Abstract
Ecstasy use is commonly combined with ethanol consumption. While combination drug use in general is correlated with a higher risk for toxicity, the risk of the specific combination of ecstasy (3,4-methylenedioxymethamphetamine (MDMA)) and ethanol is largely unknown. Therefore, we have reviewed the literature on changes in MDMA pharmacokinetics and pharmacodynamics due to concurrent ethanol exposure in human, animal and in vitro studies. MDMA pharmacokinetics appear unaffected: the MDMA blood concentration after concurrent exposure to MDMA and ethanol was comparable to lone MDMA exposure in multiple human placebo-controlled studies. In contrast, MDMA pharmacodynamics were affected: locomotor activity increased and body temperature decreased after concurrent exposure to MDMA and ethanol compared to lone MDMA exposure. Importantly, these additional ethanol effects were consistently observed in multiple animal studies. Additional ethanol effects have also been reported on other pharmacodynamic aspects, but are inconclusive due to a low number of studies or due to inconsistent findings. These investigated pharmacodynamic aspects include monoamine brain concentrations, neurological (psychomotor function, memory, anxiety, reinforcing properties), cardiovascular, liver and endocrine effects. Although only a single or a few studies were available investigating these aspects, most studies indicated an aggravation of MDMA-induced effects upon concurrent ethanol exposure. In summary, concurrent ethanol exposure appears to increase the risk for MDMA toxicity. Increased toxicity is due to an aggravation of MDMA pharmacodynamics, while MDMA pharmacokinetics is largely unaffected. Although a significant attenuation of the MDMA-induced increase of body temperature was observed in animal studies, its relevance for human exposure remains unclear.
Introduction
Ecstasy, with its active component 3,4-methylenedioxymethamphetamine (MDMA), is one of the most popular recreational drugs. Prevalence of lifetime use in the adult population is low (<5%) (EMCDDA Citation2019; WDR Citation2019) although in specifics groups (e.g. clubbers) last year prevalence of use increases up to 50–60% (Monshouwer et al. Citation2016; Palamar Citation2020; Palamar and Acosta Citation2020). As a (psycho)stimulant, MDMA affects the central nervous system by increasing brain concentrations of monoamines, particularly of serotonin, but also norepinephrine and dopamine (for reviews see Green et al. Citation2003; Dunlap et al. Citation2018). These increases are due to several mechanisms of action. Firstly, MDMA inhibits the intracellular uptake of extracellular monoamines by inhibiting monoamine reuptake transporters. In particular the serotonin reuptake transporter (SERT) is inhibited, but also the norepinephrine and dopamine reuptake transporter (NET and DAT). Secondly, MDMA increases the cytosolic concentration of monoamines by decreasing their intracellular vesicular storage. Thirdly, cytosolic monoamines can be transported outwards, because MDMA reverses the transport direction of monoamine reuptake transporters from outside-inside (reuptake), to inside-outside (transporter-mediated release), further increasing brain concentrations of monoamines (for review see Dunlap et al. Citation2018). Subsequently these extracellular monoamines can activate receptors. High extracellular monoamine concentrations, as a result of MDMA use, can overstimulate these receptors. In addition, MDMA itself also modulates receptor function, although mostly at a higher MDMA concentration than necessary for transporter inhibition. However, MDMA does modulate serotonin receptor function at submicromolar concentrations (for reviews see Rietjens et al. Citation2012; Hondebrink et al. Citation2018; Dunlap et al. Citation2018). Due to these biochemical changes, MDMA increases alertness and also causes entactogenic effects such as warm, euphoric and loving feelings, and an increased closeness to others (Meyer Citation2013; Dunlap et al. Citation2018).
However, adverse effects can also occur following MDMA exposure, including an increase in heart rate (tachycardia), blood pressure (hypertension) and body temperature (hyperthermia). Adverse effects range from mild to life-threatening effects and occasionally deaths are reported (Green et al. Citation2003; Hall and Henry Citation2006). Based on the prevalence of MDMA use and a registry of MDMA-related health incidents in the Netherlands, the risk for moderate to severe effects is estimated at 0.11% (1 in 900 pills) (van Amsterdam et al. Citation2020). Although the risk appears low, MDMA is the third most common stimulant drug reported in drug-related emergency department (ED) visits in Europe (EMCDDA Citation2020). Integral worldwide or European figures on MDMA-related incidents are absent. However, the Euro-DEN Plus network, consisting of 31 sentinel centers, reported thousands of MDMA-related ED visits between 2014 and 2017 (EMCDDA Citation2020; Noseda et al. Citation2020), indicating a substantial impact on health care facilities.
MDMA users frequently combine MDMA and ethanol; 91% of Australian clubbers (experienced MDMA users) reported concurrent use (Morefield et al. Citation2011). Also, the combination of MDMA and ethanol was the second most prevalent combination reported among past year Dutch clubbers, after the combination of ethanol and cannabis (Monshouwer et al. Citation2016).
While the risk of combining MDMA and ethanol is mostly unknown, an early review already reported interactions that affected the pharmacokinetic and dynamic properties of MDMA (Mohamed et al. Citation2009). Also, intoxication and mortality data indicate that combining MDMA and ethanol could worsen health outcome (Calle et al. Citation2019; Schürmann et al. Citation2019). For example, in 70% of MDMA-related ED visits in Europe, ethanol was also used (Noseda et al. Citation2020).
In addition, there are indications that MDMA-related health incidents with concurrent ethanol exposure are more severe compared to lone MDMA incidents (Dutch registry, Schürmann et al. Citation2019). Furthermore, a higher proportion of MDMA and ethanol intoxicated patients at a Belgium dance event required hospital care, compared to lone MDMA intoxicated patients (Calle et al. Citation2018).
In addition to recreational MDMA use, its therapeutic potential for ethanol use disorder is under investigation (Sessa et al. Citation2019). Consequently, additional populations could be at risk for exposure to a combination of MDMA and ethanol.
While several studies indicate that concurrent use of MDMA and ethanol results in health incidents (Jones et al. Citation2016; Schürmann et al. Citation2019; EMCDDA Citation2020; Noseda et al. Citation2020), there are only few indications that these incidents are more severe than those following lone MDMA use (Calle et al. Citation2018; Schürmann et al. Citation2019). Therefore, a systematic review was performed to investigate if concurrent use could worsen adverse effects.
Methods
PubMed was queried for all English-written literature up to 11 August 2020, using the following string: ((3,4-methylenedioxymethamphetamine[Title]) OR MDMA[Title] OR ecstasy[Title] OR xtc[Title]) AND (ethanol[Title/Abstract] OR alcohol[Title/Abstract])). The string resulted in 222 articles of which title and abstract were manually screened for relevance. Human, animal and in vitro studies were included if the dose or concentration of all exposures was reported and at least two exposure conditions were investigated: (1) MDMA and (2) MDMA and ethanol. In total, 37 articles were included.
