipids in human health has been demonstrated in several areas and critical review indicated that brain, immune and effects on lipid metabolism are best substantiated areas. Moreover, insights related to the milk fat globule membrane structure-function relation as well as superior activity of milk derived polar lipid compared to plant-derived sources are emerging areas of interest regarding future research and food innovations.
1. Introduction
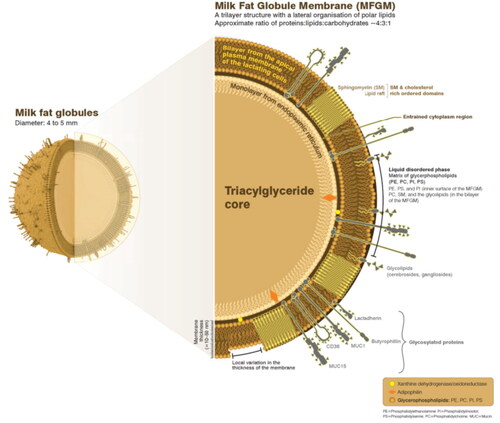
Milk is a rich source of polar lipids, which are known as essential building blocks for all biological membranes. Milk polar lipid classes include glycerophospholipids (PL) and sphingophospholipids (SL) where the latter are especially rich in milk as compared to vegetable sources. Milk PL include phosphatidylcholine (PC), phosphatidylethalomine (PE), phosphatidylserine (PS) and phosphatidylinositol (PI) species, their lyso-forms, and, particularly for PC and PE, plasmalogen forms. SL include sphingomyelin (SM), gangliosides, cerebrosides, sulfatides, ceramides and sphingosines (Vesper et al. Citation1999). Milk polar lipids account for only a small proportion of total milk lipids and are structurally organized at the milk fat globule (MFG) interface, the milk fat globule membrane (MFGM) (). To date, milk polar lipids and their metabolites, mainly studied in the form of MFGM and therefore also considering MFGM proteins, are recognized for their physiological activity well beyond providing structure to biological membranes, including effects on gut physiology, lipid metabolism, brain/cognition, endurance/physical performance, immune and skin conditioning/dermatitis (e.g. reviewed in Anto et al. Citation2020; L. Brink and Lönnerdal Citation2020; R. C. Da Silva, Colleran, and Ibrahim Citation2021).
As milk polar lipids play an essential role in human nutrition, it is important to understand their composition & physiological activities in human health. Bovine milk is one of the main industrial sources for polar lipids, in which infant nutrition is one of its major areas of application. Hence, a detailed understanding of compositional and structural similarities with human milk is warranted. Although the functionality of milk polar lipids has been reviewed previously (e.g. (Anto et al. Citation2020; L. Brink and Lönnerdal Citation2020; R. C. Da Silva, Colleran, and Ibrahim Citation2021)) a holistic and systematic approach is lacking. This systematic review provides an overview of the current state-of-the-art of milk polar lipid composition, structure, sources, and functionality, as well as a gap analyses with recommendations for future research.
2. Materials and methods
A systematic review on milk polar lipids was performed with scientific record retrieval using a combination of search queries. All searches were done using the Scopus database. Ultimately, search terms were classified into two terminology groups as specified in to have a broad coverage on topics. The search was narrowed to include publications in the last 30 years (1990–2021) as most of the technological advancements and literature related to milk polar lipid analyses were generated during this period. Other terms were evaluated for their inclusion in the search query but were excluded because they were either too general (glyco*, polar), too specific (sphingolipid, cerebroside, ganglioside) or did not retrieve any additional publications (comp*, infant, maternal) of relevance to the scope. The records retrieved included many research papers focusing on peptides and plant-based beverages, which were out of scope. Hence, two search terms, “plant” and “peptide” were specifically listed for exclusion in the search query to reduce the amount of non-relevant identifications. The final search query was defined as a combination of the following two searches.
Table 1. Search terms.
TITLE-ABS-KEY (milk AND phospholipid* OR phosphatid* OR sphingo* OR glycolipid* OR “polar lipid” OR cerebro* OR ganglio*) AND TITLE-ABS-KEY (bovine OR cow OR human OR breast OR mother OR lactat*) AND NOT TITLE-ABS-KEY (peptide* OR plant*) AND LANGUAGE (English) AND PUBYEAR > 1989
TITLE-ABS-KEY (milk AND phospholipid*) OR TITLE-ABS-KEY (phosphatid* OR sphingo* OR glycolipid* OR “polar lipid” OR cerebro* OR ganglio*) AND TITLE-ABS-KEY (bovine OR cow OR human OR breast OR mother OR lactat*) AND NOT TITLE-ABS-KEY (peptide* OR plant*) AND LANGUAGE (English) AND PUBYEAR > 1989
The final search was completed using Scopus to include records with a publication date (PUBDATETXT) up to May 2021. All identified articles based on the two search queries were screened for their eligibility. Authors began appraising papers based upon the title and abstract with four general themes to be discussed in this review: (i) Composition of bovine or human milk, (ii) MFG structure and structure-function relation, (iii) Milk sources and (iv) Physiological benefits. In three rounds of screening, whereby the entire selection of papers was randomly allocated to the three authors, all records were screened for eligibility. During each round of screening, authors independently excluded or included and categorized each paper based on the four themes and aims of this review. Any uncertainty was clarified in discussion at the end of the screening such that all papers included in this systematic review were mutually agreed upon by all authors.
Studies were included if they discussed bovine or human milk-derived polar lipids. Articles not in English were excluded. In addition, non-experimental publications were excluded, including conference papers, editorials and reviews. Some reviews were used for introduction. Specifically to the composition section, papers describing polar lipid levels in milk-based products including infant formula or disease related human milk levels were excluded. As for the section on structure, the biosynthesis of MFG and related synthetic systems were omitted. Specifically for the benefit section, observational cohort studies, in vitro and in vivo intervention studies describing the effect of dietary milk polar lipids were included. The benefit section focused on physiological benefits, where papers describing technological applications such as encapsulation and targeted delivery of biomolecules were excluded.
In turn, topics were assigned to different authors and full-text articles were assessed for eligibility according to the inclusion and exclusion criteria described above. For introduction purposes, and for further discussion of overall relevance of findings from individual studies, several references were added manually.
3. Results and discussion
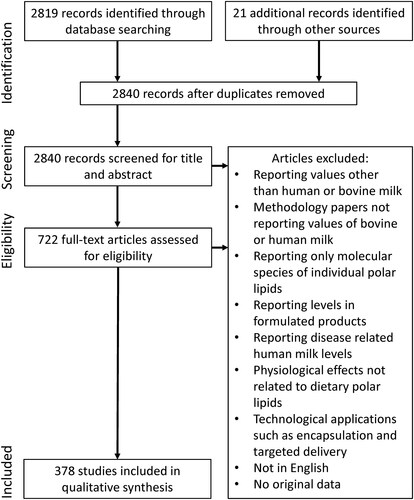
In total 2840 records were identified through database searching and manual addition. After screening and grouping of papers based upon titles and abstracts, 722 articles were selected to assess full-text eligibility. Ultimately, 378 articles were included based on the criteria described in section 2. An overview of the process is presented in .
Figure 2. Database search results according the Preferred Reporting Items for Systematic Reviews (PRISMA) statement (Moher et al. Citation2009).
A limitation of this systematic review is that search terms were biased toward polar lipids whereas the MFGM also contains proteins that may be of interest in addition to the overall MFGM composition, structure and physiological function described in this review. Due to the use of relatively broad search queries, many papers had to be excluded at a later stage. For instance, the search queries did not exclude papers that discussed effects of any intervention on blood phospholipids. Several studies included compositional information of both human and/or bovine milk polar lipids as well as information of other sources such as non-human/bovine milk or products such as infant formula. In this situation only compositional data on human or bovine milk was included. Only limited comparisons of individual study data were done, mostly as studies assessing PL levels varied in their analytical methods and outcome measures used, making it unfeasible to combine all data in a quantitative synthesis. Moreover, many confounding factors that affect concentrations have been described for both human and bovine milk which are discussed separately in this review. Nonetheless, where possible compositional data was presented in one unit and for, full breast, human milk reasonable ranges for total and individual PL were determined based on reported values.
3.1. Structure-function relation of the milk fat globule membrane
Milk lipids are secreted in fat globules, which consist of a central core of lipids (mainly triacylglycerides) surrounded by a monolayer of polar lipids, followed by a lipid bilayer with a glycosylated surface (). The tri-layer membrane is known as MFGM that acts to stabilize fat globules as an emulsion and protect lipids from coalescing (Jensen Citation2002). MFGs are important delivery vehicles of bioactive lipids such as triacyl-, di- and monoglycerides, saturated and polyunsaturated fatty acids, polar lipids, as well as fat-soluble vitamins (German and Dillard Citation2006). The composition and structure of MFGs could affect bio-functionality and variations are observed in milk from different mammalian species (Lu et al. Citation2016; Thum et al. Citation2020; Sophie Gallier, Gragson, Cabral, et al. Citation2010; Sophie Gallier, Laubscher, and Jiménez-Flores Citation2014). Although not part of the current review, these structural differences between species may be an interesting area regarding further understanding of the structural-functional relation of milk polar lipids and MFGM.
MFGM is composed of lipids, membrane-specific proteins and enzymes (Rombaut, Dewettinck, and Van Camp Citation2007; Lopez and Ménard Citation2011; L. R. Brink et al. Citation2020; Cavaletto et al. Citation1999). It is a heterogeneous mixture of macronutrients with the approximate ratio of proteins, lipids, and carbohydrates estimated to be 4:3:1 (Spence et al. Citation2009b). The lipid fraction of MFGM consists of polar and neutral lipids predominantly cholesterol (El-Loly Citation2011). The main polar lipids include PL (i.e. PC, PE, PI, PS), SL (i.e. SM and glycolipids) (Rombaut, Van Camp, and Dewettinck Citation2006; Bourlieu et al. Citation2018). In addition, polar lipids are also a major surface component of milk extracellular vesicles (Argov-Argaman et al. Citation2010; Pollott et al. Citation2016; Blans et al. Citation2017).
MFGM components are involved in regulating the biosynthesis of MFGs from the formation to the secretion of lipids droplets (Heid and Keenan Citation2005). MFGM provides key structural functionality to stabilize the lipid droplets in milk and to protect lipids from enzymatic degradation and coalescence. During digestion, MFGM components facilitate lipase docking and its emulsifying property is crucial for the uptake of lipids along the gastrointestinal tract, which is especially relevant early in life (Wooding and Mather Citation2017; Singh and Gallier Citation2017; Lopez, Cauty, et al. Citation2017; Garcia et al. Citation2014; Bourlieu et al. Citation2016; Clark and Laboda Citation1991). MFGM’s composition is highly similar to epithelial cell surfaces, and therefore is postulated to exert functions and activities beyond the delivery of the nutrients which has driven research into physiological benefits (see section 3.4 physiological benefits).
The amphipathic nature of milk polar lipids functions as the interface to encapsulate the inner neutral lipids core for dispersion in the aqueous environment (Smoczyński Citation2017). Polar lipids are not organized homogeneously in the MFGM. PE, PS, and PI are preferably located on the inner surface of the MFGM, whereas PC, SM, and glycolipids are mainly concentrated in the bilayer of the MFGM (Lopez et al. Citation2008). PC and SM are important for the structural maintenance of MFGM situated at the outer leaflet of the membrane bilayer (Lee et al. Citation2018; Lopez et al. Citation2011). An increasing PE content enhances fusion between lipid droplets and hence increases lipid droplet size during lipid synthesis (Cohen et al. Citation2017; Walter et al. Citation2020). The MFG size is the result of a balance between biosynthesis of polar and neutral lipids, lipid content, and fat to protein ratio (Couvreur and Hurtaud Citation2017; Ronit Mesilati-Stahy, Mida, and Argov-Argaman Citation2011; Duan et al. Citation2021). Changes of MFG size require reorganization of the interfacial material (Smoczyński Citation2017). The variation of MFG size and composition in milk from different species were recently reviewed by Thum and colleagues who demonstrate that many variations, including MFG size and composition, occur between human, bovine, ovine and caprine milk (Thum et al. Citation2021).
In MFGs, a localized arrangement of polar lipids is observed in which the liquid-ordered phase domains are consisted of SM-rich domains. SM together with cholesterol form lipid raft-like complexes that contribute to structural maintenance and influence the membrane fluidity of MFGs (Lee et al. Citation2018; Murthy, Guyomarc’h, and Lopez Citation2016). The acyl chain length, heterogeneity and saturation of fatty acids further affects the thermotropic phase behavior of the lipid raft (Et-Thakafy et al. Citation2018; Lopez, Cheng, and Perez Citation2018; Et-Thakafy, Guyomarc’h, and Lopez Citation2019; Chiantia and London Citation2012). The lipid raft is surrounded by a liquid-disordered phase composed of the phospholipids in which MFGM proteins are dispersed (Lopez, Madec, and Jimenez-Flores Citation2010). The phase and charge of polar lipids directly affect the interactions with proteins (Yeung et al. Citation2008; Obeid et al. Citation2019).
MFGM proteins account for 25–60% of the mass of the MFGM, 1–4% of total milk proteins, and 1% of the total globule mass (Manoni et al. Citation2020). The proteins can be classified into integral proteins and peripheral proteins, whereas others (e.g. lactadherin (H. Ye et al. Citation2013)) are partially embedded or loosely attached to the membrane. During secretion of MFGs, all constituents are rearranged within the apical plasma membrane and the MFGM. The localization of the proteins thus varies, some are associated with the inner monolayer membrane, while others are associated with the outer bilayer membrane. This includes glycosylated proteins (butyrophilin, lactadherin, MUC 1, MUC 15, CD36), non-glycosylated proteins (xanthine dehydrogenase/oxidoreductase, adipophilin), and surface proteins (β-lactoglobulin, sIgA and fatty acid-binding protein) (Manoni et al. Citation2020; Lefèvre and Subirade Citation2000; Andersen et al. Citation1997; Schroten et al. Citation2001).
Milk processing modifies the supramolecular structure and the surface composition of MFGs (Lopez Citation2005). The appreciation of the nutritional values of MFGM components in recent decades have led to the advancement in retaining the structural functionality and in optimizing the yield of MFGM. This has, amongst others evolved into efforts to mimic more closely the composition and structure of human MFGs (Sophie Gallier et al. Citation2015; Garcia and Innis Citation2013; R. C. Da Silva, Colleran, and Ibrahim Citation2021; Wei, Jin, et al. Citation2019) which is an interesting area of development regarding possible enhanced functionality compared to non-structured sources. Sources and processing optimization are further discussed in the following section.
3.2. Milk polar lipid composition
3.2.1. Phospholipid composition in human milk
3.2.1.1. Natural variation
Human milk phospholipid composition has been described by several studies. summarizes studies that have examined the composition of human milk. In these studies, careful attention was paid to homogeneity of expressed milk to ensure that within feed variation in lipid content was accounted for in data interpretation. Lactation stage appears to have a large influence on human milk composition and is the most widely studied factor influencing composition (as reviewed by (McJarrow et al. Citation2019)). Hence, data was categorized according to lactation stage and region, alongside whether it was milk obtained from preterm or term delivery. Controversy exists on whether PC or SM is the most abundant polar lipid in human milk. However, there is a consensus that choline-containing polar lipids are dominant components due to their critical role in neonatal membrane biosynthesis and organ growth (Lopez and Ménard Citation2011).
Table 2. Mean polar lipid content (mg/100 mL milk) and proportion of individual polar lipids (mg/100 mL milk and % of total polar lipid) in human milk.
Different methods for quantification were applied by different studies making an overall comparison of datasets challenging. Furthermore only a selection of studies reported used fully-expressed breastmilk (Holmes-McNary et al. Citation1996; Shunova et al. Citation2020; Wei, Jin, et al. Citation2019; Glew et al. Citation1995; Holmes, Snodgrass, and Iles Citation2000; Giuffrida et al. Citation2013; Li, Li, et al. Citation2020; Jiang et al. Citation2018; Davenport et al. Citation2015; Fischer et al. Citation2010; B.Y. Fong, Ma, and MacGibbon Citation2020; Thakkar et al. Citation2013a; Ma et al. Citation2017; Giuffrida et al. Citation2016; Benoit et al. Citation2010; Tavazzi et al. Citation2018; Selvalatchmanan et al. Citation2021), which would be preferable for quantification of total human milk composition. Other studies used only foremilk, hindmilk or did not disclose. Although LC-MS was most common among human milk studies, different analytical techniques may also affect specificity of PL analyses, with Liu et al. reviewing that hydrophilic interaction chromatography (HILIC)-MS is ideal for phospholipid quantification (Z. Liu, Rochfort, and Cocks Citation2018). Considering these points, a reasonable range of phospholipids was determined for term human milk based on all full-breast studies, specifically for colostrum and mature milk, including all regions and not differentiating for methodologies used ().
Apart from the above, inter-, and intra-individual factors also affect human milk’s phospholipid profile. One of this would be the nutritional status of the participants, with Dei Cas et al. correcting nutrient deficiencies prior to intervention (Dei Cas et al. Citation2020). Observations are discussed and the following factors may explain the reason for the differences.
3.2.1.2. Regional differences
Where similar analytical techniques were used, average PL concentrations from different regions were within typical ranges which further justifies our approach with determining reasonable human milk ranges and not differentiating further for regions. This finding is in accordance with Fong et al. who compared their dataset to recent regional studies and discussed that total PL concentration was comparable between their group of Chinese mothers and a UAE, Chinese, Malaysian and Singaporean cohort (B. Y. B. Y. Fong, Ma, and MacGibbon Citation2020)
3.2.1.3. Lactation stage (colostrum vs transitional milk vs mature milk)
Studies that examined milk polar lipid concentration generally identified colostrum as <5 days postpartum, transitional milk as ≥5 days to ≤2 weeks postpartum and mature milk as >2 weeks (Lindahl et al. Citation2019; Sala-Vila et al. Citation2005; X. Q. Zou et al. Citation2012; McJarrow et al. Citation2019). However, the lack of consistency across all studies confounded interpretation of PL concentration trends in these three types of milk. For instance, Holmes et al. considered day 7 milk as mature milk (Holmes, Snodgrass, and Iles Citation2000). Therefore, in our effort to provide reasonable ranges for, full breast, human milk only colostrum and mature milk values were considered.
As lactation stage advances and milk matures, it is hypothesized that the increased triglyceride synthesis in mammary cells results in the synthesis of larger MFGs (as reviewed by Barbas and Herrera Citation1998). This limits availability for polar lipid membrane material and hence, polar lipid concentration decreases, forming a thin membrane encapsulating the triglyceride-rich core (Bitman et al. Citation1984; Lindahl et al. Citation2019). This downward trend is notable in total phospholipids concentration as lactation stage progresses from colostrum to mature milk, but not always consistent amongst distribution of individual PL classes. This may be attributable to upward and downward trends in individual species of the five major polar lipids at different stages of lactation (McJarrow et al. Citation2019), but further review on this was beyond the scope of this review.
3.2.1.4. Gestation age (term vs preterm milk)
Lindahl et al. noted that the increased concentration of PE, PC and SM in preterm milk compared to term milk was most prominent in colostrum and became less apparent as lactation progressed (Lindahl et al. Citation2019). Polar lipid composition of preterm milk at the transitional stage appears to resemble full-term milk in early lactation. On the contrary, Holmes et al. noted no influence of gestational age (28–38 weeks) on choline content in human milk (Holmes, Snodgrass, and Iles Citation2000).
Xu et al. further explored upregulated and downregulated lipids in preterm colostrum compared to term colostrum (L. Xu, Chen, et al. Citation2020). Higher metabolic demands (Nilsson et al. Citation2018) or increased choline requirements (Bernhard, Poets, and Franz Citation2019) in the preterm infant, or immaturity of the mammary gland (Nilsson et al. Citation2018) may explain the higher polar lipid concentration in preterm colostrum. Taken together these studies thus reveal that preterm and term milk PL differences predominantly occur directly after birth and are likely related to metabolic differences between term and preterm infants.
3.2.1.5. Effect of genes, gender and diurnal variation
Single nucleotide polymorphisms in genes involved in choline metabolism (Fischer et al. Citation2010) and maternal hormone status (Ozarda, Cansev, and Ulus Citation2014) can influence PL content. Powe et al. noted that breastmilk produced for male infants had a higher energy content (Powe, Knott, and Conklin-Brittain Citation2010; Hinde Citation2007). In agreement with these findings, Thakkar et al. reported a higher mean lipid content, total PL and individual PL concentration in milk from male infants (Thakkar et al. Citation2013). Gender was found to have the highest influence at around 2 months and the role of gender on compositional differences was hypothesized to be attributable to longer nursing sessions or increased feeding volume (Thakkar et al. Citation2013).
Additionally, within-feed variation in human milk exists as well, whereby studies have noted that foremilk has a lower concentration of lipids (Thakkar et al. Citation2013; Selvalatchmanan et al. Citation2021) and polar lipids (Zeisel, Char, and Sheard Citation1986) compared to hindmilk. Likewise, evening milk is more lipid-rich than morning milk, particularly containing a 1.5-fold higher PE concentration (Selvalatchmanan et al. Citation2021). Milk polar lipid diurnal changes as well as changes during a feed are thus in line with the known differences for total milk lipids.
3.2.1.6. Effect of diet
Some studies corrected for nutrient deficiencies prior to intervention and although not examined further in this systematic review, nutrient deficiencies may affect incorporation of polar lipids into the MFGM. Roy et al. and Al-Tamer & Mahmood examined the association between socioeconomic status and dietary habits on milk polar lipid content (Roy et al. Citation2015; Y. Y. Al-Tamer and Mahmood Citation2006). Both studies noticed a difference in total polar lipid content upon stratifying according to lactation stage. SM content remained unchanged in interventions examining dietary patterns (Dei Cas et al. Citation2020) and choline intake (Davenport et al. Citation2015; Fischer et al. Citation2010). Apart from region, Glew et al. also examined the effect of malnutrition status on breastmilk composition (Glew et al. Citation1995). Total polar lipids and the concentration of individual PL classes in both moderately malnourished and severely malnourished groups were similar. In total, socioeconomic status and its influence on women’s dietary pattern may affect total milk polar lipid content but further research in a larger cohort may be warranted to conclude a definitive trend and determine if this is related to nutritional stress.
3.2.2. Phospholipid composition in bovine milk
3.2.2.1. Variation in bovine milk polar lipids
Bovine milk polar lipid composition has been studied by many studies (). Most studies reported that PC (12%–48%) and PE (3%–39%) are the most abundant polar lipid species in bovine milk. Differences in the sensitivity of the analytical techniques alongside breed, lactation stage, diet, sample size and environmental factors attribute to the variation in polar lipids quantified. Herewith, total milk lipid concentrations proved to be one of the largest factors determining variation. Moreover, Ali et al. discussed that polar lipids are impacted by technological treatment such as pasteurization, homogenization and freeze drying and as such processing of milk prior to compositional analysis can therefore be a confounder to true PL composition (Ali et al. Citation2018), discussed further in section 3.3 ‘Dairy sources and ingredients’. Attempts for a quantitative synthesis and determination of reasonable bovine milk PL ranges based upon all included studies in this systematic review emphasized that often important information about these confounding factors is missing and/or insufficient information to recalculate data into similar units is available. Therefore, no reasonable ranges for bovine are reported in this systematic review and it is recommended to focus on individual studies (e.g. selected based on bovine species and season) for estimating levels. For future analytical studies, particular for bovine milk and bovine milk derived products, more detailed information about the milk source, including at least information about the confounding factors discussed in this review, should thus be provided.
Table 3. Mean polar lipid content (mg/g of fat) and proportion of individual polar lipids (mg and % of total polar lipid) in bovine milk.
3.2.2.2. Breed and genetic differences
It is well-known that MFG size differs between cow breeds. For instance, MFG size is smaller in the milk of Holsteins compared to Jerseys (Graves, Beaulieu, and Drackley Citation2007). The size variation has been correlated to endogenous fat synthesis and hence milk fat yield (Graves, Beaulieu, and Drackley Citation2007; Lopez, Briard-Bion, and Ménard Citation2014). Smaller MFGs, for instance in the case of Holstein milk, contain a higher concentration of SM (Graves, Beaulieu, and Drackley Citation2007). Argov-Argaman et al. further examined the effect of polymorphisms in the DGAT1 enzyme’s gene and determined that there were differences in PL composition, influenced by changes in triglyceride synthesis (Argov-Argaman et al. Citation2013). Thus, genetic background of the cows will impact milk polar lipid composition, mostly related to MFG size.
3.2.2.3. Effect of parity
Although not widely examined, parity may influence milk PL levels. Cengiz et al. noted an increase in PL proportion from 1st to 3rd parity and a decline in 4th parity of Brown Swiss cows, but a dissimilar trend in Holstein cows (Cengiz et al. Citation2016). The SM concentration in both whole milk and as part of the milk fat of Jersey cows was found to increase in the third parity (Graves, Beaulieu, and Drackley Citation2007). The latter may be due to the decrease in MFG size with increasing lactation number (Campbell 1932 as cited in Graves, Beaulieu, and Drackley Citation2007).
3.2.2.4 Effect of lactation stage and within-milk differences
Studies identified in this systematic review mostly explored the effect of lactation stage on milk composition but ‘days in milk’ (DIM) of cows varied widely. Lactation stage appears to influence total PL and individual PL concentration, but the trend remains ambivalent as depicted in . Graves et al. reported that cows in late lactation (>200 DIM) had a higher milk fat content and a higher SM concentration in whole milk but SM as part of the milk fat was not affected by lactation stage (Graves, Beaulieu, and Drackley Citation2007). This may be confounded by changes in milk yield which decreases as lactation progresses (Bjerre-Harpøth et al. Citation2012). The authors also summarized findings from older literature, stating that MFG size decreases and the expression of MFGM increases with advancing lactation stage (Graves, Beaulieu, and Drackley Citation2007). Cheema et al. also reported a gradual increase in SM and PC concentration from early (DIM = 21) to late lactation (DIM = 217) in whole milk (Cheema et al. Citation2018).
Milk fat concentration is well-known to increase over the milking process (as reviewed in (Rico et al. Citation2014)). The authors discussed that the higher fat concentration in hindmilk may be explained by the secretion of more MFGs from the mammary gland (Rico et al. Citation2014). Interestingly, Wiking et al. discovered that a higher milking frequency (4 times daily versus twice) produced milk with a larger MFG size, and temporarily increased milk yield and fat percentage for 3 days (Wiking et al. Citation2006). Although not examined, this might have altered the polar lipid concentration as well. In conclusion, farm management factors related to lactation stage and milking may thus impact milk polar lipid composition.
3.2.2.5. Effect of cow feed
Studies identified in this systematic review that assessed the effect of feed on milk polar lipid composition mainly related to interventions aimed at changing the level of unsaturated fatty acids in the cow’s diet. Lashkari et al. noted an increase in SM concentration with high oleic sunflower seeds (10% of dry matter) while Lopez et al. noted a higher total PL and SM concentration with supplementation of extruded linseeds (Lashkari et al. Citation2020; Lopez et al. Citation2008). Conversely, Graves et al. found no change in SM concentration, milk fat or milk yield upon supplementing soybean oil (Graves, Beaulieu, and Drackley Citation2007) while conjugated linoleic acid reduced PE and PC concentration in milk fat (Zhang et al. Citation2021). Apart from supplementation, modification of concentrate to forage ratio also influenced concentration of PL classes whereby a high concentrate-low forage ratio increased daily yield of total phospholipids and individual phospholipid classes (Mesilati-Stahy, Malka, et al. Citation2015; Argov-Argaman et al. Citation2014). Interestingly, Mesilati-Stahy et al. examined the effect of glucogenic dietary supplementation on the composition of milk lipids and the cow’s estrous cycle, noting that PE and PS were higher in cows supplemented (R. Mesilati-Stahy, Malka, and Argov-Argaman et al. Citation2014). Thus, although mostly studied in relation to feed supplementation with unsaturated fatty acids, feed impacts the milk polar lipid profile.
