Abstract
The aryl hydrocarbon receptor (AHR) is not essential to survival, but does act as a key regulator of many normal physiological events. The role of this receptor in toxicological processes has been studied extensively, primarily employing the high-affinity ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). However, regulation of physiological responses by endogenous AHR ligands remains to be elucidated. Here, we review developments in this field, with a focus on 6-formylindolo[3,2-b]carbazole (FICZ), the endogenous ligand with the highest affinity to the receptor reported to date. The binding of FICZ to different isoforms of the AHR seems to be evolutionarily well conserved and there is a feedback loop that controls AHR activity through metabolic degradation of FICZ via the highly inducible cytochrome P450 1A1. Several investigations provide strong evidence that FICZ plays a critical role in normal physiological processes and can ameliorate immune diseases with remarkable efficiency. Low levels of FICZ are pro-inflammatory, providing resistance to pathogenic bacteria, stimulating the anti-tumor functions, and promoting the differentiation of cancer cells by repressing genes in cancer stem cells. In contrast, at high concentrations FICZ behaves in a manner similar to TCDD, exhibiting toxicity toward fish and bird embryos, immune suppression, and activation of cancer progression. The findings are indicative of a dual role for endogenously activated AHR in barrier tissues, aiding clearance of infections and suppressing immunity to terminate a vicious cycle that might otherwise lead to disease. There is not much support for the AHR ligand-specific immune responses proposed, the differences between FICZ and TCDD in this context appear to be explained by the rapid metabolism of FICZ.
1. Introduction
When first discovered, the aryl hydrocarbon receptor (AHR or dioxin receptor) was assumed to mediate metabolism of xenobiotics (Poland et al. Citation1976; Poland and Knutson Citation1982) since it bound 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and similar halogenated hydrocarbons with high affinities. However, in 1991 Nebert proposed that similar to nuclear receptors that bind hormones and regulate both cellular growth and differentiation, and the expression of particular cytochrome P450 (CYP) isozymes, the dioxin receptor plays a key role in regulating growth and the CYP1 family of enzymes metabolizes its ligands (Nebert Citation1991). In addition, he proposed that dioxins and other xenobiotic ligands of AHR mimic natural ligands.
Later, the extensive evolutionary conservation of this receptor (Hahn et al. Citation1997, Citation2017) and its role in regulating the constitutive expression of hundreds of genes (Tijet et al. Citation2006; Sartor et al. Citation2009; Dere et al. Citation2011; Salisbury et al. Citation2014) provided strong indications for important endogenous functions. The AHR belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors, some of whose members perform ancestral sensory functions and regulate circadian rhythms (McIntosh et al. Citation2010). However, the physiological roles of the AHR in vertebrates remain to be elucidated in detail.
Although this receptor is not essential for survival, AHR-deficient mice age prematurely and, furthermore, exhibit abnormal vascular development, with spontaneous development of lesions in several organs (Nebert et al. Citation1984; Schmidt et al. Citation1996; Fernandez-Salguero et al. Citation1997; Lahvis et al. Citation2000; Sauzeau et al. Citation2011; Hu et al. Citation2013). Moreover, deletion of the AHR affects endocrine, and reproductive functions (Abbott et al. Citation1999; Benedict et al. Citation2000; Baba et al. Citation2005, Citation2008; Moran et al. Citation2012); results in numerous pathologies related to defects in hematopoietic progenitor cells (Fernandez-Salguero et al. Citation1997; Bennett et al. Citation2015); disturbs hepatic energy homeostasis (Girer et al. Citation2016); and disrupts immune responses in a manner that enhances sensitivity to inflammatory stimuli (Thatcher et al. Citation2007; Sekine et al. Citation2009; Kiss et al. Citation2011). Thus, the emerging view is that this receptor plays an essential role in maintaining cellular homeostasis, control, and balancing physiological functions in response to endogenous ligands. One of its highly important functions appears to involve maintenance of progenitor cell populations, e.g. coordinating proliferation and differentiation and protecting against cellular senescence (Singh et al. Citation2009; Gasiewicz et al. Citation2014; Bennett et al. Citation2015). Indeed, persistent repression of the AHR appears to be required in order to safeguard the pluripotency of stem cells (Ko et al. Citation2016).
Most of our current knowledge in this context originates from decades of studies employing the metabolically inert, high-affinity ligand TCDD, which have clearly revealed the negative consequences of over-activating AHR. Indeed, TCDD is a highly toxic man-made chemical. Additional knowledge has been gained from the phenotypes of AHR knockout (AHR KO) and AHR-overexpressing strains of mice, extreme situations in which primarily physiological processes are altered. Interestingly, the first strains of mice carrying AHR null-alleles appeared rather normal but important functions of the AHR for development and for physiologic homeostasis are now well established (see Section 4). The present review focuses on novel information obtained using the high-affinity endogenous ligand 6-formylindolo[3,2-b]carbazole, FICZ, which indicates that the AHR is a highly dynamic regulator of numerous homeostatic processes.
2. Exogenous and endogenous activation of AHR signaling
The AHR is activated by binding of many low-molecular-weight substances, having dissociation constants (Kd) ranging from 10−13 to 10−3 M. CYP1A1, which encodes a member of the cytochrome P450 superfamily of enzymes, is typically the gene most highly up-regulated in response to activation of this receptor. CYP1A1 mediates the oxidative metabolism of a wide variety of exogenous, as well as endogenous substrates (Nebert and Dalton Citation2006).
In the absence of ligands, AHR is part of a cytoplasmic chaperone complex that also includes a dimer of Hsp90, and the co-chaperones p23 and AIP. In response to a ligand, the conformation of the receptor changes to expose a nuclear localization sequence that allows dissociation from the chaperone complex and translocation into the nucleus (Petrulis and Perdew Citation2002). Once inside the nucleus, the AHR heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT) and the resulting complex binds to response elements (AHREs) in the promotor regions of target genes, where it recruits transcription cofactors and proteins that remodel chromatin, promoting gene transcription ( reviewed in Beischlag et al. Citation2008; Jackson et al. Citation2015). Interestingly, genes involved in developmental and tumorigenic processes, including those encoding components of the Wnt/β-catenin and TGFβ signaling pathways, along with genes encoding xenobiotic-metabolizing enzymes are all targets (Chang et al. Citation2007; Sartor et al. Citation2009; Wang et al. Citation2010; Faust et al. Citation2013; Salisbury et al. Citation2014).
Identification of endogenous agonists of AHR remains the focus of intensive research. Among these, FICZ, derived from tryptophan (Trp), binds to this receptor with higher affinity than any other compound yet tested, including TCDD giving an estimated dissociation constant Kd of 0.07 nM in competitive binding assays with 1 nM TCDD using the hydroxylapatite assay (Rannug et al. Citation1987).
FICZ was initially detected as a photooxidation product of Trp (Rannug et al. Citation1987, Citation1995). The chemical structures of two Trp-derived AHR ligands were determined by means of mass spectrometry, 1H-NMR, and 13C-NMR, also fluorescence, UV, and infrared spectra were recorded. The two Trp-derived ligands were indolo[3,2-b]carbazoles identified as the monosubstituted 6-formylindolo[3,2-b]carbazole, i.e. FICZ (MW 284) and the symmetrical 6,12-diformylindolo[3,2-b]carbazole (dFICZ, MW 312), respectively (Rannug et al. Citation1995). Ultraviolet A and B radiation (UVA and UVB) and visible light can all generate FICZ upon irradiation of Trp solutions, although UVB is most efficient (Wincent et al. Citation2009). Recently it was shown that the generation of hydrogen peroxide (H2O2) during irradiation is involved in the generation of FICZ, but also that H2O2 alone in the absence of light can generate FICZ from Trp (Smirnova et al. Citation2016). Other light-independent pathways for the formation of FICZ were also described in detail by Smirnova et al. (Citation2016). Briefly, the Trp metabolites tryptamine and indole-3-pyruvic acid give rise to FICZ and its oxidation product indolo[3,2-b]carbazole-6-carboxylic acid (CICZ) via the common denominator indole-3-acetaldehyde. The human mitochondrial enzymes monoamine oxidase A and B are able to catalyze the deamination of tryptamine to give indole-3-acetaldehyde, and indole-3-pyruvate can be de-carboxylated to form indole-3-acetaldehyde that in multistep reactions yields FICZ ().
Figure 1. Formation pathways of 6-formylindolo[3,2-b]carbazole (FICZ) and indolo[3,2-b]carbazole-6-carboxylic acid (CICZ) from tryptophan directly or from the tryptophan metabolites tryptamine and indolo-3-pyruvic acid, respectively, via the common precursor indolo-3-acetaldehyde. All three pathways produce the same precursor of FICZ.
![Figure 1. Formation pathways of 6-formylindolo[3,2-b]carbazole (FICZ) and indolo[3,2-b]carbazole-6-carboxylic acid (CICZ) from tryptophan directly or from the tryptophan metabolites tryptamine and indolo-3-pyruvic acid, respectively, via the common precursor indolo-3-acetaldehyde. All three pathways produce the same precursor of FICZ.](/cms/asset/0c584692-7861-4bb7-bc19-a485dd8da253/itxc_a_1493086_f0001_c.jpg)
Microbiota, both on the human skin and in the gut, can convert Trp to several metabolites with little or no affinity for the AHR, as well as pro-ligands and high affinity ligands (reviewed in Murray and Perdew Citation2017). The lipophilic yeast Malassezia furfur, constituting a part of the normal skin microbiota can produce several Trp derived indoles and indolocarbazoles (Magiatis et al. Citation2013) (summarized in Janosik et al. Citation2018). The production of FICZ by M. furfur cultures as reported by Magiatis et al. (Citation2013) was later confirmed by Smirnova et al. (Citation2016), who also detected the oxidation product CICZ in the same cultures (Smirnova et al. Citation2016). Thus, the processes that lead to the formation of FICZ from Trp involve in addition to microbial enzymes also common mammalian/human enzymes as well as non-enzymatic reactions. Therefore, it is likely that these processes would take place in mammalian and human cells in vivo ().
The CYP1A1 enzyme is the most important enzyme for the monohydroxylation of FICZ, the primary step in the mammalian and human metabolism of FICZ. The NADPH-dependent FICZ metabolism is catalyzed with extreme efficiency by human recombinant CYP1A1, with kcat/Km of 8.1 × 107 M−1 s−1 approaching the limit of diffusion, and being 50-fold more efficient than the metabolism of the standard substrate 7-ethoxyresorufin (Wincent et al. Citation2009). CYP1A2 has an overlapping specificity with CYP1A1 and forms the same major metabolites, although with different kinetics. CYP1B1 seems to be preferentially involved in the further metabolism. By applying liquid chromatograhy-mass spectrometry (LC-MS) and NMR spectroscopy two primary metabolites, 2- and 8-hydroxyindolo[3,2-b]carbazole-6-carboxaldehyde (2-OH- and 8-OH-FICZ), respectively were identified. The further CYP-catalyzed metabolism of these two metabolites resulted in three identified dihydroxylated FICZ metabolites (Bergander et al. Citation2003, Citation2004). These metabolites are subject to further metabolism by e.g. sulfotransferases and UDP-glucuronosyltransferase, especially sulfoconjugation seems to be an important pathway (Bergander et al. Citation2004). Detailed studies with six different recombinant human sulfotransferases showed that four of them (SULT1A1, -1A2, -1B1, and -1E1) exhibited high catalytic efficiencies, especially for monohydroxylated FICZ metabolites (Wincent et al. Citation2009). Since sulfate conjugates are excreted in the urine, the authors analyzed seven human urine samples for sulfate conjugates of the mono hydroxylated metabolites 2- and 8-SO4-FICZ (the only standards available) of FICZ by LC/MS/MS. The samples contained several FICZ-derived metabolites and 8-SO4-FICZ was identified in two of the samples, demonstrating the presence of metabolites of the high affinity agonist of AHR in humans (Wincent et al. Citation2009).
Certain other indole derivatives – including indolo[3,2-b]carbazole (ICZ) (Bjeldanes et al. Citation1991), 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) (Song et al. Citation2002), the indigoids indigo and indirubin (Rannug, Bramstedt, et al. 1992; Adachi et al. Citation2001), as well as dFICZ, and two monohydroxylated FICZ metabolites (2-OH- and 8-OH-FICZ) (Wincent et al. Citation2009) – as well as lipoxin A4 (Schaldach et al. Citation1999) and the bacterial virulence factor 1-hydroxyphenazine (Moura-Alves et al. Citation2014) also bind to the AHR with high affinities.
Fecal bacteria may contribute significantly to the formation of, e.g. indigo, indirubin, indole, 3-indoxyl sulfate, indole-3-acetic acid, indole-3-acetaldehyde, indole-3-aldehyde, indole-3-lactic acid, indole-3-proprionate, indole-3-pyruvate, 3-methyl indole, and tryptamine (Kawajiri et al. Citation2009; Chung and Gadupudi Citation2011; Zelante et al. Citation2013; Jin et al. Citation2014; Murray and Perdew Citation2017). Of these, indole-3-acetaldehyde, indole-3-pyruvate, and tryptamine can be converted to FICZ (see above) and possibly other potent AHR ligands (Rannug et al. Citation1995; Bittinger et al. Citation2003; Smirnova et al. Citation2016) (). Such formation of FICZ by intestinal bacteria might explain the report by Perdew and Babbs in 1991 that diluted suspensions of rat fecal material can transform Trp into effective activators of the AHR (Perdew and Babbs Citation1991).