The effects reported in the included studies were extracted by two scientists and effects observed after concurrent ethanol and MDMA exposure were compared to effects after lone MDMA exposure. Both pharmacokinetic and pharmacodynamic effects are summarized.
Results
Pharmacokinetic effects
Several pharmacokinetic factors, such as absorption, distribution, metabolism, and elimination could be influenced by concurrent exposure to MDMA and ethanol. However, literature on such specific aspects is scarce and most studies have only investigated effects on MDMA blood concentrations. Seven human placebo-controlled studies have investigated the effect of concurrent ethanol exposure on the MDMA blood concentration (). Most studies observed a comparable MDMA blood concentration after concurrent MDMA-ethanol exposure compared to lone MDMA exposure (Kuypers et al. Citation2006; Ramaekers and Kuypers Citation2006; Dumont et al. Citation2008; Dumont, Schoemaker, et al. Citation2010; Veldstra et al. Citation2012; Spronk et al. Citation2014). One human placebo-controlled study reported a slightly, but significantly, higher MDMA blood concentration (+13%) after concurrent MDMA-ethanol exposure (Hernández-López et al. Citation2002). This finding is corroborated by one study in rats that reported a higher MDMA blood concentration at 15 min after exposure, but not after 5 and 60 min (Hamida et al. Citation2009).
Table 1. Pharmacokinetics: additional ethanol effects (MDMA + ethanol vs lone MDMA).
In addition to blood concentrations, the effect of concurrent ethanol exposure on MDMA brain concentrations was investigated in three animal studies. One mice study reported MDMA striatal concentrations increased fourfold (Johnson et al. Citation2004). One rat study reported increased MDMA concentrations in the cortex, striatum or hippocampus at 15 min after exposure, but not after 5 and 60 min (Hamida et al. Citation2009). In contrast, a comparable MDMA concentration was observed in the cortex, striatum or hippocampus of rats 45 min after concurrent MDMA and ethanol exposure compared to lone MDMA exposure (Hamida et al. Citation2007). Higher MDMA concentrations in the brain could increase toxicity. For example, inhibition of monoamine reuptake transporters increases with an increasing MDMA dose (Rickli et al. Citation2015; Zwartsen et al. Citation2017).
Although little interaction through metabolic enzymes is expected since different enzymes are involved in ethanol and MDMA metabolism, two studies reported altered metabolism during concurrent MDMA-ethanol exposure. In rats, a decreased activity was observed of the enzyme metabolizing the toxic ethanol metabolite acetaldehyde (aldehyde dehydrogenase (ALDH1), Upreti et al. Citation2009, ). Possibly, this increases the acetaldehyde concentration, resulting in increased hepatotoxic effects. Furthermore, an increase in MDMA metabolite formation was observed in primary rat hepatocytes (Pontes et al. Citation2010). Since some of these metabolites have a higher toxic potential than MDMA itself (Monks et al. Citation2004), this could aggravate hepatotoxicity. However, both studies used a high MDMA dose or concentration (10 mg/kg and 1.6 mM) and therefore, it remains unclear if these additional ethanol effects also occur at recreational MDMA doses (2–3 mg/kg).
Pharmacodynamic effects
Neurological effects
Biochemical effects
Acute MDMA exposure increases brain concentrations of monoamines, mainly due to inhibition and reversal of monoamine reuptake transporters. In humans and rats the strongest effect is observed on the serotonin concentration, although norepinephrine and dopamine concentrations also increase. Following the initial increase in the serotonin brain concentration, a serotonin depletion is observed that can last up to a week. In contrast, in mice, MDMA more strongly affects the dopaminergic system instead of the serotoninergic system (for reviews see Green et al. Citation2003; Mohamed et al. Citation2011; Meyer Citation2013). Acute ethanol exposure also increases serotonin and dopamine brain concentrations. In addition, serotonergic and dopaminergic receptors are modulated (for reviews see Lovinger Citation1997, Charlet et al. Citation2013; Trantham-Davidson and Chandler Citation2015).
Fourteen animal studies have investigated biochemical effects of concurrent MDMA-ethanol exposure, mostly focused on serotonin and dopamine brain concentrations (, , Johnson et al. Citation2004; Cassel et al. Citation2005; Hamida et al. Citation2007; Izco et al. Citation2007; Riegert et al. Citation2008; Hernandez-Rabaza et al. Citation2010; Jones et al. Citation2010; Ribeiro Do Couto et al. Citation2011; Rodríguez-Arias et al. Citation2011; Ribeiro Do Couto et al. Citation2012; Ros-Simó et al. Citation2012; Vidal-Infer et al. Citation2012; Ros-Simó et al. Citation2013; Navarro-Zaragoza et al. Citation2015). Unfortunately, additional acute effects of ethanol remain largely unknown, since most of these studies investigated effects on monoamine concentrations one to three weeks after exposure. Only one study examined acute effects and reported that concurrent ethanol exposure potentiated the MDMA-induced increase in striatal serotonin and dopamine outflow in exposed brain slices of unexposed (whole) rats (Riegert et al. Citation2008). Four studies investigated serotonin brain concentrations in rats 7–20 days after concurrent MDMA-ethanol exposure. Two observed a further decrease relative to lone MDMA exposure (Izco et al. Citation2007; Jones et al. Citation2010) and two observed no difference (Cassel et al. Citation2005; Hamida et al. Citation2007). Three studies investigated dopamine brain concentrations in mice 3–22 days after MDMA and ethanol exposure. Two showed no additional effect of ethanol (Rodríguez-Arias et al. Citation2011; Vidal-Infer et al. Citation2012) and one study reported attenuation of MDMA-induced reduction in striatal dopamine (Johnson et al. Citation2004).
Figure 1. Summary of effects of concomitant MDMA-ethanol exposure relative to lone MDMA exposure reported in human and animal studies. For individual studies and details see . CPP: conditioned place preference, indicative for reinforcing effects.
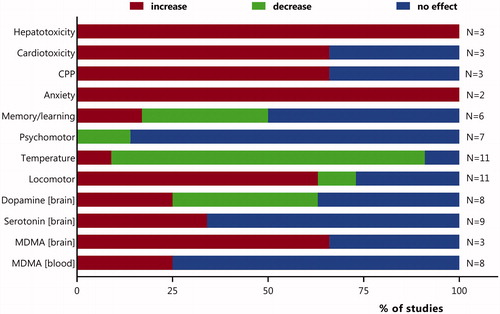
Table 2. Pharmacodynamics: additional ethanol effects on biochemical effects (MDMA + ethanol vs lone MDMA).