3.2.2.6. Effect of season and temperature
Graves et al. observed that on average SM concentration in milk fat was the greatest during summer and the lowest in winter (Graves, Beaulieu, and Drackley Citation2007). On the other hand, Liu et al. observed the highest concentration for all PL classes in autumn (Z. Liu et al. Citation2017). However, advancing lactation stage and pasture availability may have confounded seasonal differences in milk composition (Walker et al. Citation2013; Z. Liu et al. Citation2017).
Heat stress in dairy cattle frequently occurs in temperate, tropical, and sub-tropical regions (Z. Liu et al. Citation2017). Consequently, feed intake and milk yield decreases, alongside physiological changes (Tian et al. Citation2016). The latter is particularly because heat dissipation consumes a considerable amount of energy, reducing energy availability for milk production which was also demonstrated to impact MFGM composition (West Citation2003).
3.2.3. Gangliosides in human and bovine milk
For the quantification of gangliosides several methods have been described. Older literature used high-performance thin-layer chromatography (HPTLC) to analyze ganglioside content (Li Pan and Izumi Citation1999) and quantified total gangliosides as total lipid-bound sialic acid (T-LBSA) or total lipid-bound n-acetylneuraminic acid. However, HPTLC has lower sensitivity and specificity compared to high performance liquid chromatography (HPLC) and mass spectrometry used in more recent studies (Ma, Liu, et al. Citation2015). Moreover, T-LBSA is not an accurate representation of total ganglioside content due to the interference from non-ganglioside components, potentially leading to an overestimation (Perea-Sanz, Garcia-Llatas, and Lagarda Citation2018; Ma, MacGibbon, et al. Citation2015). Hence, for accurate comparison, only studies quantifying gangliosides using liquid chromatography (LC) assays were included in this systematic review ().
Table 4. Mean ganglioside content (mg/L milk) and proportion of individual gangliosides (mg and % of total gangliosides) in human and bovine milk.
Summarizing, this systematic review reveals that most studies report disialoganglioside (GD3) as the dominant ganglioside class in human colostrum. As lactation progresses from colostrum to mature milk, monosialoganglioside (GM3) concentration increases (24% to 93%) while GD3 decreases (76% to 7%) (Ma, Liu, et al. Citation2015; Giuffrida et al. Citation2014; Giuffrida et al. Citation2016). Although this trend was consistent across all studies in terms of class, the relative distribution of some GD3 species increased as lactation progressed (Ma, Liu, et al. Citation2015). However, the trend for total ganglioside content remains equivocal with some studies reporting an increase while others demonstrating a decrease. Ma et al. reviewed that studies which used the older HPTLC method identified GT3 as the third most abundant ganglioside, a detail that the current HPLC method could not provide (Ma, MacGibbon, et al. Citation2015). The increase of GM3 relative to GD3 was much slower in preterm milk than in full-term milk which may be attributable to the shift in physiological needs of the breast-fed infant (Li Pan and Izumi Citation1999).
Multiple studies demonstrated a positive linear relationship between total ganglioside concentration (GM3 + GD3) and fat % in human breastmilk across 8 months, 12 months and 4 months parturition (Thakkar et al. Citation2013; Rueda et al. Citation1995; Ma, Liu, et al. Citation2015; Ma, MacGibbon, et al. Citation2015). The physiological relationship is not well-understood but it is hypothesized that a higher milk fat percentage allows for more gangliosides to be incorporated into the MFGM (Ma, Liu, et al. Citation2015; Ma, MacGibbon, et al. Citation2015).
Although the studies assessed in this review used LC-MS for quantifying gangliosides, it is noteworthy that the mass range used for detection varied and Ma et al. commented that some studies (Giuffrida et al. Citation2014; Thakkar et al. Citation2013) did not have the mass range to include all GM3 species (Ma, Liu, et al. Citation2015). This could potentially indicate an underestimation in the content of total GM3, total gangliosides or individual GM3 species. Nonetheless, for term human milk ganglioside ranges have been determined using all studies reporting LC-MS values (). For human milk colostrum and bovine milk ranges could not be determined because of a lack of sufficient data (specifically for bovine milk when correcting for confounding factors).
Bovine milk studies report a similar trend in dominant ganglioside and relative distribution of gangliosides (GM3 and GD3) with increasing lactation stage. However, ganglioside total concentrations are lower than in human milk. Multiple factors may affect ganglioside content such as MFGM size, lactation stage, species, and season (Potočki et al. Citation2016) as with phospholipids but there is a paucity in data on ganglioside concentration in bovine milk using more reliable LC methods. Rivas-Serna et al. reported that GD3, GT3 and GM3, in order of abundance, represent 80%–90% of gangliosides in mature bovine milk (Rivas-Serna et al. Citation2015).
3.2.4. Other milk polar lipids
Levels of other minor milk polar lipid species including cerebrosides, ceramides, plasmalogens and sulfatides are, relative to PL, SM, and gangliosides, less well described. Cerebrosides (mainly glucosylceramide and galactosylceramide) and lactoceramide (also classified as a globoside having more than one sugar as the side chain) have been identified in both human and bovine milk as part of the MFGM and levels in human milk have been reported at 9.9–17.4 and 1.3–3.0 µg/ml, respectively (Ma et al. Citation2020) or 1.8 µM (non-hydroxylated fatty acid-containing cerebroside species), 1.7 µM (hydroxylated and short-chain fatty acid-containing cerebroside species) and 931 nM (lactosylceramide) (Newburg and Chaturvedi Citation1992). Bovine milk levels differed from human milk with bovine milk mainly containing non-hydroxylated fatty acid cerebrosides (reported at 8 µM) and a lower concentration of lactosylceramide (reported at 17 µM) (Newburg and Chaturvedi Citation1992). Ma et al. reported levels at 9.8–12 (cerebrosides) and 14.3–66.2 µg/ml (lactosylceramide) (Ma et al. Citation2020). Specifically for bovine milk, a comprehensive study quantified individual species of glucosylceramide, lactocylceramide and (PC and PE) plasmalogens (Z. Liu et al. Citation2020). In this study, the glycerophospholipid phosphatidylglycerol was also identified as a minor milk polar lipid species. Plasmalogens have also been detected in human milk and consisted primarily of PC, PE species and their lyso-forms (Song et al. Citation2021; Moukarzel et al. Citation2016). Thus, only a few studies describe levels of the minor phospholipids and future work should elaborate further including factors that may influence levels in both human and bovine milk.
3.3. Dairy sources and ingredients
Although polar lipids represent only 0.5%–1.0% of the total milk lipids (Bourlieu et al. Citation2018; B. Y. Fong, Norris, and MacGibbon Citation2007), bovine dairy is of particular interest because of the high content of SM and PS and levels of more complex structures such as glycolipids compared to other lecithin sources such as soybean and egg yolk (Burling and Graverholt Citation2008). These lecithin sources typically contain high a level of PC and PE and a low level of SM, where soy lecithin specifically lacks SM (Szuhaj Citation2005). Functionally these differences may have large consequences as illustrated by several studies (see section physiological benefits).
Milk polar lipid content ranges from 3.6–479.5 mg/100 g among dairy products (Rombaut, Dewettinck, and Van Camp Citation2007). A low level of polar lipids could be detected in milk powder (Ali et al. Citation2017). Specific dairy ingredients rich in polar lipids can be categorized as either MFGM-enriched ingredients or phospholipids extracts, where MFGM-enriched ingredients generally provide a broader spectrum of MFGM components and may therefore be considered as a better source for nutrition (Fontecha et al. Citation2020). MFGM-enriched ingredients can be obtained from diary streams including whey and cream (S. Gallier, MacGibbon, and McJarrow Citation2018; Rombaut, Dewettinck, and Van Camp Citation2007). Whey is derived from either cheese manufacturing or caseinate manufacturing. Sweet whey is a side stream of rennet-coagulated cheese, and acid whey is a side stream of caseinate that is produced via acid acidification of milk. During cheese manufacturing, around 20% of the phospholipids are retained in the whey (Tania Ferreiro and Rodríguez-Otero Citation2018). Therefore, whey protein concentrates and byproducts of whey protein isolates such as whey PL are good sources for milk polar lipids, especially when additional steps are taken to enrich MFGM (Levin, Burrington, and Hartel Citation2016; Zhu and Damodaran Citation2013). Acid whey however is particularly low in PL as in the production of caseinates skimmed milk is used. As a further enrichment, MFGM enriched whey was also subjected to alcohol fractionation to alter levels and ratios of polar lipid which was demonstrated to impacted overall functionality (Boyd, Drye, and Hansen Citation1999; Zhu and Damodaran Citation2013) although its application on larger scale is limited. In addition to specific enrichment of MFGM, enrichment of specific whey proteins (such as alpha-lactalbumin) has also been demonstrated to result in concurrent enrichment of milk polar lipids as compared to conventional whey (Moloney, O’Connor, and O’Regan Citation2020).
Two different processes are applied to isolate triglycerides from cream and thereby simultaneously produce MFGM rich side streams: directly from cream or reworked from butter. In the direct process, cream is first centrifuged to preconcentrate the fat, followed by a homogenization and phase inversion process to produce anhydrous milk fat. In this process, two types of serum are obtained, the so-called alpha- and beta-serum which contain the MFGM components (Fontecha et al. Citation2020). Particularly the beta-serum contain a high level of PL. Anhydrous milk fat (often called butteroil) can also be produced from butter, which is obtained via churning of cream. During churning, the mechanical agitation in the presence of air results in the aggregation of fat globules and globular membrane disruption. The membrane material along with the water-soluble components gathered in the aqueous phase are called buttermilk (Corredig, Roesch, and Dalgleish Citation2003). Melted butter can subsequently be separated into anhydrous milk fat and butter serum through a centrifugation process. Although buttermilk contains a high concentration of polar lipids as MFGM (Ren et al. Citation1992; Ali Citation2019), butter serum has a higher content of material derived from MFGM and a higher proportion of SM compared to buttermilk (Lopez, Blot, et al. Citation2017; Calvo et al. Citation2020). Thus, within dairy production several sources can be extracted that contain high levels of milk polar lipids.
Further separation of neutral and polar lipids leverages the use of solvents for extraction and fractionation as e.g. discussed above for whey polar lipids. The use of hydrophobic and hydrophilic solvents mixture is widely adopted in industry were hexane or chloroform and hydrophilic solvent propanol or ethanol are used (Rombaut, Van Camp, and Dewettinck Citation2005; Boyd, Drye, and Hansen Citation1999). Triglycerides are extracted using hydrophobic solvents, while amphiphilic polar lipids can be effectively obtained with a mixture of hydrophobic and hydrophilic solvents. Moreover, the addition of sodium phosphate buffering was demonstrated to improve the recovery of MFGM from buttermilk (Spitsberg, Ivanov, and Shritz Citation2019). Other methodologies include zinc/calcium acetate precipitation, pressurized liquid extraction, supercritical fluid extraction, and functionalized polyvinylidene fluoride membrane and microfiltration (Price et al. Citation2020; Astaire et al. Citation2003; Verma et al. Citation2020; Costa et al. Citation2010; Castro-Gómez et al. Citation2017). Supercritical fluid extraction has been reported to increase the polar lipids content by selective removal of neutral lipids like triglycerides (Costa et al. Citation2010). To increase the polar lipid content of MFGM obtained through microfiltration, the use of supercritical carbon dioxide was employed, which reduced the concentration of neutral lipids and increase the concentration of polar lipids (Astaire et al. Citation2003).
The composition of polar lipids in MFGM products is thus highly dependent on processing methodology (Spence et al. Citation2009b; El-Loly Citation2011; Spence et al. Citation2009a; Haddadian et al. Citation2018; Yao et al. Citation2015; T. Ferreiro et al. Citation2016). As discussed, during milk processing the MFG is disrupted and MFGM is released into the aqueous phase by mechanical processes such as stirring, homogenization, aeration, and heating during butter and cheese production (K. Lee et al. Citation2020). Physical forces during homogenization for example, led to the partial disruption of the MFGM and to the adsorption of milk proteins i.e. caseins and whey proteins at the milk fat globule interface (Sophie Gallier, Gragson, Jiménez-Flores, et al. Citation2010; Martins et al. Citation2008; Kiełczewska et al. Citation2021; Lopez Citation2005). Similarly, heat processing causes the incorporation of denatured whey proteins (especially β-lactoglobulin) into MFGM and thereby modifies the polar lipid content of the MFGM (Reis et al. Citation2020; S. J. Lee and Sherbon Citation2002). Heat also induces changes in the microstructure of MFGM such as the formation of SM-rich domains (Et-Thakafy, Guyomarc’h, and Lopez Citation2017). In general, rapid cooling of milk enhances nucleation and growth of the lipid domains. Whereas upon heating, diffusion of the lipid domains, coalescence and reduction in domain size were observed. In addition, Maillard reactions between PE and reducing sugars yields PE-linked Amadori products which may be formed during powder production, although the influence of glycated lipids on health is unknown to date (Kodate et al. Citation2018). Furthermore, the choice of drying method could affect the morphological characteristics, thermotropic properties and oxidative stability of phospholipids (Zhu and Damodaran Citation2011). In addition, microfiltration and ultrafiltration are used to separate polar lipids from dairy components such as lactose, whey proteins and minerals (Gassi et al. Citation2016; Jukkola et al. Citation2019). Overall, the yield, purity and composition of milk polar lipids is thus dependent on the choice of dairy streams and processing.
3.4. Physiological importance of milk polar lipids
Milk polar lipids have been associated with physiological benefits since the early 1900s. Since then, numerous studies have demonstrated the effects of milk polar lipids on human health both in vitro and in vivo. This section discusses the physiological benefits associated with milk polar lipids in different areas from clinical (infant, maternal, adult) to animal and in vitro studies.
3.4.1. Source for essential nutrients
PL and SL have a relative complex composition and contain several (conditional) essential nutrients which can be released during digestion & metabolism. A range of studies discuss the contribution of PL and SL to the overall intake of these nutrients including choline, inositol, ethanolamine, cholesterol, sialic acid and essential fatty acids including docosahexaenoic acid (DHA).
3.4.1.1 Choline
Dietary PL are a good source of choline. While choline can be obtained through endogenous synthesis, a dietary source is required as endogenous synthesis if often not sufficient, especially during development (Carver Citation2006). Choline is essential for the synthesis of neurotransmitters (acetylcholine), trimethylamine, betaine and phospholipids and thereby contributes to functioning of the liver, muscle and brain; lipid metabolism and cellular composition and repair (reviewed in (Wallace et al. Citation2018; Wiedeman et al. Citation2018)). Intervention studies with choline or PC and experiments with choline deficient diets in dams during the suckling period further revealed that choline is critical for the absorption of fat in lactating rats and that choline deficiency alters intestinal morphology (R. P. da Silva et al. Citation2015). Other studies revealed that feeding mice choline in the form of PC through the maternal diet improved T cell proliferation and improved the ability of immune cells to respond to ex vivo mitogen challenges (Dellschaft et al. Citation2018). In piglets, moderate perinatal choline deficiency induced physiological and metabolomic changes including an increased hepatic lipid content and reduced brain size (Getty and Dilger Citation2015). Among the dietary sources, PC and SM contribute to the daily intake of choline. Although determining dietary reference values for choline may be challenging because of the lack of suitable biomarkers for choline intake, adequate intake levels have been defined for adults based upon average observed intake and taking in consideration the amounts that were needed to replete deficient subjects (EFSA Citation2016). Specifically, for infants, adequate intake levels were based on upwards extrapolation from the estimated choline intake of exclusively breastfed infants and for children based upon downwards extrapolation from adult adequate intake. Specifically for preterm infants, theoretical considerations and clinical data suggest adequate intake may be higher, as discussed by Bernhard et al. (Bernhard, Poets, and Franz Citation2019).
3.4.1.2. Inositol
Inositol is a sugar alcohol present in biologic systems primarily as myo-inositol. Phosphoinositides (predominantly PI) are PL species that contain myo-inositol. Inositol is considered as an essential nutrient contributing to many key functions in biological systems including signal transduction & metabolic regulation (Carver Citation2006). Many of the inositol physiological functionalities are assigned to phosphoinositides. Historically, inositol has been of interest with respect to diabetes mellitus, renal disease and growth and development. Breastmilk supplies a high concentration of inositol and term infants can produce inositol from glucose at rates sufficient to meet requirements (Brown et al. Citation2009; Holub Citation1992). Although the role of dietary inositol in infant development is not completely understood, studies indicate that inositol supplementation may be beneficial for infants born prematurely because of a possible insufficient endogenous synthesis (Hallman et al. Citation1992). A recently updated Cochrane systematic review however concluded that there was no significant benefit, and inositol supplementation should not be routinely applied for the nutritional management of preterm infants (Howlett, Ohlsson, and Plakkal Citation2019).
3.4.1.3. Ethanolamine
In milk, ethanolamine primarily occurs as headgroup of PE although its free form also has been detected in human milk. Humans are not capable of synthesizing ethanolamine and therefore milk PE is an important dietary source of ethanolamine. Ethanolamine has been demonstrated to enhance proliferation of epithelial cells, promote intestinal development and growth in weaned piglets (Yang et al. Citation2016) as well as affect microbiome during weaning in rats (J. Zhou et al. Citation2018) thus suggesting a crucial role for ethanolamine in gut physiology.
3.4.1.5 Sialic acid
Milk is a rich source of sialic acid in the form of oligosaccharides, glycoproteins or glycolipids although levels are typically lower in cow’s milk as compared to human milk (Spichtig, Michaud, and Austin Citation2010). Glycolipids in milk are consisted primarily of gangliosides, which are composed of a glycosphingolipid with one or more sialic acid residues. Sialic acid is considered as an essential nutrient for brain development and cognition (B. Wang Citation2009; Karim and Wang Citation2006). For example, animal studies suggest that dietary sources of sialic acid influence brain cortical composition (Scholtz et al. Citation2013). Moreover, the relevance of sialic acid for neurodevelopment is illustrated by the observations that brain levels of gangliosides and glycoproteins differ between breastfed and formula fed infants (B. Wang et al. Citation2003) thereby also illustrating the need for sialic acid enrichment in formula. Milk polar lipid preparations, particularly gangliosides, are thus an important source of sialic acid.
3.4.1.6 Essential fatty acids
Proper development of the brain, retina and other tissues depends upon provision of (conditional) essential long-chain polyunsaturated fatty acids (LC-PUFA) (Uauy et al. Citation2001). Milk provides LC-PUFA in the form of triglycerides (TG) or polar lipids and several studies have investigated the effect of the chemical structure on absorption and bioavailability. Although outcomes are not consistent across all studies and likely factors such as dosing and ratios with other fatty acids will affect outcomes, some studies suggest that LC-PUFA derived from PL are better absorbed and have a higher brain availability than TG-derived forms. In rats, formula enriched with different DHA sources including egg yolk-derived PL, single-cell algae-derived TG, a DHA-ethyl ester and a sn-2 DHA monoacylglyceride revealed that, although intestinal absorption was similar to the sources tested, tissue DHA accretion and milk DHA content were higher in PL and monoacylglyceride supplemented rats (Valenzuela et al. Citation2005). In Baboon neonates, a study with stable-isotope labeled Arachidonic acid (AA) in the form of TG (AA at sn-2 position) or PC revealed that brain enrichment of AA was about 2.1-fold higher for the PL form (Wijendran et al. Citation2002). Preferential incorporation of PL-derived AA was also observed in the liver, lung, plasma and erythrocytes. In line with these observation is the study from Amate et al. who revealed that AA and DHA levels in lipoproteins were significantly higher in piglets fed LC-PUFA in the form of PL than in piglets fed LC-PUFA in the form of TG (Amate, Gil, and Ramírez Citation2001). In preterm infants, Carnielli et al. compared intestinal absorption of, amongst others, LC-PUFA-enriched formula-derived either from PL or TG and revealed that absorption of DHA and total n-3 LC-PUFA was higher from PL than from TG containing formula whereas absorption of AA and total n-6 LC-PUFA was not different between the two formula (Carnielli et al. Citation1998). On the contrary, fatty acid composition of the main plasma lipid fractions was not affected by dietary LC-PUFA source (including egg-derived PL and single cell / fungi-derived TG) in full term infants (Sala-Vila et al. Citation2004). Similarly, LC-PUFA absorption into lymph was similar for PL or TG sources in rats although sn-positioning of DHA appeared to be different (Sehl et al. Citation2019). Overall, some studies thus suggest that LC-PUFA absorption from PL may be different than that from TG sources although further human studies are required.
Although not classified as a PL or SL and not part of the chemical structure, dairy-derived polar lipid preparations contain cholesterol, as cholesterol is an integral part of the MFGM structure (). Relative to infant formula (especially those containing non-bovine milk lipid sources and formula without added milk polar lipids), human milk provides a high amount of cholesterol both in its free and esterified forms (Koletzko Citation2016; Barness Citation1994). Cholesterol is an essential building block for cellular membranes and animal studies suggest that dietary cholesterol influence brain cortical composition and behavior (Scholtz et al. Citation2013). Furthermore, it serves as a precursor for synthesis of bile acids, lipoproteins, lipophilic vitamins, steroid hormones and oxysterols that modulate cholesterol, lipid and glucose homeostasis (Koletzko Citation2016; Cartocci et al. Citation2017). Therefore, milk polar lipid preparations, in particular MFGM ingredients, have been considered as a source of cholesterol (reviewed in, amongst others, (L. Brink and Lönnerdal Citation2020)). When added to infant formula, MFGM-enriched whey increased infant serum cholesterol concentrations thereby narrowing the gap as observed between standard infant formula-fed and breastfed infants (Niklas Timby, Lönnerdal, et al. Citation2014).
3.4.2. Gut physiology benefits of PL
Many studies discuss about the role of milk polar lipids along the gastrointestinal tract including effects on lipid digestion and absorption, cell proliferation and apoptosis, gut development and maturation and microbiome. In addition, this section discusses the digestion and absorption of milk polar lipids and cholesterol.
3.4.2.1. Digestion and absorption of milk polar lipids and cholesterol
3.4.2.1.1. Phospholipids
PL digestion is thought to occur in two phases, including a pancreatic (coinciding with the lipolysis of triglycerides) and a luminal phase (reviewed in (Méndez-Sánchez et al. Citation2019; Nilsson, Duan, and Ohlsson Citation2021)). Primary digestion of PL is mediated by pancreatic phospholipase A2 IB that hydrolyzes PL at the SN-2 position to form lyso-PL and free fatty acids. Pancreatic bile salt-stimulated lipase (although likely only active in the absence of bile salts and thus not primarily contributing to the hydrolyzes of dietary PL (Venuti et al. Citation2017)) and pancreatic lipase-related protein 2 (Lowe Citation2000) have further been suggested to contribute to PL hydrolyzes. Following hydrolyzes by pancreatic enzymes, the jejuno-ileal brush-border phospholipase B (Pind and Kuksis Citation1991) and mucosal secreted phospholipase A2 X (Ortega-Anaya and Jiménez-Flores Citation2019) and phospholipase A2 IIA (Herrera-Insua et al. Citation2001) have been suggested to contribute to PL hydrolyzes at the mucosal interface. Individual difference among the PL groups may occur, e.g. pancreatic lipase related protein 2 might be more important for PE hydrolyzes because of its higher affinity for PE (reviewed in (Herrera-Insua et al. Citation2001)). Furthermore, fatty acid chain-length may determine hydrolysis efficiency of the different enzymes. Once absorbed, digestive products of PL can either be reutilized in the intestine for synthesis of PL for secretion in chyme and/or chylomicrons, used as signaling molecules or transported to the liver for hepatic PL synthesis (Ko et al. Citation2020).
3.4.2.1.2. Sphingolipids
None of the discussed pancreatic enzymes that hydrolyze PL can hydrolyze SM. In the human body most SL are digested by brush border sphingomyelinases, where the alkaline isoform, also termed NPP7, removes the phosphocholine group to form ceramide (Nilsson Citation1969). Ceramidases subsequently catalyze the formation of ceramide, free sphingoid bases, and free fatty acids from dietary SM (Nilsson and Duan Citation2006). Human milk can further contribute to ceramide hydrolyzes by human milk bile-salt stimulated lipase which, however, did not reveal activity toward SM which thus appears to be hydrolyzed by an uncharacterized human milk acid sphingomyelinase (Nyberg et al. Citation1998). Cerebrosides are degraded to ceramide and sphingosine bases by a brush-border lactase-phlorizin hydrolase, whereas the digestion and absorption of gangliosides is less well understood (Nilsson Citation2016). Similarly, the digestion and absorption of other minor SL is poorly understood.
After absorption in epithelial cells, most sphingoid bases are rapidly metabolized into palmitic acid, but a part is reincorporated into SL and, like ceramides, transported into the circulation through lymph. Compared with sphingosine, intact SM and ceramides are more slowly hydrolyzed and absorbed in lymph as molecular species of ceramide because the sequential breakdown to fatty acids and spingoid bases is slow and incomplete (Morifuji, Higashi, et al. Citation2015). Interestingly, when consumed in the form of a milk phospholipid concentrate, recovery of ceramide moieties in lymph from dietary SM was significantly higher as compared to its pure form illustrating a possible benefit of more complex milk polar lipid preparations (Morifuji, Higashi, et al. Citation2015).
In humans, ileostomy experiments with diets containing purified milk-derived SM revealed that, although there was a large person-to-person variation, ∼80% of dietary milk-derived SM was digested and absorbed (Lena Ohlsson, Burling, et al. Citation2010). The latter experiments thus suggest that most of the SM in the normal diet is digested and absorbed whereas gangliosides and their metabolites can be detected in the colon (also see section 3.2.3 Microbiome). With respect to gangliosides studies in healthy volunteers consuming a milk fat fraction over a 8 week period (43 mg ganglioside/d) plasma content of total GD3 increased by 35% (Miklavcic et al. Citation2017). These results are in line with previous studies in rats applying labeled GD3 (Reis et al. Citation2016) and in vitro bio-accessibility studies that demonstrate availability of gangliosides from breast milk, infant and follow-on formula (Lacomba et al. Citation2011) although from the latter it is evident that the matrix may affect outcomes as bio-accessibility was not identical across all tested milks. Once absorbed into the circulation both GM3 and GD3 species can be transferred across the placenta and may become available to the developing fetus as suggested from placenta transfer studies (Mitchell et al. Citation2012).
3.4.2.1.3. Cholesterol
Cholesterol in the circulation results from dietary derived and endogenously synthesized cholesterol, where digestion and absorption of cholesterol has been the subject of recent elaborate reviews (e.g. (Ko et al. Citation2020)). In the human diet, cholesterol mainly occurs in its free form although cholesterol from cholesterol-esters can be released by pancreatic cholesterol esterase (Iqbal and Hussain Citation2009). Once in its free form, cholesterol can be incorporated into bile acid mixed micelles in the intestine that facilitate luminal uptake through different transporters (Ko et al. Citation2020). In enterocytes, cholesterol is loaded into chylomicrons or high-density lipoprotein particles and secreted into the circulation through the lymphatic system.