AHR signaling can also be activated by a multitude of exogenous and endogenous factors that do not bind directly to this receptor but that transiently suppress CYP1A1. Such activation by UVB (Goerz et al. Citation1983, Citation1996; Wei et al. Citation1999; Katiyar et al. Citation2000; Fritsche et al. Citation2007; Luecke, Wincent, et al. Citation2010), hyperbaric oxygen and ozone (Okamoto et al. Citation1993; Afaq et al. Citation2009), and H2O2 (Luecke, Wincent, et al. Citation2010; Wincent et al. Citation2012) have been shown in the case of UVB and H2O2 to involve enhanced formation of FICZ from Trp (Wei et al. Citation1999; Fritsche et al. Citation2007; Wincent et al. Citation2012; Smirnova et al. Citation2016), as well as attenuated turnover of FICZ by CYP1A1 (Luecke, Wincent, et al. Citation2010; Wincent et al. Citation2012). In addition, activation by, e.g. phorbol-12-myristate-13-acetate (PMA) (Crawford et al. Citation1997), bacterial endotoxin lipopolysaccharide (LPS) (Baron et al. Citation1998; Marcus et al. Citation1998; Lee et al. Citation2015), and tumor necrosis factor-α (TNFα) (Drozdzik et al. Citation2014) may also reflect slower metabolic degradation of FICZ, since mediators of inflammation or infection such as LPS, TNFα, and the interleukins IL-6 and IL-1β repress expression and/or activity of drug metabolizing enzymes, including CYP1A1, both in vitro and in vivo (reviewed in Gerbal-Chaloin et al. Citation2013). Accordingly, induction of CYP1A1 expression in primary human monocytes by FICZ was recently shown to be inhibited by pathogen-associated molecular patterns, PAMPs, that include bacterial lipoproteins, lipopolysaccharides, peptidoglycans, and unmethylated CpG dinucleotides, important regulatory components of innate immune responses (Peres et al. Citation2017).
As illustrated in , various types of agents may attenuate the CYP1A1 enzyme and/or suppress the expression of the CYP1A1 gene and thereby slow down the CYP1A1-mediated degradation of FICZ. Many clinical drugs, metals, industrial chemicals, and phytochemicals that activate the AHR have been shown to inhibit CYP1A1 activity or gene expression (Wincent et al. Citation2012). Ke et al. (Citation2001) described suppression of cyp1a1 gene expression in mouse Hepa1c1c7 cells by TNFα and LPS in a manner dependent on activation of the nuclear factor κB (NF-κB) and chromatin remodeling in the promoter region of this gene. In accordance with such a mechanism, we have shown that reactive oxygen species (ROS), UVB, H2O2, curcumin, and sodium arsenite transiently suppress and subsequently induce CYP1A1 expression in FICZ-treated human cells and that the pleiotropic transcription factors NF-κB and Nrf2, reduced glutathione (GSH), and epigenetic modifications are involved in this process (Luecke, Wincent, et al. Citation2010; Wincent et al. Citation2012; Mohammadi-Bardbori et al. Citation2015, Citation2016). Thus, activation of the AHR by agents which bind poorly or not at all might be explained by formation of FICZ and/or attenuated metabolic degradation of FICZ. There is also an influence that comes from enhanced efficiency of CYP1A1 transctiption which occurs subsequent to elevated intracellular levels of antioxidants and chromatin remodeling (Wincent et al. Citation2012; Mohammadi-Bardbori et al. Citation2015, Citation2016).
Figure 2. The FICZ/AHR/CYP1A1 feedback loop can be blocked by various types of inhibitors. Abbreviations: AHR, aryl hydrocarbon receptor; AHRE, AHR response element; ARNT, AHR nuclear translocator; CYP1A1, cytochrome P4501A1; FICZ, 6-formylindolo[3,2-b]carbazole; kcat/Km, catalytic efficiency; Kd, dissociation constant; PAMPs, pathogen associated molecular patterns; ROS, reactive oxygen species.
![Figure 2. The FICZ/AHR/CYP1A1 feedback loop can be blocked by various types of inhibitors. Abbreviations: AHR, aryl hydrocarbon receptor; AHRE, AHR response element; ARNT, AHR nuclear translocator; CYP1A1, cytochrome P4501A1; FICZ, 6-formylindolo[3,2-b]carbazole; kcat/Km, catalytic efficiency; Kd, dissociation constant; PAMPs, pathogen associated molecular patterns; ROS, reactive oxygen species.](/cms/asset/a45fae7e-5d82-4ae8-bc56-a060601698e1/itxc_a_1493086_f0002_c.jpg)
In contrast to the binding of TCDD, which displays species differences, the binding of FICZ to different isoforms of the AHR is evolutionarily well conserved (Jonsson et al. Citation2009; Laub et al. Citation2010; Farmahin et al. Citation2014; Kim et al. Citation2016). Murine AHR containing Val375 (Val381 in the human AHR) is less responsive to TCDD than the variant with Ala at this position but both exhibit similar sensitivity to FICZ (Cho et al. Citation2015; Seok et al. Citation2018; Smith et al. Citation2018) and FICZ accelerates wound healing similarly in C57BL/6 (Ala375) and DBA/2 (Val375) mice (Morino-Koga et al. Citation2017). Work with recently developed crystal AHR structures (Schulte et al. Citation2017; Seok et al. Citation2017) have not yet revealed important FICZ-interacting residues but in studies using homology models of the AHR PAS domain several residues in the binding pocket that are critical for strong binding of FICZ have been revealed. The somewhat similar binding of TCDD and FICZ involves hydrogen bonding with residues Ser359 and Gln377 in the PASB region of murine AHR (equivalent to Ser365 and Gln383 of the human AHR) (Bisson et al. Citation2009; Nuti et al. Citation2014; Hirano et al. Citation2015; Miyagi et al. Citation2015).
Reported changes in gene expression in response to FICZ, ITE, and TCDD are very similar (Henry et al. Citation2010; Farmahin et al. Citation2016; Prochazkova et al. Citation2018). Therefore, since they elicit analogous changes in the conformation, nuclear translocation, and binding of the AHR to AHREs, ligands derived from Trp such as FICZ, ICZ, and ITE and possibly many others, most probably activate the same target genes as TCDD.
3. The FICZ/AHR/CYP1A1 feedback loop
As exemplified by the extreme toxicity of TCDD, turning off AHR-mediated signaling when it is no longer required is critical. Already in the 1980s, Nebert and coworkers (Hankinson et al. Citation1985) proposed that a feedback loop involving an endogenous AHR ligand that is also a substrate for CYP1A1 regulates this signaling. Later, some hydrophobic endogenous AHR-ligand which is metabolized by CYP1A1, 1A2, and 1B1 was proposed to be present in AHR-deficient cells (Weiss et al. Citation1996; Chang and Puga Citation1998; Roblin et al. Citation2004; Chiaro et al. Citation2007). The FICZ/AHR/CYP1A1 transcriptional-translational feedback loop () described here provides a mechanism that can explain accumulation of FICZ in the cell when AHR-mediated induction of CYP1 enzymes is blocked and how such accumulation can effectively induce CYP1 enzymes and thereby facilitate transient AHR-mediated responses (Wei et al. Citation1998, Citation2000; Bergander et al. Citation2004; Wincent et al. Citation2009, Citation2012).
Despite the efficient CYP1 enzyme-mediated metabolic clearance of FICZ, described earlier, when 10 ng FICZ (about 0.5 μg kg−1) was applied percutaneously to the back of one ear of female C57BL/6J mice, the levels of Cyp1a1 mRNA in the liver and adipose tissue rose several hundred-fold, peaking 9 h after application (Wincent et al. Citation2012). The different transcriptional responses, transient versus prolonged, observed in the liver, and adipose tissues compared to the ear illustrate the different kinetics of responses to FICZ when the compound is present at low or high concentrations. At higher concentrations, FICZ can, similar to ICZ and CICZ, efficiently inhibit the CYP1A1 enzyme and cause prolonged CYP1A1 transcription (Wei et al. Citation1998; Wincent et al. Citation2009; Smirnova et al. Citation2016). A more sustained expression was seen at the site of application in the ear as a result of the higher tissue concentration of FICZ that could inhibit its clearance.
To date, only FICZ has been demonstrated to be an efficient endogenous substrate for CYP1A1 thereby participating in the proposed CYP1A1-dependent feedback control mechanism (Wincent et al. Citation2009). To the best of our knowledge, no other high-affinity AHR ligands have yet proven to be as excellent CYP1A1 substrate as FICZ. A comparison between ICZ and FICZ showed that in the presence of human CYP1A1 the effects of FICZ disappeared faster than the effects of ICZ indicating a more efficient CYP1A1-dependent clearance of FICZ (Wei et al. Citation1998).
4. Physiological functions mediated by FICZ
4.1. Self-renewal and differentiation of stem/progenitor cells
Presently, there is considerable interest in the role of the AHR in the maintenance and development of mammalian tissues by regulating the expansion and differentiation of stem cells. A large body of evidence indicates that AHR is involved in the growth of stem cells and, in particular, that its activation may eliminate the pluripotency of and attenuate the potential for self-renewal by various types of stem cells. Thus, the AHR regulates the balance between the quiescence and proliferation of intra-thymic progenitor cells (Laiosa et al. Citation2003), hematopoietic stem cells (HSCs) (Singh et al. Citation2009; Boitano et al. Citation2010; Casado et al. Citation2011; Gasiewicz et al. Citation2014; Rentas et al. Citation2016; Unnisa et al. Citation2016), pulmonary stem cells (Morales-Hernandez et al. Citation2017), and neuro-epithelial stem cells (Latchney et al. Citation2011).
The AHR also appears to play multiple roles in connection with normal embryonic development, with reversible repression of this receptor seeming to be essential for the maintenance of embryonic stem cell (ESC) pluripotency as described by Puga and coworkers. In 2010 these investigators showed that sustained activation with TCDD disrupts the temporal expression of genes whose products are involved in diverse pathways underlying differentiation (Wang et al. Citation2010) and they later emphasized the importance of the AHR in coordinating embryonic development (Ko et al. Citation2014, Citation2016; Ko and Puga Citation2017). In agreement with such a role, the AHR is not expressed by pluripotent murine ESCs (Ko et al. Citation2014) and the Cyp1a1 promoter is intensely and constitutively active in a temporal and spatially restricted manner throughout the E7–E14 stages of embryonic development (Campbell et al. Citation2005). Moreover, in murine ESCs treated with 250 nM FICZ, the AHR and ARNT immunoprecipitate together with CHD4, the major component of the pluripotency regulator NuRD, and with the transcription factor SALL4, suggesting that these are components of the same complex (Gialitakis et al. Citation2017).
While the underlying mechanism(s) remains unclear, activation of the AHR by FICZ can apparently either attenuate or intensify the expansion of stem cells. As shown by Rentas et al. (Citation2016), expansion of early progenitor murine HSCs was promoted through down-regulation of AHR signaling by the self-renewal-stimulating RNA binding protein Musashi-2 (MSI2) and 200 nM FICZ reversed this effect. Likewise, expansion of human induced pluripotent stem cells (iPSCs) was enhanced by the AHR inhibitor CH223191 and blocked by FICZ (Leung et al. Citation2018). Administration of 30 µg FICZ kg−1 to 6-week-old female mice, by gavage every second day for 10 days significantly reduced the number of Paneth cells in the small intestine, as well as the depths of the crypts of both the small and large intestines, as well as inhibiting Wnt signaling and lowering the level of active β-catenin (Park et al. Citation2016). In contrast, AHR activation by FICZ drove an unprecedented expansion of hematopoietic cells into cells of the megakaryocyte, and erythroid linages (Smith et al. Citation2013). In addition, ex vivo treatment of rat hepatic progenitor cells with 1–100 nM FICZ led to both sustained AHR activation and triggering of cell proliferation (Harrill et al. Citation2015).
Thus, the FICZ/AHR/CYP1A1 feedback loop, with its extraordinary efficiency in clearing FICZ via enzymes whose expression is regulated by the AHR, may be involved in the regulation of stem cell niches. Interaction of FICZ with SALL proteins and the NuRD complex in ESCs indicates that transient AHR-mediated regulation of stem/progenitor cell homeostasis can be maintained by this loop. Temporary AHR activation by FICZ might potently stimulate the expansion of differentiated cells, with the loop guaranteeing the subsequent silencing of the AHR required for the self-renewal of stem cells.
Various effects of the AHR and FICZ in cancer cells and cancer stem cells (CSCs) have also been described. This receptor is expressed at high levels in a variety of human and rodent tumors and in many human tumor cell lines (reviewed in Wang, Monti, et al. Citation2017). Furthermore, it is generally believed that sustained AHR activation can be pro-oncogenic, dysregulating key physiological processes and thereby influencing tumor initiation, promotion, and progression, as well as metastasis in a cell-type specific manner (Mitchell and Elferink Citation2009; Safe et al. Citation2013; Murray et al. Citation2014; Wang, Monti, et al. Citation2017).
Treatment with 500 nM FICZ elevated the activity of the stem cell-associated factor ALDH1 in both breast cancer cells and oral squamous carcinoma cells, while also enhancing migration of the latter in scratch-wound assays, a phenomenon possibly associated with tumor metastasis (Novikov et al. Citation2016; Stanford, Ramirez-Cardenas, et al. Citation2016; Stanford, Wang, Zhou, et al. Citation2017). Such treatment also up-regulated the expression of the genes encoding the stem cell-associated factors ALDH1 and Oct4, as well as of the Snai1, Twist1, Twist2, and Vim genes, which are associated with increased migration and/or invasion by breast cancer cells (Stanford, Wang, Zhou, et al. Citation2017). In addition, exposure of head and neck squamous carcinoma cell lines to 100 nM FICZ up-regulated expression of the growth factors AREG, EREG, and PDGFA (John et al. Citation2014). Furthermore, in a validated in vitro model of the movement of breast cancer cells into and out of the lymphatic vasculature, 5 µM FICZ stimulated the synthesis of 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) and enhanced tumor breaching of the lymphatic endothelial barrier (Nguyen et al. Citation2016).
