Abstract
Flaxseed (Linum usitatissimum L.) has been associated with numerous health benefits. The flax plant synthesizes an array of biologically active compounds including peptides or linusorbs (LOs, a.k.a., cyclolinopeptides), lignans, soluble dietary fiber and omega-3 fatty acids. The LOs arise from post-translational modification of four or more ribosome-derived precursors. These compounds exhibit an array of biological activities, including suppression of T-cell proliferation, excessive inflammation, and osteoclast replication as well as induction of apoptosis in some cancer cell lines. The mechanisms of LO action are only now being elucidated but these compounds might interact with other active compounds in flaxseed and contribute to biological activity attributed to other flax compounds. This review focuses on both the biological interaction of LOs with proteins and other molecules and comprehensive knowledge of LO pharmacological and biological properties. The physicochemical and nutraceutical properties of LOs, as well as the biological effects of certain LOs, and their underlying mechanisms of action, are reviewed. Finally, strategies for producing LOs by either peptide synthesis or recombinant organisms are presented. This review will be the first to describe LOs as a versatile scaffold for the action of compounds to deliver physiochemically/biologically active molecules for developing novel nutraceuticals and pharmaceuticals.
Introduction
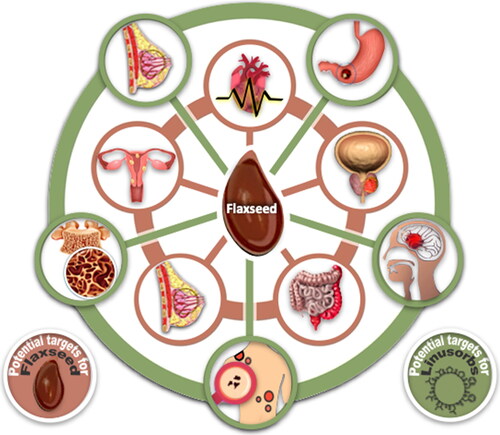
Flax (Linum usitatissimum L.) is a plant belonging to the family Linaceae family. Its seed and compounds within the seed are a known health foods that reduce risks associated with heart disease, cancer (breast cancer, prostate cancer, colon cancer, and hormone-related cancer), stroke and diabetes (Demark-Wahnefried et al. 2001; Magee Citation2011; Moghaddasi Citation2011; Chang et al. Citation2019, ). In addition, flaxseed contains over 400 times more lignan, an estrogen analog or phytoestrogen than pomegranate (Thompson et al. Citation2006). It has been shown that consuming flaxseed or flaxseed meal, or cake, can reduce proliferation and nuclear transformation of epithelial cells in mammary glands, and that diets with these ingredients are associated with reduced cancer growth (Shim et al. Citation2014). In addition, flaxseed has over 10% soluble dietary fiber, and consumption of sufficient dietary fiber can reduce plasma triglyceride, low-density lipoprotein (LDL) cholesterol and plasma cholesterol in rats and mitigate atherosclerosis (Shim, Gui, et al. Citation2015). Flaxseed water soluble gum can be used in the diet to assist in weight regulation or loss (Liu et al. Citation2018) and control or reduce serum cholesterol (Kessler, Klein, and Muller Citation1986a). Each flaxseed fraction contains several potentially useful bioactive compounds (, inner rings). In addition, peptides (linusorbs, LOs) have been isolated and purified from flaxseed oil and studies of their biological activity were conducted. Amongst effects demonstrated it was shown that LOs inhibit cholate absorption into hepatocytes, induce apoptosis in cancer cell lines (Reaney et al. Citation2013) and inhibit T-cell proliferation. The biological activity of LOs has recently been actively studied in many pathological conditions, including breast cancer (Okinyo-Owiti et al. Citation2015; Yang et al. Citation2019), gastric cancer (Zou et al. Citation2018; Zou et al. Citation2019; Zou et al. Citation2020b), glioma (Cho et al. Citation2017; Sung et al. Citation2020), gastritis, colitis, and hepatitis (Ratan et al. Citation2020), melanoma (Okinyo-Owiti et al. Citation2015), and osteoporosis (Kaneda et al. Citation2016, Citation2019) (, outer rings).
Figure 1. Schematic diagram of potential targets for flaxseed (inner rings) and LO (outer rings) treatments.
The first N to C linked cyclic peptide (orbitide) ever discovered, [1–9-NαC]-linusorb B3 (32), was isolated in 1959 from slime in flaxseed oil (Kaufmann and Tobschirbel 1959). Subsequently, the structure of 32 was further characterized by nuclear magnetic resonance (NMR) spectroscopy and circular dichroism (CD) (Naider, Benedetti, and Goodman 1971). A more rigorous nomenclature system for naming peptides was advanced by Shim, Gui, et al. (2015, Shim et al. 2019). Now 45 LOs have been identified and partially characterized in flaxseed alone ( and ). LOs are hydrophobic peptides with eight to ten amino acids (∼0.9–1.1 kDa) produced from ribosomal precursors (Arnison et al. Citation2013). Flaxseed oil and whole seed contain approximately 0.3% and 0.1% of LO, respectively, depending on genotype and environmental conditions, in addition to extraction procedures and oil treatments (Gui, Shim, Datla, et al. Citation2012; Burnett et al. Citation2016). The highest concentration of LO was found in flaxseed oil after cold-pressing (Gui, Shim, and Reaney Citation2012; Shim et al. Citation2014) (). The presence and effects of the bioactive LOs in foods are very interesting, considering research associating alpha-linolenic acid (ALA, C18:3, omega-3 fatty acid) and flaxseed oil with an array of health effects. Virtually all studies of dietary ALA bioactivity in animal models and human studies utilize flaxseed oil as a source of ALA. To our knowledge, there are no reports of the biological effects of diets containing LO depleted flaxseed oil. The question arises, are the putative biological effects of dietary ALA supplied as flaxseed oil truly attributable to the ALA in the flaxseed oil or are a portion of the biological effects due to the presence of LOs? Therefore, research associating ALA and flaxseed oil with an array of health effects might be questioned as the presence and effects of the bioactive LOs in these foods and products might induce all or a portion of the observed effects.
Figure 3. Structures of LOs. Abbreviations are Gly for glycine, Met for methionine, MetO for methionine S-oxide, and MetO2 for methionine S,S-dioxide. Structures modified from Shim et al. (Citation2019).
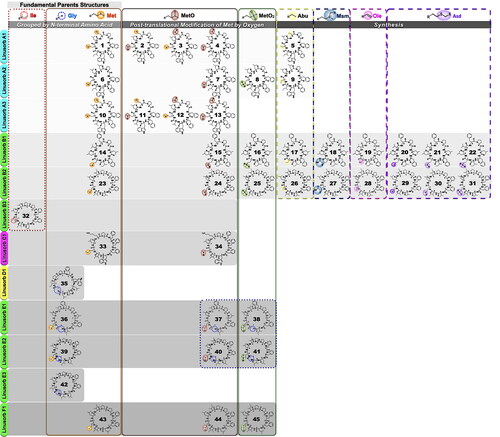
Table 1. List of forty-five LOs.
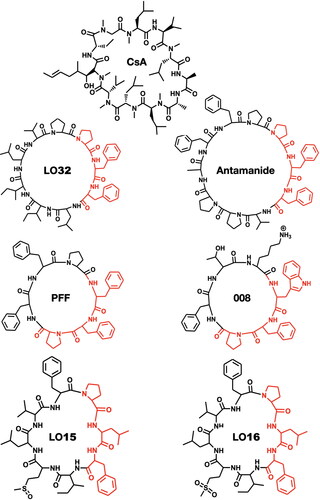
The LOs 15, 24, and 32 induce apoptosis in human lung epithelial carcinoma cells (Reaney et al. Citation2013). 32 imparts a cytoprotective action on hepatocytes, potentially through suppressing the multispecific hepatocyte cell transport system that regulates uptake of bile salts (Gaymes et al. Citation1996; Kessler, Klein, and Muller Citation1986a). Specifically, 32 inhibited cholate uptakes into hepatocytes. The tri-peptide sequence -Phe-Phe-Pro- in 32 and other major LOs, is also found in antamanide [a fungal cyclic peptide with action like cyclosporine A (CsA)] while the peptide somatostatin (a mammalian cyclic peptide and endocrine regulatory hormone) contains a -Phe-Phe- motif in its cyclic structure. This sequence is also common to many of the flaxseed LOs. The structure of 32 modulates the immune response by inhibiting T-lymphocyte activation, as occurs with both CsA and the macrolide FK506 (Gaymes et al. Citation1997). It has been suggested that the -Pro-Xxx-Phe- sequence, where Xxx indicates the presence of a hydrophobic, aliphatic, or aromatic (e.g., Phe, Trp, Tyr) residue, is the active region of antamanide (Siemion, Cebrat, and Wieczorek Citation1999), PFF, 008 (Kessler et al. Citation1986b), LOs and their analogs ( and ). The immunosuppressive activities of other LOs and their analogs have been intensively investigated. For instance, the LO [1–9-NαC]-linusorb B2 (23) inhibited concanavalin-A induced proliferation of human peripheral blood lymphocytes at therapeutic levels like CsA (Morita et al. Citation1997). Similarly, both LO23 and [1–8-NαC],[1-(Rs,Ss)-MetO]-linusorb B1 (LO15) suppressed concanavalin-A induced mouse lymphocyte proliferation (Morita et al. Citation1999). Of all the forty-five LOs, the best immunosuppressive activity against concanavalin-A induced response of human peripheral blood lymphocytes was 32 with an IC50 of 2.5 μg/mL, followed by 22 (25.2 μg/mL) and 16 (28.1 μg/mL) (Morita and Takeya Citation2010).
Figure 4. Immunosuppressive activity of natural cyclopeptides (LOs, antamanide, cycloamanides, PFF, 008) resides in their -Pro-Xxx-Phe- fragment, where: Xxx is a hydrophobic (e.g., Leu, Val) or Xxx is aromatic (e.g., Phe, Trp, Tyr). LO32 exhibited immunosuppressive activity similar to CsA (Siemion, Cebrat, and Wieczorek Citation1999; Kessler et al. Citation1986b).
LO structure is sensitive to heat and oxidizing environments. For example, treating flaxseed meal at 100 °C with a constant air pressure of 60 kPa in the heating block of an oxidative stability index instrument for 16 h produced LOs 16 and 25 (Jadhav et al. Citation2013). The oxidative conversion of LOs is potentially important in understanding which compounds contribute to flaxseed oil bioactivity. It is especially interesting considering the observation that oxidation that could occur in food processing or with storage. Some LOs might be converted to forms with increased biological activity in some assays. Also, it is known that flaxseed oil is used for frying food in some regions of China. Exposure to oxidized LOs is possible where this practice is common. It is especially interesting considering the observation that oxidation that could occur in food processing would convert some LOs into forms with altered biological activity in some assays.
Despite the above various biological effects that LOs can be utilized for commercial purposes, the role of LOs as a biologically active agent has not been fully and clearly reported in the field of food, nutrition, pharmaceuticals, and chemistry to date.
In this review, we will systematically discuss the physicochemical and nutraceutical properties of LOs and underlying molecular mechanisms and the implications of consuming a biologically active mixture of LOs as part of the diet.
Health benefits of flaxseed
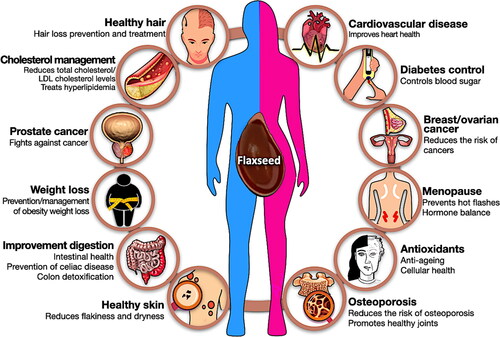
Flaxseed is a plant food that provides healthy fats, antioxidants, and fiber (Lee et al. Citation2021). Today, flaxseed is available in the form of seeds, oil, powder, and tablets (Shim, Gui, et al. Citation2015). Dietary supplements that contain flaxseed fiber and flaxseed are used to reduce constipation, and risks associated with diabetes, cholesterol, heart disease, cancer, and many other conditions (Shim et al. Citation2014). Bioactive compounds found in flaxseed include lignans, antioxidants, fiber, protein, and polyunsaturated fatty acids such as ALA (Rubilar et al. Citation2010). Consuming such nutrients might lower risks of a variety of conditions. The health benefits of flaxseed are summarized in .
Lowers cholesterol and treats hyperlipidemia
Regular consumption of flaxseed improves blood circulation while lowering both blood cholesterol and triglyceride levels (Cunnane et al. Citation1993; Prasad Citation1997). Based on these studies, Health Canada acknowledges that consuming 40 g of whole flaxseed daily helps reduce cholesterol (Health Canada Citation2014). A study from Iowa State University’s Nutrition and Wellness Research Center (NWRC) has shown that men who regularly consume flaxseed through food have significantly lower cholesterol levels (Hendrich et al. Citation2010). Kristensen’s group (Kristensen et al., Citation2012) reported that the dietary fiber in flaxseed can naturally lower cholesterol levels, partly by increasing fat excretion in feces (Kristensen et al. Citation2012). Soluble flaxseed fiber can also entrain bile so that there is less lipid reabsorption and increased excretion (Tse et al. Citation2022). In Torkan’s group study of 70 hyperlipidemic patients, an intervention group received 30 g of raw flaxseed powder daily for 40 days, while the control group did not (Torkan, Entezari, and Siavash Citation2015). At the end of the study, the group taking the flaxseed meal had lower serum lipids.