3.4.2.2. Effects on lipid digestion and absorption
Milk polar lipids are part of the MFGM that protect MFG against coalescence (). As a result of milk processing the MFG size and composition changes which will impact TG digestion (Luo et al. Citation2019). First, droplet size may affect overall TG hydrolysis because of differences in surface area, although lipolysis ability does not correspond linearly with surface area of fat droplets (Berton et al. Citation2012). Second, the interface of homogenized lipid globules is mainly covered by other surface-active molecules, predominantly milk proteins, which impact lipase activity. Whereas gastric lipase docking may be more efficient with protein stabilized emulsions resulting in an initial higher free fatty acid release during gastric digestion, overall TG hydrolysis under gastric conditions is higher with milk polar lipids stabilized emulsion which has been demonstrated by several studies applying different conditions, predominantly in vitro (Berton et al. Citation2012; Cheong et al. Citation2018; Liang et al. Citation2018; Luo et al. Citation2019; Garcia et al. Citation2014; L. Liu et al. Citation2021; Smoczyński and Kiełczewska Citation2014; Weng et al. Citation2021). In vivo, comparing breastmilk vs formula, human milk displayed an overall higher gastric lipolysis as compared to infant formula which, at least to a certain extent, may be caused by difference in MFG surface differences (Armand et al. Citation1996). In vivo, an infant formula containing large fat droplets coated with PL resulted in a different exhaled volatile organic compounds compared to a control infant formula mainly containing protein stabilized fat droplets, illustrating a possible digestive and metabolic impact of lipid droplet size and interface composition (Smolinska et al. Citation2019). Interestingly, the work from Lecomte et al. reveals that emulsions stabilized with milk PL rather than soybean PL may enhance lipid intestinal lipolysis and promote a more rapid intestinal lipid absorption and sharper kinetics of lipemia (Lecomte et al. Citation2015). More recent work further confirms these observation by demonstrating that emulsions containing bovine milk-derived polar lipids (MFGM enriched whey) relative to soy-derived PL increase postprandial TG absorption in newborn formula-fed pigs (Bach Korsholm Knudsen et al. Citation2021). When fed for 19 days to preterm piglets, these emulsions containing milk polar lipids resulted in altered plasma lipidome and hippocampal tissue diffusivity relative to emulsion containing soy-derived PL but had limited effects on other absorptive and learning abilities of preterm piglets (Henriksen et al. Citation2021).
Another mechanism through which dietary PL may affect lipid digestion and absorption is by altering biliary lipid composition which in turn full-fill a crucial role in lipid digestion and absorption (Devlin and Innis Citation1999).
Gangliosides may also possess the capacity to enhance lipid absorption as demonstrated in weaning rats fed ganglioside-enriched diets (Birecki et al. Citation2006). Although PL concentrations also differed between the diets and the mechanism of action remains to be elucidated, the ganglioside-enriched diets enhanced ex-vivo LC-PUFA uptake in both jejunal and ileal tissue isolates. In total these results demonstrate that both the size and the composition of the fat globule interface and the presence of MFGM affect lipid digestion and absorption with potential functional outcomes.
3.4.2.3 Cell proliferation and apoptosis
Although predominantly studied in vitro and far from medical application but of interest for potential anticarcinogenic activity, antiproliferative effects and apoptosis induction of milk-derived phospholipids has been investigated in several studies.
Castro-Gomez et al. isolated neutral and polar lipid fractions from buttermilk rich in PL and SL and demonstrated antiproliferative activity against human ovary and colon cancer cells in vitro (Castro-Gómez et al. Citation2016). Zanabria et al. demonstrated that MFGM isolated from raw milk displayed antiproliferative activity and induced apoptosis in a colon cancer cell line (HT-29 cells) (Zanabria et al. Citation2013). Mechanistic insights were given by Kuchta-Noctor et al. who demonstrate that a buttermilk-derived polar lipid fraction rich in SM and lactosylceramide induced caspase-independent cell death by a mechanism including downregulation of growth-signaling pathways mediated by β-catenin, phosphorylated Akt ERK1/2 and c-myc (Kuchta-Noctor et al. Citation2016). Antiproliferative activity of freshly isolated MFGM was sensitive to heat treatment, hydrolysis and/or phospholipase A2 treatment (Zanabria, Griffiths, and Corredig Citation2020), illustrating that processing may affect MFGM functionality and that overall antiproliferative activity cannot be explained by a single bioactive component. In addition, these results may also suggest that structural organization of the MFGM affects antiproliferative activity. Pulsed electric field processing, however preserved antiproliferative activity of MFGM relative to conventional heating procedures (S. Xu et al. Citation2015). The antiproliferative effect appears to be conserved to the MFGM fraction of more species, including goat and buffalo, as MFGM preparations of these species have revealed anti-proliferative effects on HT-29 cells and induced apoptosis in addition to bovine milk-derived MFGM (Ji et al. Citation2019) All treatments reduced mitochondrial membrane potential and induced apoptosis markers although goat appeared to be most effective. Thus, milk-derived polar lipids, mainly tested as MFGM, may affect cell proliferation and apoptosis in vitro and results and relevance for human health remain to be established in in vivo models and human studies.
3.4.2.4 Gut development and maturation
The interplay between nutrition, the gut microbiota, and its large number of metabolic and immune mediators plays an essential role in the development of gut homeostasis in early life as well as maintaining homeostasis later in life (Jain and Walker Citation2015). The human digestive tract is structurally mature at birth, yet maturation of gut functions such as digestion, mucosal barrier and signaling functions continues for the next 1–2 years. Although it has been demonstrated that these transitions can, to a certain extent, occur in the absence of stimulatory factors including dietary, hormonal or microbiome factors, the rate of gut development and maturation may be modulated. In artificially reared rats, which is considered as a model for gut maturation in the suckling infant, milk enriched with purified milk-derived SM accelerated enzymatic and morphological maturation of the intestine as compared to milk enriched with purified milk-derived PC (Motouri et al. Citation2003). In rat pups MFGM-enriched whey promoted intestinal proliferation and differentiation and increased tight junction protein expression (Gong et al. Citation2020). That milk polar lipids can effect gut permeability also became evident from work with an obesity animal model where gangliosides (given as a ganglioside concentrate containing 0.3% GM3 and 1.4% GD3 and formulated at 0.2 g gangliosides/kg diet,) and PL (given as a PL concentrate containing 3% PS, 31%PC, 8.7% PE and 16.5% SM and formulated at 10 g PL/kg diet) modulated tight junction expression (A. L. Zhou and Ward Citation2019). In vitro, purified milk-derived SM stimulated tight junction expression, possibly through increasing IL-8 expression and secretion whereas a whole milk polar lipid preparation (PL concentrate from buttermilk, containing >78% PL) did not show this activity (Marine Milard, Laugerette, et al. Citation2019). In vivo, SM increased expression of the murine IL-8 homologs Cxcl1 and Cxcl2 in the ileum whereas this stimulatory activity was not observed with the whole milk polar lipid preparation. Besides effects on permeability, dietary gangliosides (given as an ganglioside-enriched preparation containing 80% GD3, 9% GD1b and 5% GD3 and formulated at 0.02 % w/w of the total diet) were also demonstrated to enhance jejunal glucose uptake in weanling rats without effects on overall weight gain (Drozdowski et al. Citation2007). Although expression of major glucose transporters did not appear to explain observed maturation effects, it was suggested that stimulation of glucose uptake by gangliosides may be regulated posttranslational. In total, several studies thus demonstrate that milk polar lipids may affect gut development & maturation though different mechanisms.
3.4.2.5 Microbiota
Several studies have revealed that polar lipids can affect microbiome. In vitro experiments with MFGM preparations of different species suggested that main effects of MFGM on the intestinal piglet microbial population appears to occur in the ileum (Thum et al. Citation2020). In rat pups Gong et al. revealed that diets supplemented with MFGM-enriched whey restored a decreased intestinal mucosal barrier and microbiome alterations as observed when comparing formula vs maternal milk fed groups (Gong et al. Citation2020). In preterm infants, feeding a ganglioside-enriched formula (containing a ganglioside preparation from porcine brain) decreased fecal E. coli already at 7 days postnatal and increased fecal Bifidobacteria at day 30 postnatally (R. Rueda et al. Citation1998). Other studies also reveal a more indirect role of phospholipids on microbiome through effects on lipid emulsification and digestion (as discussed in section 3.2.2.) suggesting that both fat-type and emulsifiers in infant formula may have distinct effects on the development of the microbiome in formula-fed infants (Nejrup, Licht, and Hellgren Citation2017). With respect to fecal metabolites, human milk cohort studies associated, amongst others, breastmilk phospholipid with fecal short-chain fatty acids when comparing high and normal weight gain infants (Pekmez et al. Citation2020).
Besides effects on the intestinal microbiome, microbiota in the oral cavity of infants can also be affected by polar-lipids/MFGM which became evident from a study that revealed the oral microbiome in infants, which were fed an formula containing MFGM-enriched whey, differed from that of infants fed a non-enriched formula (Timby et al. Citation2017). As compared to differences in oral microbiota between breast and formula fed infants effects of MFGM-enriched whey were only small. However, the reduced incidence of otitis media in the MFGM-supplemented group illustrate the potential clinical significance of these small changes.
Bifidobacteria possess the capacity to degrade sialylated glycoconjugates, thus suggesting that glycolipids including gangliosides may affect microbiome (Kiyohara et al. Citation2011). B. infantis and B. bifidum substantially degraded GM3 and GD3 from bovine milk purified gangliosides in vitro, whereas B. longum subsp. longum and B. animals subsp. lactis only displayed moderate degradation (Lee et al. Citation2014). To a certain extent glycosphingolipids are also secreted in feces as demonstrated by a study that investigated differences between breastfed and formula fed infants over time (Larson et al. Citation1990). Thus, although different microbiome species poses the capacity to ferment gangliosides, degradation by microbiome does not appear to be complete.
3.4.3. Lipid metabolism
Milk-derived polar lipids have been demonstrated to affect lipid metabolism with possible relevance for the cardiovascular system, obesity, and obesity-associated complications. Clinically, butterserum derived PL (beverages formulated to deliver 975 mg SL/portion) affected postprandial lipid concentrations after consumption of an high fat breakfast in healthy adults males and as compared to a control drink containing egg PL (containing 119 mg SL/portion) (L. Ohlsson, Burling, et al. Citation2010). In this single intervention postprandial study, no differences were observed in postprandial TG, insulin, apolipoprotein (Apo)A1 or ApoB, however results suggested a lowered postprandial concentration of cholesterol in large TG-rich lipoproteins. When given over a 4-week period non-fractionated buttermilk displayed more pronounced effects in healthy adults when supplemented in the diet (Conway et al. Citation2013). In this study consumption of buttermilk over a 4-week period led to reduced serum cholesterol and TG levels. Similarly, buttermilk decreased an observed increase in serum total cholesterol and LDL-cholesterol as induced by increased egg consumption in healthy volunteers (Baumgartner et al. Citation2013). In overweight postmenopausal women, milk polar lipids derived from buttermilk (formulated in cream cheeses at 0, 3 and 5 g PL and given over a 4 week period) reduced fasting and postprandial plasma concentrations of cholesterol, total and high density lipoprotein-cholesterol and ApoB/ApoA1 ratios (Vors et al. Citation2020). PL appeared to decrease cholesterol uptake, which was evident from an increased fecal output of the intestinal cholesterol metabolite coprostanol, although no effects on major bacterial populations could be identified in fecal microbiome analyses. In a more acute setting, ingestion of milk PL in ileostomy patients further showed that milk PL decreased cholesterol absorption and increased ileal efflux of cholesterol co-excreted with SM (Vors et al. Citation2020). Later, the investigators also reported that the interventions reduced serum and chylomicron ceramide and SM species which was paralleled by a marked increase in fecal ceramide (Le Barz et al. Citation2021). Milk polar lipids thus decreased atherogenic SM and ceramide species and associated with the improvement of cardiovascular risk markers, thereby suggesting that sphingolipid metabolism in the gut contributes to the beneficial effects of milk polar lipids on lipid metabolism.
In another study, MFGM was demonstrated to modulate plasma lipoproteins, blood cell gene expression and cholesterol metabolism in healthy adults (Rosqvist et al. Citation2015). In this study interventions including either PL-rich milk fat given as whipping cream (normally having relative high level of PL and MFGM), or milk fat depleted of PL given as butter oil (∼15-fold lower PL content) were given over an 8-week intervention period. Control diets increased plasma lipids (including total cholesterol, LDL-cholesterol, non-HDL cholesterol and ApoB/ApoA1 ratio) whereas the MFGM-enriched diet did not. HDL-cholesterol, triglycerides, sitosterol, lathosterol, campesterol, and proprotein convertase subtilisin/kexin type 9 plasma concentrations and fatty acid compositions did not differ between groups. Mechanistically, gene expression profiles in peripheral blood mononuclear cells were also affected although overall meaning of these observations remain to be identified. In overweight or obese men, butterserum-derived PL relative to milk fat control reduced waist circumference and relative to milk fat or soy-derived PL decreased activity of γ-glutamyl transferase, a marker of fatty liver (Weiland et al. Citation2016). In another study with healthy adults, a formulation enriched with a buttermilk preparation did not influence fasting plasma lipid or lipoprotein levels compared to a control intervention with an approximate 2–3 times lower milk SL content (Lena Ohlsson, Burling, and Nilsson Citation2009). However, in the control group TG, cholesterol, LDL, HDL and Apo B concentrations increased over the 4-week study period which was not observed in the milk polar lipid intervention group. In conclusion, several studies applying different preparations demonstrate that milk polar lipids affect lipid metabolism in healthy and overweight individuals. Although some conflicting results have been reported, milk PL may affect surrogate markers of cardiovascular disease especially in overweight and obese adult subjects.
In infants, a formula containing MFGM-enriched whey resulted in overall higher total serum cholesterol than in infants fed a standard formula (generally depleted of cholesterol), gradually (over a 6 m period) reaching levels as observed in the breastfed reference group (Niklas Timby, Lönnerdal, et al. Citation2014). LDL to HDL ratios did not differ between formula interventions but were lower than the breastfed reference group. Studies in infants have thus mostly discussed a benefit of MFGM, delivering cholesterol, thereby narrowing the gap in serum lipid status between breastfed and formula fed infants.
Animal studies further provided insights in the effects of milk polar lipids. In mice fed a high-fat diet, a total lipid extract from buttermilk (containing 25% SM and formulated at 1.1 or 1.6% w/w) prevented body weight gain and modulated gut physiology as illustrated by altered colonic crypt depth and microbiome changes (M Milard, Laugerette, et al. Citation2019). In a similar setting, a milk-derived PL concentrate (containing 60% PL and 40% TG, formulated to deliver 1.2 % w/w PL in chow), unlike soy-derived PL in the same concentration, decreased high-fat feeding induced expression of inflammatory markers in white adipose tissue which coincided with an increased number of goblet cells in the colon (Lecomte et al. Citation2016). Functionally, effects on gut permeability and systemic inflammation have also been reported in high-fat fed mice (A. L. Zhou and Ward Citation2019). In another study employing high-fat diet fed mice, milk PL (formulated at 1.2% w/w PL and supplied by either a milk PL concentrate containing 42% PL or a concentrate containing 60% PL) reduced hepatic accumulation of intestinal cholesterol and increased fecal cholesterol excretion (Kamili et al. Citation2010). Similarly, a milk PL-enriched dairy fraction (∼50 % w/w PL) reduced hepatomegaly, hepatic steatosis and hyperlipidemia in high-fat fed mice (Wat et al. Citation2009). Furthermore, MFGM supplementation to anhydrous milk fat resulted in reduced levels of hepatic TG as compared to mice fed AMF without added PL (Albert L. Zhou et al. Citation2012). PL and SL may act differently on hepatic lipogenesis as demonstrated in a study where further fractionation of milk polar lipids was employed (Reis et al. Citation2013). The fact that total milk polar lipid preparations were effective in this and other high-fat diet studies thus suggests that different milk polar lipids may act in synergy.
In another obesity animal model, HDL receptor knockout mice fed a high fat and added-cholesterol diet, milk PL (buttermilk) strongly reduced atherogenic lipoprotein cholesterol, modulated microbiota, lowered inflammation and attenuated atherosclerosis development (Millar et al. Citation2020) further illustrating potential effects of milk PL on the cardiovascular system.
Milk SM appears to be an important factor in the observed effects given results with purified milk-derived SM and lack of effects of soy-derived PL in some of these models. Purified SM resulted in an increased fecal lipid output paralleled with decreased serum and liver cholesterol levels in obese/diabetic KK-A(y) mice (Yamauchi et al. Citation2016). In another study purified milk-derived SM reduced weight gain and improved lipid metabolism in mice fed a high-fat diet which was accompanied by an altered microbiome, predominantly reduced Gram-negative bacteria and increased Bifidobacterium (G. H. Norris et al. Citation2016). On the contrary, purified egg-derived SM did not show these improvements. The latter is in line with other mice studies demonstrating that milk-derived PL are more effective than egg PL in inhibiting cholesterol absorption (Noh and Koo Citation2004; Eckhardt et al. Citation2002). Thus, although containing SM, egg PL may be less effective than dairy derived PL to prevent obesity associated complications and impact lipid metabolism.
More complex PL structures as PL-coated lipid globules (S Gallier et al. Citation2015) have also been suggested to display similar activities, mostly in an early life nutritional programming setting. In these studies early life feeding of mice with large PL-coated lipid globules lowered body weight and fat mass later in life when challenged with a high-fat diet (Onne A.H.O. Ronda et al. Citation2019; Oosting et al. Citation2012; Oosting et al. Citation2014; Baars et al. Citation2016) At least to a certain extend these nutritional programming activity are caused by effects on post-absorptive trafficking of lipids as illustrated by reduced levels of TG in tissues (Onne A.H.O. Ronda, van de Heijning, et al. Citation2020) and altered hepatic fatty acid metabolism(O.A.H.O. Ronda, van de Heijning, et al. Citation2020).
In a maternal nutrition setting, supplementation of buttermilk-derived polar lipids in high-fat diet-fed rats during pregnancy and lactation improved body composition outcomes including a reduced white adipose tissue and body weight in off-spring (T. Li, Li, et al. Citation2020). Pups furthermore showed an increased adipose thermogenic function and improved plasma insulin and triglyceride levels. When supplemented exclusively to pups, MFGM also ameliorated metabolic disorder and improved glucose tolerance in offspring of high-fat fed mice (L. Ye et al. Citation2021). The results from these studies collectively suggest that milk polar lipids may ameliorate maternal obesity associated deleterious effects on the metabolism of their offspring either through the maternal or diet of the offspring.
In summary, both human intervention and animal studies suggest a role for milk polar lipids in controlling lipid metabolism with an overall favorable impact on the cardiovascular system and obesity associated complications.
3.4.4. Brain
Development of the brain during infant and early childhood is characterized by a rapid period of growth that coincides with the development of cognitive, behavioral, and social-emotional functions (Kadosh et al. Citation2021). During this period of development, underlying neuronal processes that include myelination, dendritic arborization and synaptogenesis and synaptic pruning can be affected by dietary factors which illustrates that nutrition may impact cognitive outcomes. Among the dietary factors affecting cognitive outcomes are polar lipids, as reviewed previously (for example (S. Gallier, MacGibbon, and McJarrow Citation2018)). The role of polar lipids is amongst others evident from observational cohort studies comparing breastfed or breastfed and formula fed infants. Children that received exclusive breastfeeding for at least 3 months had higher levels of overall myelination which was accompanied by increased general, verbal and non-verbal cognitive abilities as compared to exclusively formula fed infants (Deoni et al. Citation2018). In this study significant associations were found between formula composition (including PC and SM) and myelination trajectories. In another association study from the same investigators, early life dietary SM was also found to correlate to later cognitive development and levels of myelination in neurotypical children (Schneider et al. Citation2019). Further in vitro experiments in this study with rat derived oligodendrocyte precursor cells and coculture with primary neurons, revealed that SM treatment (bovine brain derived, applied as a liposome preparation containing PC and SM in a 3:1 ratio) resulted in increased proliferation, maturation, and differentiation of oligodendrocyte precursor cells as well as axon myelination.
Clinical intervention studies in infants related to brain benefits of polar lipids are mostly performed using MFGM preparations as the source. Most recently in combination with lactoferrin, MFGM (MFGM-enriched whey) was demonstrated to contribute to improved neurodevelopmental outcomes as assessed through the Bayley Scales of Infant Development (F. Li et al. Citation2019). This is in line with outcomes of another study that assessed effectivity of a low-energy, low-protein formula supplemented with MFGM-enriched whey where comparable neurodevelopmental outcomes were demonstrated (Niklas Timby, Domellöf, et al. Citation2014). Interestingly, further studies that focused on associated mechanisms in this study, reported effects of the intervention on oral microbiota (Timby et al. Citation2017) (see section gut physiology) and infections (Timby et al. Citation2015) (predominantly related to the immune outcomes, see section Immune), cardiovascular risk factors (see section Cardiovascular) (Niklas Timby, Lönnerdal, et al. Citation2014) and serum, plasma and erythrocyte membrane lipidome (Grip et al. Citation2018). Most recently, a follow-up analyses at 6–6.5 years of age revealed that neurodevelopment, growth, or plasma cholesterol status were no longer different between children fed the experimental or control formula during infancy (N. Timby et al. Citation2021). Work from Gurnida et al. showed that, compared to a non-enriched infant formula, a formula enriched with a complex milk lipid preparation containing gangliosides (formulated to enrich formula to a level of 2–3 mg/100 g GD3) improved hand and eye coordination, performance IQ and general IQ in 6-month old infants as assessed using the Griffith scale (Gurnida et al. Citation2012). Improved cognitive performance was accompanied by an increased concentration of serum gangliosides, both not significantly different from breast-fed infants. Results of the COGNIS study that tested an experimental formula containing MFGM, LC-PUFA, pre- and pro-biotics, gangliosides, nucleotides and sialic acid revealed that infant neurodevelopment as assessed by general movement tests was not different from infants fed with a standard formula or breastmilk (Nieto-Ruiz et al. Citation2019). Visual function as assessed by cortical visual evoked potentials differed between formula and breast-fed references and some beneficial effects of the experimental formula could be observed. Re-assessment at 2.5 y of age of these infants revealed that infants fed the experimental formula had fewer behavioral problems up to 2.5 y as assessed using the Child Behavior Checklist at 18 m and 2.5 y (A. Nieto-Ruiz et al. Citation2020). However, given the many differences between the experimental and the standard formula in this study it is difficult to conclude about the effects of milk polar lipids specifically. In healthy children (2.5–6 years old), MFGM-enriched whey (specified to have ∼9-fold higher PL levels than normal whey) decreased febrile episodes and improved behavioral outcomes as assessed using a validated questionnaire to assess behavior in 1.5- to 5-y-old children or the Achenbach System of Empirically Based Assessment (ASEBA), for the older children (Veereman-Wauters et al. Citation2012). Premature and very low birth-weight infants fed SM-fortified milk (SM 20% of total PL, milk-derived) compared to infants fed control milk (SM 13% of total PL, egg yolk lecithin) had improved neurobehavioral development as assessed by WP, Fagan, BSID-II attention and memory tests which was accompanied by increased plasma SM content (Tanaka et al. Citation2013). Several interventions with milk poler lipid preparations thus suggest a benefit of milk polar lipids for infant brain development.
With respect to maternal supplementation of complex milk lipids during pregnancy, effects on infant cognitive outcomes are currently explored (beta-serum derived, formulated in a maternal milk product supplying 8 mg gangliosides per day) (Huang et al. Citation2017). Initial results report about effects on maternal plasma ganglioside levels (Albert et al. Citation2021) and reveal that maternal MFGM supplementation during pregnancy is safe (T. Norris et al. Citation2019). However, to date no functional data about effects of maternal supplementation on infant’s cognitive performance have been reported.
In adults, a milk PL concentrate on a protein carrier (19% PL; 5% SM, 5% PC, 4% PE, 2.3% PS given as an enriched milk supplying 13.5 g PL per day) tended to shorten the reaction times in working memory tasks and positively affected stress as assessed through the Trier Social Stress Test (Hellhammer et al. Citation2010). Similarly, bovine milk-derived PL (as TG reduced cream powder enriched milk containing 0.5 or 1% PL supplying ∼1.25 and 2.5 g PL/day, respectively) attenuated stress-induced memory impairments in chronically stressed men as assessed by the Trier Social Stress Test (Schubert et al. Citation2011). Attenuated stress-responses were accompanied by an increased availability of cortisol. In another study bovine milk-derived PL (PL-enriched milk protein concentrate in water-based beverage supplying 2.7 g PL per day) improved cognitive performance in high perfectionist men under conditions of psychological stress, but failed to affect salivary cortisol stress responses (Boyle et al. Citation2019).
Animal studies provide further mechanistic insights in the role of polar lipids in brain development and cognitive performance. Although similar as with the human intervention studies, more complex polar lipid preparations like MFGM-enriched whey and cream serum-derived ingredients have been tested, a selection of studies also tested individual phospholipids. One particular study fed human milk to rats to investigate formula vs breastmilk differences and revealed differences in the brain SM class of lipids as assessed through lipidomics (Su et al. Citation2019). With respect to purified phospholipids, dietary SM (buttermilk-derived, > 90% purity) increased brain myelin and cerebroside content which was accompanied by morphological changes in developing rats challenged with L-cycloserine (inhibitor for the rate limiting enzyme for sphingolipid biosynthesize) (Oshida et al. Citation2003). In another study, rats fed a diet including prebiotics (GOS-PDX), lactoferrin and MFGM (MFGM-enriched whey protein concentrate) altered gene expression in neural circuits underlying emotion regulation and reduced anxiety-related behavior in open field tests (Mika et al. Citation2018). As the intervention included multiple active ingredients which were not present in the control, the specific role of MFGM thus remains to be identified. Another study in growing rats fed with a complex milk lipid preparation rich in PL and gangliosides (80% lipids of which 5.9% gangliosides, 50.6% PL and 23.5% neutral lipids) demonstrated improved learning behavior as illustrated by improved parameters related to novelty recognition and spatial memory (Vickers et al. Citation2009). Work from the same investigators also demonstrated that long-term supplementation of complex milk lipids (cream serum concentrate containing 13.7% PL and 0.63% gangliosides) during post-natal development improved memory in rats as illustrated by latency effects in Morris water maze, dark-light boxes and elevated plus maze tests and changes in expression of dopamine and glutamate neurotransmission pathways (Jian Guan, MacGibbon, et al. Citation2015). Similarly, a complex milk lipid preparation (containing 60% PL and 2% gangliosides) improved the memory of older (24 months) rats as assessed by Morris water maze tests (J. Guan, MacGibbon, et al. Citation2015). Functional improvement coincided with improved vascular density, dopamine output and neuroplasticity. Work from another group in aged rats revealed an improved contextual fear conditioning of rats fed an MFGM preparation (buttermilk concentrate), although other behavioral parameters were non-significant (García-Serrano et al. Citation2020). In the rat “pup in a cup” model postnatal supplementation of a formula containing MFGM-enriched whey promoted reflex development as compared to an non-enriched formula which was accompanied by differences in brain PL and metabolite composition (Moukarzel et al. Citation2018). Another preparation of PL in the form of large PL-coated lipid droplets has also been demonstrated to affect cognitive behavior in mice (Schipper et al. Citation2016). In this study, mice fed an enriched formula containing the PL-coated lipids displayed an improvement in short-term memory tasks (T-maze & novel object recognition) as compared to mice fed a formula containing a standard lipid source. In piglets, early supplementation of PL and gangliosides affected brain and cognitive development in neonatal piglets as illustrated by morphometric changes and effects on spatial T-maze tasks as compared to control animals (H. Liu et al. Citation2014). Work from the same group further studied neurodevelopment in MFGM-enriched whey fed piglets but failed to demonstrate significant differences as compared to the control group (Fil et al. Citation2019). Previously however, in the combination with prebiotics and lactoferrin, MFGM-enriched whey was demonstrated to affect structural neurodevelopment in the same piglet model (Mudd et al. Citation2016). Overall, a diverse range of milk polar lipid preparations have thus been demonstrated to affect cognitive outcomes in different animal models.