In addition, one rat study investigated the effect of concurrent MDMA-ethanol exposure on the norepinephrine brain concentration 20 days after exposure. Lone MDMA exposure as well as concurrent MDMA-ethanol exposure did not affect the norepinephrine brain concentration (Cassel et al. Citation2005, ).
Locomotor activity
In humans, acute MDMA exposure has stimulatory effects and agitation is frequently reported (Mohamed et al. Citation2011, Noseda et al. Citation2020). In animals, primarily in rats, MDMA exposure increases locomotor activity (Green et al. Citation2003). Acute effects following exposure to low ethanol doses include stimulatory effects in humans, while at higher doses sedative effects are observed (Hendler et al., Citation2013). In animals, acute ethanol exposure increases locomotor activity (Moore et al. Citation1993; Cohen et al. Citation1997 Abrahao et al. Citation2014; Filev et al. Citation2017; Hernández-Vázquez et al. Citation2018).
Table 3. Pharmacodynamics – additional ethanol effects on locomotor activity and body temperature (MDMA + ethanol vs lone MDMA).
Evidence for additional ethanol effects on MDMA-induced increases in locomotor activity is rather strong. Seven rat studies showed a consistent additional increase in locomotor activity upon concurrent MDMA-ethanol exposure (Cassel et al. Citation2004; Hamida et al. Citation2006, Citation2007, Citation2008; Riegert et al. Citation2008; Hamida et al. Citation2009, Citation2020, , ). A higher MDMA brain concentration in rats upon concurrent MDMA-ethanol exposure was suggested to contribute to the increase in locomotor activity (Hamida et al. Citation2009). Also, the increased locomotor activity is in line with higher dopaminergic and serotonergic activity (Green et al. Citation2003, Ryczko and Dubuc Citation2017).
Notably, increased locomotor activity was not observed in four mice studies, which showed variable results (Pontes, Duarte, et al. Citation2008; Rodríguez-Arias et al. Citation2011; Ros-Simó et al. Citation2012; Rostami et al. Citation2017). This lack of additional effect in mice could be due to MDMA’s primarily dopaminergic effect in mice, in contrast to its primarily serotoninergic effect in rats. Possibly, locomotor activity, which is also mediated through dopaminergic activity (Ryczko and Dubuc Citation2017), is already maximal increased in mice, hampering detection of additional ethanol effects.
Human data investigating corresponding effects of concurrent ethanol exposure, such as agitation, are lacking.
Body temperature
Following exposure to low recreational doses of MDMA (1.5–2 mg/kg oral) significant, but small, increases in body temperature (+0.4–0.8 °C) have been reported in human volunteers (for review see Liechti Citation2014). However, increases of several degrees, up to hyperthermia, can occur in humans, likely due to higher doses of MDMA, higher ambient temperature, or due to individual susceptibility (Mallick and Bodenham Citation1997; Patel et al. Citation2005; Davies et al. Citation2014). Hyperthermia is correlated to poor outcome in humans (Eyer and Zilker Citation2007). In both rat and mice studies high MDMA doses are often applied (5–20 mg/kg i.p), and large increases in body temperature have been reported (Cassel et al. Citation2004; Johnson et al. Citation2004; Hamida et al. Citation2006; Cassel et al. Citation2007; Hamida et al. Citation2007; Izco et al. Citation2007; Hamida et al. Citation2008; Pontes, Duarte, et al. Citation2008; Rodríguez-Arias et al. Citation2011; Ros-Simó et al. Citation2012, see , ). Following exposure to ethanol, most animal studies have reported a decrease in body temperature. Presumably, ethanol dysregulates the thermoregulatory system. In addition, the effect also depends on ambient temperature. At higher ambient temperatures, hyperthermia has also been reported. Changes in serotonin brain concentrations have been implicated in the hypothermic effects of ethanol (for review see Crawshaw et al. Citation1998; Wasielewski and Holloway Citation2001).
One human placebo-controlled study investigated the effect of concurrent MDMA-ethanol exposure and observed merely a trend (non-significant difference) for attenuation of the small MDMA-induced increase in body temperature (37.3 °C to 37.1 °C, Dumont, Kramers et al. Citation2010), , ). In contrast, the evidence that ethanol abolishes the MDMA-induced increase in body temperature is quite strong in animal studies. Significant attenuation was observed in eight out of ten animal studies (six in rats and four in mice) (Cassel et al. Citation2004; Johnson et al. Citation2004; Hamida et al. Citation2006; Cassel et al. Citation2007; Hamida et al. Citation2007, Citation2008; Rodríguez-Arias et al. Citation2011; Ros-Simó et al. Citation2012, see ). Some rat studies only observed this attenuation after the first combined exposure, while others observed a consistent effect, also following multiple exposures at different days (Cassel et al. Citation2004, Citation2005; Izco et al. Citation2007; Hamida et al. Citation2008). Notably, one study showed progression in the attenuation of the MDMA-induced increase in body temperature by ethanol exposure (ethanol sensitization) (Hamida et al. Citation2008). However, attenuation was not present at high ambient temperatures (32 °C, Cassel et al. Citation2007, ).
Although eight animals studies observed an attenuation of the MDMA-induced increase in body temperature by concurrent ethanol exposure (), most studies administrated high MDMA doses, far above human recreational use, but possibly relevant during overdose (). The reduction in temperature during concurrent MDMA-ethanol exposure is attributed to the ethanol-induced peripheral vasodilation and possibly due to ethanol effects on neurons in the central nervous system, which are involved in thermoregulation (Hamida et al. Citation2007; Zuniga et al. Citation2020). However, even if the attenuation of the MDMA-induced increase in temperature by ethanol proofs true in humans at relevant MDMA doses, it is unlikely to have public health consequences (e.g. prevention measures), due the ethical concerns.
Cognitive and psychiatric effects
Regular MDMA exposure has been associated with poorer memory and psychomotor performance in humans (for review see Meyer Citation2013). However, no or limited effects on human psychomotor performance were observed during acute, and shortly after, MDMA exposure (Hernández-López et al. Citation2002; Kuypers et al. Citation2006; Ramaekers and Kuypers Citation2006; Dumont et al. Citation2008; Dumont, Schoemaker, et al. Citation2010, ). Ethanol exposure, when high enough, impairs psychomotor performance in humans (Fagan et al. Citation1994; Farquhar et al. Citation2002; for review see Charlet et al. Citation2013).
Table 4. Pharmacodynamics – psychomotor effects: additional ethanol effects (MDMA + ethanol vs lone MDMA).