Rich in fiber
Flaxseed has a high content of mucilaginous gum (8–12% of seeds), a gel-forming fiber that traverses the gastrointestinal tract while remaining partially undigested (Liu et al. Citation2018). The presence of such soluble fiber can decrease nutrient absorption and increase satiety. A portion of the nutrients consumed with flaxseed is not absorbed as they are entrained in the fiber and not available for absorption or metabolism in the digestive tract. As flaxseed is low in carbohydrates but very high in soluble and insoluble fiber it can be used to increase the bulk of fecal materials and the rate of colon transit. The more rapid movement of digesta through the colon is referred to as a colon cleanse, and the effect is interpreted as detoxification. While the benefits of colon cleansing are debated, the impact of dietary fiber on fat loss and satiety is well established. Consuming just three tablespoons (30 g) of whole flaxseed provides 22% and 34% of recommended daily fiber intake for men and women, respectively (Legacy Citation2018). Eating a high-fiber flaxseed diet increases stool size and weight while promoting and maintaining soft stools and regularity.
Digestive health support
One of the best-studied benefits of flaxseed is its ability to promote digestive health. ALA reduces inflammation and protects the lining of the gastrointestinal tract. Flaxseed has been shown to be beneficial for people suffering from Crohn’s disease and other digestive disorders (Plissonneau et al. Citation2022). Dietary fiber also promotes beneficial gut flora, even for those with normal digestive function. The fiber in flaxseed is useful in occluding wastes and is said to cleanse the digestive tract of waste products. Complex carbohydrates like those in flaxseed gum provide both food and habitat for beneficial colon bacteria. Due to the excellent content of soluble and insoluble fiber, flaxseed is a well-recognized natural remedy for constipation. It is used as a dietary aid for maintaining normal bowel movements.
Lower cancer risk
Flaxseed may also help reduce risks associated with certain types of cancer, including those of breast, prostate, ovaries, and colon (Moghaddasi Citation2011). Consuming flaxseed (30 g/daily) may decrease tumor growth and reduce the risk of breast cancer (Lowcock, Cotterchio, and Boucher Citation2013). The lignans found in flaxseed are metabolized by gut bacteria to the mammalian lignans, enterolactone and enterodiol. These latter compounds exert biological effects that are like those of estrogen (Chang et al. Citation2019). The presence of phytoestrogens in the diet might reduce the risk of breast cancer and problems associated with low or variable estrogen levels in women. For similar reasons, dietary flaxseed lignans might also reduce the risk of endometriosis and ovarian cancers.
Relieves menopausal symptoms
Based on the estrogenic properties of lignans, dietary flaxseed has been proposed as an alternative to hormone replacement therapy or as a complementary approach to balancing hormones (Chang et al. Citation2019). The lignans found in flaxseed impart beneficial effects in postmenopausal women. Flaxseed may help reduce the risk of osteoporosis due to its ability to maintain estrogen balance, and consumption helps relieve mild menopausal symptoms such as hot flashes, mood disorders, and vaginal dryness (Lemay et al. Citation2002).
Reduce the risk of osteoporosis
Flaxseed aids in the prevention of bone loss and the maintenance of bone density and strength (Ragheb et al. Citation2019). Flaxseed oil consumption increases bone mineral density and reduces factors that cause osteoporosis (El-Saeed et al. Citation2018). It has been suggested that women with diabetes should eat foods containing flaxseed oil to reduce the risk of osteoporosis.
The gluten-free grain
Flaxseed provides a natural substitute for gluten in recipes that include wheat, rye, and barley flour. Grains, especially those containing gluten, can be difficult to digest for many people and may even cause severe reactions. When flaxseed powders are included with other ingredients, it is possible to formulate products with properties that are similar to those introduced by gluten. For example, flaxseed powders can absorb large amounts of liquid and help bind ingredients in cooking/baking recipes (Conforti and Davis Citation2006). As flaxseed does not contain gluten, it is a good choice for people with celiac disease or gluten sensitivity.
High in antioxidants
Flaxseed is a rich source of antioxidants, particularly lignans, which are polyphenols associated with insoluble flaxseed fiber (Lee et al. Citation2021). Dietary antioxidants like lignan help to reduce free radical damage associated with a wide range of diseases and conditions (De Silva and Alcorn Citation2019).
Healthy skin and hair
Flaxseed oil is used as a therapeutic agent that can suppress skin inflammation responses like itching, eczema, and swelling (Neukam et al. Citation2011). Flaxseed gum and oil preparations have also been reported to mitigate hair loss. In particular, the rich omega-3 fatty acids (ALA) and B-vitamins found in flaxseed help reduce skin flakiness and dryness. The consumption of flaxseed oil helps to improve various dry skin conditions such as acne, psoriasis, and atopic dermatitis as it suppresses inflammation. Roasted whole flaxseed and oil suppress inflammation, but flaxseed oil is reported to have a greater effect on dry skin. It has been suggested that the high concentration of ALA in flaxseed oil is useful in preventing skin from drying. Both consumption of 10 mL of flaxseed oil daily or applying a mixture of flaxseed oil with essential oil as a lotion has been suggested (Hubbard Citation2021). Flaxseed mucilage is a key ingredient in scalp treatments that mitigate hair loss. This component consists of a pectin-like substance and a mucin-like substance. The similarity between flaxseed pectin and the sticky component of mucin creates a mixture that adheres to the scalp and can remove dead skin cells without irritation. Mucin prevents the breakdown of proteins and has the effect of preventing the hair cuticle from being decomposed and broken. Flaxseed gum, like mucin, can also have these effects on the hair cuticle. In addition, flaxseed contains the protein conlinin, which can help to hair smoother, restore damaged hair, and provide moisture to dry scalp and hair. Thus, flaxseed is often used as a moisturizer in a hair gel formulas to maintain hair strength (Fale et al. Citation2022).
Weight loss
Several randomized studies revealed flaxseed consumption is effective in promoting weight loss. In particular, the anti-obesity effect of flaxseed supplementation was significantly observed in the participants with metabolic syndrome (Yari et al. Citation2016), obese people with pre-diabetes (Hutchins et al. Citation2013), and women with the polycystic ovarian syndrome (Heidari et al. Citation2020). Excess inflammation increases visceral fat, and pro-inflammatory cytokines decrease adiponectin, a fat cell hormone that protects against weight gain. Thus, the mechanism behind flaxseed consumption could be related to anti-inflammatory activity. Indeed, in a clinical study by Cassani et al. (Citation2015), weight loss and decreased inflammatory biomarkers, including C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) were observed in men who consumed a weight loss diet that containing flaxseed compared to a control group fed a similar diet without flaxseed (Cassani et al. Citation2015). In addition to this, flaxseeds are rich in fiber, which promotes satiety after eating (Liu et al. Citation2018). Prolonged satiety helps to prevent obesity by limiting the desire to overeat (Zarei et al. Citation2022; Yari et al. Citation2016). Consumption of forty grams of ground flaxseed with daily food helps loss weight (Dodin et al. Citation2005).
Physicochemical properties of LO
The shape of LO molecules is governed by the same types of bonds that determine the structure of larger peptides and proteins. These cyclized peptides have structural flexibility that allows them to self-organize into a minimum energy configuration that is stabilized by non-covalent bonds between atoms in an LO but this structure is also impacted by interactions with the solution and other solutes. Electrostatic, metal-ligand and hydrophobic interactions can act to stabilize complexes that include LOs. Combinations of weaker bonds can lead to significant interactions with receptors and potentially to general or specific bioactive functionality. For example, proposed applications of LOs include using these compounds for their physicochemical properties, as drug delivery agents, and as active nutraceutical ingredients.
Inorganic LO complexes
When LOs are consumed with food it is predictable that the compounds will interact with other molecules that are present. The structure of peptides and LOs suggest that these compounds would likely interact and form inorganic complexes. Considering this possibility, it has been reported that LO binds K+ ions and that this binding induces small conformational changes in the molecule (Siemion et al. Citation1997). Furthermore, 32 is reported to bind Ba2+ more tightly than K+, Na+, Mg2+ and Ca2+ (Tancredi et al. Citation1991). Moreover, Chatterji, Sankaram, and Balasubramanian (Citation1987) noted that complexes of 32 with Tb3+ and Pr3+ mimicked those formed with Ca+2. Both Tb3+ and Pr3+ induce shifts in NMR spectral peaks, and the size of the shift indicates the proximity of the metal to specific protons in the peptide. These cations interacted significantly with the protons of the peptide Phe residues (Chatterji, Sankaram, and Balasubramanian Citation1987). Jadhav, Shim, et al. (Citation2018) reported the affinity of five pure LOs (15, 16, 24, 25, and 32) and four metal salts [Zn(CH3COO)2, ZnSO4, Pb(CH3COO)2, and Cd(NO3)2]. The formation of multi-ligand complexes of these metals with the five LOs tested was confirmed by MS and NMR spectra. The binding affinity of metal ions for all LOs was highest for Cd(NO3)2 regardless of the type of LO, followed by ZnSO4, Pb(CH3COO)2, Zn(CH3COO)2 in order. Binding studies of metals with LOs indicate the involvement of multiple moieties in each LO with the available metal bonding orbitals.
While production of complexes with inorganic species might occur in foods as reported above, other investigations of the formation of peptide complexes with inorganic species have been engaged to produce useful devices. For example, organic light-emitting devices have been constructed that combine LOs with metals in diodes. Such structures have immense commercial potential. Single-molecule white emitting materials for organic light emitting diode (OLED) are unknown but a white light emitting device was constructed from a LO derivative (Bauer et al. Citation2015). Especially, [1–8-NαC],[1-(Rs,Ss)-MetO]-linusorb A2 (7) shows promise in OLED applications as an organic semiconductor. The charge transfer between a complexed metal and 7 was revealed by enhanced emission from the metal sites by ultraviolet (UV) irradiation. This is supported by X-ray absorption near-edge spectroscopy (XANES) measurements where intensity change indicates a change in the occupancy state in the presence of metal, while the peak position remains unchanged indicating chemical stability. The ability to tailor LO to act as materials in metal complexing OLEDs might be used to develop unique light emitting devices. As LO-metal systems can be very diverse in structure a wide range of chemistries is possible for OLED applications.
To date little is known of the interaction of LOs with inorganic compounds in biological systems though it is possible that these interactions could profoundly affect the shape of these compounds. Most of our knowledge of such interactions is based on simple systems. While these interactions might indicate possible mechanisms of LO action there is not sufficient information to make such a determination.
Interaction of LO with proteins and other molecules
The biological activity and properties of LOs are often compared with the antirejection drug CsA. Bovine cyclophilin A (CypA) interacts with 32 and its synthetic analogs through the amino acid sequence of -Pro-Pro-Phe-Phe that participates in cyclophilin binding (Gallo et al. Citation1998). Differences in peptide structure affect the strength of their interaction with other molecules. Binding 32 with human serum albumin (HSA) was investigated with surface plasmon resonance and CD to determine the role of this important serum carrier protein in transporting LOs (Rempel et al. Citation2010). Shim and Reaney (Citation2015) noted that the maximum binding of 32 with HSA was almost 20-fold higher that of 15. Most strikingly, oxidation of MetO to S,S-dioxide (MetO2) in 15 and 24 produced LOs (16 and 25) with stronger HSA binding. The large effect on binding by the relatively small modifications of methionine (Met) with LOs suggested that hydrophobic interactions were the main intermolecular forces stabilizing the complex between LOs and HSA (Shim and Reaney Citation2015; Jadhav, Shim, et al. Citation2018). Quantitative thermodynamic analysis of the interaction of compounds with HSA can play an important role in developing biomedical applications and determining the fate of compounds in human serum.