In animal models, maternal supplementation of milk polar lipid preparations has also been tested. In rats, maternal supplementation with a complex milk lipid preparation rich in PL and gangliosides (cream serum-derived, given at a ganglioside concentration of 0.01 and 0.05% w/w relative to measured food intake) during pregnancy and lactation resulted in higher neonatal brain weight and a changed neonatal brain lipid composition at birth (p2) (Gustavsson et al. Citation2010). However, effects on neonatal cognitive function could not be demonstrated as studied by water maze, object recognition and operant learning tasks and effects on brain composition where not observed anymore at weaning (p21). In an obesity setting, offspring born to obese (high-fat diet fed) rats displayed delayed neurological reflexes development, impaired neurogenesis before weaning and cognitive impairment in adulthood (high-fat diet feeding included after weaning) which were recovered by maternal MFGM PL supplementation (buttermilk-derived and given at 400 mg/kg body weight) (Yuan et al. Citation2021). In summary, results with maternal supplementation of milk polar lipids in animal models thus mainly suggest that offspring may be better protected against maternal obesity induced cognitive impairment.
Surprisingly few in vitro mechanistic studies have studied the role of milk polar lipid preparations on brain and cognitive health in cell culture systems, likely related to the lack of relevant cell culture models and the complex interplay between diet and brain in vivo. One particular study revealed that milk PL protect mouse-derived neuroblastoma Neur2a cells from endoplasmic reticulum stress induced cell death of possible relevance with respect to neurogenerative diseases (Nagai Citation2012), although overall clinical relevance remains to be established.
3.4.5. Endurance/physical performance
Effects of milk polar lipids on endurance, age-related frailty and physical performance has been studied clinically in several studies. An MFGM preparation (containing 33.3% PL) given as an oral supplement reduced frailty as assessed using Fried’s frailty phenotype in elderly women when given in combination with exercise and as compared to exercise with placebo intervention (milk powder containing 0.29% PL) alone or MFGM alone (Kim et al. Citation2015). In healthy adults (31–48 years), MFGM (buttermilk-derived, containing 16.6% PL) supplementation combined with exercise improved skeletal muscle strength as compared to the exercise and placebo (whole milk powder, containing 0.29% PL) intervention group (Soga, Ota, and Shimotoyodome Citation2015). In healthy elderly subjects (60–73 years) MFGM supplementation (containing 33.3% PL, 8.03% SM) together with mild exercise increased motor unit action potential conduction and improved muscle strength and physical performance as compared to placebo (containing 0.286% PL, 0.057% SM) intervention with exercise (Minegishi et al. Citation2016). Similarly home-based mild gymnastic exercise together with dietary MFGM (containing 16% PL) supplementation enhanced the effects of training as compared to a placebo (whole milk powder containing 0.32% PL) intervention (Yoshinaka et al. Citation2018). Contrary, augmented effects of MFGM with exercise could not be confirmed in community-dwelling elderly with declined walking ability (Kim et al. Citation2019).
Further mechanistic studies have been performed in animals. In mice, long term MFGM (containing 16.2% PL) intake combined with regular exercise improved endurance capacity in a dose-dependent manner via an increased lipid metabolism (Haramizu, Ota, et al. Citation2014). SM was suggested as one of the important drivers of these effects as interventions with purified SM (>98% pure) showed similar effects in this study. Also in mice, habitual exercise in combination with MFGM (containing 16.6% PL) attenuated age-related decline in muscle mass and contractile force (Haramizu, Mori, et al. Citation2014). Gene expression analyses and further in vitro analyses in this study showed that MFGM stimulated neuromuscular development and that PL and SL contribute to the effect. Later, a study in growing rats revealed that supplementation of different milk polar lipid preparations stimulated neuromuscular development independent from caloric value and an exercise stimulus (Markworth et al. Citation2017). In this study interventions were matched on ganglioside levels. Interestingly, both a beta-serum concentrate (containing 13.7% PL and 0.63% GD3) and a polar lipid-enriched complex milk lipid concentrate lacking MFGM proteins (71.7% PL and 1.8% GD3) proved effective, whereas effects where not different from control when given a complex milk lipid mixture (containing 45.2% PL and 4.8% GD3). Similarly, voluntary running exercise combined with supplementation of buttermilk-derived MFGM (containing 20.4% PL) attenuated age-related motor dysfunction in mice by suppressing neuromuscular junction abnormalities (Yano et al. Citation2017). Although further human intervention studies are required, milk polar lipids may thus impact endurance, age-related frailty, and physical performance, most likely through effects on neuromuscular junctions and when combined with exercise.
3.4.6. Immune
Immune modulatory activity, including anti-infective and anti-inflammatory activity, of milk polar lipids are well known and have been studied in different settings including human, animal and in vitro studies.
With respect to infection outcomes in adults, a milk polar lipid preparation containing 16% PL improved resistance to diarrheagenic E. coli as demonstrated in a challenge study with an attenuated diarrheagenic E. coli strain (Ten Bruggencate et al. Citation2016). Another ongoing study in adults studies assesses the effect of MFGM (as an milk beverage containing an PL-enriched lipid source, roughly 10x higher in PL than ordinary full-fat milk) on metabolic endotoxemia in adults with metabolic syndrome (Quarles et al. Citation2020). At the time of writing of this review results were however not yet available.
In 0–6 months old infants, an MFGM-enriched formula (containing MFGM-enriched whey) reduced the risk of acute otitis media and decreased overall antipyretic use to levels similar as observed in breastfed infants (Timby et al. Citation2015). Besides effects on pediatric upper respiratory infections, the overall immune modulatory effects of MFGM in this study was further illustrated by reduced serum IgG concentrations against pneumococcal serotypes 1, 5 and 14 after a first vaccination. In another ex vivo study, pre-exposure of non-inflamed bowel sections from infants that underwent surgery with bovine milk-derived gangliosides reduced bowel necrosis and proinflammatory signals upon challenges of the tissue with LPS and hypoxia (Schnabl et al. Citation2009) thereby further illustrating immunomodulatory activity of milk polar lipids. In children, the number of days with fever and the number of short febrile periods were decreased in children fed MFGM (given as enriched chocolate milk containing 500 mg PL through a 2.5% addition of MFGM-enriched whey and compared to non-enriched chocolate milk) (Veereman-Wauters et al. Citation2012). Other infectious/immune related outcomes including diarrhea, constipation, cough, doctor visit and days of school absence were similar. With respect to gastrointestinal infections in infants, Poppitt et al. studied the effect of a high-ganglioside complex milk lipid preparation on rotavirus infection and diarrhea in 8–24 months old infants (Poppitt et al. Citation2014). Despite an unseasonably low prevalence of diarrhea and rotavirus infection in the study group, mean duration of diarrhea was lower in the complex milk lipid intervention group. Assessment of overall efficacy of the intervention was however hampered because only a few end-of-trial samples were identified as rotavirus positive. In 6–11 months old infants, MFGM (given as an MFGM-enriched whey protein concentrate as complementary food and with normal skim milk proteins as control) reduced prevalence of diarrhea and bloody diarrhea (Zavaleta et al. Citation2011). Furthermore, in the combination with lactoferrin, a formula enriched with MFGM (through incorporation of an MFGM-enriched whey protein concentrate) reduced diarrhea and respiratory-associated adverse events (F. Li et al. Citation2019). Thus, in conclusion, intervention studies in infants, children and adults demonstrate a beneficial effect for milk polar lipids against infections or infection-associated complications.
A range of animal studies further support immune activity of different milk polar lipid preparations. In a mice LPS challenge model, MFGM (anhydrous milk fat with 10% MFGM) protected against LPS induced gastrointestinal leakiness which was accompanied by lower levels of serum cytokines (Snow et al. Citation2011). Similar observations came from a mouse study with LPS-challenged pups where MFGM increased growth, alleviated inflammation and increased tight junction protein and mucin expression in LPS-challenged pups (Huang et al. Citation2019). In a rat Listeria challenge model, buttermilk reduced the colonization and translocation of L. monocytogenes which, at least to certain extent, could be explained by inhibiting the adherence of L. monocytogenes to the intestinal mucosa as supported by in vitro experiments using cell lines (Sprong et al. Citation2012). A gangliosides-enriched preparation (containing 45–50 %w/w PL and 15–20 %w/w gangliosides) reduced Gardia muris parasite infection in inoculated mice as illustrated by a reduced number of released cyst and trophozoites in feces (Suh, Belosevic, and Clandinin Citation2004). In vitro growth studies further revealed that gangliosides can directly affect parasite survival. In rat models of arthritis, different fractions of MFGM (predominantly the PL-enriched MFGM isolates) reduced inflammatory responses including adjuvant-induced paw swelling which was supported by cell-based assays in vitro (Palmano et al. Citation2020). Different effects with ganglioside-enriched preparations were obtained in the same study thus suggesting that the anti-inflammatory activity of MFGM is mediated by different MFGM components that may act in concert.
In vitro, antimicrobial activity of milk polar lipid preparations has been demonstrated by different studies. Gangliosides purified from both human and bovine milk inhibited the adhesion of E. coli to human intestinal cells although human gangliosides proved more effective and GM1 was most effective when comparing efficacy of the individual milk ganglioside forms (Idota and Kawakami Citation1995). Another study showed similar anti-adhesion activity of GM3, GD3 and GM1 against enterotoxigenic E. coli (ETEC), enteropathogenic E.coli (EPEC), Listeria monocytogenes, Salmonella entericaserovartyphi, Shigella sonnei, Campylobacter jejuni and Helicobacter pylori (Salcedo et al. Citation2013). Isolated MFGs, MFGMs, lactosylceramide and gangliosides GM3 and GD3 bound to ETEC strains isolated from diarrheic calves (Sánchez-Juanes et al. Citation2009). Interestingly, adhesion to bovine milk colostrum-derived gangliosides was considerably weaker than that to gangliosides isolated from other lactational stages (Martín et al. Citation2003). Contrary to the levels of unsaturated FAs and sphingosine, the carbohydrate moiety did not differ between gangliosides isolated from different lactational stages. Hence, these results suggest that the ceramide fraction of gangliosides is the most prominent factor for binding calve-derived ETEC strains.
Digestion products of SL and to a lesser extend digestion products of PL displayed bactericidal activity against E.coli 0157:H7, Salmonella enteritidis, Campylobacter jejuni and Listeria monocytogenes in vitro suggesting that milk polar lipids, in particularly SL, may enhance the resistance to food-borne gastrointestinal infections (Sprong, Hulstein, and Van Der Meer Citation2001; Sprong, Hulstein, and Van Der Meer Citation2002). Besides, MFGM as a whole bovine milk isolate has also been demonstrated to show bactericidal effects on E.coli 0157:H7 by affecting virulence factor expression (Tellez et al. Citation2012), thereby illustrating the multifactorial action of MFGM. Similar as with pathogenic bacteria, MFGM has been demonstrated to inhibit adhesion of probiotic strains like Lactobacillus rhamnosus GG (LGG) (Guerin et al. Citation2018) although functional consequences for overall LGG functionality are unknown.
In addition to antimicrobial activity, antiviral activity of milk polar lipids has been another area of in vitro research. Rotavirus infections is one of the most important causes of severe dehydrating diarrhea in infants and children. Besides treatment with oral rehydration solutions and prevention by vaccination, nutritional interventions with bioactive compounds have been considered as a strategy against rotavirus infections (see e.g. (Kvistgaard et al. Citation2004) describing the activity of the MFGM protein lactadherin). Bovine milk-derived MFGM (isolated from buttermilk or whey cream) displayed anti-infective activity against neuraminidase-sensitive rotavirus in a dose dependent manner (Fuller et al. Citation2013). Buttermilk MFGM displayed a more prominent inhibition than cheese whey MFGM in this study which was discussed to be caused by a more complex FA composition and more abundant level of glycolipids in buttermilk- derived MFGM. Another in vitro study demonstrated activity of cerebroside against reovirus strain type 1 lang, and GD3 and GM3 against both reovirus strain type 1 lang and type 3 Dearing (Iskarpatyoti et al. Citation2012), suggesting that milk polar lipids can affect other reoviruses besides rotavirus. Other viruses that may be inhibited by milk polar lipids in vitro include HIV, inhibited by human milk-derived sulfated glycolipids (Viveros-Rogel et al. Citation2004) and respiratory syncytial virus inhibited by human milk-derived GM2 (Portelli, Gordon, and May Citation1998). The latter study revealed that human milk-derived gangliosides did not inhibit Semliki Forest virus and cytomegalovirus in vitro which is in line with another study demonstrating that both cytomegalovirus and three rhinovirus strains were not affected by human milk-derived gangliosides (Clarke and May Citation2000).
Another important antipathogenic activity of milk polar lipids is neutralization of pathogen virulence factors such as toxins by preventing binding of these factors to their target receptors along the gastrointestinal tract. The lipid fraction of human milk is able to bind shiga-toxin produced by shiga toxigenic E. coli (STEC) (Herrera-Insua et al. Citation2001) most likely through GB3 which may protect infants against diarrheal diseases (Newburg, Ashkenazi, and Cleary Citation1992). Similarly, human and bovine milk GM1 was demonstrated to neutralize cholera toxin (Iwamori et al. Citation2008; Iwamori Citation2011). On the other hand, botulinum neurotoxin did not bind to any gangliosides from human and bovine milk (Iwamori et al. Citation2008). Human milk gangliosides were further demonstrated to inhibit vacuole formation activity of Helicobacter pylori vacuolating cytotoxin (Wada et al. Citation2010). All gangliosides analyzed, GM1, GM2, GM3, GD1a, GD1b, GD3 and GT1b, revealed a significant neutralizing capacity and as tested for GM1, the lyso-form remained active suggesting that gangliosides, at least to a certain extent maintain their functionality during digestion.
Immune cell development is also affected by milk polar lipids as revealed by several in vitro studies. Treatment of bone marrow-derived dendritic cells with human milk-derived GD3 and GM3 revealed that these gangliosides could affect dendritic cell maturation, where GD3 displayed a more prominent effect than GM3 (Brønnum et al. Citation2005). Fermented milk-derived SM (predominantly having C21, C22, C23 and C24 FAs) and the digestion products sphingosine and lyso-SM enhanced human osteosarcoma interferon-β production whereas ceramide and cerebroside did not (Osada et al. Citation1993). Thus, although overall physiological relevance remains to be established, immune cell development and stimulation of interferon-β by milk polar lipid species may contribute to the observed protective effects in animal, adult, and infant studies.
With respect to allergy, and although the overall consequence for allergic reactions remains to be established, there are some indications that milk-derived polar lipids can induce invariant natural killer T-cells in food allergy in children, specifically demonstrated for α-hexosylceramides, which are present in very low amounts in bovine milk, and the more abundant SM (Brennan et al. Citation2017; Jyonouchi et al. Citation2011).
Overall, clinical (adult, children and infant) intervention studies, supported by a range of non-clinical (animal and in vitro) studies thus demonstrate that milk polar lipids in different preparations display immunomodulatory activity.
3.4.7. Dermatitis and skin conditioning
Reduced levels of epidermal ceramide content are associated with skin diseases including dermatitis, psoriasis and xerosis (Uchida and Park Citation2021) which is why several groups studied the effect of milk polar lipids and/or milk-derived SM on skin conditioning. Clinically, the effect of daily consumption of a polar lipid-enriched milk (containing a butterserum concentrate with 31.2% PL, daily administration of 3 g PL, 0.75 g SM) for 6 weeks in patients with atopic dermatitis was compared to the effect of daily administration of normal milk (Keller et al. Citation2014). From baseline, both interventions improved skin outcomes as assessed by SCORAD indices, and there appeared to be no further benefit of added PL. In healthy subjects aged 20–39 years with low skin hydration, a SM-enriched milk polar lipid preparation (buttermilk-derived, containing 5.9% SM, other PL not specified) improved skin conditioning (Higurashi et al. Citation2015).
More mechanistic insights about the possible effect of milk polar lipids on skin conditioning came from animal studies. In hairless mice a dietary PL concentrate from bovine milk (buttermilk-derived) improved epidermal function as a result of increased ceramide content resulting in a higher skin hydration (Haruta-Ono, Setoguchi, et al. Citation2012). Work from the same investigators, using radio-labeled SM administration in hairless mice further, demonstrated that SM is incorporated into skin SL where it may affect water-holding capacity of the skin (Haruta-Ono, Setoguchi, et al. Citation2012). Further studies by these investigators include SM dose-dependency studies in these mice (Haruta-Ono, Ueno, et al. Citation2012) and similar results were obtained with more complex milk PL polar lipid preparations (Morifuji, Oba, et al. Citation2015). More recent studies further demonstrate that dietary SM (≥98% purity, milk-derived) prevented ultraviolet-B irradiation induced skin barrier disruption in hairless mice (Oba et al. Citation2015). These results are in line with other ex vivo studies demonstrating that milk-derived PL can protect skin cells against ultraviolet radiation induced changes (Russell et al. Citation2010). In summary, although several preclinical studies reveal a role of dietary milk-derived polar lipids in skin conditioning, clinical studies are limited and report conflicting results.
3.4.8. Growth and safety
For infant nutrition applications it is important to demonstrate growth and safety of purified or enriched milk PL preparations, which has been demonstrated in several dedicated studies in addition to more elaborate studies also reporting about milk polar lipid functionality. Related to effects of milk polar lipids on growth in general, a preterm breastmilk cohort study that investigated the role of the human milk lipidome provided insights (Alexandre-Gouabau et al. Citation2018). In this study infants that demonstrated a faster weight Z-score between birth and hospital discharge consumed milk that was, amongst others richer in SM and dihomo-γ-linoleic acid-containing PE. The authors discuss that these lipid species may be considered as biomarkers to predict early-growth although because of the many confounding clinical factors further validation as well as mechanistic insights remain to be established.
In infants, a key study related to an experimental formula containing MFGM-enriched whey and having a reduced overall protein concentration demonstrated that infant growth, weight gain, BMI, percentage of body fat and head circumference did not differ from infants fed a standard infant formula (Niklas Timby, Domellöf, et al. Citation2014). As a result of the slightly lower caloric density of the experimental formula, infants consumed more volume as infants consuming the standard formula thereby fully compensating for the caloric density difference. Similarly, an experimental formula supplemented with functional ingredients including MFGM (described as 10% of total protein) demonstrated normal growth and neurodevelopment (Nieto-Ruiz et al. Citation2019) thus suggesting that enriching formula with bovine milk-derived MFGM results in a normal growth as compared to standard formula. Furthermore, in healthy adults a high dose of MFGM (6.5 g buttermilk-derived, containing 231 mg SM, ∼5 times the recommended dose for prevention of age-related muscle mass) given as dietary supplemental proofed safe for healthy adults (Hari et al. Citation2015). In conclusion, in addition to the many functionality studies reporting about growth, several dedicated studies in infants and adults demonstrate growth and safety of milk polar lipids in different applications.
4. Conclusions and future perspectives
This systematic review demonstrates that milk polar lipids are important nutritional components that may deliver physiological benefits for all age-groups. Natively forming a tri-layer at the milk fat globule interface, structural and compositional similarities occur between milk species including human and bovine as discussed in this systematic review. Although the native MFGM lipid globule structure is lost during processing of milks and overall composition is affected by many factors, bovine milk-derived ingredients including whey and cream derived MFGM-enriched sources are applied for nutritional supplementation of milk polar lipids in food products. Well substantiated physiological benefits supported with well-designed clinical studies and mechanistic support include brain and immune benefits and effects on lipid metabolism, although mostly tested clinically as more complex MFGM-enriched ingredients. Conflicting results are evident for virtually all areas studied and are likely related to the milk polar lipid source, dosing and/or complexity of the models used to demonstrate the benefit. An emerging area is the structure-function relation of milk polar lipids as MFGM with interesting developments including new food innovations such as structured lipid globules in infant nutrition and insights about the role of PL on lipid digestion and absorption. Furthermore, this review clearly indicates superior functional benefits of milk polar lipids in several areas as compared to non-milk polar lipid sources. This is another interesting emerging area which will be an important area of research to investigate, for example, interchangeability of PL sources in food products or designing infant formula with a PL profile inspired by human milk composition. Although, a lot of knowledge about milk polar lipids is gained to date, several aspects remain to be elucidated in future studies.
4.1. Need for consistent and coherent reporting of analytical data
This systematic review reveals that many confounding factors determine the overall milk polar lipid concentration in human and bovine milk. Combining results from all studies and attempting to determine reasonable ranges proved challenging in this systematic review, especially for bovine milk. Mainly because of the many confounding factors but also regarding the inconsistent use of units (and lack of reporting all information required to calculate data into similar units) across different studies. Therefore, a clear need regarding milk polar lipid analytics is the use of consistency in units when analyzing milk. With the rapid development of sensitive methodologies and standardization of methods this will become standard practice in future analytical studies. Considering the many confounding factors a detailed reporting of the milk origin and additional information such as total milk lipid concentration will be important.
4.2. Minor species
Only a few studies have investigated composition of the minor milk polar lipid species especially related to cerebrosides, plasmalogens and sulfatides. Although technologically challenging, future studies should elaborate further about minor polar lipid species composition and their overall physiological benefits. Of relevance for early life nutrition herewith, will be the differences between human and bovine milk composition and functionality.
4.3. Complex MFGM preparation vs single polar lipids
Some studies already demonstrated that more complex milk polar lipid preparations are more effective than individual PL, suggesting that, at least to a certain extent, milk polar lipids may act in synergy especially when also considering the protein fraction of MFGM. Future studies may focus more on effects of individual PL as well as effects of different PL acting in synergy. Moreover, although the current review focusses on polar lipids, other MFGM components such as MFGM proteins are gaining more attention because of their possible contribution to overall MFGM benefits. Although the difference between vegetable and milk sources (mainly related to SM) are well described, herewith the physiological importance of small differences in PL composition between milk polar lipid sources will become evident.
4.4. Human studies
Although certain benefits are well substantiated, overall, more clinical intervention studies are required, preferably in a setting where milk polar lipids are the only difference between test and control groups. This may particularly be important for infant formula, as the majority of reported infant studies described experimental formula in which, relative to the control formula, other changes than MFGM- enrichment were made. Herewith a clear description of the milk PL source will be important given the many sources and milk ingredients available for formulation and their differences as discussed in this review. Further, clinical studies may also unravel possible difference between different dairy sources particularly whey vs cream derived sources as from the current systematic review it is evident that compositional difference may occur.
| Abbreviations | ||
| MFG | = | milk fat globule |
| MFGM | = | milk fat globule membrane |
| PC | = | phosphatidylcholine |
| PE | = | phosphatidylethanolamine |
| PI | = | phosphatidylinositol |
| PL | = | glycerophospholipids |
| PS | = | phosphatidylserine |
| SL | = | sphingophospholipids |
| SM | = | sphingomyelin |
Disclosure statement
MV, LWC and TTL: employment FrieslandCampina.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Al-Tamer, Y. Y, and A. A. Mahmood. 2006. The influence of Iraqi mothers’ socioeconomic status on their milk-lipid content. European Journal of Clinical Nutrition 60 (12):1400–5. doi: 10.1038/sj.ejcn.1602470.
- Al-Tamer, Y. Y, and A. A. Mahmood. 2004. Fatty-acid composition of the colostrum and serum of fullterm and preterm delivering Iraqi mothers. European Journal of Clinical Nutrition 58 (8):1119–24. doi: 10.1038/sj.ejcn.1601939.
- Albert, B. B., J. G. B. Derraik, Y.-Y. Xia, T. Norris, T. Zhang, T.-L. Han, C. Chang, A. Rowan, S. Gallier, R. T. Souza, et al. 2021. Supplementation with milk enriched with complex lipids during pregnancy: A double-blind randomized controlled trial. PloS One 16 (2):e0244916. doi: 10.1371/journal.pone.0244916.
- Alexandre-Gouabau, M.-C., T. Moyon, V. Cariou, J.-P. Antignac, E. Qannari, M. Croyal, M. Soumah, Y. Guitton, A. David-Sochard, H. Billard, et al. 2018. Breast milk lipidome is associated with early growth trajectory in preterm infants. Nutrients 10 (2):164. doi: 10.3390/nu10020164.
- Ali, A. H. 2019. Current knowledge of buttermilk: Composition, applications in the food industry, nutritional and beneficial health characteristics. International Journal of Dairy Technology 72 (2):169–82. doi: 10.1111/1471-0307.12572.
- Ali, A. H., W. Wei, S. M. Abed, S. A. Korma, A. H. Mousa, H. M. Hassan, Q. Jin, and X. Wang. 2018. Impact of technological processes on buffalo and bovine milk fat crystallization behavior and milk fat globule membrane phospholipids profile. LWT - Food Science and Technology 90:424–32. doi: 10.1016/j.lwt.2017.12.058.
- Ali, A. H., X. Zou, J. Huang, S. M. Abed, G. Tao, Q. Jin, and X. Wang. 2017. Profiling of phospholipids molecular species from different mammalian milk powders by using ultra-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. Journal of Food Composition and Analysis 62:143–54. doi: 10.1016/j.jfca.2017.05.007.
- Amate, L., A. Gil, and M. Ramírez. 2001. Feeding infant piglets formula with long-chain polyunsaturated fatty acids as triacylglycerols or phospholipids influences the distribution of these fatty acids in plasma lipoprotein fractions. The Journal of Nutrition 131 (4):1250–5. doi: 10.1093/jn/131.4.1250.
- Andersen, M. H., L. Berglund, J. T. Rasmussen, and T. E. Petersen. 1997. Bovine PAS-6/7 binds α(v)Β5 integrin and anionic phospholipids through two domains. Biochemistry 36 (18):5441–6. doi: 10.1021/bi963119m.
- Anto, L., S. W. Warykas, M. Torres-Gonzalez, and C. N. Blesso. 2020. Milk polar lipids: Underappreciated lipids with emerging health benefits. Nutrients 12 (4):1001. doi: 10.3390/nu12041001.
- Argov-Argaman, N., R. Mesilati-Stahy, Y. Magen, and U. Moallem. 2014. Elevated concentrate-to-forage ratio in dairy cow rations is associated with a shift in the diameter of milk fat globules and remodeling of their membranes. Journal of Dairy Science 97 (10):6286–95. doi: 10.3168/jds.2014-8174.
- Argov-Argaman, N., K. Mida, B.-C. Cohen, M. Visker, and K. Hettinga. 2013. Milk fat content and DGAT1 genotype determine lipid composition of the milk fat globule membrane. PLoS One 8 (7):e68707. doi: 10.1371/journal.pone.0068707.
- Argov-Argaman, N., J. T. Smilowitz, D. A. Bricarello, M. Barboza, L. Lerno, J. W. Froehlich, H. Lee, A. M. Zivkovic, D. G. Lemay, S. Freeman, et al. 2010. Lactosomes: Structural and compositional classification of unique nanometer-sized protein lipid particles of human milk. Journal of Agricultural and Food Chemistry 58 (21):11234–42. doi: 10.1021/jf102495s.