No additional ethanol effects were observed upon concurrent MDMA-ethanol exposure on psychomotor function in five human studies and one rat study (Hernández-López et al. Citation2002; Cassel et al. Citation2005; Kuypers et al. Citation2006; Ramaekers and Kuypers Citation2006; Dumont et al. Citation2008; Spronk et al. Citation2014, ). However, one human study reported an increased psychomotor speed following MDMA exposure which was decreased during concurrent ethanol exposure (Dumont, Schoemaker, et al. Citation2010).
With respect to memory function, MDMA exposure lowered specific aspects of memory function (Dumont et al. Citation2008; Hernandez-Rabaza et al. Citation2010; Rodríguez-Arias et al. Citation2011; Vidal-Infer et al. Citation2012; Ros-Simó et al. Citation2013; Rostami et al. Citation2017, ). Ethanol exposure also disrupts specific aspects of memory function, including spatial and working memory (for review see Matthews and Silvers Citation2004; Charlet et al. Citation2013).
Table 5. Pharmacodynamics – cognition and psychiatric effects: additional ethanol effects (MDMA + ethanol vs lone MDMA).
The effect of concurrent MDMA-ethanol exposure on memory function was investigated in one human study (acutely) and five animal studies (3–22 days after exposure, rats and mice). Two animal studies (one rat, one mice) reported additional ethanol effects of decreased memory function (Hernandez-Rabaza et al. Citation2010; Vidal-Infer et al. Citation2012) while three other studies did not (two mice and one human, Dumont et al. Citation2008, Rodríguez-Arias et al. Citation2011; Ros-Simó et al. Citation2013).
With respect to anxiety, many human studies reported anxiety during MDMA exposure (for review see Baylen and Rosenberg Citation2006). Long-term effects have also been reported; regular ecstasy users report increased feelings of anxiety (Meyer Citation2013). In contrast, acute ethanol exposure reduces anxiety (for reviews see Charlet et al. Citation2013; Trantham-Davidson and Chandler Citation2015). Two mice studies reported increased anxiety a few days to three weeks after concurrent MDMA-ethanol exposure, compared to lone MDMA exposure (Rodríguez-Arias et al. Citation2011; Ros-Simó et al. Citation2012). MDMA exposure also reduced social animal behavior, which was not affected by concurrent ethanol exposure (Rodríguez-Arias et al. Citation2011, ).
Although there have been mixed results regarding the potential reinforcing effects of MDMA, most animal studies do show daily self-administration of low MDMA doses (for review see Schenk Citation2009). Also, many animal studies report a conditioned place preference (CPP) for MDMA (Bilsky et al. Citation1991; Aberg et al. Citation2007; Prus et al. Citation2009; Roger-Sánchez et al. Citation2013; García-Pardo et al. Citation2015). In addition, it is well known that acute ethanol exposure has rewarding and reinforcing effects (Sommer and Spanagel Citation2013; Trantham-Davidson and Chandler Citation2015).
MDMA-induced CPP was affected by concurrent ethanol exposure and depending on the specific exposure scenario, both attenuation and potentiation of CPP were observed in mice (Ribeiro Do Couto et al. Citation2011, Citation2012, ). A study in rats observed no CPP following lone MDMA exposure, but did observe CPP following concurrent MDMA-ethanol exposure (Jones et al. Citation2010, ). Possibly, the increased dopamine outflow upon concurrent MDMA-ethanol exposure observed in rat brain slices (Riegert et al. Citation2008), contributes to increases in rewarding and reinforcing effects. In addition, modulation of dopamine D1 receptors, which was shown of importance in locomotor potentiation, can also contribute (Hamida et al. Citation2020).
Cardiovascular effects
A recreational MDMA dose mildly increases heart rate and blood pressure in human volunteers (Dumont, Kramers, et al. Citation2010, Vizeli and Liechti Citation2017). This is largely mediated by increases in epinephrine and norepinephrine blood concentrations shortly after MDMA exposure (307% and 23% respectively) (Dumont, Kramers, et al. Citation2010). During high MDMA doses, tachycardia and hypertension can occur, as well as cardiac arrhythmias and myocardial infarction (Green et al. Citation2003; Dunlap et al. Citation2018). Acutely, ethanol exposure presumably has a limited effect on blood pressure, although higher doses (>5 drinks) can slightly increase blood pressure. Chronic alcohol abuse can result in hypertension (for review see Kawano Citation2010 and Piano Citation2017).
One human study investigated the acute biochemical and cardiovascular effects of concurrent MDMA-ethanol exposure. No additional increases were observed for epinephrine and norepinephrine blood concentrations, blood pressure and heart rate (Dumont, Kramers, et al. Citation2010, ). In addition, two mice studies reported potentiation of the MDMA-induced increase in biomarkers indicative for cardiotoxicity following concurrent ethanol exposure, but also an increase in protective factors (Navarro-Zaragoza et al. Citation2015, Citation2019, ).
Table 6. Pharmacodynamics – other: additional ethanol effects (MDMA + ethanol vs lone MDMA).
Hepatotoxic effects
In humans, MDMA can cause liver injury which sometimes results in acute liver failure requiring a liver transplantation (for review see Meyer Citation2013). MDMA-induced hepatotoxicity has also been reported in animals (Carvalho et al. Citation2010). In addition, it is well known that chronic ethanol exposure can lead to severe hepatotoxicity (Dinis-Oliveira et al. Citation2015). However, also acute exposure, primarily binge drinking can lead to liver damage (Massey and Arteel Citation2012).
One mice study investigated the effect of concurrent MDMA-ethanol exposure on the liver. A potentiation of MDMA-induced hepatotoxicity was reported, based on changes in biochemical and histopathological biomarkers (Pontes, Duarte, et al. Citation2008, ). In addition, two in vitro studies using primary rat hepatocytes showed ethanol aggravated the reduction in cell viability following high MDMA concentrations, especially at higher ambient temperature (Pontes, Sousa, et al. Citation2008; Pontes et al. Citation2010, ). At specific conditions (high MDMA concentration or high ambient temperature) ethanol exposure also aggravated the decrease in cell energy (ATP) and phase II metabolism (GSH, GST, GSSG), and increased oxidative stress (ROS), compared to lone MDMA exposure in primary rat and mice hepatocytes (Pontes, Santos-Marques, et al. Citation2008; Pontes, Sousa, et al. Citation2008).
Endocrine effects
In humans, MDMA exposure increased antidiuretic hormone (ADH, vasopressin) blood concentrations (Dumont, Kramers, et al. Citation2010). ADH promotes water retention, resulting in lower sodium blood concentrations and sometimes in hyponatremia. In severe cases, MDMA exposure can result in the syndrome of inappropriate antidiuretic hormone secretion (SIADH) causing cerebral edema and coma (Salathe et al. Citation2018). In contrast, acute ethanol exposure decreases ADH release, while during alcohol withdrawal ADH increases (for review see Harper et al. Citation2018).