LO structures
Edge-to-face interaction of phenylalanine
The three-dimensional (3 D) structure of peptides and peptide interactions with other molecules are governed by a range of interactions that can occur between peptides amino acids and molecules in solution. As an example, adjacent aromatic compounds can minimize their free energy by adopting an edge-to-face configuration as observed in benzene crystals (Cox, Cruickshank, and Smith Citation1958). A similar edge-to-face interaction can occur in peptides where positively charged hydrogen atoms of one aromatic group interact with the negatively charged π-electron cloud of an adjacent aromatic amino. Experiments show that weak intermolecular edge-to-face interactions between aromatic rings influence organic molecule morphology in solids and solutions. The same orientation was reported in proteins, oligopeptide crystals, and their derivatives by Burley and Petsko (Citation1986). Proteins with aromatic side chains [phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp)] all participate in edge-to-face interactions. Siemion et al. (Citation1994) suggested that the immunosuppressive activity of 32 and its analogs were, in part, related to edge-to-face interactions. Their study showed that 32 is less flexible than a linear peptide due to edge-to-face interactions. In addition, edge-to-face interactions were important in determining the 3 D structure of cyclic peptides: NαC-(Leu-Ile-Ile-Leu-Val-Pro-Pro-Tyr-Phe) ([Tyr8]32), NαC-(Leu-Ile-Ile-Leu-Val-Pro-Pro-Phe-Tyr) ([Tyr9]32), and NαC-(Leu-Ile-Ile-Leu-Val-Pro-Pro-Tyr-Tyr) ([Tyr8,9]32) (Schatte et al. Citation2012). Curiously, cyclohexyl analogs of LOs produced by Phe reduction have similar biological activity compared with their native forms (Jadhav et al. Citation2021). It is not known if these cyclohexyl analogs have similar three-dimensional structures when compared with the natural phenyl compounds. Nevertheless, the activity of these non-aromatic compounds shows that edge-to-face interactions are not essential for all LO biological activity.
LO solid and solution structures
LOs have been crystalized from several solvents including 2-propanol, water, methanol, dimethyl sulfoxide (DMSO), butanol, and acetonitrile. Typical crystal features indicate that LO crystals include voids that can encompass small solvent molecules and that these molecules are often oriented to form hydrogen bonds with the crystallized orbitides. In addition, common features of LOs are multiple internal hydrogen bonds and interorbitide bonds as well. These structures indicate that LOs bind to and interact with a range of small molecules and that some interactions can be relatively stable.
Chemical reactions of LOs
Oxidation and reduction of methionine sulfur
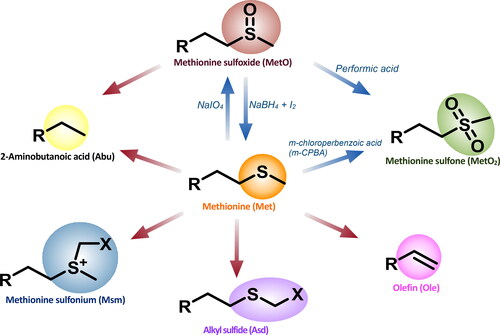
Oxidation in food is a multifaceted process that leads to changes a food’s chemical composition, and typically, a loss of nutritional quality. Oxidation of fats and oils similarly leads to rancidity and deterioration of quality. However, oxidative stability is mainly attributable to a balance between antioxidative and prooxidative factors. The main mechanism of lipid oxidation in foods is attributed to a free radical chain reaction. As some species of LOs are Met/MetO containing peptides (, ), oxidation can result in the conversion of reduced LOs to their oxidized homologues. Brühl et al. (Citation2007) identified fives LOs (4, 13, 15, 24, and 32) in cold-pressed flaxseed oil stored for up to 30 weeks, but during storage LOs with methionine were oxidized over time. Specifically, it was determined that oxidation of 14 to 15 contributed bitter flavors to flaxseed oil after long-term storage. Subsequent studies by Gui, Shim, and Reaney (Citation2012) and Olivia et al. (Citation2012) confirmed that during long-term storage of flaxseed only 4, 7, 13, 15, 24, and 32 were present. Jadhav et al. (Citation2013) detected only five LOs (1, 6, 10, 14, and 23) in freshly ground flaxseed meal. In this study of accelerated aging, the amount of Met containing LOs (23 and 14) decreased significantly while their oxidized homologues (15, 16, 24, and 25) increased in just 16 h. Burnett et al. (Citation2016) demonstrated that controlled oxidation of Met containing LOs to MetO-containing LOs can be initiated by adding H2O2 to flaxseed methanol extracts to facilitate chromatographic separation. They found that overoxidation to MetO2 could be avoided if hydrogen peroxide (H2O2) was subsequently quenched with the addition of sodium thiosulfate (Na2S2O3).
Reduction of phenylalanine
A sulfur-free analog of the 23 was prepared by direct chemical reduction of Met. The reduction converted the methionine residue to 2-amino butyrate. In addition, under more severe reduction conditions three cyclohexylalanine (CHA) analogs were formed by the reduction of Phe6 and/or Phe7 to CHA derivatives. The CHA analogs were more hydrophobic and show comparable cytotoxic activity to 32 against human breast cancer SK-BR-3 and MCF-7 cell lines (Okinyo-Owiti et al. Citation2015). As mentioned above, due to the replacement of one or both Phe groups in the CHA analogs edge-to-face interaction is not necessary for this bioactivity.
Racemization of LOs
It was discovered that LOs treated under mild alkaline conditions undergo regioselective epimerization (). While all amino acids might be subject to epimerization, methionine (Met), methionine sulfoxide (MetO) and methionine sulfone (MetO2) were found to epimerize much more rapidly than other amino acids. By selecting conditions that favored the kinetic reaction, LO products with epimerized methionine amino acid residues in Met, MetO, and MetO2 forms were produced. This reaction provides rapid access to D-amino acid-analogs where methionine was selectively isomerized. Such products are important tools for structure-activity relationship studies. LOs produced with a single epimerized amino acid retained their toxicity to human breast cancer HER2-subtype cell line SK-BR-3 and showed enhanced toxicity to a triple-negative-subtype cell line MDA-MB-231 (Jadhav et al. Citation2021).
Figure 6. Schematic representation of platform of natural (blue arrow) and synthetic (red arrow) LO analogues modified Shim et al. Citation2019.
Substitution of methionine
Jadhav et al. (Citation2021) produced a series of LOs (20–22 and 29–31) with modified Met residues that they could be bound to tags, macromolecules, and supports. When 23 is bound through Met to a resin column the LOs formed an affinity column that preferentially adsorbs apolipoprotein from Gallus serum. Orbitides bound through Met to the fluorescent moiety coumarin then mixed with erbium and terbium ions adsorb light at UV wavelengths and re-emitted the energy as light at visible wavelengths in a phenomenon called Forster Resonance Energy Transfer. The efficient transfer indicates proximity of the metal and the coumarin in a dissolved complex. Jadhav et al. (Citation2021) also reported that NMR analysis of alfa protons of reaction intermediates and coumarin tagged LOs indicate that the structures of the tagged LOs are similar to those of the unaltered species.
Nutraceutical functions of LOs
Recently, as the demand for healthy food has rapidly increased, research on the biological activity of flaxseed components is being actively conducted. In particular, more than 40% of the mass of flaxseed is oil that is rich in omega-3 fatty acids. Among the flaxseed fatty acids, ALA, is an essential fatty acid that must be consumed through food. While typical flaxseed oil contains 57% ALA, cultivars are available with over 70% ALA (Goyal et al. Citation2014; Shim et al. Citation2014). This figure is very high compared to corn and soybean oil, vegetable oils which are commonly used in food preparation, with ALA content of 1% and 8%, respectively (Peng et al. Citation2019). Through various cell and animal tests of ALA, it has been revealed that various inflammatory mediators are intimately involved in the carcinogenic process of cells (Goyal et al. Citation2014). In fact, according to the results of an epidemiological study conducted in France, it was determined that the intake of ALA was inversely correlated with the incidence of breast cancer. Interestingly, many studies of ALA biological activity were conducted with flaxseed oil and this oil contains the unique LO peptide complex. Curiously assays of isolated LOs indicate that the biological effects of these compounds are like those of whole flaxseed oil. Research on LO is ongoing from various perspectives including purification, mode of action, and pharmacokinetics, and recent studies on their health-promoting properties have been actively conducted. In the next section, we will review the biological activities of LO as a nutraceutical.
Immunomodulatory effect of LOs
Immunosuppressive activity of LOs in T cells
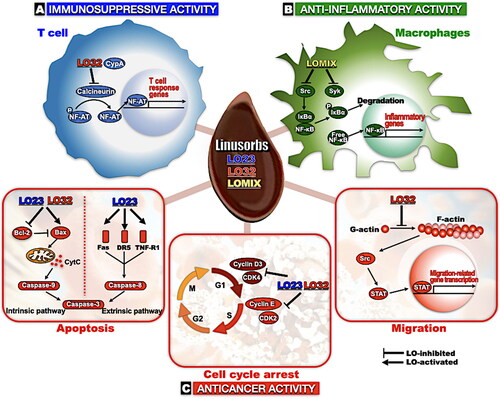
Wieczorek et al. (Citation1991) studied LO32 effects on the humoral response-mediated by T-cells and reported that it possesses immunosuppressive properties. LO32 significantly delays the rejection time after skin allograft and reduces the graft versus host (GvH) index in mice. In addition, LO32 alleviates immune-related symptoms observed in in vivo disease models, including the post-adjuvant polyarthritis-induced rats and spontaneous hemolytic anemia-induced New Zealand black (NZB) mice (Wieczorek et al. Citation1991). The mechanism of LO32 action has been studied in comparison with calcineurin inhibitors, such as CsA and FK506. Both CsA and FK506 suppress calcineurin phosphatase activity by forming complexes with CypA or FKBP12, respectively. As a result, the activity of nuclear factor of activated T cells (NF-AT) that plays a critical role for interleukin-2 (IL-2) expression is downregulated, thereby inhibiting T-lymphocyte activation (Mejia, Basu, and Shapiro Citation2014). Like immunosuppressants mentioned above, LO32 also inhibits the expression of IL-2 and calcium-dependent activation of T lymphocytes. In particular, LO32 binds to CypA, not FKBP12, and suppresses calcineurin in a manner similar to CsA (Gaymes et al. Citation1997).
Anti-inflammatory activity
Inflammation is a normal immune system’s response that protects our body from external stimuli, but an excessive and persistent inflammation can lead to chronic inflammatory diseases. Inflammatory response is primarily mediated by immune cells such as macrophages, and dendritic cells. Pathogen recognition receptors (PRRs), such as toll-like receptors (TLRs), are expressed on the surface of the immune cells, and the immune responses including inflammation are initiated when PRRs recognize pathogen-associated molecular patterns (PAMPs) (Gaymes et al. Citation1997). Stimulation of PRRs induces transcription factor activation such as nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), and IRF3 that lead to the expression of inflammatory mediators (Lawrence Citation2009; Wang et al. Citation2013; Yanai et al. Citation2018).
Nitric oxide (NO) is one of the major mediators that contributes to normal inflammatory responses by exerting a microbicidal effect that blocks pathogen replication or acting as a small molecule that modulates cytokine expression (Tripathi et al. Citation2007). However, excessive NO production by sustained stimulation is involved in inflammatory disease pathogenesis (Sharma, Al-Omran, and Parvathy Citation2007), therefore treatments that mitigate NO are considered anti-inflammatory. It was found that 23 and 32 significantly suppress NO production in lipopolysaccharide (LPS)-treated THP-1 cells with IC50 ranging from 1 to 2 µM, implying that suppression of NO production was the mechanism of anti-inflammatory activity (). In addition to suppressing NO production, the expression of key inflammatory cytokines such as TNF-α, IL-1β, and IL-6 are also significantly decreased in LPS-treated THP-1 cells treated with 23 and 32 (Zou et al. Citation2020a). LOs (7, 23, and 32) suppress NO production in LPS-stimulated RAW 264.7 cells with IC50 values of 40, 35, and 12 µM, respectively (Ratan et al. Citation2020). On the other hand, the suppressive capacity of 4/13 on NO production is relatively weak (IC50=105 µM) and 15 does not inhibit NO production,14 indicating that 7, 23, and 32 are biologically active LOs. The linusorb mixture (LOMIX), which contains 4 (18.03%), 7 (8.20%), 13 (4.92%), 15 (19.67%), 23 (26.23%), and 32 (22.95%), also significantly inhibits NO production by RAW cells after treatment with LPS, Pam3CSK4, or poly(I:C) (Ratan et al. Citation2020). LPS, Pam3CSK4, and poly(I:C) are ligands for TLR4, TLR2, and TLR3, respectively. Considering that TLR2 and TLR4 respond to bacterial infection while TLR3 senses external virus (Lester and Li Citation2014; Takeuchi et al. Citation1999), LOs are expected to exhibit anti-inflammatory activity regardless of the pathogen. Furthermore, oral administration of LOMIX (50 and 200 mg/kg) alleviates symptoms of inflammation in in vivo mouse models. LOMIX significantly reduces tissue damage and lesion size in gastritis and colitis mice experiments. In particular, it was as effective as ranitidine (40 mg/kg), an FDA-proved medication used for treating acid reflux conditions (Grant, Langtry, and Brogden Citation1989), at less than 200 mg/kg dose (Ratan et al. Citation2020). The anti-inflammatory activity of LOMIX is also observed in a hepatitis model of mice induced by injection with LPS/D-GalN (Ratan et al. Citation2020).
Table 2. IC50 values of LOs against NO release in immune cells.