- Armand, M., M. Hamosh, N. R. Mehta, P. A. Angelus, J. R. Philpott, T. R. Henderson, N. K. Dwyer, D. Lairon, and P. Hamosh. 1996. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatric Research 40 (3):429–37. doi: 10.1203/00006450-199609000-00011.
- Artegoitia, V. M., J. L. Middleton, F. M. Harte, S. R. Campagna, and M. J. De Veth. 2014. Choline and choline metabolite patterns and associations in blood and milk during lactation in dairy cows. PLoS One 9 (8):e103412. doi: 10.1371/journal.pone.0103412.
- Astaire, J. C., R. Ward, J. B. German, and R. Jiménez-Flores. 2003. Concentration of polar MFGM lipids from buttermilk by microfiltration and supercritical fluid extraction. Journal of Dairy Science 86 (7):2297–307. doi: 10.3168/jds.S0022-0302(03)73822-3.
- Avalli, A, and G. Contarini. 2005. Determination of phospholipids in dairy products by SPE/HPLC/ELSD. Journal of Chromatography. A 1071 (1–2):185–90. doi: 10.1016/j.chroma.2005.01.072.
- Baars, A., A. Oosting, E. Engels, D. Kegler, A. Kodde, L. Schipper, H. J. Verkade, and E. M. van der Beek. 2016. Milk fat globule membrane coating of large lipid droplets in the diet of young mice prevents body fat accumulation in adulthood. The British Journal of Nutrition 115 (11):1930–7. doi: 10.1017/S0007114516001082.
- Bach Korsholm Knudsen, K., C. Heerup, T. Røngaard Stange Jensen, X. Geng, N. Drachmann, P. Nordby, P. Bekker Jeppesen, I. Ifaoui, A. Müllertz, P. Torp Sangild, et al. 2021. Bovine milk-derived emulsifiers increase triglyceride absorption in newborn formula-fed pigs. Nutrients 13 (2):410–5. doi: 10.3390/nu13020410.
- Barbas, C, and E. Herrera. 1998. Lipid composition and vitamin E content in human colostrum and mature milk. Journal of Physiology and Biochemistry 54 (3):167–73.
- Barness, L. A. 1994. Nutritional requirements of infants and children with respect to cholesterol and related compounds. American Journal of Medical Genetics 50 (4):353–4. doi: 10.1002/ajmg.1320500410.
- Baumgartner, S., E. R. Kelly, S. van der Made, T. T. J. M. Berendschot, C. Husche, D. Lütjohann, and J. Plat. 2013. The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition 29 (10):1237–44. doi: 10.1016/j.nut.2013.03.020.
- Benoit, B., C. Fauquant, P. Daira, N. Peretti, M. Guichardant, and M. C. Michalski. 2010. Phospholipid species and minor sterols in french human milks. Food Chemistry 120 (3):684–91. doi: 10.1016/j.foodchem.2009.10.061.
- Bernhard, W., C. F. Poets, and A. R. Franz. 2019. Choline and choline-related nutrients in regular and preterm infant growth. European Journal of Nutrition 58 (3):931–45. doi: 10.1007/s00394-018-1834-7.
- Berton, A., S. Rouvellac, B. Robert, F. Rousseau, C. Lopez, and I. Crenon. 2012. Effect of the size and interface composition of milk fat globules on their in vitro digestion by the human pancreatic lipase: Native versus homogenized milk fat globules. Food Hydrocolloids 29 (1):123–34. doi: 10.1016/j.foodhyd.2012.02.016.
- Birecki, C. J., L. A. Drozdowski, M. Suh, J. Park Eek, M. Tom Clandinin, and A. B. Thomson. 2006. Dietary gangliosides enhance in vitro lipid uptake in weanling rats. Journal of Pediatric Gastroenterology and Nutrition 42 (1):59–65. doi: 10.1097/01.mpg.0000187567.79633.a7.
- Bitman, J., L. Wood, M. Hamosh, P. Hamosh, and N. R. Mehta. 1983. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. The American Journal of Clinical Nutrition 38 (2):300–12. doi: 10.1093/ajcn/38.2.300.
- Bitman, J., D. L. Wood, N. R. Mehta, P. Hamosh, and M. Hamosh. 1984. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. The American Journal of Clinical Nutrition 40 (5):1103–19. doi: 10.1093/ajcn/40.5.1103.
- Bitman, J, and D. L. Wood. 1990. Changes in milk fat phospholipids during lactation. Journal of Dairy Science 73 (5):1208–16. doi: 10.3168/jds.S0022-0302(90)78784-X.
- Bjerre-Harpøth, V., N. C. Friggens, V. M. Thorup, T. Larsen, B. M. Damgaard, K. L. Ingvartsen, and K. M. Moyes. 2012. Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation. Journal of Dairy Science 95 (5):2362–80. doi: 10.3168/jds.2011-4419.
- Blans, K., M. S. Hansen, L. V. Sørensen, M. L. Hvam, K. A. Howard, A. Möller, L. Wiking, L. B. Larsen, and J. T. Rasmussen. 2017. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. Journal of Extracellular Vesicles 6 (1):1294340. doi: 10.1080/20013078.2017.1294340.
- Bourlieu, C., D. Cheillan, M. Blot, P. Daira, M. Trauchessec, S. Ruet, J.-Y. Gassi, E. Beaucher, B. Robert, N. Leconte, et al. 2018. Polar lipid composition of bioactive dairy co-products buttermilk and butterserum: Emphasis on sphingolipid and ceramide isoforms. Food Chemistry 240:67–74. doi: 10.1016/j.foodchem.2017.07.091.
- Bourlieu, C., G. Paboeuf, S. Chever, S. Pezennec, J.-F. Cavalier, F. Guyomarc'h, A. Deglaire, S. Bouhallab, D. Dupont, F. Carrière, et al. 2016. Adsorption of gastric lipase onto multicomponent model lipid monolayers with phase separation. Colloids and Surfaces. B, Biointerfaces 143:97–106. doi: 10.1016/j.colsurfb.2016.03.032.
- Boyd, L. C., N. C. Drye, and A. P. Hansen. 1999. Isolation and characterization of whey phospholipids. Journal of Dairy Science 82 (12):2550–7. doi: 10.3168/jds.S0022-0302(99)75509-8.
- Boyle, N. B., L. Dye, K. Arkbåge, L. Thorell, P. D. Frederiksen, F. Croden, and C. L. Lawton. 2019. The effects of milk-based phospholipids on cognitive performance and subjective responses to psychosocial stress: A randomised, double-blind, placebo controlled trial in high-perfectionist men. Nutrition 57:183–93.
- Brennan, P. J., T.-Y. Cheng, D. G. Pellicci, G. F. M. Watts, N. Veerapen, D. C. Young, J. Rossjohn, G. S. Besra, D. I. Godfrey, M. B. Brenner, et al. 2017. Structural determination of lipid antigens captured at the CD1d–T-cell receptor interface. Proceedings of the National Academy of Sciences of the United States of America 114 (31):8348–53. doi: 10.1073/pnas.1705882114.
- Brink, L. R., A. W. Herren, S. McMillen, K. Fraser, M. Agnew, N. Roy, and B. Lönnerdal. 2020. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. Journal of Dairy Science 103 (4):3002–16. doi: 10.3168/jds.2019-17179.
- Brink, L. R, and B. Lönnerdal. 2020. Milk fat globule membrane: The role of its various components in infant health and development. The Journal of Nutritional Biochemistry 85:108465. doi: 10.1016/j.jnutbio.2020.108465.
- Brønnum, H., T. Seested, L. I. Hellgren, S. Brix, and H. Frøkiaer. 2005. Milk-derived GM3 and GD3 differentially inhibit dendritic cell maturation and effector functionalities. Scandinavian Journal of Immunology 61 (6):551–7. doi: 10.1111/j.1365-3083.2005.01566.x.
- Brown, L. D., A. Cheung, J. E. F. Harwood, and F. C. Battaglia. 2009. Inositol and mannose utilization rates in term and late-preterm infants exceed nutritional intakes. The Journal of Nutrition 139 (9):1648–52. doi: 10.3945/jn.109.109108.
- Burling, H, and G. Graverholt. 2008. Milk—A new source for bioactive phospholipids for use in food formulations. Lipid Technology 20 (10):229–31. doi: 10.1002/lite.200800058.
- Calvo, M. V., M. C. Martín-Hernández, A. García-Serrano, M. P. Castro-Gómez, L. Alonso-Miravalles, R. García-Martín, J. Megino-Tello, L. Alonso, and J. Fontecha. 2020. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. Journal of Food Composition and Analysis 86:103386. doi: 10.1016/j.jfca.2019.103386.
- Carnielli, V. P., G. Verlato, F. Pederzini, I. Luijendijk, A. Boerlage, D. Pedrotti, and P. J. Sauer. 1998. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. The American Journal of Clinical Nutrition 67 (1):97–103. doi: 10.1093/ajcn/67.1.97.
- Cartocci, V., M. Servadio, V. Trezza, and V. Pallottini. 2017. Can cholesterol metabolism modulation affect brain function and behavior? Journal of Cellular Physiology 232 (2):281–6. doi: 10.1002/jcp.25488.
- Carver, J. 2006. Conditionally essential nutrients: Choline, inositol, taurine, arginine, glutamine, and nucleotides. In Neonatal nutrition and metabolism, 2nd ed., 299–311. Cambridge, MA: Cambridge University Press. doi: 10.1017/CBO9780511544712.020.
- Castro-Gómez, P., O. Montero, J. Fontecha, D. Arráez-Román, V. Verardo, and M. Battino. 2017. In-depth lipidomic analysis of molecular species of triacylglycerides, diacylglycerides, glycerophospholipids, and sphingolipids of buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. International Journal of Molecular Science 212 (3):605. doi: 10.3390/ijms18030605.
- Castro-Gómez, P., L. M. Rodríguez-Alcalá, K. M. Monteiro, A. L. Ruiz, J. E. Carvalho, and J. Fontecha. 2016. Antiproliferative activity of buttermilk lipid fractions isolated using food grade and non-food grade solvents on human cancer cell lines. Food Chemistry 212:695–702. doi: 10.1016/j.foodchem.2016.06.030.
- Cavaletto, M., M. G. Giuffrida, C. Giunta, C. Vellano, C. Fabris, E. Bertino, J. Godovac-Zimmermann, and A. Conti. 1999. Multiple forms of lactadherin (breast antigen BA46) and butyrophilin are secreted into human milk as major components of milk fat globule membrane. The Journal of Dairy Research 66 (2):295–301. doi: 10.1017/S0022029999003507.
- Cengiz, M., O. Kaynar, O. Cannazik, M. Ileriturk, S. Cengiz, and A. Hayirli. 2016. Sampling factors causing variability in milk constituents in early lactation cows. Veterinární Medicína 60 (1):6–15. doi: 10.17221/7920-VETMED.
- Cheema, M., P. B. Smith, A. D. Patterson, A. Hristov, and F. M. Harte. 2018. The association of lipophilic phospholipids with native bovine casein micelles in skim milk: Effect of lactation stage and casein micelle size. Journal of Dairy Science 101 (10):8672–87. doi: 10.3168/jds.2017-14137.
- Cheong, L. Z., C. Jiang, X. He, S. Song, and O. M. Lai. 2018. Lipid profiling, particle size determination, and in vitro simulated gastrointestinal lipolysis of mature human milk and infant formula. Journal of Agricultural and Food Chemistry 66 (45):12042–50. doi: 10.1021/acs.jafc.8b03998.
- Chiantia, S, and E. London. 2012. Acyl chain length and saturation modulate interleaflet coupling in asymmetric bilayers: Effects on dynamics and structural order. Biophysical Journal 103 (11):2311–9. doi: 10.1016/j.bpj.2012.10.033.
- Christie, W. W., R. C. Noble, and G. Davies. 1987. Phospholipids in milk and dairy products. International Journal of Dairy Technology 40 (1):10–2. doi: 10.1111/j.1471-0307.1987.tb02385.x.
- Clark, S. B, and H. L. Laboda. 1991. Triolein-phosphatidylcholine-cholesterol emulsions as substrates for lipoprotein and hepatic lipases. Lipids 26 (1):68–73. doi: 10.1007/BF02544027.
- Clarke, N. M, and J. T. May. 2000. Effect of antimicrobial factors in human milk on rhinoviruses and milk-borne cytomegalovirus in vitro. Journal of Medical Microbiology 49 (8):719–23. doi: 10.1099/0022-1317-49-8-719.
- Claumarchirant, L., A. Cilla, E. Matencio, L. M. Sanchez-Siles, P. Castro-Gomez, J. Fontecha, A. Alegría, and M. J. Lagarda. 2016. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. International Dairy Journal 61:228–38. doi: 10.1016/j.idairyj.2016.06.005.
- Cohen, B. C., C. Raz, A. Shamay, and N. Argov-Argaman. 2017. Lipid droplet fusion in mammary epithelial cells is regulated by phosphatidylethanolamine metabolism. Journal of Mammary Gland Biology and Neoplasia 22 (4):235–49. doi: 10.1007/s10911-017-9386-7.
- Contarini, G., M. Povolo, V. Pelizzola, L. Monti, A. Bruni, L. Passolungo, F. Abeni, and L. Degano. 2014. Bovine colostrum: Changes in lipid constituents in the first 5 days after parturition. Journal of Dairy Science 97 (8):5065–72. doi: 10.3168/jds.2013-7517.
- Conway, V., P. Couture, C. Richard, S. F. Gauthier, Y. Pouliot, and B. Lamarche. 2013. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutrition, Metabolism and Cardiovascular Diseases 23 (12):1255–62. doi: 10.1016/j.numecd.2013.03.003.
- Corredig, M., R. R. Roesch, and D. G. Dalgleish. 2003. Production of a novel ingredient from buttermilk. Journal of Dairy Science 86 (9):2744–50. doi: 10.3168/jds.S0022-0302(03)73870-3.
- Costa, M. R., X. E. Elias-Argote, R. Jiménez-Flores, and M. L. Gigante. 2010. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. International Dairy Journal 20 (9):598–602. doi: 10.1016/j.idairyj.2010.03.006.
- Couvreur, S, and C. Hurtaud. 2017. Relationships between milks differentiated on native milk fat globule characteristics and fat, protein and calcium compositions. Animal 11 (3):507–18. doi: 10.1017/S1751731116001646.
- Da Silva, R. C., H. L. Colleran, and S. A. Ibrahim. 2021. Milk fat globule membrane in infant nutrition: A dairy industry perspective. The Journal of Dairy Research 88 (1):105–16. doi: 10.1017/S0022029921000224.
- da Silva, R. P., K. B. Kelly, E. D. Lewis, K. A. Leonard, S. Goruk, J. M. Curtis, D. F. Vine, S. D. Proctor, C. J. Field, and R. L. Jacobs. 2015. Choline deficiency impairs intestinal lipid metabolism in the lactating rat. The Journal of Nutritional Biochemistry 26 (10):1077–83. doi: 10.1016/j.jnutbio.2015.04.015.
- Davenport, C., J. Yan, S. Taesuwan, K. Shields, A. A. West, X. Jiang, C. A. Perry, O. V. Malysheva, S. P. Stabler, R. H. Allen, et al. 2015. Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. The Journal of Nutritional Biochemistry 26 (9):903–11. doi: 10.1016/j.jnutbio.2015.03.004.
- de Veth, M. J., V. M. Artegoitia, S. R. Campagna, H. Lapierre, F. Harte, and C. L. Girard. 2016. Choline absorption and evaluation of bioavailability markers when supplementing choline to lactating dairy cows. Journal of Dairy Science 99 (12):9732–44. doi: 10.3168/jds.2016-11382.
- Dei Cas, M., R. Paroni, P. Signorelli, A. Mirarchi, L. Cerquiglini, S. Troiani, S. Cataldi, M. Codini, T. Beccari, R. Ghidoni, et al. 2020. Human breast milk as source of sphingolipids for newborns: Comparison with infant formulas and commercial cow’s milk. Journal of Translational Medicine 18 (1):481. doi: 10.1186/s12967-020-02641-0.
- Dellschaft, N. S., C. Richard, E. D. Lewis, S. Goruk, R. L. Jacobs, J. M. Curtis, and C. J. Field. 2018. The dietary form of choline during lactation affects maternal immune function in rats. European Journal of Nutrition 57 (6):2189–99. doi: 10.1007/s00394-017-1493-0.
- Deoni, S., D. Dean, S. Joelson, J. O’Regan, and N. Schneider. 2018. Early nutrition influences developmental myelination and cognition in infants and young children. NeuroImage 178:649–59. doi: 10.1016/j.neuroimage.2017.12.056.
- Devlin, A. M, and S. M. Innis. 1999. Dietary phospholipid alters biliary lipid composition in formula-fed piglets. Lipids 34 (12):1313–8. doi: 10.1007/s11745-999-0483-4.
- Drozdowski, L. A., M. Suh, E. Park, M. T. Clandinin, and A. B. Thomson. 2007. Dietary gangliosides enhance in vitro glucose uptake in weanling rats. JPEN. Journal of Parenteral and Enteral Nutrition 31 (5):423–9. doi: 10.1177/0148607107031005423.
- Duan, B., E.-S. Hong, J.-A. Shin, Y. Qin, J.-H. Lee, C.-W. Lee, and K.-T. Lee. 2021. Correlations of fat content in human milk with fat droplet size and phospholipid species. Molecules 26 (6):1596. doi: 10.3390/molecules26061596.
- Eckhardt, E. R., D. Q. Wang, J. M. Donovan, and M. C. Carey. 2002. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 122 (4):948–56. doi: 10.1053/gast.2002.32539.
- EFSA. 2016. Dietary reference values for choline. EFSA Journal 14 (8):4484. doi: 10.2903/j.efsa.2016.4484.
- El-Loly, M. M. 2011. Composition, properties and nutritional aspects of milk fat globule membrane—A review. Polish Journal of Food and Nutrition Sciences 61 (1):7–32. doi: 10.2478/v10222-011-0001-0.
- Et-Thakafy, O., N. Delorme, F. Guyomarc’h, and C. Lopez. 2018. Mechanical properties of milk sphingomyelin bilayer membranes in the gel phase: Effects of naturally complex heterogeneity, saturation and acyl chain length investigated on liposomes using AFM. Chemistry and Physics of Lipids 210:47–59. doi: 10.1016/j.chemphyslip.2017.11.014.
- Et-Thakafy, O., F. Guyomarc’h, and C. Lopez. 2017. Lipid domains in the milk fat globule membrane: Dynamics investigated in situ in milk in relation to temperature and time. Food Chemistry 220:352–61. doi: 10.1016/j.foodchem.2016.10.017.
- Et-Thakafy, O., F. Guyomarc’h, and C. Lopez. 2019. Young modulus of supported lipid membranes containing milk sphingomyelin in the gel, fluid or liquid-ordered phase, determined using AFM force spectroscopy. Biochimica et Biophysica Acta (BBA) - Biomembranes 1861 (9):1523–32. doi: 10.1016/j.bbamem.2019.07.005.
- Ferreiro, T., S. Martínez, L. Gayoso, and J. L. Rodríguez-Otero. 2016. Evolution of phospholipid contents during the production of quark cheese from buttermilk. Journal of Dairy Science 99 (6):4154–9. doi: 10.3168/jds.2016-10861.
- Ferreiro, T, and J. L. Rodríguez-Otero. 2018. Evolution and distribution of phospholipids in cheese and whey during the manufacturing of fresh cheese from cows’ milk. International Journal of Dairy Technology 71 (3):820–3. doi: 10.1111/1471-0307.12499.
- Fil, J. E., S. A. Fleming, M. Chichlowski, G. Gross, B. M. Berg, and R. N. Dilger. 2019. Evaluation of dietary bovine milk fat globule membrane supplementation on growth, serum cholesterol and lipoproteins, and neurodevelopment in the young pig. Frontiers in Pediatrics 7:417. doi: 10.3389/fped.2019.00417.
- Fischer, L. M., K. A. da Costa, J. Galanko, W. Sha, B. Stephenson, J. Vick, and S. H. Zeisel. 2010. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. The American Journal of Clinical Nutrition 92 (2):336–46. doi: 10.3945/ajcn.2010.29459.
- Fong, B. B. Y., L. Ma, and A. K. MacGibbon. 2020. Lactational changes in phospholipid classes and molecular species concentration in human milk. International Dairy Journal 111:104830. doi: 10.1016/j.idairyj.2020.104830.
- Fong, B. Y., C. S. Norris, and A. K. MacGibbon. 2007. Protein and lipid composition of bovine milk-fat-globule membrane. International Dairy Journal 17 (4):275–88. doi: 10.1016/j.idairyj.2006.05.004.
- Fontecha, J., L. Brink, S. Wu, Y. Pouliot, F. Visioli, and R. Jiménez-Flores. 2020. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 12 (6):1607. doi: 10.3390/nu12061607.
- Fuller, K. L., T. B. Kuhlenschmidt, M. S. Kuhlenschmidt, R. Jiménez-Flores, and S. M. Donovan. 2013. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. Journal of Dairy Science 96 (6):3488–97. doi: 10.3168/jds.2012-6122.
- Gallier, S., A. K. MacGibbon, and P. McJarrow. 2018. Milk fat globule membrane (MFGM) supplementation and cognition. Agro Food Industry Hi-Tech 29 (5):3.
- Gallier, S., D. Gragson, C. Cabral, R. Jiménez-Flores, and D. W. Everett. 2010. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. Journal of Agricultural and Food Chemistry 58 (19):10503–11. doi: 10.1021/jf101878d.
- Gallier, S., D. Gragson, R. Jiménez-Flores, and D. Everett. 2010. Using confocal laser scanning microscopy to probe the milk fat globule membrane and associated proteins. Journal of Agricultural and Food Chemistry 58 (7):4250–7. doi: 10.1021/jf9032409.
- Gallier, S., A. Laubscher, and R. Jiménez-Flores. 2014. The milk fat globule membrane: Structure, methodology for its study, and functionality. In Food structures, digestion and health, 107–42. New York, NY: Elsevier Inc. doi: 10.1016/B978-0-12-404610-8.00004-9.
- Gallier, S., K. Vocking, J. A. Post, B. Van De Heijning, D. Acton, E. M. Van Der Beek, and T. Van Baalen. 2015. A novel infant milk formula concept: Mimicking the human milk fat globule structure. Colloids and Surfaces. B, Biointerfaces 136:329–39. doi: 10.1016/j.colsurfb.2015.09.024.
- García-Serrano, A., J. Tomé-Carneiro, M. Carmen Crespo, M. Visitación Calvo, I. Pereda-Pérez, S. Baliyan, E. Burgos-Ramos, O. Montero, A. Dávalos, C. Venero, et al. 2020. Concentrates of buttermilk and krill oil improve cognition in aged rats. Prostaglandins, Leukotrienes and Essential Fatty Acids 155:102077. doi: 10.1016/j.plefa.2020.102077.
- Garcia, C., C. Antona, B. Robert, C. Lopez, and M. Armand. 2014. The size and interfacial composition of milk fat globules are key factors controlling triglycerides bioavailability in simulated human gastro-duodenal digestion. Food Hydrocolloids. 35:494–504. doi: 10.1016/j.foodhyd.2013.07.005.
- Garcia, C, and S. Innis. 2013. Structure of the human milk fat globule. Lipid Technology 25 (10):223–6. doi: 10.1002/lite.201300303.
- Garcia, C., N. W. Lutz, S. Confort-Gouny, P. J. Cozzone, M. Armand, and M. Bernard. 2012. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chemistry 135 (3):1777–83. doi: 10.1016/j.foodchem.2012.05.111.
- Garcia, C., V. Millet, T. C. Coste, M. Mimoun, A. Ridet, C. Antona, U. Simeoni, and M. Armand. 2011. French mothers’ milk deficient in DHA contains phospholipid species of potential interest for infant development. Journal of Pediatric Gastroenterology and Nutrition 53 (2):206–12. doi: 10.1097/MPG.0b013e318216f1d0.
- Gassi, J. Y., M. Blot, E. Beaucher, B. Robert, N. Leconte, B. Camier, F. Rousseau, C. Bourlieu, J. Jardin, V. Briard-Bion, et al. 2016. Preparation and characterisation of a milk polar lipids enriched ingredient from fresh industrial liquid butter serum: Combination of physico-chemical modifications and technological treatments. International Dairy Journal 52:26–34. doi: 10.1016/j.idairyj.2015.08.012.
- German, J. B, and C. J. Dillard. 2006. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Critical Reviews in Food Science and Nutrition 46 (1):57–92. doi: 10.1080/10408690590957098.
- Getty, C. M, and R. N. Dilger. 2015. Moderate perinatal choline deficiency elicits altered physiology and metabolomic profiles in the piglet. PLoS One 10 (7):e0133500. doi: 10.1371/journal.pone.0133500.
- Giuffrida, F., C. Cruz-Hernandez, E. Bertschy, P. Fontannaz, I. Masserey Elmelegy, I. Tavazzi, C. Marmet, B. Sanchez-Bridge, S. Thakkar, C. De Castro, et al. 2016. Temporal changes of human breast milk lipids of chinese mothers. Nutrients 8 (11):715. doi: 10.3390/nu8110715.
- Giuffrida, F., C. Cruz-Hernandez, B. Flück, I. Tavazzi, S. K. Thakkar, F. Destaillats, and M. Braun. 2013. Quantification of phospholipids classes in human milk. Lipids 48 (10):1051–8. doi: 10.1007/s11745-013-3825-z.
- Giuffrida, F., I. M. Elmelegy, S. K. Thakkar, C. Marmet, and F. Destaillats. 2014. Longitudinal evolution of the concentration of gangliosides GM3 and GD3 in human milk. Lipids 49 (10):997–1004. doi: 10.1007/s11745-014-3943-2.
- Glew, R. H., J. A. Omene, S. Vignet, M. D. Amico, and R. W. Evans. 1995. Pergamon fatty acid composition of breast milk lipids of Nigerian women. Science 15 (4):477–89.
- Gong, H., Q. Yuan, J. Pang, T. Li, J. Li, B. Zhan, R. Chang, and X. Mao. 2020. Dietary milk fat globule membrane restores decreased intestinal mucosal barrier development and alterations of intestinal flora in infant-formula-fed rat pups. Molecular Nutrition & Food Research 64 (21):2000232. doi: 10.1002/mnfr.202000232.
- Graves, E. L., A. D. Beaulieu, and J. K. Drackley. 2007. Factors affecting the concentration of sphingomyelin in bovine milk. Journal of Dairy Science 90 (2):706–15. doi: 10.3168/jds.S0022-0302(07)71554-0.
- Grip, T., T. S. Dyrlund, L. Ahonen, M. Domellöf, O. Hernell, T. Hyötyläinen, M. Knip, B. Lönnerdal, M. Orešič, and N. Timby. 2018. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatric Research 84 (5):726–32. doi: 10.1038/s41390-018-0130-9.
- Guan, J., A. MacGibbon, R. Zhang, D. M. Elliffe, S. Moon, and D.-X. Liu. 2015. Supplementation of complex milk lipid concentrate (CMLC) improved the memory of aged rats. Nutritional Neuroscience 18 (1):22–9. doi: 10.1179/1476830513Y.0000000096.
- Guan, J., A. Macgibbon, B. Fong, R. Zhang, K. Liu, A. Rowan, and P. McJarrow. 2015. Long-term supplementation with beta serum concentrate (BSC), a complex of milk lipids, during post-natal brain development improves memory in rats. Nutrients 7 (6):4526–41. doi: 10.3390/nu7064526.