One human study showed that concurrent ethanol exposure reversed the MDMA-induced increase of the ADH concentration. However, sodium concentrations were unaffected, possibly due to insufficient power of the study (Dumont, Kramers, et al. Citation2010).
Furthermore, MDMA increases cortisol and prolactin blood concentrations in humans. Concurrent ethanol exposure did not affect these concentrations (Dumont, Schoemaker, et al. Citation2010, ). Consistent findings for cortisol concentrations were reported in a rat study (Pacifici et al. Citation2001).
Study limitations
Most studies that were available for this review involved animal studies. Importantly, pharmacokinetics and pharmacodynamics in animals can differ from those in humans. Also, differences in experimental set-up between studies, such as a controlled environment versus a real life environment, can lead to variable outcomes. For example, the ambient temperature is often higher in real life than in volunteer studies, and MDMA users are often not naïve users but also not “binge” users, two dosing regimens that are often applied in animal studies. Even between controlled animal studies, differences in experimental setting often exist, hampering comparison or confirmation of observed effects. Finally, the dose applied in animal studies is often much higher than a human recreational dose, while neurobiological effects occur at similar doses in humans and rats (for review see Baumann et al. Citation2007). To illustrate, humans typically consume 1–2 tablets orally (150–300 mg, Van der Gouwe and Vrolijk Citation2019, 2–4 mg/kg), while animal studies apply doses that are 5–10 times higher, and often administrated via intraperitoneal injection ().
Conclusion
Concurrent ethanol exposure can modulate MDMA pharmacokinetics and pharmacodynamics. For a summary of studies that have investigated effects of concurrent MDMA-ethanol exposure, see and . Primarily animal studies have shown that concurrent ethanol exposure appears to aggravate many of MDMA’s effects, but also attenuates some. With respect to MDMA pharmacokinetics, most studies indicate only a small effect, or no effect, of concurrent ethanol exposure (, ). Therefore, most changes in pharmacodynamics upon concurrent MDMA-ethanol exposure are probably not due to changes in pharmacokinetics. Notably, many studies found additional ethanol effects on MDMA pharmacodynamics. For most effects, only a few studies are available and the majority indicated an aggravation of MDMA-induced biochemical, psychological, neurological and cardiovascular effects. However, a significant number of studies showed (relatively) consistent additional ethanol effects on the MDMA-induced increases in locomotor activity and body temperature. While locomotor activity in rats further increased upon concurrent exposure, a remarkable decrease in body temperature was consistently observed in many animal studies (, ). The relevance of this observation for human exposure remains unclear. Despite the extensive evidence from animal studies and hypothesized mechanisms explaining the decrease in body temperature upon concurrent ethanol exposure, it is in contrast with the scarce epidemiological data. These indicate that severe poisonings are more frequently observed during concurrent MDMA-ethanol poisoning compared to lone MDMA poisoning (35% vs 20% severe, Schürmann et al. Citation2019). Also, is it in contrast to the common believe that multi-intoxications have a more severe clinical course compared to mono-intoxications. For example, a large Swedish study (n∼14.000) reported that multiple drugs were involved in 80% of drug fatalities and alcohol was the most prevalent drug in mono- and multidrug fatalities (Jones et al. Citation2016).
In summary, concurrent ethanol exposure appears to increase the risk for MDMA toxicity, although it also appears to attenuate some clinically relevant effects.
Acknowledgements
The authors gratefully acknowledge the critical internal review and helpful comments provided by Prof. Dylan de Lange, Dutch Poisons Information Center, University Medical Center Utrecht, Utrecht, The Netherlands; and Dr. Margriet van Laar, Department of Drug Monitoring and Policy, Netherlands Institute of Mental Health and Addiction, Trimbos Institute, Utrecht, The Netherlands. Also, the authors gratefully acknowledge the suggestions of the editor and the reviewer. Their valuable suggestions have helped to improve the quality of this manuscript.
Declaration of interest
The primary employment of the authors is shown on the cover page. The Trimbos Institute is a nonprofit organization with activities in the field of mental health and the use of addictive substances like alcohol, tobacco and drugs, for example prevention, monitoring, research and information supply to the public, professionals and the government (https://www.trimbos.nl/english/). The Trimbos Institute is partly funded by the Dutch government (Ministry of Health, Welfare and Sport) and partly relies on project-based funding. This specific study was performed with own resources. The Dutch Poisons Information Center (DPIC) is part of the University Medical Center Utrecht and its toxicovigilance function is commissioned and financially funded by the Dutch government. The review, including conception, drafting, and final conclusions, is the work of the authors, and the views are not necessarily those of their employers. Both authors have not appeared in any legal proceedings in the last 5 years related to the contents of this paper.
Both authors designed the study. EV performed the systematic search. Both authors extracted data from the selected studies. LH wrote the first draft of the manuscript, and both authors contributed substantially to its revision. Both authors have approved of and contributed to the final manuscript and declare that there is no conflict of interest regarding the publication of this article. The authors have not appeared in any legal/regulatory proceedings. The authors were not compensated for the drafting of this manuscript. The authors do not have any financial or other conflicts of interest regarding this manuscript to report. The preparation of this review was conducted during normal course of author’s employment.
References
- Aberg M, Wade D, Wall E, Izenwasser S. 2007. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 29:37–46.
- Abrahao KP, Oliveira Goeldner F, Souza-Formigoni MLO. 2014. Individual differences in ethanol locomotor sensitization are associated with dopamine D1 receptor intra-cellular signaling of DARPP-32 in the nucleus accumbens. PloS One. 9:e98296.
- van Amsterdam J, Pennings E, van den Brink W. 2020. Fatal and non-fatal health incidents related to recreational ecstasy use. J Psychopharmacol. 34:591–599.
- Baumann M, Wang X, Rothman R. 2007. 4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacol. 3:407–424. 189.
- Baylen C, Rosenberg H. 2006. A review of the acute subjective effects of MDMA/ecstasy. Addiction. 101:933–947.
- Bilsky E, Hubbell C, Delconte J, Reid L. 1991. MDMA produces a conditioned place preference and elicits ejaculation in male rats: a modulatory role for the endogenous opioids. Pharmacol Biochem Behav. 40:443–447.
- Calle P, Maudens K, Lemoyne S, Geerts S, Van Sassenbroeck D, Jensen P, Van Overloop J, Deconinck E, Blanckaert P. 2019. Lessons to be learned from toxicological analyses in intoxicated patients and seized materials at an electronic music dance festival. Forensic Sci Int. 299:174–179.