The molecular mode of action of LOs in the molecular level is closely associated with NF-κB pathway. At concentrations between 1–4 µM 23 and 32 significantly decrease the phosphorylation of the NF-κB subunit p-65 (Zou et al. Citation2020a). LOMIX (200 µg/mL) also decreases the transcriptional activity of NF-κB and translocation of NF-κB subunits (p65 and p50) to the nucleus in RAW264.7 cells. In parallel, suppression of p-p65 was also confirmed in stomach and liver lysates of inflammatory mice models after administering LOMIX. According to the in vitro kinase assay, LOMIX inhibits activity of Src and Syk kinases. As these are the most upstream molecules in LPS-induced NF-κB pathway (Byeon et al. Citation2012; Ratan et al. Citation2020), Src and Syk might be direct targets of LOs in NF-κB signaling.
Anticancer activity of LOs
Anticancer activity of LOs (particularly, 23 and 32) has been investigated in several tumor cell types including breast cancer (Okinyo-Owiti et al. Citation2015; Yang et al. Citation2019), gastric cancer (Zou et al. Citation2018; Zou et al. Citation2019; Zou et al. Citation2020b), brain cancers (glioma, the most common type; glioblastoma, the most aggressive type) (Sung et al. Citation2020), and skin cancer (melanoma, the most dangerous type) (Okinyo-Owiti et al. Citation2015). Most recently, the antitumor efficacy of 32 was evaluated in C6 glioma cells and U251 glioblastoma cells. It was found that 32 shows cytotoxic effect against C6 glioma cells in dose-dependent manner at 20–40 µM, whereas doses less than 10 μM do not exert cytotoxic effects (IC50=22 µM). Above 20 µM concentration, 32 reduces cell viability of U251 glioblastoma cells by 70% (IC50=13 µM) (Sung et al. Citation2020) (). Cell viability of A375 human melanoma cells, is reduced by 56% after 48 h treatment with 500 µM of 32 (IC50=454 µM), but cytotoxicity is not detected for either 23 or LOs after a 24 h treatment (Okinyo-Owiti et al. Citation2015). Cytotoxicity of 32 (400 µg/mL) and 23 (400 µg/mL) is 75% and 42% in SK-BR-3, and HER-2 positive breast cancer cells, respectively, after a 24 h treatment (IC50 of 32 = 262 µM and IC50 of 23 = 420 µM) (Okinyo-Owiti et al. Citation2015). For MCF-7 cells [estrogen receptor (ER), progesterone receptor (PR) positive and HER2 negative breast cancer cells], exposure to 32 (192 µM) and 23 (189 µM) for 24 h induces cytotoxicity at 18% and 19%, respectively (Okinyo-Owiti et al. Citation2015). In addition, 32 reduces cell viability of MDA-MB-231, triple negative breast cancer cells, by 60% (IC50=10 µM) (Sung et al. Citation2020). Meanwhile, the anticancer activity of LOs is improved when the solvent-based treatment is replaced with a polyethylene glycol (PGE)-based formulation. In the PEG-based formulation, 25 nM of 32 exhibits cytotoxicity of 45% and 80% in MDA-MB-231 cells after 96- and 120-hours treatments, respectively, but the effect of 23 is not as significant as 32. On the other hand, SK-BR-3 is not affected by 23 and 32 treatments (Yang et al. Citation2019). These results suggest that 32 may have specificity for triple negative types of breast cancers. Cell viability of a SGC-7901 gastric cancer cell line is inhibited by both 32 and 23 in 0.6% ethanol with IC50 values of 153 μM and 171 μM, respectively (Zou et al. Citation2018). When comparing IC50 values in parallel, 32 is more effective than 23 across all cancer cell types examined (). L-isomers (14, 15, 16, 23, 24, and 25) are naturally available LO forms while peptides with D-isomers of methionine (21, 29, 30, and 31) can be chemically synthesized by treatment with mild base. There are cytotoxicity differences between L- and D-isomers in MDA-MB-231 cells, and notable is that 29 (D-isomer) shows higher cytotoxicity than 23 (L-isomer) (Jadhav et al. Citation2021). However, most D-isomer modified LOs show lower cytotoxicity compared to natural L-isomers (Jadhav et al. Citation2021) (). These small differences in activity arise from differences in the 3-dimensional structure of the LOs. Such chemical modifications might be used to explore interactions between LOs and their receptors.
Table 3. Cytotoxicity (IC50) of LOs in cancer.
The underlying molecular mechanisms of LOs are closely associated with apoptosis. Apoptosis, a type of programmed cell death, is an essential event in a variety of normal physiological processes including embryonic development, appropriate immune system, and the elimination of cells with genetic abnormalities (e.g., cancer cells) (Elmore Citation2007). Apoptosis can occur through two pathways: an extrinsic pathway [a.k.a., death receptor (DR) pathway] and an intrinsic pathway (a.k.a., mitochondrial pathway) (Hengartner Citation2000). The extrinsic pathway can be triggered by death ligands. The death ligands bind to their cognate death receptors, including Fas receptors, TNF receptors, TNF-related apoptosis-inducing ligand (TRAIL) receptors, and TNF receptor-related apoptosis-mediating protein (TRAMP), which cause a conformational alteration in the death receptors (Guicciardi and Gores Citation2009) Consequently, adaptor proteins [e.g., Fas-associating protein with death domain (FADD) and TNF receptor-associated protein with death domain (TRADD)] are recruited and apoptotic signaling composed of caspases is activated (Pobezinskaya and Liu Citation2012; Sun et al. Citation2000). Meanwhile, the intrinsic pathway is triggered by internal stresses including DNA damage, oxidative stress, and hypoxia (Sinha et al. Citation2013). Once the intrinsic pathway is activated, Bax, a proapoptotic protein, in cytoplasm is converted to its oligomer form and migrates to the mitochondria, where cytochrome c (CytC) is released (Jurgensmeier et al. Citation1998). CytC mediates the formation of apoptosomes consisting of CytC, apoptosis-protease activating factor 1 (Apaf-1), and deoxy adenosine triphosphate (dATP) (Bao and Shi Citation2007). Subsequently, the apoptosome activates the caspase pathway and induces apoptosis. Contrary to Bax, B-cell lymphoma 2 (Bcl-2) plays an antiapoptotic role by inhibiting CytC release (Czabotar et al. Citation2014). In cancer cells, the apoptosis pathway is often defective, therefore triggering apoptosis in malignant cells can be good anticancer strategy. Both 23 and 32 also cause apoptosis in SGC-7901 gastric cancer cells, but their target pathways are different (). 23 and 32 increase the expression of Fas receptor, Fas Ligand (FasL), TNF receptor1 (TNFR1), TNF-α, and TRAIL receptor 2 (TRAILR2, DR5), which are associated with extrinsic apoptosis pathway. However, intrinsic pathway is only affected by 32. 32 increases depolarization of mitochondrial membrane, but 23 does not. Furthermore, 32, but not 23, alters mitochondrial protein expression (Wang and Youle Citation2009). Specifically, 32 increases expression of pro-apoptotic protein Bax and CytC, an apoptogenic factor, while anti-apoptotic protein Bcl-2 expression is decreased (Zou et al. Citation2018). 32 also activates the intrinsic apoptotic pathway in C6 glioma cells via regulating Bcl-2, caspase-9, and caspase-3 (Sung et al. Citation2020). In addition, LOMIX which contains large amounts of 32 also increase apoptotic factors-related to the intrinsic pathway, such as CytC, Bax, and caspases (Zou et al. Citation2020b). The lack of effects of 23 on mitochondrial pathways might be explained by the presence of the –SCH3 functional group at the methionine residue (Zou et al. Citation2018). Rupture of mitochondria, an essential initial event in the activation of intrinsic apoptosis, is known to be caused primarily by excessive reactive oxygen species (ROS) (Kowaltowski and Vercesi Citation1999). The –SCH3 could scavenge ROS, which might determine the lack of effect of 23 on mitochondria (Zou et al. Citation2018).
Table 4. Underlying molecular mechanisms of LOs in cancer cells.
Cell cycle arrest is another possible mechanism for anticancer effects of LOs. Zou et al. (Citation2019) reported that 23 and 32 induced G1 cell cycle arrest in SGC-7901 cells. Exposure to LOs downregulates the expression of positive cell cycle regulators including cyclin-dependent kinases (CDK2 and CDK4) and cyclins (D3 and E) (Malumbres and Barbacid Citation2009), and upregulates the expression levels of CDK inhibitors such as p21 (WAF1/CIP1) and p27 (KIP1) (Zou et al. Citation2019). Arrest of cells in the G1 phase of the cell cycle is closely correlated with AKT (Georgakis et al. Citation2006; Liang and Slingerland Citation2003; Sugikawa et al. Citation1999), and it was found that 23 and flaxseed-derived orbitides significantly suppress AKT phosphorylation (Zou et al. Citation2019; Zou et al. Citation2020b). 32 also suppresses AKT, but its effect is weaker than that of 23. In parallel, 23 is more effective in arresting the cell cycle than 32 (Zou et al. Citation2019). 32 also suppresses the migration and invasion capacity of C6 glioma cells (Sung et al. Citation2020). Sung et al. (Citation2020) reported that 32 inhibits actin polymerization, which plays a pivotal role in overall cancer progression including metastasis (Izdebska et al. Citation2018; Olson and Sahai Citation2009; Yamaguchi and Condeelis Citation2007), thereby blocking the activation of Src kinase, a notorious proto-oncogene tyrosine-protein kinase.
Low cytotoxicity of LOs on normal cells and organs
Safety and toxicity are important considerations for nutraceuticals. Natural products have been widely used over the centuries, where the safety and efficacy in human are verified based on the experiences of traditional practitioners. For this reason, ingredients derived from natural products are being used as an early resource in the process of functional foods or drug development. In single-dose toxicity studies, neither mortality nor injury is observed in mice administered high oral doses of LOMIX (5 g/kg) (Ratan et al. Citation2020). The weight and histological status of liver and colon, as well as those of immune system organs such as thymus and spleen, are also not altered in mice administered LOMIX. This finding indicates LOMIX does not cause acute in vivo toxicity upon oral exposure (Ratan et al. Citation2020). Consistently, the cell viability of RAW 264.7 cell and HEK 293 cells (human embryonic kidney cells) are not influenced by LOMIX (200 µg/mL). In the case of 32, toxicity was investigated with various routes of administration. Oral administration of 32 in a 2% gelatin solution was not toxic to mice and rats at dose of to 4 g/kg and 3 g/kg doses, respectively. Intravenous injection of 32 at 230 mg/kg is also not toxic to rats (Wieczorek et al. Citation1991).
Both 32 and 23 are not toxic to nontumor cells. While 32 and 23 exert cytotoxicity with IC50 of 153 µM and 171 µM in gastric cancer cell lines, GES-1 cells (human normal gastric cells) are not affected by treatment with less than 200 µM 32 and 23 (Zou et al. Citation2018). The selective toxic effect on cancer cells is also observed in other natural cyclic peptides such as [1–9-NαC]-crourorb A1 (orbitide from the latex of Croton urucurana) (Cândido-Bacani et al. Citation2015), and this phenomenon suggests that LOs may be advantageous for the development of ingredients for functional foods or drug targeting cancers as these compounds have minimal adverse effects in normal tissues.
Anti-osteoporosis effect of LOs
Kaneda et al. (Citation2016) reported the inhibitory activity of LOs in osteoclast differentiation. The osteoclast differentiation inhibitory activity is observed in most LOs including LOs (2, 4, 7, 12, 13, 15, 23, 24, and 32) without cytotoxicity, and in particular, the inhibitory efficacy of 2, 12, and 13 is stronger than that of curcumin and ipriflavone used as positive controls (IC50 of 13 = 0.58 µM, IC50 of 2 = 0.83 µM, IC50 of 12 = 0.47 µM, IC50 of curcumin = 7.5 µM, and IC50 of ipriflavone = 17.2 µM) (Kaneda et al. Citation2016). The underlying molecular mechanism for inhibition of osteoclastogenesis by LOs has been studied with 13. Osteoclast is a bone-resorbing cell and differentiated from monocyte-macrophage (M/Mφ) lineage cells (Takayanagi Citation2007). In the process of osteoclast differentiation, M/Mφ lineage precursors stimulated with macrophage colony-stimulating factor (M-CSF) express c-fos, a subunit of activator protein 1 (AP-1), resulting in the promotion of RANK expression. Sequentially, RANKL, primarily expressed by osteoblast cells, activates the RANK on osteoclast precursors, and nuclear factor of activated T cells 1 (NFATc1), a master regulator of osteoclastogenesis, is activated via c-fos (Kim and Kim Citation2014; Yavropoulou and Yovos Citation2008). In M-CSF induced bone marrow macrophages (BMMs), 13 suppresses the RANK expressions through down-regulation of c-fos expression, which is thought to be a crucial transcriptional factor for M-CSF and RANK/RANKL signaling (Kaneda et al. Citation2019).