- Guerin, J., C. Soligot, J. Burgain, M. Huguet, G. Francius, S. El-Kirat-Chatel, F. Gomand, S. Lebeer, Y. Le Roux, F. Borges, et al. 2018. Adhesive interactions between milk fat globule membrane and Lactobacillus rhamnosus GG inhibit bacterial attachment to Caco-2 TC7 intestinal cell. Colloids and Surfaces. B, Biointerfaces 167:44–53. doi: 10.1016/j.colsurfb.2018.03.044.
- Gurnida, D. A., A. M. Rowan, P. Idjradinata, D. Muchtadi, and N. Sekarwana. 2012. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Human Development 88 (8):595–601. doi: 10.1016/j.earlhumdev.2012.01.003.
- Gustavsson, M., S. C. Hodgkinson, B. Fong, C. Norris, J. Guan, C. U. Krageloh, B. H. Breier, M. Davison, P. McJarrow, and M. H. Vickers. 2010. Maternal supplementation with a complex milk lipid mixture during pregnancy and lactation alters neonatal brain lipid composition but lacks effect on cognitive function in rats. Nutrition Research 30 (4):279–89. doi: 10.1016/j.nutres.2010.04.005.
- Haddadian, Z., G. T. Eyres, P. Bremer, and D. W. Everett. 2018. Polar lipid composition of the milk fat globule membrane in buttermilk made using various cream churning conditions or isolated from commercial samples. International Dairy Journal 81:138–42. doi: 10.1016/j.idairyj.2018.01.012.
- Hallman, M., K. Bry, K. Hoppu, M. Lappi, and M. Pohjavuori. 1992. Inositol supplementation in premature infants with respiratory distress syndrome. The New England Journal of Medicine 326 (19):1233–9. doi: 10.1056/nejm199205073261901.
- Haramizu, S., T. Mori, M. Yano, N. Ota, K. Hashizume, A. Otsuka, T. Hase, and A. Shimotoyodome. 2014. Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice. SpringerPlus 3 (1):1–17. doi: 10.1186/2193-1801-3-339.
- Haramizu, S., N. Ota, A. Otsuka, K. Hashizume, S. Sugita, T. Hase, T. Murase, and A. Shimotoyodome. 2014. Dietary milk fat globule membrane improves endurance capacity in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 307:1009–17. doi: 10.1152/ajpregu.00004.2014.-Milk.
- Hari, S., R. Ochiai, Y. Shioya, and Y. Katsuragi. 2015. Safety evaluation of the consumption of high dose milk fat globule membrane in healthy adults: A double-blind, randomized controlled trial with parallel group design. Bioscience, Biotechnology, and Biochemistry 79 (7):1172–7. doi: 10.1080/09168451.2015.1012150.
- Haruta-Ono, Y., S. Setoguchi, H. M. Ueno, S. Higurashi, N. Ueda, K. Kato, T. Saito, K. Matsunaga, and J. Takata. 2012. Orally administered sphingomyelin in bovine milk is incorporated into skin sphingolipids and is involved in the water-holding capacity of hairless mice. Journal of Dermatological Science 68 (1):56–62. doi: 10.1016/j.jdermsci.2012.07.006.
- Haruta-Ono, Y., H. Ueno, N. Ueda, K. Kato, and T. Yoshioka. 2012. Investigation into the dosage of dietary sphingomyelin concentrate in relation to the improvement of epidermal function in hairless mice. Animal Science Journal = Nihon Chikusan Gakkaiho 83 (2):178–83. doi: 10.1111/j.1740-0929.2011.00940.x.
- Heid, H. W, and T. W. Keenan. 2005. Intracellular origin and secretion of milk fat globules. European Journal of Cell Biology 84 (2–3):245–58. doi: 10.1016/j.ejcb.2004.12.002.
- Hellhammer, J., A. R. Waladkhani, T. Hero, and C. Buss. 2010. Effects of milk phospholipid on memory and psychological stress response. British Food Journal 112 (10):1124–37. doi: 10.1108/00070701011080258.
- Henriksen, N. L., K. Aasmul-Olsen, R. Venkatasubramanian, M. K. E. Nygaard, R. R. Sprenger, A. B. Heckmann, M. S. Ostenfeld, C. S. Ejsing, S. F. Eskildsen, A. Müllertz, et al. 2021. Dairy-derived emulsifiers in infant formula show marginal effects on the plasma lipid profile and brain structure in preterm piglets relative to soy lecithin. Nutrients 13 (3):718. doi: 10.3390/nu13030718.
- Herrera-Insua, I., H. F. Gomez, V. A. Diaz-Gonzalez, P. Chaturvedi, D. S. Newburg, and T. G. Cleary. 2001. Human milk lipids bind shiga toxin. Advances in Experimental Medicine and Biology 501:333–9. doi: 10.1007/978-1-4615-1371-1_41.
- Higurashi, S., Y. Haruta-Ono, H. Urazono, T. Kobayashi, and Y. Kadooka. 2015. Improvement of skin condition by oral supplementation with sphingomyelin-containing milk phospholipids in a double-blind, placebo-controlled, randomized trial. Journal of Dairy Science 98 (10):6706–12. doi: 10.3168/jds.2015-9529.
- Hinde, K. 2007. First-time macaque mothers bias milk composition in favor of sons. Current Biology 17 (22):R958–9. doi: 10.1016/j.cub.2007.09.029.
- Holmes-McNary, M. Q., W. L. Cheng, M. H. Mar, S. Fussell, and S. H. Zeisel. 1996. Choline and choline esters in human and rat milk and in infant formulas. The American Journal of Clinical Nutrition 64 (4):572–6. doi: 10.1093/ajcn/64.4.572.
- Holmes, H. C., G. J. Snodgrass, and R. A. Iles. 2000. Changes in the choline content of human breast milk in the first 3 weeks after birth. European Journal of Pediatrics 159 (3):198–204. doi: 10.1007/s004310050050.
- Holub, B. J. 1992. The nutritional importance of inositol and the phosphoinositides. The New England Journal of Medicine 326 (19):1285–7. doi: 10.1056/NEJM199205073261909.
- Howlett, A., A. Ohlsson, and N. Plakkal. 2019. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database of Systematic Reviews 2020 (1):CD00366. doi: 10.1002/14651858.CD000366.pub4.
- Huang, S., Z. Wu, C. Liu, D. Han, C. Feng, S. Wang, J. Wang, and D. Song. 2019. Milk fat globule membrane supplementation promotes neonatal growth and alleviates inflammation in low-birth-weight mice treated with lipopolysaccharide. BioMed Research International 2019:1–10. doi: 10.1155/2019/4876078.
- Huang, S., T. T. Mo, T. Norris, S. Sun, T. Zhang, T. Li Han, A. Rowan, et al. 2017. The CLIMB (Complex Lipids in Mothers and Babies) study: Protocol for a multicentre, three-group, parallel randomised controlled trial to investigate the effect of supplementation of complex lipids in pregnancy, on maternal ganglioside status and subsequent cognitive outcomes in the offspring. BMJ Open 7 (10):e016637. doi: 10.1136/bmjopen-2017-016637.
- Idota, T, and H. Kawakami. 1995. Inhibitory effects of milk gangliosides on the adhesion of Escherichia coli to human intestinal carcinoma cells. Bioscience, Biotechnology, and Biochemistry 59 (1):69–72. doi: 10.1271/bbb.59.69.
- Ilcol, Y. O., R. Ozbek, E. Hamurtekin, and I. H. Ulus. 2005. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. The Journal of Nutritional Biochemistry 16 (8):489–99. doi: 10.1016/j.jnutbio.2005.01.011.
- Iqbal, J, and M. M. Hussain. 2009. Intestinal lipid absorption. American Journal of Physiology - Endocrinology and Metabolism 296 (6):E1183–94. doi: 10.1152/ajpendo.90899.2008.
- Iskarpatyoti, J. A., E. A. Morse, R. P. McClung, M. Ikizler, J. D. Wetzel, N. Contractor, and T. S. Dermody. 2012. Serotype-specific differences in inhibition of reovirus infectivity by human-milk glycans are determined by viral attachment protein Σ1. Virology 433 (2):489–97. doi: 10.1016/j.virol.2012.08.036.
- Iwamori, M. 2011. Ability to neutralize cholera toxin of mammalian milk through expression of its receptor, ganglioside GM1. In Cholera: Symptoms, diagnosis and treatment, 135–45. Hauppauge, NY: Nova Science Publishers, Inc.
- Iwamori, M., K. Takamizawa, M. Momoeda, Y. Iwamori, and Y. Taketani. 2008. Gangliosides in human, cow and goat milk, and their abilities as to neutralization of cholera toxin and botulinum type A neurotoxin. Glycoconjugate Journal 25 (7):675–83. doi: 10.1007/s10719-008-9128-6.
- Jain, N, and W. A. Walker. 2015. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nature Reviews. Gastroenterology & Hepatology 12 (1):14–25. doi: 10.1038/NRGASTRO.2014.153.
- Jensen, R. G. 2002. The composition of bovine milk lipids: January 1995 to December 2000. Journal of Dairy Science 85 (2):295–350. doi: 10.3168/jds.S0022-0302(02)74079-4.
- Ji, X., W. Xu, J. Cui, Y. Ma, and S. Zhou. 2019. Goat and buffalo milk fat globule membranes exhibit better effects at inducing apoptosis and reduction the viability of HT-29 cells. Scientific Reports 9 (1):2577. doi: 10.1038/s41598-019-39546-y.
- Jiang, C., B. Ma, S. Song, O. M. Lai, and L. Z. Cheong. 2018. Fingerprinting of phospholipid molecular species from human milk and infant formula using HILIC-ESI-IT-TOF-MS and discriminatory analysis by principal component analysis. Journal of Agricultural and Food Chemistry 66 (27):7131–8. doi: 10.1021/acs.jafc.8b01393.
- Jukkola, A., S. Hokkanen, T. Kämäräinen, R. Partanen, A. Heino, and O. J. Rojas. 2019. Changes in milk fat globules and membrane lipids under the shear fields of microfiltration and centrifugation. Journal of Membrane Science 573:218–25. doi: 10.1016/j.memsci.2018.12.007.
- Jyonouchi, S., V. Abraham, J. S. Orange, J. M. Spergel, L. Gober, E. Dudek, R. Saltzman, K. E. Nichols, and A. Cianferoni. 2011. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. The Journal of Allergy and Clinical Immunology 128 (1):102–9.e13. doi: 10.1016/J.JACI.2011.02.026.
- Kadosh, K. C., L. Muhardi, P. Parikh, M. Basso, H. J. Jan Mohamed, T. Prawitasari, F. Samuel, G. Ma, and J. M. Geurts. 2021. Nutritional support of neurodevelopment and cognitive function in infants and young children—An update and novel insights. Nutrients 13 (1):199–26. doi: 10.3390/nu13010199.
- Kamili, A., E. Wat, R. W. Chung, S. Tandy, J. M. Weir, P. J. Meikle, and J. S. Cohn. 2010. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutrition and Metabolism 7:90. doi: 10.1186/1743-7075-7-90.
- Karim, M, and B. Wang. 2006. Is sialic acid in milk food for the brain? CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 1:18. doi: 10.1079/PAVSNNR20061018.
- Keller, S., H.-Y. Le, C. Rödiger, U.-C. Hipler, R. Kertscher, A. Malarski, L.-M. Hunstock, M. Kiehntopf, M. Kaatz, J. Norgauer, et al. 2014. Supplementation of a dairy drink enriched with milk phospholipids in patients with atopic dermatitis—A double-blind, placebo-controlled, randomized, cross-over study. Clinical Nutrition 33 (6):1010–6. doi: 10.1016/j.clnu.2014.01.014.
- Kiełbowicz, G., P. Micek, and C. Wawrzeńczyk. 2013. A new liquid chromatography method with charge aerosol detector (CAD) for the determination of phospholipid classes. Application to milk phospholipids. Talanta 105:28–33. doi: 10.1016/j.talanta.2012.11.051.
- Kiełczewska, K., K. Ambroziak, D. Krzykowska, and M. Aljewicz. 2021. The effect of high-pressure homogenisation on the size of milk fat globules and MFGM composition in sweet buttermilk and milk. International Dairy Journal 113:104898. doi: 10.1016/j.idairyj.2020.104898.
- Kim, H., T. Suzuki, M. Kim, N. Kojima, N. Ota, A. Shimotoyodome, T. Hase, E. Hosoi, and H. Yoshida. 2015. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: A randomized double blind, placebo-controlled, follow-up trial. PLoS One 10 (2):e0116256. doi: 10.1371/journal.pone.0116256.
- Kim, H., C. Won Won, M. Kim, N. Kojima, K. Fujino, Y. Osuka, E. Hosoi, and T. Suzuki. 2019. The effects of exercise and milk-fat globule membrane (MFGM) on walking parameters in community-dwelling elderly Japanese women with declines in walking ability: A randomized placebo controlled trial. Archives of Gerontology and Geriatrics 83:106–13. doi: 10.1016/j.archger.2019.03.029.
- Kiyohara, M., K. Tanigawa, T. Chaiwangsri, T. Katayama, H. Ashida, and K. Yamamoto. 2011. An exo-α-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 21 (4):437–47. doi: 10.1093/glycob/cwq175.
- Ko, C., Wei, J. Qu, D. D. Black, and P. Tso. 2020. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nature Reviews Gastroenterology and Hepatology 17 (3):169–83. doi: 10.1038/s41575-019-0250-7.
- Kodate, A., Y. Otoki, N. Shimizu, J. Ito, S. Kato, N. Umetsu, T. Miyazawa, and K. Nakagawa. 2018. Development of quantitation method for glycated aminophospholipids at the molecular species level in powdered milk and powdered buttermilk. Scientific Reports 8 (1):1–10. doi: 10.1038/s41598-018-27010-2.
- Koletzko, B. 69. 2016. Human milk lipids. Annals of Nutrition and Metabolism 69 (Suppl 2):27–40. doi: 10.1159/000452819.
- Kuchta-Noctor, A. M., B. A. Murray, C. Stanton, R. Devery, and P. M. Kelly. 2016. Anticancer activity of buttermilk against SW480 colon cancer cells is associated with caspase-independent cell death and attenuation of Wnt, Akt, and ERK signaling. Nutrition and Cancer 68 (7):1234–46. doi: 10.1080/01635581.2016.1206580.
- Kvistgaard, A. S., L. T. Pallesen, C. F. Arias, S. López, T. E. Petersen, C. W. Heegaard, and J. T. Rasmussen. 2004. Inhibitory effects of human and bovine milk constituents on rotavirus infections. Journal of Dairy Science 87 (12):4088–96. doi: 10.3168/jds.S0022-0302(04)73551-1.
- Lacomba, R., J. Salcedo, A. Alegría, R. Barberá, P. Hueso, E. Matencio, and M. J. Lagarda. 2011. Effect of simulated gastrointestinal digestion on sialic acid and gangliosides present in human milk and infant formulas. Journal of Agricultural and Food Chemistry 59 (10):5755–62. doi: 10.1021/jf200663k.
- Larson, G., P. Falk, L. Hynsjö, A. C. Midtvedt, and T. Midtvedt. 1990. Faecal excretion of glycosphingolipids of breast-fed and formula-fed infants. Microbial Ecology in Health and Disease 3 (6):305–19. doi: 10.3109/08910609009140253.
- Lashkari, S., J. W. Moller, S. K. Jensen, L. I. Hellgren, M. T. Sørensen, P. K. Theil, and K. Sejrsen. 2020. Changes in long-chain fatty acid composition of milk fat globule membrane and expression of mammary lipogenic genes in dairy cows fed sunflower seeds and rumen-protected choline. Journal of Animal Physiology and Animal Nutrition 104 (6):1606–19. doi: 10.1111/jpn.13386.
- Le Barz, M., C. Vors, E. Combe, L. Joumard-Cubizolles, M. Lecomte, F. Joffre, M. Trauchessec, S. Pesenti, E. Loizon, A.-E. Breyton, et al. 2021. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI Insight 6 (10):e146161. doi: 10.1172/jci.insight.146161.
- Lecomte, M., C. Bourlieu, E. Meugnier, A. Penhoat, D. Cheillan, G. Pineau, E. Loizon, M. Trauchessec, M. Claude, O. Ménard, et al. 2015. Milk polar lipids affect in vitro digestive lipolysis and postprandial lipid metabolism in mice. The Journal of Nutrition 145 (8):1770–7. doi: 10.3945/jn.115.212068.
- Lecomte, M., L. Couëdelo, E. Meugnier, P. Plaisancié, M. Létisse, B. Benoit, L. Gabert, A. Penhoat, A. Durand, G. Pineau, et al. 2016. Dietary emulsifiers from milk and soybean differently impact adiposity and inflammation in association with modulation of colonic goblet cells in high-fat fed mice. Molecular Nutrition & Food Research 60 (3):609–20. doi: 10.1002/mnfr.201500703.
- Lee, H., E. Padhi, Y. Hasegawa, J. Larke, M. Parenti, A. Wang, O. Hernell, B. Lönnerdal, and C. Slupsky. 2018. Compositional dynamics of the milk fat globule and its role in infant development. Frontiers in Pediatrics 6:313. doi: 10.3389/fped.2018.00313.
- Lee, H., D. Garrido, D. A. Mills, and D. Barile. 2014. Hydrolysis of milk gangliosides by infant-gut associated bifidobacteria determined by microfluidic chips and high-resolution mass spectrometry. Electrophoresis 35 (11):1742–50. doi: 10.1002/elps.201300653.
- Lee, H., J. B. German, R. Kjelden, C. B. Lebrilla, and D. Barile. 2013. Quantitative analysis of gangliosides in bovine milk and colostrum-based dairy products by ultrahigh performance liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry 61 (40):9689–96. doi: 10.1021/jf402255g.
- Lee, K., A. Kim, K. B. Hong, H. J. Suh, and K. Jo. 2020. Preparation and characterization of a polar milk lipid-enriched component from whey powder. Food Science of Animal Resources 40 (2):209–20. doi: 10.5851/kosfa.2020.e5.
- Lee, S. J, and J. W. Sherbon. 2002. Chemical changes in bovine milk fat globule membrane caused by heat treatment and homogenization of whole milk. The Journal of Dairy Research 69 (4):555–67. doi: 10.1017/S002202990200571X.
- Lefèvre, T, and M. Subirade. 2000. Interaction of β-lactoglobulin with phospholipid bilayers: A molecular level elucidation as revealed by infrared spectroscopy. International Journal of Biological Macromolecules 28 (1):59–67. doi: 10.1016/S0141-8130(00)00149-5.
- Levin, M. A., K. J. Burrington, and R. W. Hartel. 2016. Whey protein phospholipid concentrate and delactosed permeate: Applications in caramel, ice cream, and cake. Journal of Dairy Science 99 (9):6948–60. doi: 10.3168/jds.2016-10975.
- Li, F., S. S. Wu, C. L. Berseth, C. L. Harris, J. D. Richards, J. L. Wampler, W. Zhuang, et al. 2019. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. Journal of Pediatrics 215:24–31.e8. doi: 10.1016/j.jpeds.2019.08.030.
- Li, K., J. Jiang, H. Xiao, K. Wu, C. Qi, J. Sun, and D. Li. 2018. Changes in the metabolite profile of breast milk over lactation stages and their relationship with dietary intake in Chinese women: HPLC-QTOFMS based metabolomic analysis. Food & Function 9 (10):5189–97. doi: 10.1039/c8fo01005f.
- Li, M., Q. Li, S. Kang, X. Cao, Y. Zheng, J. Wu, R. Wu, J. Shao, M. Yang, and X. Yue. 2020. Characterization and comparison of lipids in bovine colostrum and mature milk based on UHPLC-QTOF-MS lipidomics. Food Research International (Ottawa, Ont.) 136:109490. doi: 10.1016/j.foodres.2020.109490.
- Li Pan, X, and T. Izumi. 1999. Chronological changes in the ganglioside composition of human milk during lactation. Early Human Development 55 (1):1–8. doi: 10.1016/S0378-3782(98)00105-4.
- Li, S., Y. Chen, B. Han, T. Xu, T. Liu, H. Yi, X. Zhou, L. Zhang, P. Liu, C. Ma, et al. 2020. Composition and variability of phospholipids in Chinese human milk samples. International Dairy Journal 110:104782. doi: 10.1016/j.idairyj.2020.104782.
- Li, T., H. Gong, Q. Yuan, M. Du, F. Ren, and X. Mao. 2020. Supplementation of polar lipids-enriched milk fat globule membrane in high-fat diet-fed rats during pregnancy and lactation promotes brown/beige adipocyte development and prevents obesity in male offspring. FASEB Journal 34 (3):1.
- Liang, L., X. Zhang, X. Wang, Q. Jin, and D. J. McClements. 2018. Influence of dairy emulsifier type and lipid droplet size on gastrointestinal fate of model emulsions: In vitro digestion study. Journal of Agricultural and Food Chemistry 66 (37):9761–9. doi: 10.1021/acs.jafc.8b02959.
- Lindahl, I. E. I., V. M. Artegoitia, E. Downey, J. A. O’mahony, C. Anne O’shea, C. A. Ryan, A. L. Kelly, H. C. Bertram, and U. K. Sundekilde. 2019. Quantification of human milk phospholipids: The effect of gestational and lactational age on phospholipid composition. Nutrients 11 (2):1–14. doi: 10.3390/nu11020222.
- Liu, H., E. C. Radlowski, M. S. Conrad, Y. Li, R. N. Dilger, and R. W. Johnson. 2014. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. The Journal of Nutrition 144 (12):1903–9. doi: 10.3945/jn.114.199828.
- Liu, L., Y. Pan, X. Zhang, Y. Zhang, and X. Li. 2021. Effect of particle size and interface composition on the lipid digestion of droplets covered with membrane phospholipids. Journal of Agricultural and Food Chemistry 69 (1):159–69. doi: 10.1021/acs.jafc.0c04945.
- Liu, Z., C. Li, J. Pryce, and S. Rochfort. 2020. Comprehensive characterization of bovine milk lipids: Phospholipids, sphingolipids, glycolipids, and ceramides. Journal of Agricultural and Food Chemistry 68 (24):6726–38. doi: 10.1021/acs.jafc.0c01604.
- Liu, Z., A. Logan, B. G. Cocks, and S. Rochfort. 2017. Seasonal variation of polar lipid content in bovine milk. Food Chemistry 237:865–9. doi: 10.1016/j.foodchem.2017.06.038.
- Liu, Z., S. Rochfort, and B. Cocks. 2018. Milk lipidomics: What we know and what we don’t. Progress in Lipid Research 71:70–85. doi: 10.1016/j.plipres.2018.06.002.
- Lopez, C. 2005. Focus on the supramolecular structure of milk fat in dairy products. Reproduction, Nutrition, Development 45 (4):497–511. doi: 10.1051/rnd:2005034.
- Lopez, C., M. Blot, V. Briard-Bion, C. Cirié, and B. Graulet. 2017. Butter serums and buttermilks as sources of bioactive lipids from the milk fat globule membrane: Differences in their lipid composition and potentialities of cow diet to increase n-3 PUFA. Food Research International (Ottawa, Ont.) 100 (Pt 1):864–72. doi: 10.1016/j.foodres.2017.08.016.
- Lopez, C., V. Briard-Bion, and O. Ménard. 2014. Polar lipids, sphingomyelin and long-chain unsaturated fatty acids from the milk fat globule membrane are increased in milks produced by cows fed fresh pasture based diet during spring. Food Research International 58:59–68. doi: 10.1016/j.foodres.2014.01.049.
- Lopez, C., V. Briard-Bion, O. Ménard, E. Beaucher, F. Rousseau, J. Fauquant, N. Leconte, and B. Robert. 2011. Fat globules selected from whole milk according to their size: Different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chemistry 125 (2):355–68. doi: 10.1016/j.foodchem.2010.09.005.
- Lopez, C., V. Briard-Bion, O. Menard, F. Rousseau, P. Pradel, and J. M. Besle. 2008. Phospholipid, sphingolipid, and fatty acid compositions of the milk fat globule membrane are modified by diet. Journal of Agricultural and Food Chemistry 56 (13):5226–36. doi: 10.1021/jf7036104.
- Lopez, C., C. Cauty, F. Rousseau, M. Blot, A. Margolis, and M. H. Famelart. 2017. Lipid droplets coated with milk fat globule membrane fragments: Microstructure and functional properties as a function of PH. Food Research International (Ottawa, Ont.) 91:26–37. doi: 10.1016/j.foodres.2016.11.025.
- Lopez, C., K. Cheng, and J. Perez. 2018. Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chemistry and Physics of Lipids 215:46–55. doi: 10.1016/j.chemphyslip.2018.07.008.
- Lopez, C., M. N. Madec, and R. Jimenez-Flores. 2010. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chemistry 120 (1):22–33. doi: 10.1016/j.foodchem.2009.09.065.
- Lopez, C, and O. Ménard. 2011. Human milk fat globules: Polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids and Surfaces. B, Biointerfaces 83 (1):29–41. doi: 10.1016/j.colsurfb.2010.10.039.
- Lowe, M. E. 2000. Properties and function of pancreatic lipase related protein 2. Biochimie 82 (11):997–1004. doi: 10.1016/S0300-9084(00)01184-6.
- Lu, J., N. Argov-Argaman, J. Anggrek, S. Boeren, T. van Hooijdonk, J. Vervoort, and K. A. Hettinga. 2016. The protein and lipid composition of the membrane of milk fat globules depends on their size. Journal of Dairy Science 99 (6):4726–38. doi: 10.3168/jds.2015-10375.
- Luo, J., Z. Wang, Y. Li, C. Chen, F. Ren, and H. Guo. 2019. The simulated in vitro infant gastrointestinal digestion of droplets covered with milk fat globule membrane polar lipids concentrate. Journal of Dairy Science 102 (4):2879–89. doi: 10.3168/jds.2018-15044.
- Ma, L., B. Y. Fong, A. K. MacGibbon, and G. Norris. 2020. Qualitative and quantitative study of glycosphingolipids in human milk and bovine milk using high performance liquid chromatography–data-dependent acquisition–mass spectrometry. Molecules 25 (17):4024. doi: 10.3390/molecules25174024.
- Ma, L., X. Liu, A. K. MacGibbon, A. Rowan, P. McJarrow, and B. Y. Fong. 2015. Lactational changes in concentration and distribution of ganglioside molecular species in human breast milk from Chinese mothers. Lipids 50 (11):1145–54. doi: 10.1007/s11745-015-4073-1.
- Ma, L., A. K. MacGibbon, H. J. B. Jan, Mohamed, S. L. Loy, A. Rowan, P. McJarrow, and B. Y. Fong. 2015. Determination of ganglioside concentrations in breast milk and serum from malaysian mothers using a high performance liquid chromatography-mass spectrometry-multiple reaction monitoring method. International Dairy Journal 49:62–71. doi: 10.1016/j.idairyj.2015.05.006.
- Ma, L., A. K. MacGibbon, H. J. B. Jan Mohamed, S. L. Loy, A. Rowan, P. McJarrow, and B. Y. Fong. 2017. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography–mass spectrometry–multiple reaction monitoring method. International Dairy Journal 71:50–9. doi: 10.1016/j.idairyj.2017.03.005.
- Maas, C., A. R. Franz, A. Shunova, M. Mathes, C. Bleeker, C. F. Poets, E. Schleicher, and W. Bernhard. 2017. Choline and polyunsaturated fatty acids in preterm infants’ maternal milk. European Journal of Nutrition 56 (4):1733–42. doi: 10.1007/s00394-016-1220-2.