However, recent evidence suggests that the AHR can attenuate the proliferation of CSCs by down-regulating the activity of the stem cell-associated factors Oct4 and ALDH1 (Prud'homme et al. Citation2010; Bunaciu and Yen Citation2011). FICZ (100 nM) augmented retinoic acid (RA)-induced cytostatic mechanisms that drive the differentiation of human myeloblastic leukemia HL-60 cells (Bunaciu and Yen Citation2013; Bunaciu et al. Citation2015). Furthermore, in B16F10 murine melanoma cells exposed to 5 nM FICZ the level of Aldh1a1 mRNA was reduced and an inverse correlation between the levels of AHR and ALDH1 was observed (Contador-Troca et al. Citation2015). In addition, Morales-Hernandez et al. (Citation2016) found that 10 nM FICZ stimulated the differentiation of human embryonic teratocarcinoma (NTERA) cells, possibly by inhibiting the expression of the stem cell-associated POU5F1 (Oct4) and NANOG genes.
In yet another study on human cancer cells, AHR ligands were shown to inhibit cell expansion. When Cheng et al. (Citation2015) added 50 nM FICZ or 10 µM of the AHR ligand ITE to human U87 glioblastoma cells, both repressed expression of the POU5F1 gene. In addition, ITE reduced the size of tumors obtained by injecting U87 cells into nude mice but, unfortunately, similar experiments were not performed with FICZ (Cheng et al. Citation2015). FICZ (100 nM) down-regulated cyclin D1, and up-regulated p27 and caused cell cycle and proliferation arrest in colon cancer cells (Yin et al. Citation2016). Moreover, in 2013, Shin and coworkers reported that in mice implanted subcutaneously with murine T-cell lymphoma or B16 melanoma cells, three intraperitoneal (i.p.) injections of 3 µg FICZ (approximately 150 μg kg−1) at two day intervals starting on the day of implantation inhibited tumor growth in a manner dependent on the AHR and natural killer (NK) cells (Shin et al. Citation2013). In another study, 5 nM FICZ raised the level of E-cadherin in murine NMuMG epithelial cells and repressed their migration in a wound healing assay suggesting that FICZ can modulate the epithelial to mesenchymal transition (EMT) (Rico-Leo et al. Citation2013).
In conclusion, investigations in which FICZ has been employed to elucidate mechanisms of cancer cell growth have reported different outcomes. Therefore, endogenously activated AHR can be postulated to play a dual role in tumorigenesis: (1) stimulation of tumor cell migration and invasiveness by up-regulating the expression of genes associated with growth and migration and (2) inhibition of cancer cell proliferation through inhibition of the expression of factors associated with CSC, repression of EMT and epithelial cell migration, and promotion of the control of tumor development by immune cells.
4.2. Differentiation of immune cells and immune responses
The levels of the AHR expressed by cells of the immune system are particularly high and two high-profile publications in 2008 describing an important role for AHR in a murine model of multiple sclerosis have intensified focus on the involvement of this receptor in infection and autoimmunity (Quintana et al. Citation2008; Veldhoen et al. Citation2008). With respect to the adaptive immune system, AHR activation by FICZ was reported in these two studies to enhance pro-inflammatory responses only by stimulating the differentiation of CD4+ T helper 17 cells (Th17) that express the cytokines IL-17, IL-17F, and IL-22 and blocking the formation of regulatory T cells (Tregs) that express the transcriptional regulator forkhead box P3 (Foxp3) (). In addition, oral administration of 1 µg TCDD to each mouse induced Tregs and markedly reduced disease severity (Quintana et al. Citation2008). Quintana et al. (Citation2008) proposed that TCDD diverts T cells toward regulator T cells and FICZ generates effector T cells. Support for differential effects of FICZ and TCDD was provided in several subsequent studies (Mezrich et al. Citation2010; Singh et al. Citation2011; Schulz et al. Citation2012; Singh et al. Citation2016). TCDD also suppressed sensitization of mice to peanuts, at least in part by elevating the percentage of Treg cells, whereas FICZ did not increase this percentage or influence the cytokine production induced by peanut extract (Schulz et al. Citation2012) (). However, both FICZ and TCDD enhanced the severity of collagen-induced arthritis in transgenic, shared epitope (SE)-positive mice and elevated the numbers of cells expressing IL-17 both in popliteal lymph nodes and the spleen, without influencing the abundance of FoxP3 expressing cells (Fu et al. Citation2018) ().
Table 1. Effects by FICZ on disease severity in murine models of human diseases.
With immune cells in vitro, FICZ stimulated the production of IL-22 in human cells (Trifari et al. Citation2009; Ramirez et al. Citation2010; Dorgham et al. Citation2015; Effner et al. Citation2017) and both IL-17 and IL-22 in mouse cells (Rutz et al. Citation2011; Pauly et al. Citation2012; Hayes et al. Citation2014; Schiering et al. Citation2017) and the inability of FICZ to promote the formation of Foxp3-expressing Tregs under the conditions employed was noted in some studies (Vogel et al. Citation2008; Mezrich et al. Citation2010; Singh et al. Citation2011; Pauly et al. Citation2012; Duarte et al. Citation2013). Under conditions that induce Th17 cells, both FICZ and TCDD up-regulated the expression of a microRNA-132/212 cluster that enhances the Th17 differentiation of naïve murine T cells (Nakahama et al. Citation2013). Additionally, in their assessment of the responses of Th17 cells to treatment with FICZ, TCDD, ITE, β-naphthoflavone (βNF) or 3-methylcholanthrene (3MC), Duarte et al. (Citation2013) found no indications of any intrinsic difference in the mode of action of these ligands and, moreover, a high dose of FICZ administered systemically proved to suppress experimental autoimmune encephalomyelitis (EAE) substantially. Thus, chronic activation of the AHR can prevent Th17-mediated inflammation in the central nervous system and the different regulation of Treg and Th17 cell differentiation by FICZ and TCDD proposed by Quintana and coworkers in 2008 was refuted ().
Confirmation that the dose and duration of AHR activation by high-affinity ligands are the primary drivers of T cell differentiation was provided by a recent examination of allogenic responses in mice (Ehrlich et al. Citation2018) ().
Activation of anti-inflammatory responses and tolerogenic cell phenotypes by FICZ, as well as TCDD, is now firmly established and several laboratories have detected induction of Foxp3-expressing Tregs by FICZ (Kimura et al. Citation2008; Abu-Rezq and Millar Citation2013; Mohinta et al. Citation2015; Jurado-Manzano et al. Citation2017; Ye et al. Citation2017; Ehrlich et al. Citation2018). Such induction attenuated disease severity in murine models of diabetes and graft-versus host disease (Abu-Rezq and Millar Citation2013; Ehrlich et al. Citation2018) (). Treatment of murine CD4+ T cells originally expressing the proinflammatory IL-17 with 100 nM FICZ in the presence of TGFβ caused these cells to acquire an anti-inflammatory phenotype (Gagliani et al. Citation2015). Furthermore, when 100 nM FICZ or 100 nM TCDD was added in the presence of IL-27 and TGFβ to murine CD4+ T cells, these cells acquired an anti-inflammatory Tr1 phenotype, with enhanced secretion of the anti-inflammatory IL-10 (Apetoh et al. Citation2010). In addition, expression of IL-10 by naïve human T cells was enhanced by differentiation in the presence of FICZ (Gandhi et al. Citation2010).
Wheeler et al. (Citation2014) observed no influence of FICZ on the immune response of wild-type mice with functional CYP1A1 to influenza virus A. However, when FICZ was administered to CYP1A1-defective mice via micro-osmotic pumps (10 µg FICZ kg−1 h−1), like TCDD, this compound diminished the virus-specific CD8+ T cell response (Wheeler et al. Citation2014). In other mice infected with the influenza A virus, ten daily i.p. administrations of 100 μg FICZ kg−1 attenuated virus-specific immunoglobulin M (IgM) levels and elevated the proportion of Treg cells, without reducing CD8+ T cell responses (Boule et al. Citation2018). In other studies, treatment of mice with 2 µg FICZ (about 100 µg kg−1) suppressed immune responses to the influenza A, vesicular stomatitis, and Sendai viruses (Yamada et al. Citation2016), whereas treatment of mice infected with ocular herpes simplex virus intraperitoneally with 1 µg FICZ each (about 50 µg kg−1) once every day for eleven consecutive days had no effect on the occurrence of ocular lesions (Veiga-Parga et al. Citation2011).
There are recent indications that AHR activation by FICZ may help reduce inflammation via activation of the Trp-degrading enzyme indoleamine 2,3-dioxidase (IDO) in the dendritic cells (DCs) present in various epithelial tissues. Vogel et al. (Citation2008) found that both TCDD and FICZ activated IDO in human DCs derived from the monocytic U937 cell line, whereas only TCDD (10 nM), but not FICZ (100 nM) stimulated the formation of Foxp3-expressing Tregs. In several subsequent investigations, FICZ was also shown to elicit phenotypical alterations in DCs similar to those caused by TCDD, including attenuation of the responses of Th17 cells (Bankoti et al. Citation2010; Simones and Shepherd Citation2011; Kado et al. Citation2017; Thatcher et al. Citation2016). Expression of IDO1 was elevated in murine bone marrow DCs (BMDCs) by exposure to 100 nM FICZ (Pauly et al. Citation2012) and in Langerhans cells obtained from hematopoietic stem cells derived from the umbilical cord by treatment with 3.5 µM (1 µg/ml) FICZ (Koch et al. Citation2017).
Exposure of DCs derived from human monocytes to 50 nM or 300 nM FICZ elevated the expression of the IDO enzyme, induced a tolerogenic phenotype by promoting Treg-like cell differentiation and suppressed the activation of naïve T lymphocytes (Jurado-Manzano et al. Citation2017). Such activation of IDO does not appear to be AHR ligand-specific, since TCDD, kynurenine, ITE, and the synthetic compound SU5416 all had this same effect (Simones and Shepherd Citation2011; Mezrich et al. Citation2012; Bessede et al. Citation2014). The absence of IDO-activation by FICZ in certain cases (Ilchmann et al. Citation2012; Mezrich et al. Citation2012) may reflect its rapid CYP1A1-mediated degradation.
Therefore, taking rapid metabolic degradation of FICZ into account, there appears to be no intrinsic difference in the effects of FICZ and TCDD on T cell differentiation and T cell-mediated adaptive immune responses.
A potential role for the AHR and its ligands in maintaining activation of B cells by antigen was indicated by the findings by Villa et al. (Citation2017) that treating anti-IgM- and IL-4-stimulated B cells with 250 nM FICZ potently elevated the expression of the Ccno gene the product of which is involved in controlling the cell cycle and, thereby, probably B cell proliferation.
Mast cells (MCs), which regulate murine and human mucosal innate immunity and allergic and anaphylactic responses by releasing pro-inflammatory mediators (including histamine, mast cell proteases, and pro-inflammatory cytokines) were recently found to express the AHR constitutively (Maaetoft-Udsen et al. Citation2012; Sibilano et al. Citation2012) as well as to be highly responsive to FICZ (Sibilano et al. Citation2012; Zhou et al. Citation2013). Another study did, however, not confirm any role of the AHR in mast cell homeostasis (Pilz et al. Citation2016). In their studies on the degranulation (i.e. release of the granule-associated enzyme β-hexosaminase (Hex)) of mast cells derived from murine bone marrow (BMMCs) in response to a challenge with dinitrophenyl-specific IgE, Sibilano et al. (Citation2012) found that 300 nM FICZ enhanced the levels of cyclic adenosine monophosphate (cAMP) and ROS in the absence of this antigen and promoted degranulation when added together with antigen. However, treatment with 300 nM FICZ both 3 and 16 h prior to the antigen challenge lowered the cAMP/ROS response and impaired degranulation. Thus, depending on the timing, administration of FICZ to mice could either attenuate or enhance the severity of passive systemic anaphylactic responses (Sibilano et al. Citation2012) ().
Importantly, Zhou et al. (Citation2013) described the critical role of AHR signaling in maintaining MC homeostasis. When FICZ was added to BMMCs during sensitization with anti-ovalbumin (OVA) IgE, inverted U-shaped dose–response curves were obtained. FICZ alone was unable to promote release of Hex, but when added together with IgE low (0.1–1 nM), but not higher concentrations (10–100 nM) of this compound increased both degranulation and cytokine responses when challenged with antigen. When sensitized in the presence of 1 nM FICZ, the MCs responded with increased intracellular levels of Ca2+ and ROS and the higher levels of ROS oxidized the tyrosine phosphatase SHP-2 protein, thereby enhancing MC signaling. Likewise, these same investigators showed that in a murine model of IgE-mediated passive cutaneous anaphylaxis, a single intra-dermal administration of FICZ (0.03 or 0.3 µg FICZ kg−1) together with IgE during the sensitization phase aggravated responses to a subsequent OVA challenge (Zhou et al. Citation2013) (). In other cases, IgE-mediated degranulation, and changes in the levels of ROS/Ca2+, leukotriene C4 (LTC4), IL-13, and PKR-like ER kinase (PERK) signaling in BMMCs were all augmented by 1 nM FICZ (Kawasaki et al. Citation2014; Wang, Zhou, et al. Citation2017). Alltogether, these observations indicate that endogenous activation of the AHR plays a key role in the homeostasis of MCs, a role that depends on the extent and timing of activation.
In the case of primary murine, microglial cells co-treatment with 20 nM FICZ influenced pro-inflammatory responses to LPS, suggesting that the AHR could be involved in regulating anti-inflammatory processes in the CNS (Lee et al. Citation2015). Multiple investigations have observed a potential association between environmental AHR ligands and an increased risk for developing ALS. In this context Ash et al. (Citation2017) reported that AHR ligands elevated the amount of aggregated TAR DNA-Binding Protein-43 (TDP-43) in human cell lines, in iPSCs, and even in the murine brain. FICZ (100 nM) enhanced the level of TDP-43 protein in iPSCs derived from a patient with ALS that had differentiated into neurons. Moreover, in human neuroblastoma M17 cells 500 nM FICZ raised TDP-43 levels and stabilized this protein; while in H4 neuroglioma cells this same concentration increased the expression of the TARDBP, SOD1, PON2, and C9ORF72 genes associated with ALS (Ash et al. Citation2017). Accordingly, environmental chemicals that lead to sustained activation of AHR signaling or block metabolic degradation of FICZ might be significant risk factors for ALS.