Potential application and alternate LO sources
LO applications
Since the potential or immediate health benefits of functional foods cannot be explained by a single traditional nutrient, research to elucidate the various roles and interactions of these biological components is very important. The LOs described above were found to have a variety of activities such as anti-inflammatory, anticancer, and anti-osteoporosis effects, in both in vitro and/or in vivo models. Furthermore, the molecular mechanisms and target biomarkers of LOs in improving health problems have also been well-characterized (Ratan et al. Citation2020; Zou et al. Citation2020a; Zou et al. Citation2018; Zou et al. Citation2020b; Sung et al. Citation2020; Kaneda et al. Citation2019). The potential of LO as a novel functional food, along with clear and conclusive evidence from a biological basis, is expected to enable a more accurate understanding of various bioactive mechanisms. However, further studies are still needed, including formulation-related food processing, industrial-scale production/isolation of LOs, and toxicological considerations.
In recent years, the field of nutritional epigenetics research has been rapidly expanding. Nutritional epigenetics is the study of the effects of nutrition (such as food and diet) on the epigenome (Landecker Citation2011). Epigenetics is a term used to describe the regulation of gene expression by epigenetic markers such as DNA methylation and histone modifications. Epigenetic markers modulate the access of transcriptional factors or RNA polymerases to DNA by altering the spatial conformation of chromatin, thereby regulating gene expressions regardless of the DNA sequence (Tiffon Citation2018). Adding and removing the epigenetic markers are mediated by enzymes, such as DNA methyltransferases (DMTs), histone methyltransferases (HMTs), histone acetyltransferases (HATs), and histone deacetylases (HDACs) (Han et al. Citation2019). Since epigenetic defects are closely related to the onset and exacerbation of most chronic diseases including cancer, autoimmune diseases, and metabolic diseases, modulation of epigenetic machinery via dietary supplements is being attempted from the perspective of nutritional epigenetics (Sedley Citation2020). Folic acid and vitamin B12 are essential for DNA methylation because they are carbon sources for s-adenosylmethionine (AdoMet), a methyl donor in the methylation reaction, and a diet deficient in folic acid and vitamin B12 has been reported to be highly correlated with cancer. Phytochemicals such as epigallocatechin-gallate (EGCG), quercetin, resveratrol, curcumin have been also reported to inhibit tumorigenic responses by suppressing activities of DMTs and HDACs (Carlos-Reyes et al. Citation2019). Therefore, the above-mentioned nutritional factors are suggested as ideal functional food ingredients for cancer prevention. Cyclic peptides have higher affinity and selectivity for target molecules than their linear counterparts and are resistant to exopeptidases. Due to these structural advantages, cyclic peptides have the potential to be effective inhibitors. Indeed, cyclic peptides derived from natural products (e.g., romidepsin) have been reported as effective HDAC inhibitors (Mwakwari et al. Citation2010). Likewise, LOs, cyclic peptide, has the potential to modulate epigenetic enzymes due to their structural advantages. If the epigenetic regulatory effects are proven, LOs could be classified as epigenetic bioactive substances.
From a therapeutic point of view, LOs are also attractive compounds. Several studies revealed that LOs inhibit various signal pathways including AKT, JNK, NF-κB, Syk, and Src. Among them, the kinase activities of Syk and Src are directly suppressed by LOMIX. Syk and Src have been highlighted as therapeutic targets because they are implicated in the pathogenesis of a multitude of diseases, such as autoimmune diseases, allergic disorders, and tumors (Wong et al. Citation2004). For example, R406 and R788, which are small molecule-inhibitors for Syk kinase, showed preventive effects against arthritis in mice, and they achieved a 20% improvement in the ACR criteria (American College of Rheumatology), which is an indicator of the degree of reduction in symptoms of rheumatoid arthritis (RA) in clinical trials (Coffey et al. Citation2012; Genovese et al. 2014; Pine et al. Citation2007; Weinblatt et al. Citation2008). In addition, entospletinib, an oral Syk inhibitor, exerts anti-tumor activity against chronic lymphoid leukemia (Liu and Mamorska-Dyga Citation2017). Artemisia asiatica ethanol extract (Stillen™), approved as a treatment for gastric mucosal ulcers in Korea, is also found to exhibit anti-inflammatory activities through inhibition of Src and Syk (Jeong et al. Citation2014). Likewise, LOMIX relieves the symptoms of gastritis, colitis, and hepatitis in mice, which suggests the potential of LOMIX as an anti-inflammatory agent (Ratan et al. Citation2020). Moreover, LOs (in particular, 23 and 32) shows anticancer efficacy at the cellular level, which implies they have the potential to be applied as anticancer drugs, still further studies are needed to evaluate efficacy in vivo (Sung et al. Citation2020; Yang et al. Citation2019; Zou et al. Citation2018). Collectively, LOs are expected to provide a promising scaffold for novel drug development.
Other LO sources
While it is attractive to use flaxseed LO extracts as nutraceuticals it is desirable to identify improved sources of these cyclic peptides. In principle, cyclic peptides can be synthesized by solid phase synthesis followed by cyclization (Kates et al. Citation1994), but peptide synthesis on resins is a multistep process that incurs greater losses and lower yields with each step in the synthesis. Alternatively, orbitides might be produced in living cells that are used as orbitide factories (Sardar, Lin, and Schmidt Citation2015). Shortly after Covello et al. (Citation2012) and Condie et al. (Citation2011) identified sequences encoding orbitides in both Saponaria officinalis L. and L. usitatissimum L., they proposed that these sequences could be used for producing novel orbitides in recombinant plants. This group later identified and characterized fractions containing two processing enzymes, an oligopeptidase (OLP1) and a cyclase (PCY1) (Barber et al. Citation2013). A recombinant organism that expresses the sequences OLP1 and PCY1 acquires the ability to synthesize orbitides from protein precursors produced by members of Caryophyllaceae. The Caryophyllaceae system of orbitide production is likely less efficient than that of Linaceae as most precursor proteins observed in Linum appear to express multiple copies of each orbitide while Caryophyllaceae only express a single orbitide. In our research, we have found that flaxseed (L. usitatissimum L.), a member of the genus Linum in the family Linaceae, accumulated the highest concentration of orbitides (LOs) (Gui, Shim, Datla, et al. Citation2012; Shim, Gui, et al. Citation2015).
Recombinant plants could be transformed with vectors that induce the expression of LOs to produce organisms that share the protective or functional properties that the orbitides provide. Conversely, the system that produces orbitides in flaxseed could be used to produce a recombinant plant or other cell type that could be used in the commercial manufacture of bulk orbitide products. In the latter case it could prove desirable to make modifications that enable greater total accumulation of LOs or the production of novel LOs using the LO producing sequences from flaxseed
Conclusions
Flaxseed contains a significant amount of LOs, and biologically active agents of flaxseed LOs have been actively investigated. This review offers a summary of the comprehensive properties of LOs, as well as intracellular biological effects of certain LOs, including LO23, LO32, and LOMIX, and their underlying action mechanisms. LOs exert their biological activity by interacting with target molecules based on their circular structure. The target molecules and signals expressed after exposure to LOs in each cellular response are summarized in . We propose that LOs may be promising food or therapeutic ingredients for the prevention and alleviation of chronic diseases, including inflammatory diseases and cancer. Although great progress has been made in understanding the biological activity of LOs, much remains unknown. To extend their use in the food and pharmaceutical industry, further research, including animal experiments and synthesis of LOs with unique structures, is needed.
Figure 7. Schematic diagram of the molecular mechanisms that mediate biological effects of LOs. (A) In T cells, calcineurin, a serine/threonine phosphatase, activates NF-AT. Activated NF-AT is translocated into nuclear, then promotes expression of genes related to T cell response. LO32-CypA complex inhibits calcineurin and thereby inactivates T cells. (B) Early in the inflammatory response, Src and Syk kinases, the most upstream molecules in NF-κB signal pathway, are activated then IκBα is phosphorylated by a series of kinase cascades. Phosphorylated IκBα is degraded, and free NF-κB released from IκBα moves into the nucleus, promoting the expression of inflammatory genes. LOMIX suppresses NF-κB signaling by directly inhibiting Src and Syk kinases. (C) LO23 and LO32 exert antitumor activity via inducing apoptosis (left), arresting cell cycle (center), or inhibiting migration (right). Apoptosis is activated by two distinct mechanisms: intrinsic and extrinsic pathways. In the intrinsic pathway, Bax, a pro-apoptotic protein, binds to and forms pore in mitochondrial outer membranes and release of CytC from mitochondria. Released CytC activates caspase-9 and caspase-3, and eventually kills the cell. In healthy cells, Bcl-2, a member of the Bcl-2 family, acts as a key regulator by inhibiting pro-apoptotic protein Bax, thereby preventing apoptosis. LO23 and LO32 induce apoptosis by suppressing Bcl-2 and upregulating Bax in cancer cells. The extrinsic pathway is mediated by death receptors, including Fas and DR5 (a.k.a., TRAILR2), and TNF receptors. Death receptors activate caspase-8 and caspase-3, leading to apoptosis. LO23 upregulates Fas, DR5, and TNF-R1 in cancer cells, thereby inducing apoptosis (left). Cyclin D3/CDK4 complex and cyclin E/CDK2 complex are essential cell cycle regulators that play pivotal roles in G1 phase and transition from G1 to S phase, respectively. Both LO23 and LO32 downregulates cyclin D3/CDK4 complex and cyclin E/CDK2 complex, leading to cell cycle arrest (Zou et al. Citation2019, modified, center). LO32 suppresses Src-STAT signal pathway via inhibiting F-actin formation in a C6 glioma cell, thereby reducing the expression of metastasis-related genes (Sung et al. Citation2020, modified, right). CypA: cyclophilin A; NF-AT: nuclear factor of activated T cells; NF-κB: nuclear factor kappa B; IκBα: inhibitor kappa B alpha; Bcl-2: B-cell lymphoma 2; CytC: cytochrome c; DR5: death receptor 5; TRAILR2: TNF-related apoptosis-inducing ligand receptor 2; TNF: tumor necrosis factor; CDK4: cyclin-dependent kinase 4.
Suggestions for further research
We propose to study the target of LOs by expanding it to various molecular mechanisms. There is substantial evidence that the NF-κB pathway is a LO target in anti-inflammatory activity, but NF-κB does not appear to be the only target. For example, treatment with either LO23 and LO32 with 4 µM and 1 µM, respectively, dramatically diminishes the production of inflammatory mediators and cytokines, including NO, TNF-α. However, these treatments do not alter phosphorylation levels of NF-κB (Zou et al. Citation2020a). This observation implies the existence of another pathway involved in the anti-inflammatory mechanisms of LOs. AP-1, like NF-κB, acts as a major transcriptional factor in TLR4-mediated inflammatory responses (Lu, Yeh, and Ohashi Citation2008) and is, therefore, considered as a potential target for anti-inflammatory treatments. Interestingly, flaxseed intake had been found to reduce the phosphorylated levels of MAPKs (ERK and JNK), which are upstream kinases of AP-1 signaling, in lung tissue of A/J mice (Chikara et al. Citation2018). Moreover, it has been reported that cyclic peptide extract from Pseudostellaria heterophylla suppressed the expression of cytokines (e.g., TNF-α and IL-10), and inhibited along with inhibition of activator protein (AP)-1, JNK, and p38, in LPS-stimulated pulmonary alveolar macrophages derived from chronic obstructive pulmonary disease (COPD) rat model (Lu et al. Citation2020). These studies suggest that the MAPK/AP-1 signaling could be an important candidate target for LOs.
Furthermore, kahalalide F, a natural cyclic peptide that possesses antitumor activity and is under a phase I clinical trial, shows cytotoxicity against prostate and breast cancer cells, with IC50 values ranging from 0.07 to 0.28 µM (IC50 for PC3 cells = 0.07 µM, IC50 for DU145 cells = 0.18 µM, IC50 for LNCaP = 0.26 µM, IC50 for SKBR-3 = 0.23 µM, IC50 for BT474 = 0.26 µM, and IC50 for MCF7 = 0.28 µM) (Salazar et al. Citation2013; Suarez et al. Citation2003). Wewakazole B, a novel cyclopeptide from cyanobacterium Lyngbya majuscule, also exhibits a cytotoxicity against MCF-7 breast cancer cells and H460 lung cancer cells with IC50 of 0.58 µM and 1.0 µM, respectively (Abdalla and McGaw Citation2018). However, the anticancer effects of LOs dissolved in DMSO are only apparent at relatively high concentrations (∼400 µg/mL) to exert anticancer effects (Okinyo-Owiti et al. Citation2015). This phenomenon might be caused by the aggregation of LOs at high concentrations. LOs 23 and 32 have been reported to aggregate in breast cancer cells due to their hydrophobicity (Okinyo-Owiti et al. Citation2015). Zou et al. (Citation2020a) reported that LOs (23 and 32) dissolved in DMSO do not show dose-dependence at high doses in THP-1 cells, which may be due to LO aggregation. Interestingly, PEG-based formulation significantly improves the solubility of LOs, and consequently, the IC50 value of 23 and 32 for breast cancer cells is decreased to the nanomolar range in () (Yang et al. Citation2019). This result suggests that it is necessary to select the most suitable solvents that maximize LO solubility and toxicity for future in vitro and in vivo experiments.