- Manoni, M., C. Di Lorenzo, M. Ottoboni, M. Tretola, and L. Pinotti. 2020. Comparative proteomics of milk fat globule membrane (MFGM) proteome across species and lactation stages and the potentials of MFGM fractions in infant formula preparation. Foods 9 (9):1251. doi: 10.3390/foods9091251.
- Markworth, J. F., B. Durainayagam, V. C. Figueiredo, K. Liu, J. Guan, A. K. MacGibbon, B. Y. Fong, et al. 2017. Dietary supplementation with bovine-derived milk fat globule membrane lipids promotes neuromuscular development in growing rats. Nutrition and Metabolism 14 (1):1–14. doi: 10.1186/s12986-017-0161-y.
- Martín, M. J., S. Martín-Sosa, J. M. Alonso, and P. Hueso. 2003. Enterotoxigenic escherichia coli strains bind bovine milk gangliosides in a ceramide-dependent process. Lipids 38 (7):761–8. doi: 10.1007/s11745-003-1124-7.
- Martins, P. A., F. Gomes, W. L. Vaz, and M. J. Moreno. 2008. Binding of phospholipids to β-lactoglobulin and their transfer to lipid bilayers. Biochimica et Biophysica Acta 1778 (5):1308–15. doi: 10.1016/j.bbamem.2008.02.011.
- McJarrow, P., H. Radwan, L. Ma, A. K. MacGibbon, M. Hashim, H. Hasan, R. S. Obaid, F. Naja, H. J. J. Mohamed, H. Al Ghazal, et al. 2019. Human milk oligosaccharide, phospholipid, and ganglioside concentrations in breast milk from United Arab Emirates mothers: Results from the MISC cohort. Nutrients 11 (10):2400. doi: 10.3390/nu11102400.
- Méndez-Sánchez, N., M. Dibildox-Martinez, J. Sosa-Noguera, R. Sánchez-Medal, and F. J. Flores-Murrieta. 2019. Superior silybin bioavailability of silybin-phosphatidylcholine complex in oily-medium soft-gel capsules versus conventional silymarin tablets in healthy volunteers. BMC Pharmacology & Toxicology 20 (1):5. doi: 10.1186/s40360-018-0280-8.
- Mesilati-Stahy, R., H. Malka, and N. Argov-Argaman. 2015. Influence of glucogenic dietary supplementation and reproductive state of dairy cows on the composition of lipids in milk. Animal 9 (6):1008–15. doi: 10.1017/S1751731115000099.
- Mesilati-Stahy, R., K. Mida, and N. Argov-Argaman. 2011. Size-dependent lipid content of bovine milk fat globule and membrane phospholipids. Journal of Agricultural and Food Chemistry 59 (13):7427–35. doi: 10.1021/jf201373j.
- Mesilati-Stahy, R., U. Moallem, Y. Magen, and N. Argov-Argaman. 2015. Altered concentrate to forage ratio in cows ration enhanced bioproduction of specific size subpopulation of milk fat globules. Food Chemistry 179:199–205. doi: 10.1016/j.foodchem.2015.01.138.
- Micek, P., Z. M. Kowalski, M. Sady, J. Oprządek, J. Domagała, and P. Wanat. 2019. An energy-protein feed additive containing different sources of fat improves feed intake and milk performance of dairy cows in mid-lactation. The Journal of Dairy Research 86 (1):55–62. doi: 10.1017/S0022029919000062.
- Mika, A., M. Gaffney, R. Roller, A. Hills, C. A. Bouchet, K. A. Hulen, R. S. Thompson, M. Chichlowski, B. M. Berg, and M. Fleshner. 2018. Feeding the developing brain: Juvenile rats fed diet rich in prebiotics and bioactive milk fractions exhibit reduced anxiety-related behavior and modified gene expression in emotion circuits. Neuroscience Letters 677:103–9. doi: 10.1016/j.neulet.2018.01.052.
- Miklavcic, J. J., G. K. Shoemaker, K. L. Schnabl, B. M. Larsen, A. B. Thomson, V. C. Mazurak, and M. T. Clandinin. 2017. Ganglioside intake increases plasma ganglioside content in human participants. Journal of Parenteral and Enteral Nutrition 41 (4):657–66. doi: 10.1177/0148607115620093.
- Milard, M., F. Laugerette, A. Durand, C. Buisson, E. Meugnier, E. Loizon, and C. Louche-Pelissier. 2019. Milk polar lipids in a high-fat diet can prevent body weight gain: Modulated abundance of gut bacteria in relation with fecal loss of specific fatty acids. Molecular Nutrition & Food Research 63 (4):e1801078. doi: 10.1002/MNFR.201801078.
- Milard, M., A. Penhoat, A. Durand, C. Buisson, E. Loizon, E. Meugnier, K. Bertrand, F. Joffre, D. Cheillan, L. Garnier, et al. 2019. Acute effects of milk polar lipids on intestinal tight junction expression: Towards an impact of sphingomyelin through the regulation of IL-8 secretion? The Journal of Nutritional Biochemistry 65:128–38. doi: 10.1016/j.jnutbio.2018.12.007.
- Millar, C. L., C. Jiang, G. H. Norris, C. Garcia, S. Seibel, L. Anto, J. Y. Lee, and C. N. Blesso. 2020. Cow’s milk polar lipids reduce atherogenic lipoprotein cholesterol, modulate gut microbiota and attenuate atherosclerosis development in LDL-receptor knockout mice fed a western-type diet. The Journal of Nutritional Biochemistry 79:108351. doi: 10.1016/j.jnutbio.2020.108351.
- Minegishi, Y., N. Ota, S. Soga, and A. Shimotoyodome. 2016. Effects of nutritional supplementation with milk fat globule membrane on physical and muscle function in healthy adults aged 60 and over with semiweekly light exercise: A randomized double-blind, placebo-controlled pilot trial. Journal of Nutritional Science and Vitaminology 62 (6):409–15. doi: 10.3177/jnsv.62.409.
- Mitchell, M. D., K. Henare, B. Balakrishnan, E. Lowe, B. Y. Fong, and P. McJarrow. 2012. Transfer of gangliosides across the human placenta. Placenta 33 (4):312–6. doi: 10.1016/j.placenta.2011.12.018.
- Moher, D., A. Liberati, J. Tetzlaff, and D. G. Altman. 2009. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. BMJ 339 (jul21 1):b2535. doi: 10.1136/bmj.b2535.
- Moloney, C., D. O’Connor, and J. O’Regan. 2020. Polar lipid, ganglioside and cholesterol contents of infant formulae and growing up milks produced with an alpha lactalbumin-enriched whey protein concentrate. International Dairy Journal 107:104716. doi: 10.1016/j.idairyj.2020.104716.
- Morifuji, M., S. Higashi, C. Oba, S. Ichikawa, K. Kawahata, T. Yamaji, H. Itoh, Y. Manabe, and T. Sugawara. 2015. Milk phospholipids enhance lymphatic absorption of dietary sphingomyelin in lymph-cannulated rats. Lipids 50 (10):987–96. doi: 10.1007/s11745-015-4054-4.
- Morifuji, M., C. Oba, S. Ichikawa, K. Ito, K. Kawahata, Y. Asami, S. Ikegami, H. Itoh, and T. Sugawara. 2015. A novel mechanism for improvement of dry skin by dietary milk phospholipids: Effect on epidermal covalently bound ceramides and skin inflammation in hairless mice. Journal of Dermatological Science 78 (3):224–31. doi: 10.1016/J.JDERMSCI.2015.02.017.
- Motouri, M., H. Matsuyama, J. Ichi Yamamura, M. Tanaka, S. Aoe, T. Iwanaga, and H. Kawakami. 2003. Milk sphingomyelin accelerates enzymatic and morphological maturation of the intestine in artificially reared rats. Journal of Pediatric Gastroenterology and Nutrition 36 (2):241–7. doi: 10.1097/00005176-200302000-00016.
- Moukarzel, S., R. A. Dyer, C. Garcia, A. M. Wiedeman, G. Boyce, J. Weinberg, B. O. Keller, R. Elango, and S. M. Innis. 2018. Milk fat globule membrane supplementation in formula-fed rat pups improves reflex development and may alter brain lipid composition. Scientific Reports 8 (1):15277. doi: 10.1038/s41598-018-33603-8.
- Moukarzel, S., R. A. Dyer, B. O. Keller, R. Elango, and S. M. Innis. 2016. Human milk plasmalogens are highly enriched in long-chain PUFAs. The Journal of Nutrition 146 (11):2412–7. doi: 10.3945/JN.116.236802.
- Mudd, A. T., L. S. Alexander, K. Berding, R. V. Waworuntu, B. M. Berg, S. M. Donovan, and R. N. Dilger. 2016. Dietary prebiotics, milk fat globule membrane, and lactoferrin affects structural neurodevelopment in the young piglet. Frontiers in Pediatrics 4:4. doi: 10.3389/FPED.2016.00004.
- Murthy, A. V. R., F. Guyomarc’h, and C. Lopez. 2016. Cholesterol decreases the size and the mechanical resistance to rupture of sphingomyelin rich domains, in lipid bilayers studied as a model of the milk fat globule membrane. Langmuir: The ACS Journal of Surfaces and Colloids 32 (26):6757–65. doi: 10.1021/acs.langmuir.6b01040.
- Nagai, K. 2012. Bovine milk phospholipid fraction protects Neuro2a cells from endoplasmic reticulum stress via PKC activation and autophagy. Journal of Bioscience and Bioengineering 114 (4):466–71. doi: 10.1016/j.jbiosc.2012.05.009.
- Nejrup, R. G., T. R. Licht, and L. I. Hellgren. 2017. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Scientific Reports 7 (1):1–11. doi: 10.1038/s41598-017-04298-0.
- Newburg, D. S., S. Ashkenazi, and T. G. Cleary. 1992. Human milk contains the shiga toxin and shiga-like toxin receptor glycolipid Gb3. The Journal of Infectious Diseases 166 (4):832–6. doi: 10.1093/INFDIS/166.4.832.
- Newburg, D. S, and P. Chaturvedi. 1992. Neutral glycolipids of human and bovine milk. Lipids 27 (11):923–7. doi: 10.1007/BF02535874.
- Nieto-Ruiz, A., E. Diéguez, N. Sepúlveda-Valbuena, F. Herrmann, T. Cerdó, F. López-Torrecillas, R. De-Castellar, J. Jiménez, M. Pérez-García, M. T. Miranda, et al. 2020. The effects of an infant formula enriched with milk fat globule membrane, long-chain polyunsaturated fatty acids and synbiotics on child behavior up to 2.5 years old: The COGNIS study. Nutrients 12 (12):3825–16. doi: 10.3390/nu12123825.
- Nieto-Ruiz, A., J. Antonio García-Santos, M. G. Bermúdez, F. Herrmann, E. Diéguez, N. Sepúlveda-Valbuena, and S. García. 2019. Cortical visual evoked potentials and growth in infants fed with bioactive compounds-enriched infant formula: Results from COGNIS randomized clinical trial. Nutrients 11 (10):2456. doi: 10.3390/nu11102456.
- Nilsson, Å. 1969. The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 176 (2):339–47. doi: 10.1016/0005-2760(69)90192-1.
- Nilsson, Å. 2016. Role of sphingolipids in infant gut health and immunity. The Journal of Pediatrics 173:S53–S9. doi: 10.1016/j.jpeds.2016.02.076.
- Nilsson, A. K., C. Löfqvist, S. Najm, G. Hellgren, K. Sävman, M. X. Andersson, L. E. Smith, and A. Hellström. 2018. Long-chain polyunsaturated fatty acids decline rapidly in milk from mothers delivering extremely preterm indicating the need for supplementation. Acta Paediatrica (Oslo, Norway : 1992) 107 (6):1020–7. doi: 10.1111/apa.14275.
- Nilsson, A, and R.-D. Duan. 2006. Absorption and lipoprotein transport of sphingomyelin. Journal of Lipid Research 47 (1):154–71. doi: 10.1194/jlr.M500357-JLR200.
- Nilsson, Å., R.-D. Duan, and L. Ohlsson. 2021. Digestion and absorption of milk phospholipids in newborns and adults. Frontiers in Nutrition 8:724006. doi: 10.3389/fnut.2021.724006.
- Noh, S. K, and S. I. Koo. 2004. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. The Journal of Nutrition 134 (10):2611–6. doi: 10.1093/jn/134.10.2611.
- Norris, G. H., C. Jiang, J. Ryan, C. M. Porter, and C. N. Blesso. 2016. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. The Journal of Nutritional Biochemistry 30:93–101. doi: 10.1016/j.jnutbio.2015.12.003.
- Norris, T., R. Souza, Y. Xia, T. Zhang, A. Rowan, S. Gallier, H. Zhang, H. Qi, and P. Baker. 2019. Effect of supplementation of complex milk lipids in pregnancy on fetal growth: Results from the complex lipids in mothers and babies (CLIMB) randomized controlled trial. Journal of Maternal-Fetal and Neonatal Medicine 34 (20):3313–22. doi: 10.1080/14767058.2019.1683539.
- Nyberg, L., A. Farooqi, L. Bläckberg, R.-D. Duan, Å. Nilsson, and O. Hernell. 1998. Digestion of ceramide by human milk bile salt-stimulated lipase. Journal of Pediatric Gastroenterology and Nutrition 27 (5):55.
- Oba, C., M. Morifuji, S. Ichikawa, K. Ito, K. Kawahata, T. Yamaji, Y. Asami, H. Itou, and T. Sugawara. 2015. Dietary milk sphingomyelin prevents disruption of skin barrier function in hairless mice after UV-B irradiation. PLoS One 10 (8):e0136377. doi: 10.1371/journal.pone.0136377.
- Obeid, S., F. Guyomarc'h, E. David-Briand, F. Gaucheron, A. Riaublanc, and C. Lopez. 2019. The phase and charge of milk polar lipid membrane bilayers govern their selective interactions with proteins as demonstrated with casein micelles. Journal of Colloid and Interface Science 534:279–90. doi: 10.1016/j.jcis.2018.09.033.
- Ohlsson, L., H. Burling, R. D. Duan, and A. Nilsson. 2010. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. European Journal of Clinical Nutrition 64 (11):1344–9. doi: 10.1038/ejcn.2010.164.
- Ohlsson, L., H. Burling, and Å. Nilsson. 2009. Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids in Health and Disease 8 (1):44. doi: 10.1186/1476-511X-8-44.
- Ohlsson, L., E. Hertervig, B. A. Jönsson, R. D. Duan, L. Nyberg, R. Svernlöv, and Å. Nilsson. 2010. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. The American Journal of Clinical Nutrition 91 (3):672–8. doi: 10.3945/ajcn.2009.28311.
- Oosting, A., N. van Vlies, D. Kegler, L. Schipper, M. Abrahamse-Berkeveld, S. Ringler, H. J. Verkade, and E. M. van der Beek. 2014. Effect of dietary lipid structure in early postnatal life on mouse adipose tissue development and function in adulthood. The British Journal of Nutrition 111 (2):215–26. doi: 10.1017/S0007114513002201.
- Oosting, A., D. Kegler, H. J. Wopereis, I. C. Teller, B. J. Van De Heijning, H. J. Verkade, and E. M. Van Der Beek. 2012. Size and phospholipid coating of lipid droplets in the diet of young mice modify body fat accumulation in adulthood. Pediatric Research 72 (4):362–9. doi: 10.1038/PR.2012.101.
- Ortega-Anaya, J, and R. Jiménez-Flores. 2019. Symposium review: The relevance of bovine milk phospholipids in human nutrition—Evidence of the effect on infant gut and brain development. Journal of Dairy Science 102 (3):2738–48. doi: 10.3168/jds.2018-15342.
- Osada, K., K. Nagira, K. Teruya, H. Tachibana, S. Shirahata, and H. Murakami. 1993. Enhancement of interferon-β production with sphingomyelin from fermented milk. Biotherapy (Dordrecht, Netherlands) 7 (2):115–23. doi: 10.1007/BF01877735.
- Oshida, K., T. Shimizu, M. Takase, Y. Tamura, T. Shimizu, and Y. Yamashiro. 2003. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatric Research 53 (4):589–93. doi: 10.1203/01.PDR.0000054654.73826.AC.
- Ozarda, Y., M. Cansev, and I. H. Ulus. 2014. Relations of human breastmilk choline content with maternal hormonal status. Breastfeeding Medicine 9 (1):39–41. doi: 10.1089/bfm.2013.0029.
- Palmano, K. P., A. K. Macgibbon, C. A. Gunn, and L. M. Schollum. 2020. In vitro and in vivo anti-inflammatory activity of bovine milkfat globule (MFGM)-derived complex lipid fractions. Nutrients 12 (7):2089. doi: 10.3390/nu12072089.
- Pekmez, C. T., M. W. Larsson, M. V. Lind, N. V. Manjarrez, C. Yonemitsu, A. Larnkjaer, L. Bode, C. Mølgaard, K. F. M. Ichaelsen, and L. O. Dragsted. 2020. Breastmilk lipids and oligosaccharides influence branched short-chain fatty acid concentrations in infants with excessive weight gain. Molecular Nutrition and Food Research 64 (3):e1900977. doi: 10.1002/mnfr.201900977.
- Perea-Sanz, L., G. Garcia-Llatas, and M. J. Lagarda. 2018. Gangliosides in human milk and infant formula: A review on analytical techniques and contents. Food Reviews International 34 (6):511–38. doi: 10.1080/87559129.2017.1347671.
- Pind, S, and A. Kuksis. 1991. Further characterization of a novel phospholipase B (phospholipase A2-lysophospholipase) from intestinal brush-border membranes. Biochemistry and Cell Biology = Biochimie et Biologie Cellulaire 69 (5–6):346–57. doi: 10.1139/o91-054.
- Pollott, G., A. Brito, C. Gardiner, and C. Lawson. 2016. A comparison of different methodologies for the measurement of extracellular vesicles and milk-derived particles in raw milk from cows. Biomarker Insights 11:147–55. doi: 10.4137/BMI.S38438.
- Poppitt, S. D., R. A. McGregor, K. R. Wiessing, V. K. Goyal, A. J. Chitkara, S. Gupta, K. Palmano, B. Kuhn-Sherlock, and M. A. McConnell. 2014. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in Northern Indian infants. Journal of Pediatric Gastroenterology & Nutrition 59 (2):167–71. doi: 10.1097/MPG.0000000000000398.
- Portelli, J., A. Gordon, and J. T. May. 1998. Effect of compounds with antibacterial activities in human milk on respiratory syncytial virus and cytomegalovirus in vitro. Journal of Medical Microbiology 47 (11):1015–8. doi: 10.1099/00222615-47-11-1015.
- Potočki, S. 2016. Gangliosides in dairy products: Milk sphingolipids. Acta Alimentaria 45 (4): 572–7. doi: 10.1556/066.2016.45.4.15.
- Powe, C. E., C. D. Knott, and N. Conklin-Brittain. 2010. Infant sex predicts breast milk energy content. American Journal of Human Biology 22 (1): 50–4. doi: 10.1002/ajhb.20941.
- Price, N., T. Fei, S. Clark, and T. Wang. 2020. Application of zinc and calcium acetate to precipitate milk fat globule membrane components from a dairy by-product. Journal of Dairy Science 103 (2):1303–14. doi: 10.3168/jds.2019-16892.
- Quarles, W. R., A. Pokala, E. L. Shaw, J. Ortega-Anaya, L. Hillmann, R. Jimenez-Flores, and R. S. Bruno. 2020. Alleviation of metabolic endotoxemia by milk fat globule membrane: Rationale, design, and methods of a double-blind, randomized, controlled, crossover dietary intervention in adults with metabolic syndrome. Current Developments in Nutrition 4 (9):nzaa130. doi: 10.1093/cdn/nzaa130.
- Reis, M. G., N. C. Roy, E. N. Bermingham, L. Ryan, R. Bibiloni, W. Young, L. Krause, B. Berger, M. North, K. Stelwagen, et al. 2013. Impact of dietary dairy polar lipids on lipid metabolism of mice fed a high-fat diet. Journal of Agricultural and Food Chemistry 61 (11):2729–38. doi: 10.1021/jf303795b.
- Reis, M. G., R. Bibiloni, P. McJarrow, A. MacGibbon, B. Fong, S. Bassett, N. Roy, and M. M. dos Reis. 2016. Isotopic labeling of milk disialogangliosides (GD3). Chemistry and Physics of Lipids 200:104–12. doi: 10.1016/j.chemphyslip.2016.08.003.
- Reis, M. G., P. Harris, C. Berry, H. Nguyen, P. Maclean, and M. Weeks. 2020. Tracking changes in volatile components and lipids after homogenisation and thermal processing of milk. International Dairy Journal 103:104624. doi: 10.1016/j.idairyj.2019.104624.
- Ren, S., J. N. Scarsdale, T. Ariga, Y. Zhang, R. A. Klein, R. Hartmann, Y. Kushi, H. Egge, and R. K. Yu. 1992. O-acetylated gangliosides in bovine buttermilk. characterization of 7-O-acetyl, 9-O-acetyl, and 7,9-di-O-acetyl G(D3). Journal of Biological Chemistry 267 (18):12632–8. doi: 10.1016/S0021-9258(18)42324-1.
- Rico, D. E., E. R. Marshall, J. Choi, K. E. Kaylegian, C. D. Dechow, and K. J. Harvatine. 2014. Within-milking variation in milk composition and fatty acid profile of holstein dairy cows. Journal of Dairy Science 97 (7):4259–68. doi: 10.3168/jds.2013-7731.
- Rivas-Serna, I. M., R. Polakowski, G. K. Shoemaker, V. C. Mazurak, and M. T. Clandinin. 2015. Profiling gangliosides from milk products and other biological membranes using LC/MS. Journal of Food Composition and Analysis 44:45–55. doi: 10.1016/j.jfca.2015.06.006.
- Rodríguez-Alcalá, L. M, and J. Fontecha. 2010. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. Journal of Chromatography. A 1217 (18):3063–6. doi: 10.1016/j.chroma.2010.02.073.
- Rombaut, R., J. Van Camp, and K. Dewettinck. 2005. Analysis of phospho- and sphingolipids in dairy products by a new HPLC method. Journal of Dairy Science 88 (2):482–8. doi: 10.3168/jds.S0022-0302(05)72710-7.
- Rombaut, R., J. Van Camp, and K. Dewettinck. 2006. Phospho- and sphingolipid distribution during processing of milk, butter and whey. International Journal of Food Science and Technology 41 (4):435–43. doi: 10.1111/j.1365-2621.2005.01091.x.
- Rombaut, R., K. Dewettinck, and J. Van Camp. 2007. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC-ELSD). Journal of Food Composition and Analysis 20 (3-4):308–12. doi: 10.1016/j.jfca.2006.01.010.
- Ronda, O. A. H. O., B. J. M. van de Heijning, I. Martini, A. Gerding, J. C. Wolters, Y. T. van der Veen, M. Koehorst, A. Jurdzinski, R. Havinga, E. M. van der Beek, et al. 2020. Effects of an early life diet containing large phospholipid-coated lipid globules on hepatic lipid metabolism in mice. Scientific Reports 10 (1):16128. doi: 10.1038/s41598-020-72777-y.
- Ronda, O. A., B. J. Van De Heijning, A. De Bruin, A. Jurdzinski, F. Kuipers, and H. J. Verkade. 2019. Programming effects of an early life diet containing large phospholipid-coated lipid globules are transient under continuous exposure to a high-fat diet. The British Journal of Nutrition 122 (12):1321–8. doi: 10.1017/S0007114519002083.
- Ronda, O. A., B. J. Van De Heijning, I. A. Martini, M. Koehorst, R. Havinga, A. Jurdzinski, and V. W. Bloks. 2020. An early-life diet containing large phospholipid-coated lipid globules programs later-life postabsorptive lipid trafficking in high-fat diet but not in low-fat dietfed mice. British Journal of Nutrition 2020:1–11. doi: 10.1017/S0007114520002421.
- Rosqvist, F., A. Smedman, H. Lindmark-Månsson, M. Paulsson, P. Petrus, S. Straniero, M. Rudling, I. Dahlman, and U. Risérus. 2015. Potential role of milk fat globule membrane in modulating plasma lipoproteins, gene expression, and cholesterol metabolism in humans: A randomized study. The American Journal of Clinical Nutrition 102 (1):20–30. doi: 10.3945/ajcn.115.107045.
- Roy, S., A. Basu, P. Dhar, and M. Ghosh. 2015. Calcium, iron and essential fatty acid composition of Bengali mother’s milk: A population based cross-sectional study. Indian Journal of Community Health 26:310–7.
- Rueda, R., J. L. Sabatel, J. Maldonado, J. A. Molina-Font, and A. Gil. 1998. Addition of gangliosides to an adapted milk formula modifies levels of fecal escherichia coli in preterm newborn infants. The Journal of Pediatrics 133 (1):90–4. doi: 10.1016/S0022-3476(98)70184-2.
- Rueda, R., R. Puente, P. Hueso, J. Maldonado, and A. Gil. 1995. New data on content and distribution of gangliosides in human milk. Biological Chemistry Hoppe-Seyler 376 (12):723–8. doi: 10.1515/bchm3.1995.376.12.723.
- Russell, A., A. Laubscher, R. Jimenez-Flores, and L. H. Laiho. 2010. Investigating the protective properties of milk phospholipids against ultraviolet light exposure in a skin equivalent model. Multiphoton Microscopy in the Biomedical Sciences X 2010:7569. doi: 10.1117/12.845803.
- Sala-Vila, A., A. I. Castellote, C. Campoy, M. Rivero, M. Rodriguez-Palmero, and M. C. López-Sabater. 2004. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. The Journal of Nutrition 134 (4):868–73. doi: 10.1093/jn/134.4.868.
- Sala-Vila, A., A. I. Castellote, M. Rodriguez-Palmero, C. Campoy, and M. C. López-Sabater. 2005. Lipid composition in human breast milk from Granada (Spain): Changes during lactation. Nutrition 21 (4):467–73. doi: 10.1016/j.nut.2004.08.020.
- Salcedo, J., R. Barbera, E. Matencio, A. Alegría, and M. J. Lagarda. 2013. Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: An in vitro study. Food Chemistry 136 (2):726–34. doi: 10.1016/j.foodchem.2012.08.078.
- Sánchez-Juanes, F., J. M. Alonso, L. Zancada, and P. Hueso. 2009. Glycosphingolipids from bovine milk and milk fat globule membranes: A comparative study. Adhesion to enterotoxigenic Escherichia coli strains. Biological Chemistry 390 (1):31–40. doi: 10.1515/BC.2009.003.
- Schipper, L., G. van Dijk, L. M. Broersen, M. Loos, N. Bartke, A. J. Scheurink, and E. M. van der Beek. 2016. A postnatal diet containing phospholipids, processed to yield large, phospholipid-coated lipid droplets, affects specific cognitive behaviors in healthy male mice. The Journal of Nutrition 146 (6):1155–61. doi: 10.3945/jn.115.224998.
- Schnabl, K. L., B. Larsen, J. E. Van Aerde, G. Lees, M. Evans, M. Belosevic, C. Field, A. B. Thomson, and M. T. Clandinin. 2009. Gangliosides protect bowel in an infant model of necrotizing enterocolitis by suppressing proinflammatory signals. Journal of Pediatric Gastroenterology and Nutrition 49 (4):382–92. doi: 10.1097/MPG.0b013e3181b6456d.