In conclusion, AHR activation by FICZ can promote the development of Th17 cells, which may lead to tissue inflammation and autoimmunity; but FICZ can also stimulate immunosuppressive activity by expanding the population of regulatory T cells. Similarly, FICZ can stimulate or inhibit cytokine production and the maturation and homeostasis of MCs in vitro, as well as anaphylactic responses in vivo, depending on the dose and timing of exposure.
5. Effects of FICZ on immune barriers
Dysregulation of immune barriers is associated with many common diseases and the AHR is expressed at high levels in cells of these barriers, which are routinely exposed to environmental ligands (Esser et al. Citation2009; Esser and Rannug Citation2015). In response to secretion of cytokine IL-22 by hematopoietic cells, probably in combination with IL-17, the epithelial cells of the gut, lung, and skin produce inflammatory cytokines, chemokines that recruit neutrophils (e.g. CXCL1 and CXCL5), anti-microbial proteins and mucins to maintain homeostasis, fight invading pathogens, and accelerate regeneration of the barrier whenever required (Sonnenberg et al. Citation2011; Stockinger and Omenetti Citation2017). Thus, IL-22 and IL-17 appear to have both pro-inflammatory and protective functions.
In a model is proposed that explains how FICZ through activation of the IL-22–IL-22 receptor (IL-22R) pathway in immune barriers can reduce the severity in animal models of several long-term diseases when administered systemically. Under such conditions, FICZ escapes the CYP1A1-dependent clearance in the non-hematopoietic cells in the epithelial cell lining. This model can also explain how FICZ in the presence of CYP1A1 inhibitors can act pro-inflammatory and provide resistance to pathogenic bacteria.
Figure 3. Activation of the interleukin 22 (IL-22)–IL-22 receptor (IL-22R) pathway by FICZ can regulate immunity, inflammation and tissue homeostasis at immune barriers of, e.g. the skin, lung, and intestine. (1). FICZ formed externally from tryptophan, e.g. by enzyme-catalyzed conversions in microorganisms and by UV-light exposure or taken up from the diet, is efficiently metabolized and cleared in epithelial cells at barrier surfaces after AHR-mediated upregulation of the CYP1A1 enzyme. (2). FICZ formed from tryptophan through host metabolism or added systemically, e.g. via intraperitoneal injection, activates AHR-mediated formation of IL-22 in various immune cells. IL-22 regulates immune responses via ligation of the IL-22R on epithelial cells leading to release of tissue specific cytokines, chemokines and antimicrobial agents. (3). FICZ formed externally may also activate the IL-22–IL-22R pathway if CYP1A1 inhibitors are present that inhibit the clearance of FICZ. For abbreviations, see .
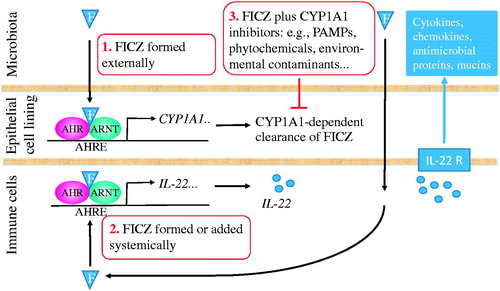
5.1. Gut immunity
The intestinal epithelium constitutes a relatively impermeable physical and immunological barrier against the contents in the lumen, while the intestinal epithelial cells (IECs) take up nutrients and participate in the inflammatory and immune responses when activated by IL-22 and IL-17 (Sonnenberg et al. Citation2011; Conti and Gaffen Citation2015). The general sources of IL-22 and IL-17 in the gut include innate lymphoid cells (ILCs), Th17 and Th22 cells, and various types of intraepithelial lymphocytes (IELs) (e.g. T cell receptor (TCR) γδ cells). One dominant endogenous source of IL-22 appears to be the heterogeneous population of ILCs that includes NK cells and lymphoid tissue-inducer (LTi) cells (Sonnenberg et al. Citation2011).
AHR-deficient mice have strongly reduced numbers of intestinal IELs and ILCs and are therefore unable to mount an IL-22 response, succumbing to the intestinal pathogen Citrobacter rodentium (Kiss et al. Citation2011; Lee et al. Citation2011; Li et al. Citation2011; Qiu et al. Citation2012). Qiu et al. (Citation2012) found that in wild-type mice, FICZ increased the production of IL-22 by the ILCs. Moreover, i.p. injection of 12-day-old mice with 0.5 µg FICZ (about 50 µg kg−1) for 6 days promoted accumulation of ILCs expressing the transcription factor RORγt required for production of IL-22 (Qiu et al. Citation2012). Daily i.p. injections of 100 µg kg−1 FICZ to adult mice dramatically reduced their mortality following infection with the intestinal pathogen Listeria monocytogenes (Kimura et al. Citation2014). Furthermore, in an earlier study on adult mice, 1 µg FICZ (50 µg kg−1) injected i.p. together with heat-killed Mycobacterium tuberculosis markedly increased the subset of IL-17-expressing γδ T cells in the peritoneum, as well as their production of IL-22 (Martin et al. Citation2009). Thus, FICZ appears to enhance gut protection against intestinal pathogens.
Intestinal epithelial cells can be viewed as gatekeepers that regulate the passage of CYP1A1-degradable AHR ligands from the lumen into the blood (). The role of AHR/ARNT in metabolic degradation of AHR agonists by the gut epithelium was first demonstrated in 2007 by Gonzalez and coworkers, who disrupted the Arnt gene in mice predominantly in this epithelium, and found loss of intestinal Cyp1a1 expression, in addition to an endogenous factor that caused systemic expression of Cyp1a1 (Ito et al. Citation2007). This factor only activated the AHR when the mice were fed natural diets containing phytochemicals or synthetic diets supplemented with indole-3-carbinol (I3C), derived from the glucosinolate glucobrassicin.
Recently, Stockinger and coworkers demonstrated that CYP1A1 in the IECs plays a vital role in intestinal immunity by controlling endogenous availability of FICZ (Schiering et al. Citation2017). Employing their mouse strain that expressed Cyp1a1 constitutively, these investigators found that Th17 cells differentiated in vitro metabolized FICZ rapidly, and compared to wild-type Th17 cells, showed a lower production of IL-22 in response to 0.01 nM FICZ. Furthermore, overexpression of Cyp1a1 specifically by IECs showed that these cells performed an important CYP1A1-mediated feed-back function involved in regulating intestinal immunity. Actually, the metabolic clearance of natural AHR ligands in mice that overexpress Cyp1a1 in the gut epithelium led to a pseudo AHR-deficient state. Upon infection with Citrobacter rodentium, these animals exhibited markedly reduced numbers of group 3 ILCs and Th17 cells and succumbed rapidly, with augmented intestinal pathology. Conversely, mice deficient in CYP1A1, CYP1A2, and CYP1B1 (CYP1 triple-KO mice), as well as wild-type mice fed diets free of phytochemicals, but supplemented with I3C demonstrated enhanced IL-22 production, as well as less histopathology upon bacterial infection. Likewise, a follow-up in vitro study with Th17 cells derived from CYP1 triple-KO mice or wild-type murine Th17 cells exposed to the polycyclic aromatic hydrocarbons (PAHs) fluoranthene, pyrene, or phenanthrene that competitively inhibit CYP1A1 (Shimada and Guengerich Citation2006) led to the conclusion that IL-22 production by Th17 cells can be enhanced profoundly by inhibiting CYP1 activity (Schiering et al. Citation2018). Both of these investigations indicate strongly that inhibitors of CYP1A1 activity can promote AHR-mediated protection against pathogens by elevating the endogenous level of FICZ (Schiering et al. Citation2017, Citation2018).
In addition, AHR acted as a negative regulator of the inflammasome, another component of host defenses against microbial pathogens, by inhibiting induction of the pattern recognition receptor NLRP3 by LPS (Huai et al. Citation2014). FICZ (100 nM) also suppressed recruitment of inflammatory cells and consequently peritonitis upon exposure to alum ().
The composition of the murine commensal microbiota influences susceptibility to gastrointestinal infections and induced colitis (Ivanov et al. Citation2008; Gao et al. Citation2018). Recently, specific components of this microbiota were found to regulate gut immunity by promoting production of AHR ligands from Trp and thereby transcription of genes encoding anti-inflammatory cytokines, resulting in protection against intestinal damage induced by dextran sulfate sodium (DSS) (Zelante et al. Citation2013; Lamas et al. Citation2016; Hou et al. Citation2018). Lamas et al. (Citation2016) showed that mice with dysbiotic microbiota due to their lack of the caspase recruitment domain 9 (CARD9) produced lower levels of endogenous AHR agonists and recovered more poorly from DSS-induced colitis (). When 1 μg FICZ (50 µg kg−1) was injected i.p. one day after DSS administration, the severity of colitis in these animals was reduced significantly. Moreover, the effects of defects in the colonic expression of IL-22 and in genes coding for antimicrobial proteins in Card9 KO mice could be reversed by FICZ (Lamas et al. Citation2016).
Following the first report by Monteleone et al. (Citation2011) that FICZ can alleviate DSS-induced colitis in a murine model of human inflammatory bowel disease (IBD), several studies involving single or repeated i.p. injections of 1 µg FICZ per mouse (50 µg kg−1) have confirmed that this ligand can trigger regulatory signals in the gut that promote production of IL-22 and suppress colitis (Ji et al. Citation2015; Monteleone, Marafini, et al. Citation2016; Monteleone, Zorzi, et al. Citation2016; Chen et al. Citation2017; Wang et al. Citation2018; Yu et al. Citation2018). Moreover, upon exposure to 2–200 nM FICZ, a dose-dependent increase in IL-22 with a reduction in the level of interferon-γ (IFNγ) occurred in lamina propria mononuclear cells from both patients with Crohn’s disease and healthy individuals (Monteleone et al. Citation2011). As mentioned earlier, IL-22 is important for the regeneration of intestinal stem cells and, consequently, in several cases FICZ restored the crypt architecture and lengthened the colon of mice treated with DSS.
In addition to IL-22, the anti-inflammatory cytokine IL-10 plays a key role in regulating autoimmune diseases and FICZ doubled the number of Tr1 cells producing this cytokine, and augmented its secretion (Apetoh et al. Citation2010). In addition, FICZ stimulated the homing of FoxP3-expressing Tregs in the large intestine of mice (Ye et al. Citation2017), and up-regulated epithelial expression of the IL-10 receptor (IL-10R) (Lanis et al. Citation2017), which helps protect the epithelial integrity. Thus, in addition to stimulating formation of IL-22, FICZ might also suppress colitis by regulating the expression of the IL-10R.
Altogether, these data reveal that control of FICZ metabolism in epithelial cells by the FICZ/AHR/CYP1A1 feedback loop can explain the effects of this compound on gut immunity.
5.2. Pulmonary and eye immunity
Experiments by Thatcher et al. (Citation2016) indicated that endogenous AHR ligands might be involved in the physiological regulation of Th2-mediated immunity in the lung via a dendritic cell-dependent mechanism. They found that after murine pulmonary dendritic cells were incubated overnight with LPS and OVA, addition of 100 or 200 nM FICZ markedly inhibited the proliferation of T cells (Thatcher et al. Citation2016). FICZ was also suggested to exert anti-asthmatic effects on the basis of the observation that when injected i.p. to a murine model of allergic asthma during sensitization to OVA, 3 or 30 μg of FICZ kg−1 reduced pulmonary eosinophilia and expression of Th2 cytokines (Jeong et al. Citation2012) (). In another examination of allergic asthma in mice, 30 nM FICZ activated Jagged 1 (Jag1), a component of the pro-inflammatory Notch pathway, in BMDCs (Xia et al. Citation2015).
In thyroid eye disease (TED) mast cells, together with infiltrating T cells, cause chronic orbital inflammation and promote production of myofibroblasts. In orbital fibroblasts from patients with TED, several of the detrimental effects, such as remodelning of orbital fibroblasts (including TGFβ-induced proliferation), production of collagen, contraction of myofibroblasts, and formation of α-smooth muscle actin (α-SMA) filament were blocked by 100 nM–10 µM FICZ. FICZ (100 nM) prevented TGFβ-induced profibrotic Wnt signaling by inhibiting phosphorylation of glycogen synthase kinase 3β (GSK-3β), destabilizing β-catenin and blocking transcription of genes involved in myofibroblast production (Woeller et al. Citation2016).
5.3. Skin immunity
In addition to being formed from Trp upon exposure of human keratinocytes to light and oxidation by H2O2 in the skin of patients with vitiligo Schallreuter et al. Citation2012, FICZ can be synthesized by the microbial flora on the skin (Magiatis et al. Citation2013). Malassezia yeasts, a normal component of this flora, interact with melanocytes, fibroblasts, keratinocytes, and dendritic cells to modulate the immune responses against them (Ashbee Citation2006). In extracts of skin scale from patients with Malassezia-associated seborrheic dermatitis, the capacity to activate AHR was 10- to 1000-fold higher than in healthy controls, a difference ascribed to FICZ and other highly potent AHR agonists present in these extracts (Magiatis et al. Citation2013).
The key roles played by dynamic AHR signaling in skin immunity have been emphasized by Stockinger and coworkers (Di Meglio et al. Citation2014). They found that the AHR, primarily in keratinocytes, prevented excessive skin inflammation by controlling the expression of certain chemokine ligands involved in neutrophil attraction (Csf2, Csf3, Cxcl1, Cxcl5) and of antimicrobial peptides (S100a7a, S100a8) in response to inflammatory stimuli. When AHR signaling in full-thickness biopsies from the lesional skin of patients with psoriasis was activated by 250 nM FICZ, 29 genes belonging to the so-called psoriasis transcriptome were down-regulated. Furthermore, activation of the AHR pathway in a murine model of psoriasis by i.p. injection of 100 µg FICZ kg−1 for 6 days over the course of treatment with imiquimod (IMQ) ameliorated skin pathology () and lowered the expression of several mediators of inflammation, including Csf2, Csf3, Cxcl5, S100a7a, and S100a8, as well as IL-17 and IL-22 (Di Meglio et al. Citation2014). A later murine study confirmed that FICZ decreased IL-17 expression and lessened the severity of psoriasis (Smith, Jayawickreme, et al. Citation2017) (). Moreover, low concentrations of FICZ attenuated the expression of the chemokine ligand 5 (CCL5) by HaCaT cells (Morino-Koga et al. Citation2013) and augmented the expression of filaggrin, an epithelial barrier protein, by normal human keratinocytes in vitro (Tsuji et al. Citation2017; Kiyomatsu-Oda et al. Citation2018). However, in primary mouse keratinocytes treated simultaneously with IL-1β and 100 nM FICZ, expression of the Cxcl5 chemokine was enhanced (Smith, Boyer, et al. Citation2017).