- Calle P, Sundahl N, Maudens K, Wille SM, Van Sassenbroeck D, De Graeve K, Gogaert S, De Paepe P, Devriese D, Arno G, et al. 2018. Medical emergencies related to ethanol and illicit drugs at an annual, nocturnal, indoor, electronic dance music event. Prehosp Disaster Med. 33:71–76.
- Carvalho M, Pontes H, Remião F, Bastos M, Carvalho F. 2010. Mechanisms underlying the hepatotoxic effects of ecstasy. Curr Pharm Biotechnol. 11:476–495.
- Cassel JC, Hamida S, Ben Jones BC. 2007. Attenuation of MDMA-induced hyperthermia by ethanol in rats depends on ambient temperature. Eur J Pharmacol. 571:152–155.
- Cassel JC, Jeltsch H, Koenig J, Jones BC. 2004. Locomotor and pyretic effects of MDMA-ethanol associations in rats. Alcohol. 34:285–289.
- Cassel JC, Riegert C, Rutz S, Koenig J, Rothmaier K, Cosquer B, Lazarus C, Birthelmer A, Jeltsch H, Jones BC, et al. 2005. Ethanol, 3,4-methylenedioxymethamphetamine (Ecstasy) and their combination: long-term behavioral, neurochemical and neuropharmacological effects in the rat. Neuropsychopharmacology. 30:1870–1882.
- Charlet K, Beck A, Heinz A. 2013. The dopamine system in mediating alcohol effects in humans. Curr Top Behav Neurosci. 13:461–488.
- Cohen C, Perrault G, Sanger DJ. 1997. Evidence for the involvement of dopamine receptors in ethanol-induced hyperactivity in mice. Neuropharmacology. 36:1099–1108.
- Crawshaw LI, Wallace H, Crabbe J. 1998. Ethanol, body temperature and thermoregulation. Clin Exp Pharmacol Physiol. 25:150–154.
- Davies O, Batajoo-Shrestha B, Sosa-Popoteur J, Olibrice M. 2014. Full recovery after severe serotonin syndrome, severe rhabdomyolysis, multi-organ failure and disseminated intravascular coagulopathy from MDMA. Heart Lung. 43:117–119.
- Dinis-Oliveira JR, Magalhaes T, Queiros O, Proenca JB, Moreira R, de Lourdes Bastos M, Carvalho F. 2015. Signs and related mechanisms of ethanol hepatotoxicity. Curr Drug Abuse Rev. 8:86–103.
- Dumont GJ, Kramers C, Sweep F, Willemsen J, Touw D, Schoemaker R, van Gerven J, Buitelaar J, Verkes R. 2010. Ethanol co-administration moderates 3,4-methylenedioxymethamphetamine effects on human physiology. J Psychopharmacol. 24:165–174.
- Dumont GJ, Schoemaker R, Touw D, Sweep F, Buitelaar J, van Gerven J, Verkes R. 2010. Acute psychomotor effects of MDMA and ethanol (co-) administration over time in healthy volunteers. J Psychopharmacol. 24:155–164.
- Dumont GJH, Wezenberg E, Valkenberg MMGJ, de Jong CAJ, Buitelaar JK, van Gerven JMA, Verkes RJ. 2008. Acute neuropsychological effects of MDMA and ethanol (co-)administration in healthy volunteers. Psychopharmacology (Berl). 197:465–474.
- Dunlap L, Andrews A, Olson D. 2018. Dark classics in chemical neuroscience: 3,4-methylenedioxymethamphetamine. ACS Chem Neurosci. 9:2408–2427.
- [EMCDDA] European Monitoring Centre for Drugs and Drug addiction. 2019. European drug report 2019: trends and developments. Luxembourg: Publications Office of the European Union.
- [EMCDDA] European Monitoring Centre for Drugs and Drug addiction. 2020. Drug-related hospital emergency presentations in Europe: update from the Euro-DEN Plus expert network, technical report. Luxembourg: Publications Office of the European Union.
- Eyer F, Zilker T. 2007. Bench-to-bedside review: mechanisms and management of hyperthermia due to toxicity. Crit Care. 11:236.
- Fagan D, Tiplady B, Scott DB. 1994. Effects of ethanol on psychomotor performance under steady-state conditions. J Psychopharmacol. 8:75–80.
- Farquhar K, Lambert K, Drummond GB, Tiplady B, Wright P. 2002. Effect of ethanol on psychomotor performance and on risk taking behaviour. J Psychopharmacol. 16:379–384.
- Filev R, Engelke DS, Da Silveira DX, Mello LE, Santos-Junior JG. 2017. THC inhibits the expression of ethanol-induced locomotor sensitization in mice. Alcohol. 65:31–35.
- García-Pardo M, Escobar-Valero C, Rodríguez-Arias M, Miñarro J, Aguilar M. 2015. Involvement of NMDA glutamate receptors in the acquisition and reinstatement of the conditioned place preference induced by MDMA. Behav Pharmacol. 26:411–417.
- Van der Gouwe D, Vrolijk R. 2019. Jaarlijks rapport 2018: drugs informatie en monitoring system [Annual report 2018 drug information and monitoring system]. Trimbos Institute. Dutch.
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. 2003. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev. 55:463–508.
- Hall AP, Henry JA. 2006. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 96:678–685.
- Hamida SB, Bach S, Plute E, Jones BC, Kelche C, Cassel JC. 2006. Ethanol-ecstasy (MDMA) interactions in rats: preserved attenuation of hyperthermia and potentiation of hyperactivity by ethanol despite prior ethanol treatment. Pharmacol Biochem Behav. 84:162–168.
- Hamida SB, Plute E, Bach S, Lazarus C, Tracqui A, Kelche C, de Vasconcelos AP, Jones BC, Cassel JC. 2007. Ethanol–MDMA interactions in rats: the importance of interval between repeated treatments in biobehavioral tolerance and sensitization to the combination. Psychopharmacology (Berl). 192:555–569.
- Hamida SB, Plute E, Cosquer B, Kelche C, Jones BC, Cassel JC. 2008. Interactions between ethanol and cocaine, amphetamine, or MDMA in the rat: thermoregulatory and locomotor effects. Psychopharmacology (Berl). 197:67–82.
- Hamida SB, Tracqui A, de Vasconcelos AP, Szwarc E, Lazarus C, Kelche C, Jones BC, Cassel JC. 2009. Ethanol increases the distribution of MDMA to the rat brain: possible implications in the ethanol-induced potentiation of the psychostimulant effects of MDMA. Int J Neuropsychopharmacol. 12:749–759.
- Hamida SB, Lecourtier L, Loureiro M, Cosquer B, Tracqui A, Simmoneaux V, Nehlig A, Jones B, Pereira de Vasconcelos A, Cassel J. 2020. Ventral striatum regulates behavioral response to ethanol and MDMA combination. Addict Biol. e12938.