Author contributions
JYC and MJTR supervised, conceptualized, and designed the manuscript. YYS and JHK wrote the original draft of the manuscript. JYC and MJTR edited and revised the manuscript critically. YYS and MJTR revised the final written manuscript. YYS and JHK prepared the figures and tables. YYS, MJTR and JYC acquired the funding for this project. YYS and JHK contributed equally as first authors of the paper, preparing first drafts of the paper. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
Dr. Martin J. T. Reaney is the founder of and has an equity interest in, Prairie Tide Diversified Inc. Dr. Youn Young Shim is the Korea Branch Representative for PTD (Seoul, Korea).
Additional information
Funding
References
- Abdalla, M. A., and L. J. McGaw. 2018. Natural cyclic peptides as an attractive modality for therapeutics: A mini review. Molecules 23 (8):2080. doi: 10.3390/molecules23082080.
- Arnison, P. G., M. J. Bibb, G. Bierbaum, A. A. Bowers, T. S. Bugni, G. Bulaj, J. A. Camarero, D. J. Campopiano, G. L. Challis, J. Clardy, et al. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Natural Product Reports 30 (1):108–60. doi: 10.1039/c2np20085f.
- Bao, Q, and Y. Shi. 2007. Apoptosome: A platform for the activation of initiator caspases. Cell Death and Differentiation 14 (1):56–65. doi: 10.1038/sj.cdd.4402028.
- Barber, C. J., P. T. Pujara, D. W. Reed, S. Chiwocha, H. Zhang, and P. S. Covello. 2013. The two-step biosynthesis of cyclic peptides from linear precursors in a member of the plant family Caryophyllaceae involves cyclization by a serine protease-like enzyme. The Journal of Biological Chemistry 288 (18):12500–10. doi: 10.1074/jbc.M112.437947.
- Bauer, R., P. Bazylewski, P. D. Jadhav, J. Shen, D. P. Okinyo-Owiti, J. Yang, G. S. Chang, M. J. T. Reaney, and R. Sammynaiken. 2015. Characterization of metal-functionalized flax orbitide as a new candidate for light-emitting semiconductor. Journal of Physics: Conference Series 619:012026–4. doi: 10.1088/1742-6596/619/1/012026.
- Brühl, L., B. Matthäus, E. Fehling, B. Wiege, B. Lehmann, H. Luftmann, K. Bergander, K. Quiroga, A. Scheipers, O. Frank, et al. 2007. Identification of bitter off-taste compounds in the stored cold pressed linseed oil. Journal of Agricultural and Food Chemistry 55 (19):7864–8. doi: 10.1021/jf071136k.
- Burley, S. K., and G. A. Petsko. 1986. Amino-aromatic interactions in proteins. FEBS Letters 203 (2):139–43. doi: 10.1016/0014-5793(86)80730-X.
- Burnett, P.-G. G., C. M. Olivia, D. P. Okinyo-Owiti, and M. J. T. Reaney. 2016. Orbitide composition of the flax core collection (FCC). Journal of Agricultural and Food Chemistry 64 (25):5197–206. doi: 10.1021/acs.jafc.6b02035.
- Byeon, S. E., Y.-S. Yi, J. Oh, B. C. Yoo, S. Hong, and J. Y. Cho. 2012. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators of Inflammation 2012:512926. doi: 10.1155/2012/512926.
- Cândido-Bacani, P. d. M., P. d. O. Figueiredo, M. d F. C. Matos, F. R. Garcez, and W. S. Garcez. 2015. Cytotoxic orbitide from the latex of Croton urucurana. Journal of Natural Products 78 (11):2754–60. doi: 10.1021/acs.jnatprod.5b00724.
- Carlos-Reyes, A., J. S. Lopez-Gonzalez, M. Meneses-Flores, D. Gallardo-Rincon, E. Ruiz-Garcia, L. A. Marchat, H. Astudillo-de la Vega, O. N. Hernandez de la Cruz, and C. Lopez-Camarillo. 2019. Dietary compounds as epigenetic modulating agents in cancer. Frontiers in Genetics 10 (79):79. doi: 10.3389/fgene.2019.00079.
- Cassani, R. S. L., P. G. Fassini, J. H. Silvah, C. M. M. Lima, and J. S. Marchini. 2015. Impact of weight loss diet associated with flaxseed on inflammatory markers in men with cardiovascular risk factors: A clinical study. Nutrition Journal 14:5–8. doi: 10.1186/1475-2891-14-5.
- Chang, V. C., M. Cotterchio, B. A. Boucher, D. J. A. Jenkins, L. Mirea, S. E. McCann, and L. U. Thompson. 2019. Effect of dietary flaxseed intake on circulating sex hormone levels among postmenopausal women: A randomized controlled intervention trial. Nutrition and Cancer 71 (3):385–98. doi: 10.1080/01635581.2018.1516789.
- Chatterji, D., M. B. Sankaram, and D. Balasubramanian. 1987. Conformational and ion-binding properties of cyclolinopeptide A isolated from linseed. Journal of Biosciences 11 (1-4):473–84. doi: 10.1007/BF02704696.
- Chikara, S., S. Mamidi, A. Sreedasyam, K. Chittem, R. Pietrofesa, A. Zuppa, G. Moorthy, N. Dyer, M. Christofidou-Solomidou, and K. M. Reindl. 2018. Flaxseed consumption inhibits chemically induced lung tumorigenesis and modulates expression of phase II enzymes and inflammatory cytokines in A/J mice. Cancer Prevention Research (Philadelphia, Pa.) 11 (1):27–37. doi: 10.1158/1940-6207.CAPR-17-0119.
- Cho, J. Y., N. Y. Sung, D. Jung, Z. A. Ratan, Y. Y. Shim, and M. J. T. Reaney. 2017. Composition for treating inflammatory disease comprising cyclic peptide mixture. Korean Patent 10-101763475B1.
- Coffey, G., F. DeGuzman, M. Inagaki, Y. Pak, S. M. Delaney, D. Ives, A. Betz, Z. J. Jia, A. Pandey, D. Baker, et al. 2012. Specific inhibition of spleen tyrosine kinase suppresses leukocyte immune function and inflammation in animal models of rheumatoid arthritis. The Journal of Pharmacology and Experimental Therapeutics 340 (2):350–9. doi: 10.1124/jpet.111.188441.
- Condie, J. A., G. Nowak, D. W. Reed, J. J. Balsevich, M. J. T. Reaney, P. G. Arnison, and P. S. Covello. 2011. The biosynthesis of Caryophyllaceae-like cyclic peptides in Saponaria vaccaria L. from DNA-encoded precursors. The Plant Journal 67 (4):682–90. doi: 10.1111/j.1365-313X.2011.04626.x.
- Conforti, F. D, and S. F. Davis. 2006. The effect of soya flour and flaxseed as a partial replacement for bread flour in yeast bread. International Journal of Food Science and Technology 41 (s2):95–101. doi: 10.1111/j.1365-2621.2006.01410.x.
- Covello, P. S., R. S. S. Datla, S. L. Stone, J. J. Balsevich, M. J. T. Reaney, P. G. Arnison, and J. A. Condie. 2012. DNA sequences encoding Caryophyllaceae and Caryophyllaceae-like cyclopeptide precursors and methods of use. US Patent Application 2012/0058905A1.
- Cox, E. G., D. W. J. Cruickshank, and J. A. S. Smith. 1958. The crystal structure of benzene at –3 0C. Proceedings of the Royal Society A: Mathematical, Physical, & Engineering Sciences 247:1–21. doi: 10.1098/rspa.1958.0167.
- Cunnane, S. C., S. Ganguli, C. Menard, A. C. Liede, M. J. Hamadeh, Z. Y. Chen, T. M. Wolever, and D. J. Jenkins. 1993. High alpha-linolenic acid flaxseed (Linum usitatissimum): Some nutritional properties in humans. The British Journal of Nutrition 69 (2):443–53. doi: 10.1079/bjn19930046.
- Czabotar, P. E., G. Lessene, A. Strasser, and J. M. Adams. 2014. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nature Reviews. Molecular Cell Biology 15 (1):49–63. doi: 10.1038/nrm3722.
- De Silva, S. F, and J. Alcorn. 2019. Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals 12 (2):68. doi: 10.3390/ph12020068.
- Demark-Wahnefried, W., D. T. Price, T. J. Polascik, C. N. Robertson, E. E. Anderson, D. F. Paulson, P. J. Walther, M. Gannon, and R. T. Vollmer. 2001. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: Exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology 58 (1):47–52. doi: 10.1016/S0090-4295(01)01014-7.
- Dodin, S., A. Lemay, H. Jacques, F. Légaré, J. C. Forest, and B. Mâsse. 2005. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: A randomized, double-blind, wheat germ placebo-controlled clinical trial. The Journal of Clinical Endocrinology & Metabolism 90 (3):1390–7. doi: 10.1210/jc.2004-1148.
- Elmore, S. 2007. Apoptosis: A review of programmed cell death. Toxicologic Pathology 35 (4):495–516. doi: 10.1080/01926230701320337.
- El-Saeed, G. S. M., E. A. Elghoroury, S. Morsy, H. M. Aly, and H. Wafaey. 2018. Phenotype of vitamin D receptor gene polymorphisms, impact of feeding flaxseed oil, and osteoporosis in ovariectomised diabetic rats. Bulletin of the National Research Centre 42 (1):11. doi: 10.1186/s42269-018-0003-8.
- Fale, S. K., M. J. Umekar, R. Das, and M. R. Alaspure. 2022. A comprehensive study of herbal cosmetics prepared from flaxseed. Multidisciplinary International Research Journal of Gujarat Technological University 4:106–12.
- Gallo, P., F. Rossi, M. Saviano, C. Pedone, G. Colonna, and R. Ragone. 1998. Specific interaction between bovine cyclophilin A and synthetic analogues of cyclolinopeptide A. Journal of Biochemistry 124 (5):880–5. doi: 10.1093/oxfordjournals.jbchem.a022202.
- Gaymes, T. J., N. J. Carrett, N. Patel, J. E. Kay, and I. Z. Siemion. 1996. Effects of cyclolinopeptide A on T lymphocyte activation and peptidyl prolyl isomerase activity. Biochemical Society Transactions 24 (1):90S. doi: 10.1042/bst024090s.
- Gaymes, T. J., M. Cebrat, I. Z. Siemion, and J. E. Kay. 1997. Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Letters 418 (1–2):224–7. doi: 10.1016/S0014-5793(97)01345-8.
- Genovese, M. C., D. M. van der Heijde, E. C. Keystone, A. J. Spindler, C. Benhamou, A. Kavanaugh, E. Fudman, K. Lampl, C. O’Brien, E. L. Duffield, et al. 2014. A phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 2 dosing regimens of fostamatinib in patients with rheumatoid arthritis with an inadequate response to a tumor necrosis factor-alpha antagonist. The Journal of Rheumatology 41 (11):2120–8. doi: 10.3899/jrheum.140238.
- Georgakis, G. V., Y. Li, G. Z. Rassidakis, L. J. Medeiros, G. B. Mills, and A. Younes. 2006. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. British Journal of Haematology 132 (4):503–11. doi: 10.1111/j.1365-2141.2005.05881.x.
- Goyal, A., V. Sharma, N. Upadhyay, S. Gill, and M. Sihag. 2014. Flax and flaxseed oil: An ancient medicine and modern functional food. Journal of Food Science and Technology 51 (9):1633–53. doi: 10.1007/s13197-013-1247-9.
- Grant, S. M., H. D. Langtry, and R. N. Brogden. 1989. Ranitidine. An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in peptic ulcer disease and other allied diseases. Drugs 37 (6):801–70. doi: 10.2165/00003495-198937060-00003.
- Guicciardi, M. E, and G. J. Gores. 2009. Life and death by death receptors. FASEB Journal 23 (6):1625–37. doi: 10.1096/fj.08-111005.
- Gui, B., Y. Y. Shim, R. S. S. Datla, R. S. Covello, S. L. Stone, and M. J. T. Reaney. 2012. Identification and quantification of cyclolinopeptides in five flaxseed cultivars. Journal of Agricultural and Food Chemistry 60 (35):8571–9. doi: 10.1021/jf301847u.
- Gui, B., Y. Y. Shim, and M. J. T. Reaney. 2012. Distribution of cyclolinopeptides in flaxseed fractions and products. Journal of Agricultural and Food Chemistry 60 (35):8580–9. doi: 10.1021/jf3023832.
- Han, M., L. Jia, W. Lv, L. Wang, and W. Cui. 2019. Epigenetic enzyme mutations: Role in tumorigenesis and molecular inhibitors. Frontiers in Oncology 9:194. doi: 10.3389/fonc.2019.00194.
- Health Canada. 2014. Summary of health Canada’s assessment of a health claim about ground whole flaxseed and blood cholesterol lowering. Accessed August 24, 2022. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/flaxseed-graines-de-lin-eng.pdf.
- Heidari, Z., H. Ghasemi-Tehrani, H. Fallahzadeh, and A. Nadjarzadeh. 2020. The effects of flaxseed on weight loss in women with polycystic ovarian syndrome: A randomized controlled trial. Iranian Journal of Diabetes and Obesity 11:79–86. doi: 10.18502/ijdo.v11i2.2652.