Similarly, FICZ and AHR may be involved in the etiology of cutaneous systemic lupus erythematosus (SLE) (Dorgham et al. Citation2015) and atopic dermatitis (AD) (Kiyomatsu-Oda et al. Citation2018). In a study on chronic eczema induced by mites, application of 2.5 μg FICZ kg−1 reduced the AD-like inflammation of the skin and transepidermal water loss, and as in the model of psoriasis involving induction by IMQ, the expression of IL-22 was elevated (Kiyomatsu-Oda et al. Citation2018) ().
More evidence for an important physiological role for FICZ and AHR-dependent expression of IL-22 in the skin has been obtained by Cibrian et al. (Citation2016), who noted that uptake of Trp and intracellular accumulation of FICZ in skin γδ T cells is regulated by the activation marker CD69 in combination with the aromatic-amino-acid-transporter complex LAT1-CD98. These results revealed the importance of Trp uptake for AHR-dependent secretion of IL-22 by γδ T cells during the development of psoriasis (Cibrian et al. Citation2016).
6. The toxicity of FICZ
The median lethal concentrations (LC50) of FICZ, in the case of fibroblast-like monkey kidney COS-7 cells and primary chicken hepatocytes, were estimated to be 9.4 and 14 µM, respectively (Farmahin et al. Citation2014, Citation2016).
Treatment with 0.1–1 µM FICZ reduced the proliferation of several human tumor cell lines (Yin et al. Citation2016; Mohammadi-Bardbori et al. Citation2017). Release of Ca2+, depletion of GSH, an increase in the level of intracellular ROS, impairment of mitochondrial membrane integrity, stimulation of caspase 3 activity, and release of cytochrome c all occurred in HepG2 cells and mouse hepatoma (Hepa-1) cells exposed to 1 µM FICZ for 24 h (Mohammadi-Bardbori et al. Citation2017). Increase in ROS and elevated apoptosis signaling were also obtained when 1 nM FICZ was added together with 50 nM of 3′-methoxy-4′-nitroflavone (MNF), which inhibits CYP1A1. Interestingly, Mohammadi-Bardbori and coworkers also showed that the growth of HepG2 and Hepa-1 cells was stimulated by 0.01 nM FICZ, the level ordinarily present in cell culture medium (Oberg et al. Citation2005; Wincent et al. Citation2012). Exposure of BMMCs to as little as 1 nM FICZ led to accumulation of Ca2+ in the cytosol and higher levels of intracellular ROS that peaked 2 h after treatment, along with a decline in intracellular GSH (Wang, Zhou, et al. Citation2017). In addition, there was a rapid but transient decrease in mitochondrial ATP production and reduction of the mitochondrial membrane potential, as well as up-regulation of Nrf2, a strong inducer of antioxidant genes. Altogether, these thought-provoking findings indicate that high levels of FICZ can exert ROS-dependent toxicity, whereas under certain conditions low doses of FICZ can transiently elevate local levels of second messengers such as ROS/Ca2+, thereby promoting cellular adaptation, survival, and proliferation.
Recently, FICZ has proven to be potently embryotoxic toward fish and birds. FICZ is a strong agonist of fish AHR2, inducing CYP1A and CYP1B1 in zebrafish embryos approximately fivefold more potently than the polybrominated biphenyl PCB126 (Jonsson et al. Citation2009). Wincent et al. (Citation2016) demonstrated that FICZ dramatically increased mortality and the incidence and severity of pericardial edema and circulatory failure, while reducing hatching and blocking inflation of the swim bladder in zebrafish embryos. Importantly, these toxic manifestations were only seen when CYP1A was inhibited by morpholino antisense oligonucleotides or α-naphthoflavone (αNF), this latter compound augmenting the mortality caused by FICZ even at concentrations as low as 5 pM (Wincent et al. Citation2016). Proper development of zebrafish embryos appears to require crosstalk between the AHR and the Wnt/β-catenin pathway (Yin et al. Citation2011; Yoshioka et al. Citation2011) and this latter pathway was a key target for FICZ during early zebrafish development (Wincent et al. Citation2015).
FICZ was recently also tested for embryo toxicity in chicken and Japanese quail embryos (Jonsson et al. Citation2016). Injection of FICZ into the air sac of chicken embryos was found to cause dose-dependent mortality with LD50 values below 20 μg kg−1. Notably, when 4 μg FICZ kg−1 was injected into the yolk of a chicken egg together with the antifungal drug ketoconazole, which inhibits metabolism of FICZ by chicken CYP1A4- and CYP1A5, its toxic potency was similar to that of PCB126 (Jonsson et al. Citation2016).
Routine rodent toxicity studies with high doses of FICZ have yet to be performed, but certain reports involving exposure of adult mice to relatively high concentrations of FICZ have appeared with no mention of signs of pathology (Schulz et al. Citation2012; Wheeler et al. Citation2014).
One conclusion based on the relevant in vivo studies to date is that FICZ (unless its metabolic clearance is compromised) is not toxic toward adult vertebrates, since the rapid turnover of FICZ, regulated by the FICZ/AHR/CYP1A1 feedback loop, efficiently controls endogenous AHR functions. It is, however, apparent that FICZ can disrupt the embryonic development of fish and birds.
7. Other effects of FICZ
7.1. FICZ acts as a powerful sensitizer to blue light, which may result in photo-aging of the skin and the retinal epithelium
FICZ has been found to be an endogenous chromophore that acts as a photosensitizer, by potentiating UVA-induced oxidative stress even at nanomolar concentrations. Park et al. (Citation2015) concluded that the photodynamic potency of FICZ exceeds that of the well-known endogenous photosensitizers riboflavin (vitamin B2) and protoporphyrin IX and described oxidative damage (including 8-oxo-dG the major DNA lesion induced by ROS) caused by combinations of FICZ and UVA in HaCaT human keratinocytes. Phototoxicity was also seen in primary human epidermal keratinocytes, organotypic epidermal skin reconstructs, and mouse skin. Repair of 8-oxo-dG by base excision repair (BER) was not affected, while the more versatile repair system, nucleotide excision repair (NER) was impaired (Brem et al. Citation2017). Thus, if not rapidly degraded, FICZ could cause dermal photocarcinogenesis, as well as photo-aging. Indeed, 100 nM FICZ inhibited TGFβ-induced up-regulation of collagen I synthesis (Murai et al. Citation2018). In addition, the AHR protects against blue light-induced degeneration of retinal pigmented epithelial cells (Kim et al. Citation2014).
The AHR regulates the stress response to UVB-light in human keratinocytes and in wild-type mice (Fritsche et al. Citation2007), as well as melanogenesis in human melanocytes and mouse skin (Luecke, Backlund, et al. Citation2010; Jux et al. Citation2011). Treatment of primary human melanocytes with 100 nM FICZ doubled the activity of the melanin-producing enzyme tyrosinase, an effect weaker and more transient than that of 10 nM TCDD (Luecke, Backlund, et al. Citation2010).
7.2. FICZ stimulates intrachromosomal exchange and retrotransposition
Concentration-dependent increases in sister chromatid conversion and gene conversion in the RS112 strain of Saccharomyces cerevisiae occurred upon exposure to 7.5–15 μM FICZ, with analogous increases in sister chromatid exchanges (SCE) in Chinese hamster ovary cells treated with 0.12–1.2 μM FICZ (Rannug, Agurell, et al. 1992). Since neither type of cells express AHR or CYP1A1 the effects are not AHR-dependent or generated by CYP-metabolites of FICZ. However, to suggest a plausible mechanism more data are needed.
Transposable elements (TEs), including LINEs and SINEs are estimated to comprise 45% of the human genome (Hancks and Kazazian Citation2012). Picomolar levels of FICZ induced retrotransposition (RTP) of long interspersed nucleotide element-1 (LINE-1 or L1) in human hepatocellular carcinoma HuH-7 cells, in a manner dependent on the activation of mitogen-activated protein kinase (MAPK) and ARNT, but not the AHR (Okudaira et al. Citation2010). In addition, FICZ (10 nM) regulated the transcription of Alu retrotransposons located in the upstream promoter regions of the pluripotency genes NANOG and OCT4 (Morales-Hernández et al. Citation2016). When administered by injection into mouse testes, 4 μg FICZ kg−1 also repressed the germ-line specific genes MVH, Mili, and Miwi, effects mediated by the AHR and proposed to control the expression of small non-coding TEs involved in the maturation of germ cells (Rico-Leo et al. Citation2016).
7.3. FICZ activates nuclear hormone receptors
In addition to being a high affinity ligand for the AHR, FICZ activates receptors belonging to the superfamily of nuclear hormone receptors (NRs). In human HepG2 cells carrying expression vectors for full-length receptors, low micromolar amounts of FICZ activated most of the vertebrate vitamin D receptors (VDRs) studied, the invertebrate pregnane X receptor (Ciona VDR/PXR), and invertebrate and vertebrate liver X receptors (LXRs) (including the human LXRα and LXRβ) (Reschly et al. Citation2007, Citation2008; Ekins et al. Citation2008). Utilizing a reporter system that allows quantitative assessment of the activities of multiple transcription factors in HepG2 cells, 1 µM FICZ was also shown to activate vertebrate peroxisome proliferator-activated receptors (PPARs) (Romanov et al. Citation2008).
7.4. FICZ influences behavior
The expression of cyp1a1 exhibits circadian rhythmicity, with an acrophase that peaks several hours after the peak AHR expression under light–dark (LD) conditions (Huang et al. Citation2002), as well as in continuous darkness (DD) (Carmona-Antonanzas et al. Citation2017). TCDD and FICZ altered the expression of the clock genes Per1, Cry1, Cry2, and Bmal1 and attenuated phase shifts in response to light (Garrett and Gasiewicz Citation2006; Mukai and Tischkau Citation2007; Mukai et al. Citation2008; Xu et al. Citation2010).
Similar to the avoidance of novel items of food by rats exposed to TCDD (Lensu et al. Citation2011), Sprague-Dawley rats exposed to 100 μg FICZ kg−1 by gavage avoided chocolate almost totally for only a few hours, although this avoidance was still clearly present two weeks later (Mahiout and Pohjanvirta Citation2016).
8. Conclusions and future perspective
Collectively, the findings reviewed here reveal that at high concentrations, various AHR ligands behave similarly, i.e. sustained AHR activation by high doses of the endogenous ligand FICZ resembles that caused by toxic ligands such as TCDD. However, at pico- or nanomolar concentrations, FICZ is different, because of its high affinity for the AHR in combination with its being an almost perfect substrate for CYP1A1, thus creating a FICZ/AHR/CYP1A1 negative feedback loop that makes FICZ an ideal regulator of transient and dynamic cellular processes. It is mandatory that this loop be regulated, because both endogenous and exogenous compounds that inhibit CYP1A1 might otherwise lead to accumulation of toxic levels of FICZ. Combustion products in the air we breathe, a major threat to human health, include the PAHs fluoranthene, pyrene, and phenanthrene that can cause such inhibition and thereby most likely dysregulate the growth and differentiation of embryonic, immune, and cancer cells.
The investigations described here also highlight the impact of FICZ on pathological processes, which motivates its further assessment as a potential therapeutic agent. In particular, the realization that FICZ can regulate immune functions suggests that this compound might be used in treating, e.g. IBD, MS, psoriasis, diabetes, allergies, and cancer. FICZ might also be valuable in connection with photodynamic therapies, wound healing, and regenerative therapies, due to its ability to expand populations of stem cells. At the same time, the observations that FICZ can also aggravate diseases such as MS, ALS, and rheumatoid arthritis in the animal models of these diseases underscores the importance of appropriate dose regimens.
Acknowledgements
The authors gratefully acknowledge the review comments provided by three external reviewers selected by the Editor and anonymous to the authors. These comments were very helpful in strengthening the manuscript and clarifying key points.
Declaration of interest
The employment affiliations of the authors are shown on the cover page. The authors report no conflicts of interest. The preparation of the manuscript was financially supported by departmental grants from The Department of Molecular Biosciences, The Wenner-Gren Institute, Stockholm University and Institute of Environmental Medicine, Karolinska Institutet. The manuscript has not been sent to any other journal. It has not reviewed by others. The authors have not appeared in any regulatory or legal proceedings in the last 5 years that draw on the contents of the paper. The paper and its conclusions are the exclusive professional work product of the authors.
References
- Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JJ. 1999. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol. 155:62–70.
- Abu-Rezq HA, Millar DG. 2013. Streptozotocin-induced diabetes depends on the balance of Th17/Treg responses and is modulated by ligands of the aryl-hydrocarbon receptor. Int J Immunol Res. 3:40–49.
- Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA III, Kato T, Saeki K, Matsuda T. 2001. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 276:31475–31478.
- Afaq F, Zaid MA, Pelle E, Khan N, Syed DN, Matsui MS, Maes D, Mukhtar H. 2009. Aryl hydrocarbon receptor is an ozone sensor in human skin. J Invest Dermatol. 129:2396–2403.
- Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. 2010. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 11:854–861.
- Ash PEA, Stanford EA, Al Abdulatif A, Ramirez-Cardenas A, Ballance HI, Boudeau S, Jeh A, Murithi JM, Tripodis Y, Murphy GJ, et al. 2017. Dioxins and related environmental contaminants increase TDP-43 levels. Mol Neurodegener. 12:35.
- Ashbee HR. 2006. Recent developments in the immunology and biology of Malassezia species. FEMS Immunol Med Microbiol. 47:14–23.