- Harper KM, Knapp DJ, Criswell HE, Breese GR. 2018. Vasopressin and alcohol: a multifaceted relationship. Psychopharmacology (Berl). 235:3363–3379.
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. 2013. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 13:489–509.
- Hernández-López C, Farré M, Roset PN, Menoyo E, Pizarro N, Ortuño J, Torrens M, Camí J, de La Torre R. 2002. 3,4-Methylenedioxymethamphetamine (Ecstasy) and alcohol interactions in humans: psychomotor performance, subjective effects, and pharmacokinetics. J Pharmacol Exp Ther. 300:236–244.
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, Renau-Piqueras J, Canales JJ. 2010. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addict Biol. 15:413–423.
- Hernández-Vázquez F, Reyes-Guzmán C, Méndez M. 2018. Impact of a novel environment on alcohol-induced locomotor activity in Wistar rats. Alcohol. 71:5–13.
- Hondebrink L, Zwartsen A, Westerink RHS. 2018. Effect fingerprinting of new psychoactive substances (NPS): what can we learn from in vitro data? Pharmacol Ther. 182:193–224.
- Izco M, Orio L, O'Shea E, Colado MI. 2007. Binge ethanol administration enhances the MDMA-induced long-term 5-HT neurotoxicity in rat brain. Psychopharmacology (Berl). 189:459–470.
- Johnson EA, O'Callaghan JP, Miller DB. 2004. Brain concentrations of d-MDMA are increased after stress. Psychopharmacology (Berl). 173:278–286.
- Jones A, Holmgren A, Ahlner J. 2016. Post-mortem concentrations of drugs determined in femoral blood in single-drug fatalities compared with multi-drug poisoning deaths. Forensic Sci Int. 267:96–103.
- Jones B, Hamida S, Ben Pereira de Vasconcelos A, Kelche C, Lazarus C, Jackisch R, Cassel J. 2010. Effects of ethanol and ecstasy on conditioned place preference in the rat. J Psychopharmacol. 24:275–279.
- Kawano K. 2010. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 33:181–191.
- Kuypers KPC, Samyn N, Ramaekers JG. 2006. MDMA and alcohol effects, combined and alone, on objective and subjective measures of actual driving performance and psychomotor function. Psychopharmacology (Berl). 187:467–475.
- Liechti ME. 2014. Effects of MDMA on body temperature in humans. Temperature (Austin). 1:192–200.
- Lovinger DM. 1997. Serotonin’s role in alcohol’s effects on the brain. Alcohol Health Res World. 21:114–120.
- Mallick A, Bodenham AR. 1997. MDMA induced hyperthermia: a survivor with an initial body temperature of 42.9 degrees C. J Accid Emerg Med. 14:336–338.
- Massey VL, Arteel GE. 2012. Acute alcohol-induced liver injury. Front Physiol. 3:193.
- Matthews DB, Silvers JR. 2004. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem. 82:299–308.
- Meyer J. 2013. 3,4-methylenedioxymethamphetamine (MDMA): current perspectives. Subst Abuse Rehabil. 4:83–99.
- Mohamed W, Hamida S, Ben Cassel J, de Vasconcelos A, Jones B. 2011. MDMA: interactions with other psychoactive drugs. Pharmacol Biochem Behav. 99:759–774.
- Mohamed WMY, Hamida S, Ben Pereira de Vasconcelos A, Cassel JC, Jones BC. 2009. Interactions between 3,4-methylenedioxymethamphetamine and ethanol in humans and rodents. Neuropsychobiology. 60:188–194.
- Monks TJ, Jones DD, Bai F, Lau S. 2004. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit. 26:132–136.
- Monshouwer K, van der Pol P, Drost YC, van Laar MW. 2016. Het Grote Uitgaansonderzoek 2016 [The major nightlife study]. Trimbos Institute. Dutch.
- Moore TO, June HL, Lewis MJ. 1993. Ethanol-induced stimulation and depression on measures of locomotor activity: effects of basal activity levels in rats. Alcohol. 10:537–540.
- Morefield K, Keane M, Felgate P, White J, Irvine R. 2011. Pill content, dose and resulting plasma concentrations of 3,4-methylendioxymethamphetamine (MDMA) in recreational ‘ecstasy’ users. Addiction. 106:1293–1300.
- Navarro-Zaragoza J, Ros-Simó C, Milanés MV, Valverde O, Laorden ML. 2015. Binge ethanol and MDMA combination exacerbates toxic cardiac effects by inducing cellular stress. PLoS One. 10:e0141502.
- Navarro-Zaragoza J, Ros-Simó C, Milanés MV, Valverde O, Laorden ML. 2019. Binge ethanol and MDMA combination exacerbates HSP27 and Trx-1 (biomarkers of toxic cardiac effects) expression in right ventricle. Life Sci. 220:50–57.
- Noseda R, Schmid Y, Scholz I, Liakoni E, Liechti M. 2020. MDMA-related presentations to the emergency departments of the European Drug Emergencies Network plus (Euro-DEN Plus) over the four-year period 2014–2017. Clin Toxicol.
- Pacifici R, Zuccaro P, Hernandez López C, Pichini S, Di Carlo S, Farré M, Roset PN, Ortuño J, Segura J, Torre RL. 2001. Acute effects of 3, 4-methylenedioxymethamphetamine alone and in combination with ethanol on the immune system in humans. J Pharmacol Exp Ther. 296:207–215.
- Palamar JJ. 2020. Diffusion of ecstasy in the electronic dance music scene. Subst Use Misuse. 55:2243–2250.
- Palamar JJ, Acosta P. 2020. Virtual raves and happy hours during COVID-19: new drug use contexts for electronic dance music partygoers. Int J Drug Policy.
- Patel M, Belson M, Longwater A, Olson K, Miller M. 2005. Methylenedioxymethamphetamine (ecstasy)-related hyperthermia. J Emerg Med. 29:451–454.
- Piano MR. 2017. Alcohol’s effects on the cardiovascular system. Alcohol Res. 38:219–241.
- Pontes H, Duarte JA, de Pinho PG, Soares ME, Fernandes E, Dinis-Oliveira RJ, Sousa C, Silva R, Carmo H, Casal S, et al. 2008. Chronic exposure to ethanol exacerbates MDMA-induced hyperthermia and exposes liver to severe MDMA-induced toxicity in CD1 mice. Toxicology. 252:64–71.
- Pontes H, de Pinho PG, Fernandes E, Branco PS, Ferreira LM, Carmo H, Remião F, Carvalho F, Bastos ML. 2010. Metabolic interactions between ethanol and MDMA in primary cultured rat hepatocytes. Toxicology. 270:150–157.