- Hendrich, S., S. Ewing-Blount, C. Curtis, and M. Ferlazzo. 2010. Iowa State NWRC study finds flaxseed lowers high cholesterol in men. Iowa State University. Accessed August 24, 2022. https://www.news.iastate.edu/news/2010/mar/flaxseed.
- Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407 (6805):770–6. doi: 10.1038/35037710.
- Hubbard, A. 2021. Can flaxseed gel tame your frizz and smooth your curls? Healthline Media a Red Ventures Company. Accessed August 24, 2022. https://www.healthline.com/health/beauty-skin-care/flaxseed-gel-for-hair#recipes.
- Hutchins, A. M., B. D. Brown, S. C. Cunnane, S. G. Domitrovich, E. R. Adams, and C. E. Bobowiec. 2013. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: A randomized study. Nutrition Research (New York, N.Y.) 33 (5):367–75. doi: 10.1016/j.nutres.2013.02.012.
- Izdebska, M., W. Zielińska, D. Grzanka, and M. Gagat. 2018. The role of actin dynamics and actin-binding proteins expression in epithelial-to-mesenchymal transition and its association with cancer progression and evaluation of possible therapeutic targets. BioMed Research International 2018:4578373. doi: 10.1155/2018/4578373.
- Jadhav, P. D., D. P. Okinyo-Owiti, P. W. K. Ahiahonu, and M. J. T. Reaney. 2013. Detection, isolation and characterisation of cyclolinopeptides J and K in ageing flax. Food Chemistry 138 (2-3):1757–63. doi: 10.1016/j.foodchem.2012.10.126.
- Jadhav, P. D., J. Shen, P.-G G. Burnett, J. Yang, R. Sammynaiken, and M. J. T. Reaney. 2021. Methionine epimerization in cyclic peptides. RSC Advances 11 (34):20859–64. doi: 10.1039/D1RA04260B.
- Jadhav, P. D., Y. Y. Shim, and M. J. T. Reaney. 2018. Affinity binding of chicken apolipoprotein A1 to a novel flax orbitide (linusorb). RSC Advances 8 (32):17702–9. doi: 10.1039/C8RA01757C.
- Jadhav, P. D., Y. Zuo, Y. Y. Shim, J. Shen, M. J. T. Reaney, N. Zhang, and Y. Wang. 2018. Metal binding novel flaxseed peptides (linusorbs). International Journal of Food Science & Technology 53 (8):1972–82. doi: 10.1111/ijfs.13786.
- Jeong, D., Y.-S. Yi, G. H. Sung, W. S. Yang, J. G. Park, K. Yoon, D. H. Yoon, C. Song, Y. Lee, M. H. Rhee, et al. 2014. Anti-inflammatory activities and mechanisms of Artemisia asiatica ethanol extract. Journal of Ethnopharmacology 152 (3):487–96. doi: 10.1016/j.jep.2014.01.030.
- Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proceedings of the National Academy of Sciences of the United States of America 95 (9):4997–5002. doi: 10.1073/pnas.95.9.4997.
- Kaneda, T., Y. Nakajima, S. Koshikawa, A. E. Nugroho, and H. Morita. 2019. Cyclolinopeptide F, a cyclic peptide from flaxseed inhibited RANKL-induced osteoclastogenesis via downergulation of RANK expression. Journal of Natural Medicines 73 (3):504–12. doi: 10.1007/s11418-019-01292-w.
- Kaneda, T., H. Yoshida, Y. Nakajima, M. Toishi, A. E. Nugroho, and H. Morita. 2016. Cyclolinopeptides, cyclic peptides from flaxseed with osteoclast differentiation inhibitory activity. Bioorganic & Medicinal Chemistry Letters 26 (7):1760–1. doi: 10.1016/j.bmcl.2016.02.040.
- Kates, S. A., N. A. Solé, F. Albericio, and G. Barany. 1994. Solid-phase synthesis of cyclic peptides. In Peptides, eds. C. Basava, and G. M. Anantharamaiah, 39–58. Boston: Birkhäuser. doi: 10.1007/978-1-4615-8176-5_4.
- Kaufmann, H. P, and A. Tobschirbel. 1959. Über ein Oligopeptid aus Leinsamen. Chemische Berichte 92 (11):2805–9. doi: 10.1002/cber.19590921122.
- Kessler, H., M. Gehrke, A. Haupt, M. Klein, A. Müller, and K. Wagner. 1986b. Common structural features for cytoprotection activities of somatostatin, antamanide and related peptides. Wiener Klinische Wochenschrift 64:74–8.
- Kessler, H., M. Klein, A. Muller. 1986a. Conformational prerequisites for the in vitro inhibition of cholate uptake in hepatocytes by cyclic analogues of antamanide and somatostatin. Angewandte Chemie International Edition in English 25 (11):997–9. doi: 10.1002/anie.198609971.
- Kim, J. H, and N. Kim. 2014. Regulation of NFATc1 in osteoclast differentiation. Journal of Bone Metabolism 21 (4):233–41. doi: 10.11005/jbm.2014.21.4.233.
- Kowaltowski, A. J, and A. E. Vercesi. 1999. Mitochondrial damage induced by conditions of oxidative stress. Free Radical Biology & Medicine 26 (3–4):463–71. doi: 10.1016/S0891-5849(98)00216-0.
- Kristensen, M., M. G. Jensen, J. Aarestrup, K. E. Petersen, L. Søndergaard, M. S. Mikkelsen, and A. Astrup. 2012. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type. Nutrition & Metabolism 9 (1):8.doi:10.1186/1743-7075-9-8.
- Landecker, H. 2011. Food as exposure: Nutritional epigenetics and the new metabolism. BioSocieties 6 (2):167–94. doi: 10.1057/biosoc.2011.1.
- Lawrence, T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology 1 (6):a001651. doi: 10.1101/cshperspect.a001651.
- Lee, C. G., Y. Y. Shim, M. J. T. Reaney, and H. J. Chang. 2021. Food puree for seniors: The effects of XanFlax as a thickener on physicochemical and antioxidant properties. Foods 10 (5):1100. doi: 10.3390/foods10051100.
- Legacy, S. R. 2018. Food data central, United States Department of Agriculture. Accessed February 26, 2022. https://fdc.nal.usda.gov/fdc-app.html#/food-details/169414/nutrients.
- Lemay, A., S. Dodin, N. Kadri, H. Jacques, and J. C. Forest. 2002. Flaxseed dietary supplement versus hormone replacement therapy in hypercholesterolemic menopausal women. Obstetrics and Gynecology 100 (3):495–504. doi: 10.1016/S0029-7844(02)02123-3.
- Lester, S. N, and K. Li. 2014. Toll-like receptors in antiviral innate immunity. Journal of Molecular Biology 426 (6):1246–64. doi: 10.1016/j.jmb.2013.11.024.
- Liang, J, and J. M. Slingerland. 2003. Multiple roles of the PI3K/PKB (AKT) pathway in cell cycle progression. Cell Cycle (Georgetown, Tex.) 2 (4):339–45. doi: 10.4161/cc.2.4.433.
- Liu, D, and A. Mamorska-Dyga. 2017. Syk inhibitors in clinical development for hematological malignancies. Journal of Hematology & Oncology 10 (1):145. doi: 10.1186/s13045-017-0512-1.[PMC].[28754125.
- Liu, J., Y. Y. Shim, T. J. Tse, Y. Wang, and M. J. T. Reaney. 2018. Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends in Food Science & Technology 75:146–57. doi: 10.1016/j.tifs.2018.01.011.
- Lowcock, E. C., M. Cotterchio, and B. A. Boucher. 2013. Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes & Control: CCC 24 (4):813–6. doi: 10.1007/s10552-013-0155-7.
- Lu, F., H. Yang, S.-D. Lin, L. Zhao, C. Jiang, Z.-B. Chen, Y.-Y. Liu, Y.-J. Kan, J. Hu, and W.-S. Pang. 2020. Cyclic peptide extracts derived from Pseudostellaria heterophylla ameliorates COPD via regulation of the TLR4/MyD88 pathway proteins. Frontiers in Pharmacology 11:850. doi: 10.3389/fphar.2020.00850.
- Lu, Y. C., W. C. Yeh, and P. S. Ohashi. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42 (2):145–51. doi: 10.1016/j.cyto.2008.01.006.
- Magee, E. 2011. The benefits of flaxseed. WebMD. Accessed August 26, 2022. http://www.webmd.com/diet/features/benefits-of-flaxseed.
- Malumbres, M, and M. Barbacid. 2009. Cell cycle, CDKs and cancer: A changing paradigm. Nature Reviews. Cancer 9 (3):153–66. doi: 10.1038/nrc2602.
- Mejia, J. C., A. Basu, and R. Shapiro. 2014. Calcineurin inhibitors. In Kidney transplantation-principles and practice, eds. P. J. Morris, and S. J. Knechtle, 231–49. London, UK: Elsevier.
- Moghaddasi, M. S. 2011. Linseed and usages in humanlife. Advances in Environmental Biology 5:1380–92. https://link.gale.com/apps/doc/A260791716/AONE?u=googlescholar&sid=bookmark-AONE&xid=ec68aeee.
- Morita, H., A. Shishido, T. Matsumoto, H. Itokawa, and K. Takeya. 1999. Cyclolinopeptides B–E, new cyclic peptides from Linum usitatissimum. Tetrahedron 55 (4):967–76. doi: 10.1016/S0040-4020(98)01086-2.
- Morita, H., A. Shishido, T. Matsumoto, K. Takeya, H. Itokawa, T. Hirano, and K. Oka. 1997. A new immunosuppressive cyclic nonapeptide, cycloinopeptide B from Linum usitatissimum. Bioorganic & Medicinal Chemistry Letters 7 (10):1269–72. doi: 10.1016/S0960-894X(97)00206-0.
- Morita, H, and K. Takeya. 2010. Bioactive cyclic peptides from higher plants. Heterocycles 80 (2):739–64. doi: 10.3987/REV-09-SR(S)7.
- Mwakwari, S. C., V. Patil, W. Guerrant, and A. K. Oyelere. 2010. Macrocyclic histone deacetylase inhibitors. Current Topics in Medicinal Chemistry 10 (14):1423–40. doi: 10.2174/156802610792232079.
- Naider, F., E. Benedetti, and M. Goodman. 1971. Conformation of cyclolinopeptide A observed by circular dichroism. Proceedings of the National Academy of Sciences of the United States of America 68 (6):1195–8. doi: 10.1073/pnas.68.6.1195.
- Neukam, K., S. De Spirt, W. Stahl, M. Bejot, J.-M. Maurette, H. Tronnier, and U. Heinrich. 2011. Supplementation of flaxseed oil diminishes skin sensitivity and improves skin barrier function and condition. Skin Pharmacology and Physiology 24 (2):67–74. doi: 10.1159/000321442.
- Okinyo-Owiti, D. P., Q. Dong, B. Ling, P. D. Jadhav, R. Bauer, J. M. Maley, M. J. T. Reaney, J. Yang, and R. Sammynaiken. 2015. Evaluating the cytotoxicity of flaxseed orbitides for potential cancer treatment. Toxicology Reports 2:1014–8. doi: 10.1016/j.toxrep.2015.06.011.
- Olivia, C. M., P.-G G. Burnett, D. P. Okinyo-Owiti, J. Shen, and M. J. T. Reaney. 2012. Rapid reversed-phase liquid chromatography separation of cyclolinopeptides with monolithic and microparticulate columns. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 904:128–34. doi: 10.1016/j.jchromb.2012.07.037.
- Olson, M. F, and E. Sahai. 2009. The actin cytoskeleton in cancer cell motility. Clinical & Experimental Metastasis 26 (4):273–87. doi: 10.1007/s10585-008-9174-2.
- Peng, L.-P., S.-Q. Men, Z.-A. Liu, N.-N. Tong, M. Imran, and Q.-Y. Shu. 2019. Fatty acid composition, phytochemistry, antioxidant activity on seed coat and kernel of Paeonia ostii from main geographic production areas. Foods 9 (1):30. doi: 10.3390/foods9010030.
- Pine, P. R., B. Chang, N. Schoettler, M. L. Banquerigo, S. Wang, A. Lau, F. Zhao, E. B. Grossbard, D. G. Payan, and E. Brahn. 2007. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clinical Immunology (Orlando, Fla.) 124 (3):244–57. doi: 10.1016/j.clim.2007.03.543.
- Plissonneau, C., A. Sivignon, B. Chassaing, F. Capel, V. Martin, M. Etienne, I. Wawrzyniak, P. Chausse, F. Dutheil, G. Mairesse, et al. 2022. Beneficial effects of linseed supplementation on gut mucosa-associated microbiota in a physically active mouse model of Crohn’s disease. International Journal of Molecular Sciences 23 (11):5891. doi: 10.3390/ijms23115891.
- Pobezinskaya, Y. L, and Z. Liu. 2012. The role of TRADD in death receptor signaling. Cell Cycle (Georgetown, Tex.) 11 (5):871–6. doi: 10.4161/cc.11.5.19300.