- Schneider, N., J. Hauser, M. Oliveira, E. Cazaubon, S. C. Mottaz, B. V. O’Neill, P. Steiner, Sean, and C. L. Deoni. 2019. Sphingomyelin in brain and cognitive development: Preliminary data. eneuro 6 (4):ENEURO.0421-18.2019. doi: 10.1523/ENEURO.0421-18.2019.
- Scholtz, S. A., B. S. Gottipati, B. J. Gajewski, and S. E. Carlson. 2013. Dietary sialic acid and cholesterol influence cortical composition in developing rats. The Journal of Nutrition 143 (2):132–5. doi: 10.3945/jn.112.169508.
- Schroten, H., M. Bosch, R. Nobis-Bosch, H. Koehler, F. G. Hanisch, and R. Plogmann. 2001. Anti-infectious properties of the human milk fat globule membrane. Advances in Experimental Medicine and Biology 501:189–92. doi: 10.1007/978-1-4615-1371-1_24.
- Schubert, M., C. Contreras, N. Franz, and J. Hellhammer. 2011. Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutrition Research (New York, N.Y.) 31 (6):413–20. doi: 10.1016/j.nutres.2011.05.012.
- Sehl, A., L. Couëdelo, I. Chamekh-Coelho, C. Vaysse, and M. Cansell. 2019. In vitro lipolysis and lymphatic absorption of N-3 long-chain PUFA in the rat: Influence of the molecular lipid species as carrier. The British Journal of Nutrition 122 (6):639–47. doi: 10.1017/S0007114519001491.
- Selvalatchmanan, J., A. V. Rukmini, S. Ji, A. Triebl, L. Gao, A. K. Bendt, M. R. Wenk, J. J. Gooley, and F. Torta. 2021. Variability of lipids in human milk. Metabolites 11 (2):1–18. doi: 10.3390/metabo11020104.
- Sharma, K. C., V. K. Sachdeva, and S. Singh. 2000. A comparative gross and lipid composition of murrah breed of buffalo and cross-bred cow’s milk during different lactation stages. Archives Animal Breeding 43 (2):123–30. doi: 10.5194/aab-43-123-2000.
- Shoji, H., T. Shimizu, N. Kaneko, K. Shinohara, S. Shiga, M. Saito, K. Oshida, T. Shimizu, M. Takase, and Y. Yamashiro. 2006. Comparison of the phospholipid classes in human milk in Japanese mothers of term and preterm infants. Acta Paediatrica (Oslo, Norway : 1992) 95 (8):996–1000. doi: 10.1080/08035250600660933.
- Shunova, A., K. A. Böckmann, M. Minarski, A. R. Franz, C. Wiechers, C. F. Poets, and W. Bernhard. 2020. Choline content of term and preterm infant formulae compared to expressed breast milk—How do we justify the discrepancies? Nutrients 12 (12):3815–2. doi: 10.3390/nu12123815.
- Singh, H, and S. Gallier. 2017. Nature’s complex emulsion: The fat globules of milk. Food Hydrocolloids. 68:81–9. doi: 10.1016/j.foodhyd.2016.10.011.
- Smoczyński, M. 2017. Role of phospholipid flux during milk secretion in the mammary gland. Journal of Mammary Gland Biology and Neoplasia 22 (2):117–29. doi: 10.1007/s10911-017-9376-9.
- Smoczyński, M, and K. Kiełczewska. 2014. Fractal and physico-chemical analysis of cows’ milk fat globules after lipolysis. Journal of Food and Nutrition Research 53 (3):207–16.
- Smolinska, A., A. Baranska, J. W. Dallinga, R. P. Mensink, S. Baumgartner, B. J. van de Heijning, and F. J. van Schooten. 2019. Comparing patterns of volatile organic compounds exhaled in breath after consumption of two infant formulae with a different lipid structure: A randomized trial. Scientific Reports 9 (1):554. doi: 10.1038/s41598-018-37210-5.
- Snow, D. R., R. E. Ward, A. Olsen, R. Jimenez-Flores, and K. J. Hintze. 2011. Membrane-rich milk fat diet provides protection against gastrointestinal leakiness in mice treated with lipopolysaccharide. Journal of Dairy Science 94 (5):2201–12. doi: 10.3168/jds.2010-3886.
- Soga, S., N. Ota, and A. Shimotoyodome. 2015. Dietary milk fat globule membrane supplementation combined with regular exercise improves skeletal muscle strength in healthy adults: A randomized double-blind, placebo-controlled, crossover trial. Nutrition Journal 14 (1):85. doi: 10.1186/s12937-015-0073-5.
- Song, S., T. T. Liu, X. Liang, Z. Y. Liu, D. Yishake, X. T. Lu, M. T. Yang, Q. Q. Man, J. Zhang, and H. L. Zhu. 2021. Profiling of phospholipid molecular species in human breast milk of chinese mothers and comprehensive analysis of phospholipidomic characteristics at different lactation stages. Food Chemistry 348:129091. doi: 10.1016/j.foodchem.2021.129091.
- Sørensen, L. K. 2006. A liquid chromatography/tandem mass spectrometric approach for the determination of gangliosides GD3 and GM3 in bovine milk and infant formulae. Rapid Communications in Mass Spectrometry: RCM 20 (24):3625–33. doi: 10.1002/rcm.2775.
- Spence, A. J., R. Jimenez-Flores, M. Qian, and L. Goddik. 2009a. The influence of temperature and pressure factors in supercritical fluid extraction for optimizing nonpolar lipid extraction from buttermilk powder. Journal of Dairy Science 92 (2):458–68. doi: 10.3168/jds.2008-1278.
- Spence, A. J., R. Jimenez-Flores, M. Qian, and L. Goddik. 2009b. Phospholipid enrichment in sweet and whey cream buttermilk powders using supercritical fluid extraction. Journal of Dairy Science 92 (6):2373–81. doi: 10.3168/jds.2008-1534.
- Spichtig, V., J. Michaud, and S. Austin. 2010. Determination of sialic acids in milks and milk-based products. Analytical Biochemistry 405 (1):28–40. doi: 10.1016/j.ab.2010.06.010.
- Spitsberg, V. L., L. Ivanov, and V. Shritz. 2019. Recovery of milk fat globule membrane (MFGM) from buttermilk: Effect of Ca-binding salts. The Journal of Dairy Research 86 (3):374–6. doi: 10.1017/S002202991900061X.
- Sprong, R. C., M. F. Hulstein, and R. Van Der Meer. 2001. Bactericidal activities of milk lipids. Antimicrobial Agents and Chemotherapy 45 (4):1298–301. doi: 10.1128/AAC.45.4.1298-1301.2001.
- Sprong, R. C., M. F. Hulstein, and R. Van Der Meer. 2002. Bovine milk fat components inhibit food-borne pathogens. International Dairy Journal 12 (2–3):209–15. doi: 10.1016/S0958-6946(01)00139-X.
- Sprong, R. C., M. F. E. Hulstein, T. T. Lambers, and R. Van Der Meer. 2012. Sweet buttermilk intake reduces colonisation and translocation of listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. The British Journal of Nutrition 108 (11):2026–33. doi: 10.1017/S0007114512000165.
- Su, M., A. K. Subbaraj, K. Fraser, X. Qi, H. Jia, W. Chen, M. Gomes Reis, M. Agnew, L. Day, N. C. Roy, et al. 2019. Lipidomics of brain tissues in rats fed human milk from Chinese mothers or commercial infant formula. Metabolites 9 (11):253. doi: 10.3390/metabo9110253.
- Suh, M., M. Belosevic, and M. T. Clandinin. 2004. Dietary lipids containing gangliosides reduce giardia muris infection in vivo and survival of giardia lamblia trophozoites in vitro. Parasitology 128 (Pt 6):595–602. doi: 10.1017/S0031182004005128.
- Szuhaj, B. F. 2005. Lecithins. In Bailey’s industrial oil and fat products, ed. F. Shadidi, 6th ed. Hoboken, NJ: John Wiley & Sons, Inc.
- Tanaka, K., M. Hosozawa, N. Kudo, N. Yoshikawa, K. Hisata, H. Shoji, K. Shinohara, and T. Shimizu. 2013. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain & Development 35 (1):45–52. doi: 10.1016/j.braindev.2012.03.004.
- Tavazzi, I., P. Fontannaz, L. Y. Lee, and F. Giuffrida. 2018. Quantification of glycerophospholipids and sphingomyelin in human milk and infant formula by high performance liquid chromatography coupled with mass spectrometer detector. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 1072:235–43. doi: 10.1016/j.jchromb.2017.10.067.
- Tellez, A., M. Corredig, A. Guri, R. Zanabria, M. W. Griffiths, and V. Delcenserie. 2012. Bovine Milk fat globule membrane affects virulence expression in Escherichia coli O157:H7. Journal of Dairy Science 95 (11):6313–9. doi: 10.3168/jds.2012-5560.
- Ten Bruggencate, S. J., P. D. Frederiksen, S. M. Pedersen, E. G. Floris-Vollenbroek, E. L. van De Bos, E. Van Hoffen, and P. L. Wejse. 2016. Dietary milk-fat-globule membrane affects resistance to diarrheagenic escherichia coli in healthy adults in a randomized, placebo-controlled, double-blind study. The Journal of Nutrition 146 (2):249–55. doi: 10.3945/jn.115.214098.
- Thakkar, S. K., F. Giuffrida, C. H. Cristina, C. A. De Castro, R. Mukherjee, L. A. Tran, P. Steenhout, L. Y. Lee, and F. Destaillats. 2013. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. American Journal of Human Biology 25 (6):770–9. doi: 10.1002/ajhb.22446.
- Thum, C., N. C. Roy, D. W. Everett, and W. C. McNabb. 2021. Variation in milk fat globule size and composition: A source of bioactives for human health. Critical Reviews in Food Science and Nutrition 2021:1–27. doi: 10.1080/10408398.2021.1944049.
- Thum, C., W. Young, C. A. Montoya, N. C. Roy, and W. C. McNabb. 2020. In vitro fermentation of digested milk fat globule membrane from ruminant milk modulates piglet ileal and caecal microbiota. Frontiers in Nutrition 7:91. doi: 10.3389/fnut.2020.00091.
- Timby, N., M. Adamsson, E. Domellöf, T. Grip, O. Hernell, B. Lönnerdal, and M. Domellöf. 2021. Neurodevelopment and growth until 6.5 years of infants who consumed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. The American Journal of Clinical Nutrition 113 (3):586–92. doi: 10.1093/ajcn/nqaa354.
- Tian, H., N. Zheng, W. Wang, J. Cheng, S. Li, Y. Zhang, and J. Wang. 2016. Integrated Metabolomics Study of the Milk of Heat-stressed Lactating Dairy Cows. Scientific Reports 6 (1). doi: 10.1038/srep24208.
- Timby, N., E. Domellöf, O. Hernell, B. Lönnerdal, and M. Domellöf. 2014. Neurodevelopment, nutrition, and growth until 12 months of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. The American Journal of Clinical Nutrition 99 (4):860–8. doi: 10.3945/ajcn.113.064295.
- Timby, N., M. Domellöf, P. L. Holgerson, C. E. West, B. Lönnerdal, O. Hernell, and I. Johansson. 2017. Oral microbiota in infants fed a formula supplemented with bovine milk fat globule membranes—A randomized controlled trial. PLoS One 12 (1):e0169831. doi: 10.1371/journal.pone.0169831.
- Timby, N., O. Hernell, O. Vaarala, M. Melin, B. Lönnerdal, and M. Domellöf. 2015. Infections in infants fed formula supplemented with bovine milk fat globule membranes. Journal of Pediatric Gastroenterology & Nutrition 60 (3):384–9. doi: 10.1097/MPG.0000000000000624.
- Timby, N., B. Lönnerdal, O. Hernell, and M. Domellöf. 2014. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatric Research 76 (4):394–400. doi: 10.1038/pr.2014.110.
- Uauy, R., D. R. Hoffman, P. Peirano, D. G. Birch, and E. E. Birch. 2001. Essential fatty acids in visual and brain development. Lipids 36 (9):885–95. doi: 10.1007/S11745-001-0798-1.
- Uchida, Y, and P. Park. 2021. Ceramides in skin health and disease: An update. American Journal of Clinical Dermatology 22 (6):853–66. doi: 10.1007/s40257-021-00619-2.
- Valenzuela, A., S. Nieto, J. Sanhueza, M. J. Nuñez, and C. Ferrer. 2005. Tissue accretion and milk content of docosahexaenoic acid in female rats after supplementation with different docosahexaenoic acid sources. Annals of Nutrition & Metabolism 49 (5):325–32. doi: 10.1159/000087337.
- Veereman-Wauters, G., S. Staelens, R. Rombaut, K. Dewettinck, D. Deboutte, R. J. Brummer, M. Boone, and P. Le Ruyet. 2012. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 28 (7–8):749–52. doi: 10.1016/j.nut.2011.10.011.
- Venuti, E., D. Shishmarev, P. W. Kuchel, S. Dutt, C. S. Blumenthal, and K. J. Gaskin. 2017. Bile salt stimulated lipase: Inhibition by phospholipids and relief by phospholipase A2. Journal of Cystic Fibrosis 16 (6):763–70. doi: 10.1016/j.jcf.2017.07.005.
- Verma, A., A. K. Sharma, A. Agarwal, S. Datta, and K. Ambatipudi. 2020. Selective enrichment of milk fat globules using functionalized polyvinylidene fluoride membrane. Preparative Biochemistry & Biotechnology 50 (1):18–27. doi: 10.1080/10826068.2019.1658117.
- Vesper, H., E. M. Schmelz, M. N. Nikolova-Karakashian, D. L. Dillehay, D. V. Lynch, and A. H. Merrill. 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. The Journal of Nutrition 129 (7):1239–50. doi: 10.1093/JN/129.7.1239.
- Vickers, M. H., J. Guan, M. Gustavsson, C. U. Krägeloh, B. H. Breier, M. Davison, B. Fong, C. Norris, P. McJarrow, and S. C. Hodgkinson. 2009. Supplementation with a mixture of complex lipids derived from milk to growing rats results in improvements in parameters related to growth and cognition. Nutrition Research 29 (6):426–35. doi: 10.1016/j.nutres.2009.06.001.
- Viveros-Rogel, M., L. Soto-Ramirez, P. Chaturvedi, D. S. Newburg, and G. M. Ruiz-Palacios. 2004. Inhibition of HIV-1 infection in vitro by human milk sulfated glycolipids and glycosaminoglycans. Advances in Experimental Medicine and Biology 554:481–7. doi: 10.1007/978-1-4757-4242-8_69.
- Vors, C., L. Joumard-Cubizolles, M. Lecomte, E. Combe, L. Ouchchane, J. Drai, K. Raynal, F. Joffre, L. Meiller, M. Le Barz, et al. 2020. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: Towards a gut sphingomyelin-cholesterol interplay. Gut 69 (3):487–501. doi: 10.1136/gutjnl-2018-318155.
- Wada, A., M. Hasegawa, P.-F. Wong, E. Shirai, N. Shirai, L.-J. Tan, R. Llanes, H. Hojo, E. Yamasaki, A. Ichinose, et al. 2010. Direct binding of gangliosides to Helicobacter pylori vacuolating cytotoxin (VacA) neutralizes its toxin activity. Glycobiology 20 (6):668–78. doi: 10.1093/glycob/cwq014.
- Walker, G. P., C. Wijesundera, F. R. Dunshea, and P. T. Doyle. 2013. Seasonal and stage of lactation effects on milk fat composition in Northern Victoria. Animal Production Science 53 (6):560. doi: 10.1071/AN11363.
- Wallace, T. C., J. K. Blusztajn, M. A. Caudill, K. C. Klatt, E. Natker, S. H. Zeisel, and K. M. Zelman. 2018. The underconsumed and underappreciated essential nutrient. Nutrition Today 53 (6):240–53. doi: 10.1097/NT.0000000000000302.
- Walter, L., V. K. Narayana, R. Fry, A. Logan, D. Tull, and B. Leury. 2020. Milk fat globule size development in the mammary epithelial cell: A potential role for ether phosphatidylethanolamine. Scientific Reports 10 (1):12299. doi: 10.1038/s41598-020-69036-5.
- Wang, B. 2009. Sialic acid is an essential nutrient for brain development and cognition. Annual Review of Nutrition 29 (1):177–222. doi: 10.1146/annurev.nutr.28.061807.155515.
- Wang, B., P. McVeagh, P. Petocz, and J. Brand-Miller. 2003. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. The American Journal of Clinical Nutrition 78 (5):1024–9. doi: 10.1093/ajcn/78.5.1024.
- Wang, L., Y. Shimizu, S. Kaneko, S. Hanaka, T. Abe, H. Shimasaki, H. Hisaki, and H. Nakajima. 2000. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatrics International 42 (1):14–20. doi: 10.1046/j.1442-200X.2000.01169.x.
- Wat, E., S. Tandy, E. Kapera, A. Kamili, R. W. Chung, A. Brown, M. Rowney, and J. S. Cohn. 2009. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 205 (1):144–50. doi: 10.1016/j.atherosclerosis.2008.12.004.
- Wei, W., Q. Jin, and X. Wang. 2019. Human milk fat substitutes: Past achievements and current trends. Progress in Lipid Research 74:69–86. doi: 10.1016/j.plipres.2019.02.001.
- Wei, W., J. Yang, D. Yang, X. Wang, Z. Yang, Q. Jin, M. Wang, J. Lai, and X. Wang. 2019. Phospholipid composition and fat globule structure I: Comparison of human milk fat from different gestational ages, lactation stages, and infant formulas. Journal of Agricultural and Food Chemistry 67 (50):13922–8. doi: 10.1021/acs.jafc.9b04247.
- Weiland, A., A. Bub, S. W. Barth, J. Schrezenmeir, and M. Pfeuffer. 2016. Effects of dietary milk- and soya-phospholipids on lipid-parameters and other risk indicators for cardiovascular diseases in overweight or obese men—Two double-blind, randomised, controlled, clinical trials. Journal of Nutritional Science 5:1–9. doi: 10.1017/jns.2016.9.
- Weng, J., R. Lin, C. Jiang, W. Wei, X. Wang, and Q. Jin. 2021. O/W emulsion stabilized by bovine milk phospholipid-protein nanoemulsions: Preparation, stability, and in vitro digestion. Journal of Agricultural and Food Chemistry 69 (17):5003–12. doi: 10.1021/acs.jafc.0c05617.
- West, J. W. 2003. Effects of heat-stress on production in dairy cattle. Journal of Dairy Science 86 (6):2131–44. doi: 10.3168/jds.S0022-0302(03)73803-X.
- Wiedeman, A. M., S. I. Barr, T. J. Green, Z. Xu, S. M. Innis, and D. D. Kitts. 2018. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients 10 (10):1513. doi: 10.3390/nu10101513.
- Wijendran, V., M. C. Huang, G. Y. Diau, G. Boehm, P. W. Nathanielsz, and J. Thomas Brenna. 2002. Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatric Research 51 (3):265–72. doi: 10.1203/00006450-200203000-00002.
- Wiking, L., J. H. Nielsen, A. K. Båvius, A. Edvardsson, and K. Svennersten-Sjaunja. 2006. Impact of milking frequencies on the level of free fatty acids in milk, fat globule size, and fatty acid composition. Journal of Dairy Science 89 (3):1004–9. doi: 10.3168/jds.S0022-0302(06)72166-X.
- Wooding, F. B. P., and I. H. Mather. 2017. Ultrastructural and immunocytochemical evidence for the reorganisation of the milk fat globule membrane after secretion. Cell and Tissue Research 367 (2):283–95. doi: 10.1007/s00441-016-2505-8.
- Xu, L., W. Chen, X. Wang, Z. Yu, and S. Han. 2020. Comparative lipidomic analyses reveal different protections in preterm and term breast milk for infants. Frontiers in Pediatrics 8:590. doi: 10.3389/fped.2020.00590.
- Xu, S., M. Walkling-Ribeiro, M. W. Griffiths, and M. Corredig. 2015. Pulsed electric field processing preserves the antiproliferative activity of the milk fat globule membrane on colon carcinoma cells. Journal of Dairy Science 98 (5):2867–74. doi: 10.3168/jds.2014-8839.
- Xu, W., A. Van Knegsel, E. Saccenti, R. Van Hoeij, B. Kemp, and J. Vervoort. 2020. Metabolomics of milk reflects a negative energy balance in cows. Journal of Proteome Research 19 (8):2942–9. doi: 10.1021/acs.jproteome.9b00706.
- Yamauchi, I., M. Uemura, M. Hosokawa, A. Iwashima-Suzuki, M. Shiota, and K. Miyashita. 2016. The dietary effect of milk sphingomyelin on the lipid metabolism of obese/diabetic KK-Ay mice and wild-type C57BL/6J mice. Food & Function 7 (9):3854–67. doi: 10.1039/c6fo00274a.
- Yang, H. S., X. Xiong, Q. Q. Wen, and Y. L. Yin. 2016. Effects of dietary supplementation with ethanolamine on intestine development and growth performance of weaned piglets. Journal of Animal Science 94 (Suppl 3):79–81. doi: 10.2527/jas.2015-9560.
- Yano, M., Y. Minegishi, S. Sugita, and N. Ota. 2017. Milk fat globule membrane supplementation with voluntary running exercise attenuates age-related motor dysfunction by suppressing neuromuscular junction abnormalities in mice. Experimental Gerontology 97:29–37. doi: 10.1016/j.exger.2017.07.012.
- Yao, Y., G. Zhao, J. Xiang, X. Zou, Q. Jin, and X. Wang. 2016. Lipid composition and structural characteristics of bovine, caprine and human milk fat globules. International Dairy Journal 56:64–73. doi: 10.1016/j.idairyj.2015.12.013.
- Yao, Y., G. Zhao, X. Zou, L. Huang, and X. Wang. 2015. Microstructural and lipid composition changes in milk fat globules during milk powder manufacture. RSC Advances 5 (77):62638–46. doi: 10.1039/C5RA08247A.
- Ye, H., B. Li, V. Subramanian, B. H. Choi, Y. Liang, A. Harikishore, G. Chakraborty, K. Baek, and H. S. Yoon. 2013. NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochimica et Biophysica Acta 1828 (3):1083–93. doi: 10.1016/j.bbamem.2012.12.009.
- Ye, L., Q. Zhang, F. Xin, B. Cao, L. Qian, and Y. Dong. 2021. Neonatal milk fat globule membrane supplementation during breastfeeding ameliorates the deleterious effects of maternal high-fat diet on metabolism and modulates gut microbiota in adult mice offspring in a sex-specific way. Frontiers in Cellular and Infection Microbiology 11:621957. doi: 10.3389/fcimb.2021.621957.
- Yeung, T., G. E. Gilbert, J. Shi, J. Silvius, A. Kapus, and S. Grinstein. 2008. Membrane phosphatidylserine regulates surface charge and protein localization. Science (New York, N.Y.) 319 (5860):210–3. doi: 10.1126/science.1152066.
- Yoshinaka, Y., S. Soga, N. Ota, K. Yokoyama, Y. Yamada, and M. Kimura. 2018. Light rhythmic exercise with dietary milk fat globule membrane improves physical fitness in an elderly Japanese population: A double-blind randomized placebo-controlled trial. Bioscience, Biotechnology, and Biochemistry 82 (4):677–82. doi: 10.1080/09168451.2017.1412248.
- Yuan, Q.-C., H. Gong, M. Du, and X.-Y. Mao. 2021. Supplementation of milk polar lipids to obese dams improves neurodevelopment and cognitive function in male offspring. FASEB Journal 35 (4):e21454. doi: 10.1096/fj.202001974RRR.
- Zanabria, R., M. W. Griffiths, and M. Corredig. 2020. Does structure affect biological function? Modifications to the protein and phospholipids fraction of the milk fat globule membrane after extraction affect the antiproliferative activity of colon cancer cells. Journal of Food Biochemistry 44 (2):e13104. doi: 10.1111/jfbc.13104.
- Zanabria, R., A. M. Tellez, M. Griffiths, and M. Corredig. 2013. Milk fat globule membrane isolate induces apoptosis in HT-29 human colon cancer cells. Food & Function 4 (2):222–30. doi: 10.1039/c2fo30189j.
- Zavaleta, N., A. S. Kvistgaard, G. Graverholt, G. Respicio, H. Guija, N. Valencia, and B. Lönnerdal. 2011. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. Journal of Pediatric Gastroenterology & Nutrition 53 (5):561–8. doi: 10.1097/MPG.0b013e318225cdaf.
- Zeisel, S. H., D. Char, and N. F. Sheard. 1986. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. The Journal of Nutrition 116 (1):50–8. doi: 10.1093/jn/116.1.50.
- Zhang, M., Z. Xing, Q. Huang, and L. Han. 2021. Effect of conjugated linoleic acid supplementation on fat globule size in raw milk. International Dairy Journal 115:104919. doi: 10.1016/j.idairyj.2020.104919.
- Zheng, H., R. Jiménez-Flores, and D. W. Everett. 2014. Lateral lipid organization of the bovine milk fat globule membrane is revealed by washing processes. Journal of Dairy Science 97 (10):5964–74. doi: 10.3168/jds.2014-7951.
- Zhou, A. L, and R. E. Ward. 2019. Milk polar lipids modulate lipid metabolism, gut permeability, and systemic inflammation in high-fat-fed C57BL/6J Ob/Ob mice, a model of severe obesity. Journal of Dairy Science 102 (6):4816–31. doi: 10.3168/jds.2018-15949.
- Zhou, A. L., K. J. Hintze, R. Jimenez-Flores, and R. E. Ward. 2012. Dietary fat composition influences tissue lipid profile and gene expression in fischer-344 rats. Lipids 47 (12):1119–30. doi: 10.1007/S11745-012-3729-3.
- Zhou, J., X. Xiong, K. X. Wang, L. J. Zou, P. Ji, and Y. L. Yin. 2018. Ethanolamine enhances intestinal functions by altering gut microbiome and mucosal anti-stress capacity in weaned rats. British Journal of Nutrition 120 (3):241–9. doi: 10.1017/S0007114518001101.
- Zhu, D, and S. Damodaran. 2011. Composition, thermotropic properties, and oxidative stability of freeze-dried and spray-dried milk fat globule membrane isolated from cheese whey. Journal of Agricultural and Food Chemistry 59 (16):8931–8. doi: 10.1021/jf201688w.
- Zhu, D, and S. Damodaran. 2013. Dairy lecithin from cheese whey fat globule membrane: Its extraction, composition, oxidative stability, and emulsifying properties. Journal of the American Oil Chemists’ Society 90 (2):217–24. doi: 10.1007/s11746-012-2152-5.
- Zou, X. Q., Z. Guo, J. H. Huang, Q. Z. Jin, L. Z. Cheong, X. G. Wang, and X. B. Xu. 2012. Human Milk fat globules from different stages of lactation: A lipid composition analysis and microstructure characterization. Journal of Agricultural and Food Chemistry 60 (29):7158–67. doi: 10.1021/jf3013597.
- Zou, X., Z. Guo, Q. Jin, J. Huang, L. Cheong, X. Xu, and X. Wang. 2015. Composition and microstructure of colostrum and mature bovine milk fat globule membrane. Food Chemistry 185:362–70. doi: 10.1016/j.foodchem.2015.03.145.
- Zou, X., J. Huang, Q. Jin, Z. Guo, Y. Liu, L. Cheong, X. Xu, and X. Wang. 2013. Lipid composition analysis of milk fats from different mammalian species: Potential for use as human milk fat substitutes. Journal of Agricultural and Food Chemistry 61 (29):7070–80. doi: 10.1021/jf401452y.