- Pontes H, Santos-Marques M, Fernandes E, Duarte J, Remião F, Carvalho F, Bastos M. 2008. Effect of chronic ethanol exposure on the hepatotoxicity of ecstasy in mice: an ex vivo study. Toxicol Vitr. 22:910–920.
- Pontes H, Sousa C, Silva R, Fernandes E, Carmo H, Remião F, Carvalho F, Bastos ML. 2008. Synergistic toxicity of ethanol and MDMA towards primary cultured rat hepatocytes. Toxicology. 254:42–50.
- Prus AJ, James JR, Rosecrans JA. 2009. Methods of behavior analysis in neuroscience. 2nd ed. Boca Raton (FL): Buccafusco JJ. Chapter 4, Conditioned place preference.
- Ramaekers JG, Kuypers KPC. 2006. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on behavioral measures of impulsivity: Alone and in combination with alcohol. Neuropsychopharmacology. 31:1048–1055.
- Ribeiro Do Couto B, Daza-Losada M, Rodríguez-Arias M, Nadal R, Guerri C, Summavielle T, Miñarro J, Aguilar MA. 2012. Adolescent pre-exposure to ethanol and 3,4-methylenedioxymethylamphetamine (MDMA) increases conditioned rewarding effects of MDMA and drug-induced reinstatement. Addict Biol. 17:588–600.
- Ribeiro Do Couto B, Rodríguez-Arias M, Fuentes S, Gagliano H, Armario A, Miñarro J, Aguilar MA. 2011. Adolescent pre-exposure to ethanol or MDMA prolongs the conditioned rewarding effects of MDMA. Physiol Behav. 103:585–593.
- Rickli A, Hoener MC, Liechti ME. 2015. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. 25:365–376.
- Riegert C, Wedekind F, Hamida S, Ben Rutz S, Rothmaier AK, Jones BC, Cassel JC, Jackisch R. 2008. Effects of ethanol and 3,4-methylenedioxymethamphetamine (MDMA) alone or in combination on spontaneous and evoked overflow of dopamine, serotonin and acetylcholine in striatal slices of the rat brain. Int J Neuropsychopharmacol. 11:743–763.
- Rietjens SJ, Hondebrink L, Westerink RHS, Meulenbelt J. 2012. Pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine (MDMA): interindividual differences due to polymorphisms and drug-drug interactions. Crit Rev Toxicol. 42:854–876.
- Rodríguez-Arias M, Maldonado C, Vidal-Infer A, Guerri C, Aguilar MA, Miñarro J. 2011. Intermittent ethanol exposure increases long-lasting behavioral and neurochemical effects of MDMA in adolescent mice. Psychopharmacology (Berl). 218:429–442.
- Roger-Sánchez C, Rodríguez-Arias M, Miñarro J, Aguilar M. 2013. Involvement of 5-hydroxytryptamine 5-HT3 serotonergic receptors in the acquisition and reinstatement of the conditioned place preference induced by MDMA. Eur J Pharmacol. 714:132–141.
- Ros-Simó C, Ruiz-Medina J, Valverde O. 2012. Behavioural and neuroinflammatory effects of the combination of binge ethanol and MDMA in mice. Psychopharmacology (Berl). 221:511–525.
- Ros-Simó C, Moscoso-Castro M, Ruiz-Medina J, Ros J, Valverde O. 2013. Memory impairment and hippocampus specific protein oxidation induced by ethanol intake and 3, 4-Methylenedioxymethamphetamine (MDMA) in mice. J Neurochem. 125:736–746.
- Rostami M, Rezayof A, Alijanpour S, Sharifi KA. 2017. Hippocampal nicotinic receptors have a modulatory role for ethanol and MDMA interaction in memory retrieval. Brain Res. 1669:11–17.
- Ryczko D, Dubuc R. 2017. Dopamine and the brainstem locomotor networks: from lamprey to human. Front Neurosci. 11:295.
- Salathe C, Blanc A, Tagan D. 2018. SIADH and water intoxication related to ecstasy. BMJ Case Rep.
- Schenk S. 2009. MDMA self-administration in laboratory animals: a summary of the literature and proposal for future research. Neuropsychobiology. 60:130–136.
- Schürmann L, Croes E, Lameijer M, Valkenberg H. 2019. Monitor drugsincidenten 2018 [Monitor drugs incidents 2018]. Trimbos Institute. Dutch.
- Sessa B, Sakal C, O’Brien S, Nutt D. 2019. First study of safety and tolerability of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy in patients with ethanol use disorder: preliminary data on the first four participants. BMJ Case Rep. 12:e230109.
- Sommer WH, Spanagel R. 2013. Behavioral neurobiology of alcohol addiction. Berlin (Germany): Springer.
- Spronk DB, Dumont GJH, Verkes RJ, De Bruijn ERA. 2014. The acute effects of MDMA and ethanol administration on electrophysiological correlates of performance monitoring in healthy volunteers. Psychopharmacology (Berl). 231:2877–2888.
- Trantham-Davidson H, Chandler LJ. 2015. Alcohol-induced alterations in dopamine modulation of prefrontal activity. Alcohol. 49:773–779.
- Upreti VV, Eddington ND, Moon KH, Song BJ, Lee IJ. 2009. Drug interaction between ethanol and 3,4-methylenedioxymethamphetamine (“ecstasy”). Toxicol Lett. 188:167–172.
- Veldstra JL, Brookhuis KA, de Waard D, Molmans BHW, Verstraete AG, Skopp G, Jantos R. 2012. Effects of alcohol (BAC 0.5‰) and ecstasy (MDMA 100 mg) on simulated driving performance and traffic safety. Psychopharmacology (Berl). 222:377–390.
- Vidal-Infer A, Aguilar MA, Miñarro J, Rodríguez-Arias M. 2012. Effect of intermittent exposure to ethanol and MDMA during adolescence on learning and memory in adult mice. Behav Brain Funct. 8:32.
- Vizeli P, Liechti ME. 2017. Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol. 31:576–588.
- Wasielewski JA, Holloway FA. 2001. Alcohol’s interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 25:94–100.
- WDR. World Drug Report 2019. United Nations publication; 2019. Sales No. E.19.XI.8.
- Zuniga A, Ryabinin AE, Cunningham CL. 2020. Effects of pharmacological inhibition of the centrally-projecting Edinger-Westphal nucleus on ethanol-induced conditioned place preference and body temperature. Alcohol. 87:121–131.
- Zwartsen A, Verboven AHA, van Kleef RGDM, Wijnolts FMJ, Westerink RHS, Hondebrink L. 2017. Measuring inhibition of monoamine reuptake transporters by new psychoactive substances (NPS) in real-time using a high-throughput, fluorescence-based assay. Toxicol in Vitro. 45:60–71.