- Prasad, K. 1997. Dietary flaxseed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 132 (1):69–76. doi: 10.1016/S0021-9150(97)06110-8.
- Ragheb, E. M., R. M. Bahnasy, E. E-S. Abd-Elhady, and R. M. Saad. 2019. The potential effect of flax seeds against osteoporosis in experimental female rats. World Journal of Dairy & Food Sciences 14:185–95. doi: 10.5829/idosi.wjdfs.2019.185.195.
- Ratan, Z. A., D. Jeong, N. Y. Sung, Y. Y. Shim, M. J. T. Reaney, Y.-S. Yi, and J. Y. Cho. 2020. LOMIX, a mixture of flaxseed linusorbs, exerts anti-inflammatory effects through Src and Syk in the NF-κB pathway. Biomolecules 10 (6):859. doi: 10.3390/biom10060859.
- Reaney, M. J. T., Y. Jia, J. Shen, C. Schock, N. Tyler, J. Elder, and S. Singh. 2013. Recovery of hydrophobic peptides from oils. US Patent. No. 8,383,172 B2.
- Rempel, B., B. Gui, J. Maley, M. J. T. Reaney, and R. Sammynaiken. 2010. Biomolecular interaction study of cyclolinopeptide a with human serum albumin. Journal of Biomedicine & Biotechnology 2010:737289–8. doi: 10.1155/2010/737289.
- Rubilar, M., C. Gutiérrez, M. Verdugo, C. Shene, and J. Sineiro. 2010. Flaxseed as a source of functional ingredients. Journal of Soil Science and Plant Nutrition 10 (3):373–7. doi: https://dx.doi.org/10.4067/S0718-95162010000100010.
- Salazar, R., H. Cortés-Funes, E. Casado, B. Pardo, A. López-Martín, C. Cuadra, J. Tabernero, C. Coronado, M. García, A. Soto Matos-Pita, et al. 2013. Phase I study of weekly kahalalide F as prolonged infusion in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology 72 (1):75–83. doi: 10.1007/s00280-013-2170-5.
- Sardar, D., Z. Lin, and E. W. Schmidt. 2015. Modularity of RiPP enzymes enables designed synthesis of decorated Peptides. Chemistry & Biology 22 (7):907–16. doi: 10.1016/j.chembiol.2015.06.014.
- Schatte, G., S. Labiuk, B. Li, P.-G. Burnett, M. Reaney, P. Grochulski, M. Fodje, J. Yang, and R. Sammynaiken. 2012. Cyclolinopeptide B methanol trisolvate. Acta Crystallographica. Section E, Structure Reports Online 68 (Pt 1):o50–1. doi: 10.1107/S1600536811051488.
- Sedley, L. 2020. Advances in nutritional epigenetics-A fresh perspective for an old idea. Lessons learned, limitations, and future directions. Epigenetics Insights 13:2516865720981924–0. doi: 10.1177/2516865720981924.
- Sharma, J. N., A. Al-Omran, and S. S. Parvathy. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15 (6):252–9. doi: 10.1007/s10787-007-0013-x.
- Shim, Y. Y., B. Gui, P. G. Arnison, Y. Wang, and M. J. T. Reaney. 2014. Flaxseed (Linum usitatissimum L.) bioactive compounds and peptide nomenclature: A review. Trends in Food Science & Technology 38 (1):5–20. doi: 10.1016/j.tifs.2014.03.011.
- Shim, Y. Y., B. Gui, Y. Wang, and M. J. T. Reaney. 2015. Flaxseed (Linum usitatissimum L.) oil processing and selected products. Trends in Food Science & Technology 43 (2):162–77. doi: 10.1016/j.tifs.2015.03.001.
- Shim, Y. Y, and M. J. T. Reaney. 2015. Kinetic interactions between cyclolinopeptides and immobilized human serum albumin by surface plasmon resonance. Journal of Agricultural and Food Chemistry 63 (4):1099–106. doi: 10.1021/jf504811x.
- Shim, Y. Y., Z. Song, P. D. Jadhav, and M. J. T. Reaney. 2019. Orbitides from flaxseed (Linum usitatissimum L.): A comprehensive review. Trends in Food Science & Technology 93:197–211. doi: 10.1016/j.tifs.2019.09.007.
- Shim, Y. Y., L. W. Young, P. G. Arnison, E. Gilding, and M. J. T. Reaney. 2015. Proposed systematic nomenclature for orbitides. Journal of Natural Products 78 (4):645–52. doi: 10.1021/np500802p.
- Siemion, I. Z., M. Cebrat, A. Jankowski, M. Lisowski, A. Pedyczak, and A. Wysłouch. 1994. Does the edge-to-face interaction between aromatic rings occur in cyclolinopeptide A analogues? International Journal of Peptide and Protein Research 44 (1):61–9. doi: 10.1111/j.1399-3011.1994.tb00405.x.
- Siemion, I. Z., M. Cebrat, and Z. Wieczorek. 1999. Cyclolinopeptides and their analogs – A new family of peptide immunosuppressants affecting the calcineurin system. Archivum Immunologiae et Therapiae Experimentalis 47 (3):143–53.
- Siemion, I. Z., W. A. Klis, A. Sucharda-Sobczyk, and R. Obermeier. 1997. The conformation of cyclolinopeptide A in solution. Roczniki Chemii 51:1489–98.
- Sinha, K., J. Das, P. B. Pal, and P. C. Sil. 2013. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Archives of Toxicology 87 (7):1157–80. doi: 10.1007/s00204-013-1034-4.
- Suarez, Y., L. Gonzalez, A. Cuadrado, M. Berciano, M. Lafarga, and A. Munoz. 2003. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Molecular Cancer Therapeutics 2 (9):863–72.
- Sugikawa, E., T. Hosoi, N. Yazaki, M. Gamanuma, N. Nakanishi, and M. Ohashi. 1999. Mutant p53 mediated induction of cell cycle arrest and apoptosis at G1 phase by 9-hydroxyellipticine. Anticancer Research 19:3099–108.
- Sun, B. H., X. P. Zhao, B. J. Wang, D. L. Yang, and L. J. Hao. 2000. FADD and TRADD expression and apoptosis in primary hepatocellular carcinoma. World Journal of Gastroenterology 6:223–7. doi: 10.3748/wjg.v6.i2.223.
- Sung, N. Y., D. Jeong, Y. Y. Shim, Z. A. Ratan, Y.-J. Jang, M. J. T. Reaney, S. Lee, B.-H. Lee, J.-H. Kim, Y.-S. Yi, et al. 2020. The anti-cancer effect of linusorb B3 from flax seed oil through the promotion of apoptosis, inhibition of actin polymerization, and suppression of Src activity in glioblastoma cells. Molecules 25 (24):5881. doi: 10.3390/molecules25245881.
- Takayanagi, H. 2007. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nature Reviews. Immunology 7 (4):292–304. doi: 10.1038/nri2062.
- Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11 (4):443–51. doi: 10.1016/S1074-7613(00)80119-3.
- Tancredi, T., E. Benedetti, M. Grimaldi, C. Pedone, F. Rossi, M. Saviano, P. A. Temussi, and G. Zanotti. 1991. Ion binding of cyclolinopeptide A: An NMR and CD conformational study. Biopolymers 31 (6):761–7. doi: 10.1002/bip.360310621.
- Thompson, L. U., B. A. Boucher, Z. Liu, M. Cotterchio, and N. Kreiger. 2006. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutrition and Cancer 54 (2):184–201. doi: 10.1207/s15327914nc5402_5.
- Tiffon, C. 2018. The impact of nutrition and environmental epigenetics on human health and disease. International Journal of Molecular Sciences 19 (11):3425. doi: 10.3390/ijms19113425.
- Torkan, M., M. H. Entezari, and M. Siavash. 2015. Effect of flaxseed on blood lipid level in hyperlipidemic patients. Reviews on Recent Clinical Trials 10 (1):61–7. doi: 10.2174/1574887110666150121154334.
- Tripathi, P., P. Tripathi, L. Kashyap, and V. Singh. 2007. The role of nitric oxide in inflammatory reactions. FEMS Immunology and Medical Microbiology 51 (3):443–52. doi: 10.1111/j.1574-695X.2007.00329.x.
- Tse, T. J., Y. Guo, Y. Y. Shim, S. K. Purdy, J. H. Kim, J. Y. Cho, J. Alcorn, and M. J. T. Reaney. 2022. Availability of bioactive flax lignan from foods and supplements. Critical Reviews in Food Science and Nutrition 1–16. doi: 10.1080/10408398.2022.2072807.
- Wang, A., M. Al-Kuhlani, S. C. Johnston, D. M. Ojcius, J. Y. Chou, and D. Dean. 2013. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cellular Microbiology 15 (5):779–94. doi: 10.1111/cmi.12071.
- Wang, C, and R. J. Youle. 2009. The role of mitochondria in apoptosis. Annual Review of Genetics 43:95–118. doi: 10.1146/annurev-genet-102108-134850.
- Weinblatt, M. E., A. Kavanaugh, R. Burgos-Vargas, A. H. Dikranian, G. Medrano-Ramirez, J. L. Morales-Torres, F. T. Murphy, T. K. Musser, N. Straniero, A. V. Vicente-Gonzales, et al. 2008. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: A twelve-week, randomized, placebo-controlled trial. Arthritis & Rheumatism 58 (11):3309–18. doi: 10.1002/art.23992.
- Wieczorek, Z., B. Bengtsson, J. Trojnar, and I. Z. Siemion. 1991. Immunosuppressive activity of cyclolinopeptide A. Peptide Research 4:275–83.
- Wong, B. R., E. B. Grossbard, D. G. Payan, and E. S. Masuda. 2004. Targeting Syk as a treatment for allergic and autoimmune disorders. Expert Opinion on Investigational Drugs 13 (7):743–62. doi: 10.1517/13543784.13.7.743.
- Yamaguchi, H, and J. Condeelis. 2007. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta 1773 (5):642–52. doi: 10.1016/j.bbamcr.2006.07.001.
- Yanai, H., S. Chiba, S. Hangai, K. Kometani, A. Inoue, Y. Kimura, T. Abe, H. Kiyonari, J. Nishio, N. Taguchi-Atarashi, et al. 2018. Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice. Proceedings of the National Academy of Sciences of the United States of America 115 (20):5253–8. doi: 10.1073/pnas.1803936115.
- Yang, J., P. D. Jadhav, M. J. T. Reaney, R. Sammynaiken, and J. Yang. 2019. A novel formulation significantly increases the cytotoxicity of flaxseed orbitides (linusorbs) LOB3 and LOB2 towards human breast cancer MDA-MB-231 cells. Die Pharmazie-An International Journal of Pharmaceutical Sciences 74:520–2. doi: 10.1691/ph.2019.9055.
- Yari, Z., M. Rahimlou, H. Poustchi, and A. Hekmatdoost. 2016. Flaxseed supplementation in metabolic syndrome management: A pilot randomized, open-labeled, controlled study. Phytotherapy Research : PTR 30 (8):1339–44. doi: 10.1002/ptr.5635.
- Yavropoulou, M. P, and J. G. Yovos. 2008. Osteoclastogenesis-current knowledge and future perspectives. Journal of Musculoskeletal & Neuronal Interactions 8 (3):204–16.
- Zarei, M., S. Adeli, S. Hosseini, and E. Daneshzad. 2022. The effect of flaxseed intake on appetite reduction: A systematic review of randomized clinical trials. Phytotherapy Research 1–3. doi: 10.1002/ptr.7570.
- Zou, X.-G., J.-N. Hu, J. Li, J.-Y. Yang, Y.-X. Du, Y.-F. Yu, and Z.-Y. Deng. 2018. iCellular uptake of [1–9-NαC]-linusorb B2 and [1–9-NαC]-linusorb B3 isolated from flaxseed, and their antitumor activities in human gastric SGC-7901 cells. Journal of Functional Foods 48:692–703. doi: 10.1016/j.jff.2018.08.008.
- Zou, X.-G., J.-N. Hu, M. Wang, Y.-X. Du, J. Li, Q.-Y. Mai, and Z.-Y. Deng. 2019. [1–9-NαC]-linusorb B2 and [1–9-NαC]-linusorb B3 isolated from flaxseed induce G1 cell cycle arrest on SGC-7901 cells by modulating the AKT/JNK signaling pathway. Journal of Functional Foods 52:332–9. doi: 10.1016/j.jff.2018.11.002.
- Zou, X.-G., Y. Y. Shim, J. Y. Cho, D. Jeong, J. Yang, Z.-Y. Deng, and M. J. T. Reaney. 2020a. Flaxseed orbitides, linusorbs, inhibit LPS-induced THP-1 macrophage inflammation. RSC Advances 10 (38):22622–30. doi: 10.1039/C9RA09058D.
- Zou, X.-G., J. Li, P.-L. Sun, Y.-W. Fan, J.-Y. Yang, and Z.-Y. Deng. 2020b. Orbitides isolated from flaxseed induce apoptosis against SGC-7901 adenocarcinoma cells. International Journal of Food Sciences and Nutrition 71 (8):929–39. doi: 10.1080/09637486.2020.1750573.