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y. 2005. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 25:10040–10051.
- Baba T, Shima Y, Owaki A, Mimura J, Oshima M, Fujii-Kuriyama Y, Morohashi KI. 2008. Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex Dev. 2:1–11.
- Bankoti J, Rase B, Simones T, Shepherd DM. 2010. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 246:18–28.
- Baron JM, Zwadlo-Klarwasser G, Jugert F, Hamann W, Rubben A, Mukhtar H, Merk HF. 1998. Cytochrome P450 1B1: a major P450 isoenzyme in human blood monocytes and macrophage subsets. Biochem Pharmacol. 56:1105–1110.
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 18:207–250.
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. 2000. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 56:382–388.
- Bennett JA, Singh KP, Unnisa Z, Welle SL, Gasiewicz TA. 2015. Deficiency in aryl hydrocarbon receptor (AHR) expression throughout aging alters gene expression profiles in murine long-term hematopoietic stem cells. PLoS One. 10:e0133791.
- Bergander L, Wahlstrom N, Alsberg T, Bergman J, Rannug A, Rannug U. 2003. Characterization of in vitro metabolites of the aryl hydrocarbon receptor ligand 6-formylindolo[3,2-b]carbazole by liquid chromatography-mass spectrometry and NMR. Drug Metab Dispos. 31:233–241.
- Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. 2004. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 149:151–164.
- Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C, et al. 2014. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 511:184–190.
- Bisson WH, Koch DC, O'Donnell EF, Khalil SM, Kerkvliet NI, Tanguay RL, Abagyan R, Kolluri SK. 2009. Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J Med Chem. 52:5635–5641.
- Bittinger MA, Nguyen LP, Bradfield CA. 2003. Aspartate aminotransferase generates proagonists of the aryl hydrocarbon receptor. Mol Pharmacol. 64:550–556.
- Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. 1991. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA. 88:9543–9547.
- Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, et al. 2010. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 329:1345–1348.
- Boule LA, Burke CG, Jin GB, Lawrence BP. 2018. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep. 8:1826.
- Brem R, Macpherson P, Guven M, Karran P. 2017. Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Sci Rep. 7:4310.
- Bunaciu RP, Jensen HA, MacDonald RJ, LaTocha DH, Varner JD, Yen A. 2015. 6-Formylindolo(3,2-b)carbazole (FICZ) modulates the signalsome responsible for RA-induced differentiation of HL-60 myeloblastic leukemia cells. PLoS One. 10:e0135668.
- Bunaciu RP, Yen A. 2013. 6-Formylindolo (3,2-b)carbazole (FICZ) enhances retinoic acid (RA)-induced differentiation of HL-60 myeloblastic leukemia cells. Mol Cancer. 12:39.
- Bunaciu RP, Yen A. 2011. Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 71:2371–2380.
- Campbell SJ, Henderson CJ, Anthony DC, Davidson D, Clark AJ, Wolf CR. 2005. The murine Cyp1a1 gene is expressed in a restricted spatial and temporal pattern during embryonic development. J Biol Chem. 280:5828–5835.
- Carmona-Antonanzas G, Santi M, Migaud H, Vera LM. 2017. Light- and clock-control of genes involved in detoxification. Chronobiol Int. 34:1026–1041.
- Casado FL, Singh KP, Gasiewicz TA. 2011. Aryl hydrocarbon receptor activation in hematopoietic stem/progenitor cells alters cell function and pathway-specific gene modulation reflecting changes in cellular trafficking and migration. Mol Pharmacol. 80:673–682.
- Chang CY, Puga A. 1998. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 18:525–535.
- Chang X, Fan Y, Karyala S, Schwemberger S, Tomlinson CR, Sartor MA, Puga A. 2007. Ligand-independent regulation of transforming growth factor beta1 expression and cell cycle progression by the aryl hydrocarbon receptor. Mol Cell Biol. 27:6127–6139.
- Chen W, Pu A, Sheng B, Zhang Z, Li L, Liu Z, Wang Q, Li X, Ma Y, Yu M, et al. 2017. Aryl hydrocarbon receptor activation modulates CD8alphaalpha + TCRalphabeta + IELs and suppression of colitis manifestations in mice. Biomed Pharmacother. 87:127–134.
- Cheng J, Li W, Kang B, Zhou Y, Song J, Dan S, Yang Y, Zhang X, Li J, Yin S, et al. 2015. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 6:7209.
- Chiaro CR, Patel RD, Marcus CB, Perdew GH. 2007. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 72:1369–1379.
- Cho SW, Suzuki K, Miura Y, Miyazaki T, Nose M, Iwata H, Kim EY. 2015. Novel role of hnRNP-A2/B1 in modulating aryl hydrocarbon receptor ligand sensitivity. Arch Toxicol. 89:2027–2038.
- Chung KT, Gadupudi GS. 2011. Possible roles of excess tryptophan metabolites in cancer. Environ Mol Mutagen. 52:81–104.
- Cibrian D, Saiz ML, de la Fuente H, Sanchez-Diaz R, Moreno-Gonzalo O, Jorge I, Ferrarini A, Vazquez J, Punzon C, Fresno M, et al. 2016. CD69 controls the uptake of l-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat Immunol. 17:985–996.
- Contador-Troca M, Alvarez-Barrientos A, Merino JM, Morales-Hernandez A, Rodriguez MI, Rey-Barroso J, Barrasa E, Cerezo-Guisado MI, Catalina-Fernandez I, Saenz-Santamaria J, et al. 2015. Dioxin receptor regulates aldehyde dehydrogenase to block melanoma tumorigenesis and metastasis. Mol Cancer. 14:148.
- Conti HR, Gaffen SL. 2015. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 195:780–788.
- Crawford RB, Holsapple MP, Kaminski NE. 1997. Leukocyte activation induces aryl hydrocarbon receptor up-regulation, DNA binding, and increased Cyp1a1 expression in the absence of exogenous ligand. Mol Pharmacol. 52:921–927.
- Dere E, Forgacs AL, Zacharewski TR, Burgoon LD. 2011. Genome-wide computational analysis of dioxin response element location and distribution in the human, mouse, and rat genomes. Chem Res Toxicol. 24:494–504.
- Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, Tosi I, Hirota K, Nestle FO, Mrowietz U, et al. 2014. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 40:989–1001.
- Dorgham K, Amoura Z, Parizot C, Arnaud L, Frances C, Pionneau C, Devilliers H, Pinto S, Zoorob R, Miyara M, et al. 2015. Ultraviolet light converts propranolol, a nonselective β-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur J Immunol. 45:3174–3187.
- Drozdzik A, Dziedziejko V, Kurzawski M. 2014. IL-1 and TNF-alpha regulation of aryl hydrocarbon receptor (AhR) expression in HSY human salivary cells. Arch Oral Biol. 59:434–439.
- Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. 2013. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One. 8:e79819.
- Effner R, Hiller J, Eyerich S, Traidl-Hoffmann C, Brockow K, Triggiani M, Behrendt H, Schmidt-Weber CB, Buters JT. 2017. Cytochrome P450s in human immune cells regulate IL-22 and c-Kit via an AHR feedback loop. Sci Rep. 7:44005.
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI. 2018. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol Sci. 161:310–320.
- Ekins S, Reschly EJ, Hagey LR, Krasowski MD. 2008. Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol Biol. 8:103.
- Esser C, Rannug A. 2015. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 67:259–279.
- Esser C, Rannug A, Stockinger B. 2009. The aryl hydrocarbon receptor in immunity. Trends Immunol. 30:447–454.
- Farmahin R, Crump D, Kennedy SW. 2014. Sensitivity of avian species to the aryl hydrocarbon receptor ligand 6-formylindolo [3,2-b] carbazole (FICZ). Chem Biol Interact. 221:61–69.
- Farmahin R, Crump D, O'Brien JM, Jones SP, Kennedy SW. 2016. Time-dependent transcriptomic and biochemical responses of 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are explained by AHR activation time. Biochem Pharmacol. 115:134–143.
- Faust D, Vondráček J, Krčmář P, Smerdová L, Procházková J, Hrubá E, Hulinková P, Kaina B, Dietrich C, Machala M. 2013. AhR-mediated changes in global gene expression in rat liver progenitor cells. Arch Toxicol. 87:681–698.
- Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. 1997. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 34:605–614.
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, et al. 2007. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 104:8851–8856.
- Fu J, Nogueira SV, Drongelen VV, Coit P, Ling S, Rosloniec EF, Sawalha AH, Holoshitz J. 2018. Shared epitope-aryl hydrocarbon receptor crosstalk underlies the mechanism of gene-environment interaction in autoimmune arthritis. Proc Natl Acad Sci USA. 115:4755–4760.
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. 2015. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 523:221–225.
- Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. 2010. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 11:846–853.
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. 2018. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 8:13.
- Garrett RW, Gasiewicz TA. 2006. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 69:2076–2083.
- Gasiewicz TA, Singh KP, Bennett JA. 2014. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann NY Acad Sci. 1310:44–50.
- Gerbal-Chaloin S, Iankova I, Maurel P, Daujat-Chavanieu M. 2013. Nuclear receptors in the cross-talk of drug metabolism and inflammation. Drug Metab Rev. 45:122–144.
- Gialitakis M, Tolaini M, Li Y, Pardo M, Yu L, Toribio A, Choudhary JS, Niakan K, Papayannopoulos V, Stockinger B. 2017. Activation of the aryl hydrocarbon receptor interferes with early embryonic development. Stem Cell Rep. 9:1377–1386.
- Girer NG, Murray IA, Omiecinski CJ, Perdew GH. 2016. Hepatic aryl hydrocarbon receptor attenuates fibroblast growth factor 21 expression. J Biol Chem. 291:15378–15387.
- Goerz G, Barnstorf W, Winnekendonk G, Bolsen K, Fritsch C, Kalka K, Tsambaos D. 1996. Influence of UVA and UVB irradiation on hepatic and cutaneous P450 isoenzymes. Arch Dermatol Res. 289:46–51.
- Goerz G, Merk H, Bolsen K, Tsambaos D, Berger H. 1983. Influence of chronic UV-light exposure on hepatic and cutaneous monooxygenases. Experientia. 39:385–386.
- Hahn ME, Karchner SI, Merson RR. 2017. Diversity as opportunity: insights from 600 million years of AHR evolution. Curr Opin Toxicol. 2:58–71.
- Hahn ME, Karchner SI, Shapiro MA, Perera SA. 1997. Molecular evolution of two vertebrate aryl hydrocarbon (dioxin) receptors (AHR1 and AHR2) and the PAS family. Proc Natl Acad Sci USA. 94:13743–13748.
- Hancks DC, Kazazian HH Jr. 2012. Active human retrotransposons: variation and disease. Curr Opin Genet Dev. 22:191–203.
- Hankinson O, Andersen RD, Birren BW, Sander F, Negishi M, Nebert DW. 1985. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 260:1790–1795.
- Harrill JA, Parks BB, Wauthier E, Rowlands JC, Reid LM, Thomas RS. 2015. Lineage-dependent effects of aryl hydrocarbon receptor agonists contribute to liver tumorigenesis. Hepatology. 61:548–560.
- Hayes MD, Ovcinnikovs V, Smith AG, Kimber I, Dearman RJ. 2014. The aryl hydrocarbon receptor: differential contribution to T helper 17 and T cytotoxic 17 cell development. PLoS One. 9:e106955.
- Henry EC, Welle SL, Gasiewicz TA. 2010. TCDD and a putative endogenous AhR ligand, ITE, elicit the same immediate changes in gene expression in mouse lung fibroblasts. Toxicol Sci. 114:90–100.
- Hirano M, Hwang JH, Park HJ, Bak SM, Iwata H, Kim EY. 2015. In silico analysis of the interaction of avian aryl hydrocarbon receptors and dioxins to decipher isoform-, ligand-, and species-specific activations. Environ Sci Technol. 49:3795–3804.
- Hou Q, Ye L, Liu H, Huang L, Yang Q, Turner JR, Yu Q. 2018. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. https://doi.org/10.1038/s41418-018-0070-2
- Hu P, Herrmann R, Bednar A, Saloupis P, Dwyer MA, Yang P, Qi X, Thomas RS, Jaffe GJ, Boulton ME, et al. 2013. Aryl hydrocarbon receptor deficiency causes dysregulated cellular matrix metabolism and age-related macular degeneration-like pathology. Proc Natl Acad Sci USA. 110:E4069–E4078.
- Huai W, Zhao R, Song H, Zhao J, Zhang L, Zhang L, Gao C, Han L, Zhao W. 2014. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun. 5:4738.
- Huang P, Ceccatelli S, Rannug A. 2002. A study on diurnal mRNA expression of CYP1A1, AHR, ARNT, and PER2 in rat pituitary and liver. Environ Toxicol Pharmacol. 11:119–126.
- Ilchmann A, Krause M, Heilmann M, Burgdorf S, Vieths S, Toda M. 2012. Impact of culture medium on maturation of bone marrow-derived murine dendritic cells via the aryl hydrocarbon receptor. Mol Immunol. 51:42–50.
- Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. 2007. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 117:1940–1950.
- Ivanov II, Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 4:337–349.
- Jackson DP, Joshi AD, Elferink CJ. 2015. Ah receptor pathway intricacies; signaling through diverse protein partners and DNA-motifs. Toxicol Res (Camb). 4:1143–1158.
- Janosik T, Rannug A, Rannug U, Wahlström N, Slätt J, Bergman J. 2018. The chemistry and properties of indolocarbazoles. Chem Rev. http://dx.doi.org/10.1021/acs.chemrev.8b00186.
- Jeong KT, Hwang SJ, Oh GS, Park JH. 2012. FICZ, a tryptophan photoproduct, suppresses pulmonary eosinophilia and Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma. Int Immunopharmacol. 13:377–385.
- Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y, Xiao W, Yang H. 2015. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci. 60:1958–1966.
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. 2014. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 85:777–788.
- John K, Lahoti TS, Wagner K, Hughes JM, Perdew GH. 2014. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol Carcinogen. 53:765–776.
- Jonsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, Hahn ME, Stegeman JJ. 2009. The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chem Biol Interact. 181:447–454.
- Jonsson ME, Mattsson A, Shaik S, Brunstrom B. 2016. Toxicity and cytochrome P450 1A mRNA induction by 6-formylindolo[3,2-b]carbazole (FICZ) in chicken and Japanese quail embryos. Comp Biochem Physiol C Toxicol Pharmacol. 179:125–136.
- Jurado-Manzano BB, Zavala-Reyes D, Turrubiartes-Martinez EA, Portales-Perez DP, Gonzalez-Amaro R, Layseca-Espinosa E. 2017. FICZ generates human tDCs that induce CD4+ CD25high Foxp3+ Treg-like cell differentiation. Immunol Lett. 190:84–92.
- Jux B, Kadow S, Luecke S, Rannug A, Krutmann J, Esser C. 2011. The aryl hydrocarbon receptor mediates UVB radiation-induced skin tanning. J Investig Dermatol. 131:203–210.
- Kado S, Chang WL, Chi AN, Wolny M, Shepherd DM, Vogel CF. 2017. Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells. Arch Toxicol. 91:2209–2221.
- Katiyar SK, Matsui MS, Mukhtar H. 2000. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 114:328–333.
- Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, Hirokawa T, et al. 2009. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA. 106:13481–13486.
- Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, Zhou Y, Huang SK. 2014. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 69:445–452.
- Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. 2001. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 276:39638–39644.
- Kim IS, Hwang JH, Hirano M, Iwata H, Kim EY. 2016. In vitro and in silico evaluation of transactivation potencies of avian AHR1 and AHR2 by endogenous ligands: implications for the physiological role of avian AHR2. Comp Biochem Physiol C Toxicol Pharmacol. 187:1–9.
- Kim SY, Yang HJ, Chang YS, Kim JW, Brooks M, Chew EY, Wong WT, Fariss RN, Rachel RA, Cogliati T, et al. 2014. Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Invest Ophthalmol Vis Sci. 55:6031–6040.
- Kimura A, Abe H, Tsuruta S, Chiba S, Fujii-Kuriyama Y, Sekiya T, Morita R, Yoshimura A. 2014. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int Immunol. 26:209–220.
- Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. 2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 105:9721–9726.
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 334:1561–1565.
- Kiyomatsu-Oda M, Uchi H, Morino-Koga S, Furue M. 2018. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J Dermatol Sci. 90:284–294.
- Ko C-I, Puga A. 2017. Does the aryl hydrocarbon receptor regulate pluripotency? Curr Opin Toxicol. 2:1–7.
- Ko CI, Fan Y, de Gannes M, Wang Q, Xia Y, Puga A. 2016. Repression of the aryl hydrocarbon receptor is required to maintain mitotic progression and prevent loss of pluripotency of embryonic stem cells. Stem Cells. 34:2825–2839.
- Ko CI, Wang Q, Fan Y, Xia Y, Puga A. 2014. Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem Cell Res. 12:296–308.
- Koch S, Stroisch TJ, Vorac J, Herrmann N, Leib N, Schnautz S, Kirins H, Forster I, Weighardt H, Bieber T. 2017. AhR mediates an anti-inflammatory feedback mechanism in human Langerhans cells involving FcepsilonRI and IDO. Allergy. 72:1686–1693.
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. 2000. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 97:10442–10447.
- Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. 2003. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 171:4582–4591.
- Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. 2016. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 22:598–605.
- Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, Gerich ME, Glover LE, Kominsky DJ, Colgan SP. 2017. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 10:1133–1144.
- Latchney SE, Lioy DT, Henry EC, Gasiewicz TA, Strathmann FG, Mayer-Proschel M, Opanashuk LA. 2011. Neural precursor cell proliferation is disrupted through activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Stem Cells Dev. 20:313–326.
- Laub LB, Jones BD, Powell WH. 2010. Responsiveness of a Xenopus laevis cell line to the aryl hydrocarbon receptor ligands 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Chem Biol Interact. 183:202–211.
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. 2011. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 13:144–151.
- Lee YH, Lin CH, Hsu PC, Sun YY, Huang YJ, Zhuo JH, Wang CY, Gan YL, Hung CC, Kuan CY, et al. 2015. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia. 63:1138–1154.
- Lensu S, Tuomisto JT, Tuomisto J, Pohjanvirta R. 2011. Characterization of the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-provoked strong and rapid aversion to unfamiliar foodstuffs in rats. Toxicology. 283:140–150.
- Leung A, Zulick E, Skvir N, Vanuytsel K, Morrison TA, Naing ZH, Wang Z, Dai Y, Chui DHK, Steinberg MH, et al. 2018. Notch and aryl hydrocarbon receptor signaling impact definitive hematopoiesis from human pluripotent stem cells. Stem Cells. 36:1004–1019.
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 147:629–640.
- Luecke S, Backlund M, Jux B, Esser C, Krutmann J, Rannug A. 2010. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigm Cell Melanoma Res. 23:828–833.
- Luecke S, Wincent E, Backlund M, Rannug U, Rannug A. 2010. Cytochrome P450 1A1 gene regulation by UVB involves crosstalk between the aryl hydrocarbon receptor and nuclear factor kappaB. Chem Biol Interact. 184:466–473.
- Maaetoft-Udsen K, Shimoda LMN, Frøkiaer H, Turner H. 2012. Aryl hydrocarbon receptor ligand effects in RBL2H3 cells. J Immunotoxicol. 9:327–337.
- Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, Vlachos C, Stathopoulou K, Skaltsounis AL, Marselos M, et al. 2013. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol. 133:2023–2030.
- Mahiout S, Pohjanvirta R. 2016. Aryl hydrocarbon receptor agonists trigger avoidance of novel food in rats. Physiol Behav. 167:49–59.
- Marcus RS, Holsapple MP, Kaminski NE. 1998. Lipopolysaccharide activation of murine splenocytes and splenic B cells increased the expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator. J Pharmacol Exp Ther. 287:1113–1118.
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. 2009. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 31:321–330.
- McIntosh BE, Hogenesch JB, Bradfield CA. 2010. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 72:625–645.
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 185:3190–3198.
- Mezrich JD, Nguyen LP, Kennedy G, Nukaya M, Fechner JH, Zhang X, Xing Y, Bradfield CA. 2012. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PLoS One. 7:e44547.
- Mitchell KA, Elferink CJ. 2009. Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol. 77:947–956.
- Miyagi S, Murata K, Sashino K, Sawamura S, Uruno S, Yoshimura S, Akahoshi E, Ishihara-Sugano M, Itoh S, Kurita N. 2015. Binding affinity between AhR and exogenous/endogenous ligands: molecular simulations and biological experiment. Mol Simul. 41:555–563.
- Mohammadi-Bardbori A, Akbarizadeh AR, Delju F, Rannug A. 2016. Chromatin remodeling by curcumin alters endogenous aryl hydrocarbon receptor signaling. Chem Biol Interact. 252:19–27.
- Mohammadi-Bardbori A, Bastan F, Akbarizadeh AR. 2017. The highly bioactive molecule and signal substance 6-formylindolo[3,2-b]carbazole (FICZ) plays bi-functional roles in cell growth and apoptosis in vitro. Arch Toxicol. 91:3365–3372.
- Mohammadi-Bardbori A, Vikstrom Bergander L, Rannug U, Rannug A. 2015. NADPH oxidase-dependent mechanism explains how arsenic and other oxidants can activate aryl hydrocarbon receptor signaling. Chem Res Toxicol. 28:2278–2286.
- Mohinta S, Kannan AK, Gowda K, Amin SG, Perdew GH, August A. 2015. Differential regulation of Th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. Toxicol Sci. 145:233–243.
- Monteleone I, Marafini I, Zorzi F, Di Fusco D, Dinallo V, Rizzo A, Sileri P, Sica G, Monteleone G. 2016. Smad7 Knockdown Restores Aryl Hydrocarbon Receptor-mediated Protective Signals in the Gut. J Crohns Colitis. 10:670–677.
- Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. 2011. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 141:237–248.
- Monteleone I, Zorzi F, Marafini I, Di Fusco D, Dinallo V, Caruso R, Izzo R, Franze E, Colantoni A, Pallone F, et al. 2016. Aryl hydrocarbon receptor-driven signals inhibit collagen synthesis in the gut. Eur J Immunol. 46:1047–1057.
- Morales-Hernández A, González-Rico FJ, Román AC, Rico-Leo E, Alvarez-Barrientos A, Sánchez L, Macia Á, Heras SR, García-Pérez JL, Merino JM, et al. 2016. Alu retrotransposons promote differentiation of human carcinoma cells through the aryl hydrocarbon receptor. Nucleic Acids Res. 44:4665–4683.
- Morales-Hernandez A, Nacarino-Palma A, Moreno-Marin N, Barrasa E, Paniagua-Quinones B, Catalina-Fernandez I, Alvarez-Barrientos A, Bustelo XR, Merino JM, Fernandez-Salguero PM. 2017. Lung regeneration after toxic injury is improved in absence of dioxin receptor. Stem Cell Res. 25:61–71.
- Moran TB, Brannick KE, Raetzman LT. 2012. Aryl-hydrocarbon receptor activity modulates prolactin expression in the pituitary. Toxicol Appl Pharmacol. 265:139–145.
- Morino-Koga S, Uchi H, Mitoma C, Wu Z, Kiyomatsu M, Fuyuno Y, Nagae K, Yasumatsu M, Suico MA, Kai H, et al. 2017. 6-Formylindolo[3,2-b]carbazole accelerates skin wound healing via activation of ERK, but not aryl hydrocarbon receptor. J Invest Dermatol. 137:2217–2226.
- Morino-Koga S, Uchi H, Tsuji G, Takahara M, Kajiwara J, Hirata T, Furue M. 2013. Reduction of CC-chemokine ligand 5 by aryl hydrocarbon receptor ligands. J Dermatol Sci. 72:9–15.
- Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, et al. 2014. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 512:387–392.
- Mukai M, Lin TM, Peterson RE, Cooke PS, Tischkau SA. 2008. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms. 23:200–210.
- Mukai M, Tischkau SA. 2007. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci. 95:172–181.
- Murai M, Tsuji G, Hashimoto-Hachiya A, Kawakami Y, Furue M, Mitoma C. 2018. An endogenous tryptophan photo-product, FICZ, is potentially involved in photo-aging by reducing TGF-beta-regulated collagen homeostasis. J Dermatol Sci. 89:19–26.
- Murray IA, Patterson AD, Perdew GH. 2014. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 14:801–814.
- Murray IA, Perdew GH. 2017. Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis. Curr Opin Toxicol. 2:15–23.
- Nakahama T, Hanieh H, Nguyen NT, Chinen I, Ripley B, Millrine D, Lee S, Nyati KK, Dubey PK, Chowdhury K, et al. 2013. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc Natl Acad Sci USA. 110:11964–11969.
- Nebert DW. 1991. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 5:1203–1214.
- Nebert DW, Brown DD, Towne DW, Eisen HJ. 1984. Association of fertility, fitness and longevity with the murine Ah locus among (C57BL/6N) (C3H/HeN) recombinant inbred lines. Biol Reprod. 30:363–373.
- Nebert DW, Dalton TP. 2006. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 6:947–960.
- Nguyen CH, Brenner S, Huttary N, Atanasov AG, Dirsch VM, Chatuphonprasert W, Holzner S, Stadler S, Riha J, Krieger S, et al. 2016. AHR/CYP1A1 interplay triggers lymphatic barrier breaching in breast cancer spheroids by inducing 12(S)-HETE synthesis. Hum Mol Genet. 25:5006–5016.
- Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, Laklouk I, Sarita-Reyes C, Gusenleitner D, Li A, et al. 2016. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2-human breast cancer cells. Mol Pharmacol. 90:674–688.
- Nuti R, Gargaro M, Matino D, Dolciami D, Grohmann U, Puccetti P, Fallarino F, Macchiarulo A. 2014. Ligand binding and functional selectivity of L-tryptophan metabolites at the mouse aryl hydrocarbon receptor (mAhR). J Chem Inf Model. 54:3373–3383.
- Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. 2005. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 85:935–943.
- Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y. 1993. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun. 197:878–885.
- Okudaira N, Iijima K, Koyama T, Minemoto Y, Kano S, Mimori A, Ishizaka Y. 2010. Induction of long interspersed nucleotide element-1 (L1) retrotransposition by 6-formylindolo[3,2-b]carbazole (FICZ), a tryptophan photoproduct. Proc Natl Acad Sci USA. 107:18487–18492.
- Park JH, Choi AJ, Kim SJ, Cheong SW, Jeong SY. 2016. AhR activation by 6-formylindolo[3,2-b]carbazole and 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibit the development of mouse intestinal epithelial cells. Environ Toxicol Pharmacol. 43:44–53.
- Park SL, Justiniano R, Williams JD, Cabello CM, Qiao S, Wondrak GT. 2015. The tryptophan-derived endogenous aryl hydrocarbon receptor ligand 6-formylindolo[3,2-b]carbazole is a nanomolar UVA photosensitizer in epidermal keratinocytes. J Invest Dermatol. 135:1649–1658.
- Pauly SK, Fechner JH, Zhang X, Torrealba J, Bradfield CA, Mezrich JD. 2012. The aryl hydrocarbon receptor influences transplant outcomes in response to environmental signals. Toxicol Environ Chem. 94:1175–1187.
- Perdew GH, Babbs CF. 1991. Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer. 16:209–218.
- Peres AG, Zamboni R, King IL, Madrenas J. 2017. Suppression of CYP1 members of the AHR response by pathogen-associated molecular patterns. J Leukoc Biol. 102:1471–1480.
- Petrulis JR, Perdew GH. 2002. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 141:25–40.
- Pilz C, Feyerabend T, Sonner J, Redaelli C, Peter K, Kunze A, Haas K, Esser C, Schakel K, Wick W, et al. 2016. Normal mast cell numbers in the tissues of AhR-deficient mice. Exp Dermatol. 25:62–63.
- Poland A, Glover E, Kende AS. 1976. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 251:4936–4946.
- Poland A, Knutson JC. 1982. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 22:517–554.
- Prochazkova J, Strapacova S, Svrzkova L, Andrysik Z, Hyzdalova M, Hruba E, Pencikova K, Libalova H, Topinka J, Klema J, et al. 2018. Adaptive changes in global gene expression profile of lung carcinoma A549 cells acutely exposed to distinct types of AhR ligands. Toxicol Lett. 292:162–174.
- Prud'homme GJ, Glinka Y, Toulina A, Ace O, Subramaniam V, Jothy S. 2010. Breast cancer stem-like cells are inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS One. 5:e13831.
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 36:92–104.
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453:65–71.
- Ramirez JM, Brembilla NC, Sorg O, Chicheportiche R, Matthes T, Dayer JM, Saurat JH, Roosnek E, Chizzolini C. 2010. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur J Immunol. 40:2450–2459.
- Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. 1987. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 262:15422–15427.
- Rannug U, Agurell E, Rannug A, Cederberg H. 1992. Certain tryptophan photoproducts are inhibitors of cytochrome P450-dependent mutagenicity. Environ Mol Mutagen. 20:289–296.
- Rannug U, Bramstedt H, Nilsson U. 1992. The presence of genotoxic and bioactive components in indigo dyed fabrics-a possible health risk? Mutat Res. 282:219–225.
- Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. 1995. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol. 2:841–845.
- Rentas S, Holzapfel N, Belew MS, Pratt G, Voisin V, Wilhelm BT, Bader GD, Yeo GW, Hope KJ. 2016. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 532:508–511.
- Reschly EJ, Ai N, Welsh WJ, Ekins S, Hagey LR, Krasowski MD. 2008. Ligand specificity and evolution of liver X receptors. J Steroid Biochem Mol Biol. 110:83–94.
- Reschly EJ, Bainy AC, Mattos JJ, Hagey LR, Bahary N, Mada SR, Ou J, Venkataramanan R, Krasowski MD. 2007. Functional evolution of the vitamin D and pregnane X receptors. BMC Evol Biol. 7:222.
- Rico-Leo EM, Alvarez-Barrientos A, Fernandez-Salguero PM. 2013. Dioxin receptor expression inhibits basal and transforming growth factor β-induced epithelial-to-mesenchymal transition. J Biol Chem. 288:7841–7856.
- Rico-Leo EM, Moreno-Marin N, Gonzalez-Rico FJ, Barrasa E, Ortega-Ferrusola C, Martin-Munoz P, Sanchez-Guardado LO, Llano E, Alvarez-Barrientos A, Infante-Campos A, et al. 2016. piRNA-associated proteins and retrotransposons are differentially expressed in murine testis and ovary of aryl hydrocarbon receptor deficient mice. Open Biol. 6:160186.
- Roblin S, Okey AB, Harper PA. 2004. AH receptor antagonist inhibits constitutive CYP1A1 and CYP1B1 expression in rat BP8 cells. Biochem Biophys Res Commun. 317:142–148.
- Romanov S, Medvedev A, Gambarian M, Poltoratskaya N, Moeser M, Medvedeva L, Gambarian M, Diatchenko L, Makarov S. 2008. Homogeneous reporter system enables quantitative functional assessment of multiple transcription factors. Nat Methods. 5:253–260.
- Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. 2011. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol. 12:1238–1245.
- Safe S, Lee SO, Jin UH. 2013. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 135:1–16.
- Salisbury TB, Tomblin JK, Primerano DA, Boskovic G, Fan J, Mehmi I, Fletcher J, Santanam N, Hurn E, Morris GZ, Denvir J. 2014. Endogenous aryl hydrocarbon receptor promotes basal and inducible expression of tumor necrosis factor target genes in MCF-7 cancer cells. Biochem Pharmacol. 91:390–399.
- Sartor MA, Schnekenburger M, Marlowe JL, Reichard JF, Wang Y, Fan Y, Ma C, Karyala S, Halbleib D, Liu X, et al. 2009. Genomewide analysis of aryl hydrocarbon receptor binding targets reveals an extensive array of gene clusters that control morphogenetic and developmental programs. Environ Health Perspect. 117:1139–1146.
- Sauzeau V, Carvajal-Gonzalez JM, Riolobos AS, Sevilla MA, Menacho-Marquez M, Roman AC, Abad A, Montero MJ, Fernandez-Salguero P, Bustelo XR. 2011. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem. 286:2896–2909.
- Schaldach CM, Riby J, Bjeldanes LF. 1999. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 38:7594–7600.
- Schallreuter KU, Salem MA, Gibbons NC, Maitland DJ, Marsch E, Elwary SM, Healey AR. 2012. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: Epidermal H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling. FASEB J. 26:2471?2485.
- Schiering C, Vonk A, Das S, Stockinger B, Wincent E. 2018. Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells. Biochem Pharmacol. 151:47–58.
- Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, et al. 2017. Feedback control of AHR signalling regulates intestinal immunity. Nature. 542:242–245.
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. 1996. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 93:6731–6736.
- Schulte KW, Green E, Wilz A, Platten M, Daumke O. 2017. Structural basis for aryl hydrocarbon receptor-mediated gene activation. Structure. 25:1025–1033 e3.
- Schulz VJ, Smit JJ, Huijgen V, Bol-Schoenmakers M, van Roest M, Kruijssen LJ, Fiechter D, Hassing I, Bleumink R, Safe S, et al. 2012. Non-dioxin-like AhR ligands in a mouse peanut allergy model. Toxicol Sci. 128:92–102.
- Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. 2009. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 29:6391–6400.
- Seok SH, Lee W, Jiang L, Molugu K, Zheng A, Li Y, Park S, Bradfield CA, Xing Y. 2017. Structural hierarchy controlling dimerization and target DNA recognition in the AHR transcriptional complex. Proc Natl Acad Sci USA. 114:5431–5436.
- Seok SH, Ma ZX, Feltenberger JB, Chen H, Chen H, Scarlett C, Lin Z, Satyshur KA, Cortopassi M, Jefcoate CR, et al. 2018. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J Biol Chem. 293:1994–2005.
- Shimada T, Guengerich FP. 2006. Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem Res Toxicol. 19:288–294.
- Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, Sunwoo JB. 2013. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 110:12391–12396.
- Sibilano R, Frossi B, Calvaruso M, Danelli L, Betto E, Dall'Agnese A, Tripodo C, Colombo MP, Pucillo CE, Gri G. 2012. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol. 189:120–127.
- Simones T, Shepherd DM. 2011. Consequences of AhR activation in steady-state dendritic cells. Toxicol Sci. 119:293–307.
- Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. 2009. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 77:577–587.
- Singh NP, Singh UP, Rouse M, Zhang J, Chatterjee S, Nagarkatti PS, Nagarkatti M. 2016. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J Immunol. 196:1108–1122.
- Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. 2011. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 6:e23522.
- Smirnova A, Wincent E, Vikstrom Bergander L, Alsberg T, Bergman J, Rannug A, Rannug U. 2016. Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist FICZ. Chem Res Toxicol. 29:75–86.
- Smith BW, Rozelle SS, Leung A, Ubellacker J, Parks A, Nah SK, French D, Gadue P, Monti S, Chui DH, et al. 2013. The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation. Blood. 122:376–385.
- Smith KJ, Boyer JA, Muku GE, Murray IA, Gowda K, Desai D, Amin SG, Glick AB, Perdew GH. 2017. Editor's highlight: Ah receptor activation potentiates neutrophil chemoattractant (C-X-C Motif) ligand 5 expression in keratinocytes and skin. Toxicol Sci. 160:83–94.
- Smith KJ, Murray IA, Boyer JA, Perdew GH. 2018. Allelic variants of the aryl hydrocarbon receptor differentially influence UVB-mediated skin inflammatory responses in SKH1 mice. Toxicology. 394:27–34.
- Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, Coquery CM, Neil J, Pryor WM, Mayhew D, et al. 2017. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 137:2110–2119.
- Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. 2002. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 99:14694–14699.
- Sonnenberg GF, Fouser LA, Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 12:383–390.
- Stanford EA, Ramirez-Cardenas A, Wang Z, Novikov O, Alamoud K, Koutrakis P, Mizgerd JP, Genco CA, Kukuruzinska M, Monti S, et al. 2016. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol Cancer Res. 14:696–706.
- Stanford EA, Wang Z, Novikov O, Mulas F, Landesman-Bollag E, Monti S, Smith BW, Seldin DC, Murphy GJ, Sherr DH. 2016. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 14:20.
- Stockinger B, Omenetti S. 2017. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 17:535–544.
- Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. 2007. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. Am J Pathol. 170:855–864.
- Thatcher TH, Williams MA, Pollock SJ, McCarthy CE, Lacy SH, Phipps RP, Sime PJ. 2016. Endogenous ligands of the aryl hydrocarbon receptor regulate lung dendritic cell function. Immunology. 147:41–54.
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. 2006. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 69:140–153.
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 10:864–871.
- Tsuji G, Hashimoto-Hachiya A, Kiyomatsu-Oda M, Takemura M, Ohno F, Ito T, Morino-Koga S, Mitoma C, Nakahara T, Uchi H, et al. 2017. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 8:e2931.
- Unnisa Z, Singh KP, Henry EC, Donegan CL, Bennett JA, Gasiewicz TA. 2016. Aryl hydrocarbon receptor deficiency in an Exon 3 deletion mouse model promotes hematopoietic stem cell proliferation and impacts endosteal niche cells. Stem Cells Int. 2016:4536187.
- Veiga-Parga T, Suryawanshi A, Rouse BT. 2011. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 7:e1002427.
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453:106–109.
- Villa M, Gialitakis M, Tolaini M, Ahlfors H, Henderson CJ, Wolf CR, Brink R, Stockinger B. 2017. Aryl hydrocarbon receptor is required for optimal B-cell proliferation. EMBO J. 36:116–128.
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. 2008. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 375:331–335.
- Wang HC, Zhou Y, Huang SK. 2017. SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Arch Toxicol. 91:1739–1748.
- Wang Q, Yang K, Han B, Sheng B, Yin J, Pu A, Li L, Sun L, Yu M, Qiu Y, et al. 2018. Aryl hydrocarbon receptor inhibits inflammation in DSS induced colitis via the MK2/pMK2/TTP pathway. Int J Mol Med. 41:868–876.
- Wang Y, Fan Y, Puga A. 2010. Dioxin exposure disrupts the differentiation of mouse embryonic stem cells into cardiomyocytes. Toxicol Sci. 115:225–237.
- Wang Z, Monti S, Sherr DH. 2017. The diverse and important contributions of the AHR to cancer and cancer immunity. Curr Opin Toxicol. 2:93–102.
- Wei YD, Bergander L, Rannug U, Rannug A. 2000. Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch Biochem Biophys. 383:99–107.
- Wei YD, Helleberg H, Rannug U, Rannug A. 1998. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 110:39–55.
- Wei YD, Rannug U, Rannug A. 1999. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 118:127–140.
- Weiss C, Kolluri SK, Kiefer F, Gottlicher M. 1996. Complementation of Ah receptor deficiency in hepatoma cells. Negative feedback regulation and cell cycle control by the Ah receptor. Exp Cell Res. 226:154–163.
- Wheeler JL, Martin KC, Resseguie E, Lawrence BP. 2014. Differential consequences of two distinct AhR ligands on innate and adaptive immune responses to influenza A virus. Toxicol Sci. 137:324–334.
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. 2009. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 284:2690–2696.
- Wincent E, Bengtsson J, Mohammadi Bardbori A, Alsberg T, Luecke S, Rannug U, Rannug A. 2012. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 109:4479–4484.
- Wincent E, Kubota A, Timme-Laragy A, Jonsson ME, Hahn ME, Stegeman JJ. 2016. Biological effects of 6-formylindolo[3,2-b]carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem Pharmacol. 110–111:117–129.
- Wincent E, Stegeman JJ, Jonsson ME. 2015. Combination effects of AHR agonists and Wnt/β-catenin modulators in zebrafish embryos: implications for physiological and toxicological AHR functions. Toxicol Appl Pharmacol. 284:163–179.
- Woeller CF, Roztocil E, Hammond CL, Feldon SE, Phipps RP. 2016. the aryl hydrocarbon receptor and its ligands inhibit myofibroblast formation and activation: implications for thyroid eye disease. Am J Pathol. 186:3189–3202.
- Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C, Chatila TA. 2015. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 136:441–453.
- Xu CX, Krager SL, Liao DF, Tischkau SA. 2010. Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of Period1 gene. Toxicol Sci. 115:98–108.
- Yamada T, Horimoto H, Kameyama T, Hayakawa S, Yamato H, Dazai M, Takada A, Kida H, Bott D, Zhou AC, et al. 2016. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat Immunol. 17:687–694.
- Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, Jobin C, Chen ZE, Zhou L. 2017. The Aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. 21:2277–2290.
- Yin A, Korzh S, Winata CL, Korzh V, Gong Z. 2011. Wnt signaling is required for early development of zebrafish swimbladder. PLoS One. 6:e18431.
- Yin J, Sheng B, Han B, Pu A, Yang K, Li P, Wang Q, Xiao W, Yang H. 2016. The AhR is involved in the regulation of LoVo cell proliferation through cell cycle-associated proteins. Cell Biol Int. 40:560–568.
- Yoshioka W, Peterson RE, Tohyama C. 2011. Molecular targets that link dioxin exposure to toxicity phenotypes. J Steroid Biochem Mol Biol. 127:96–101.
- Yu M, Wang Q, Ma Y, Li L, Yu K, Zhang Z, Chen G, Li X, Xiao W, Xu P, Yang H. 2018. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int J Biol Sci. 14:69–77.
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. 2013. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 39:372–385.
- Zhou Y, Tung HY, Tsai YM, Hsu SC, Chang HW, Kawasaki H, Tseng HC, Plunkett B, Gao P, Hung CH, et al. 2013. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 121:3195–3204.
