Abstract
During the past century, discoveries of microorganisms as causes of infections and antibiotics as effective therapeutic agents have contributed to significant gains in public health in many parts of the world. Health agencies worldwide are galvanizing attention toward antibiotic resistance, which is a major threat to public health (Centers for Disease Control and Prevention, 2013; World Health Organization, 2014). Some life scientists believe that we are approaching the post-antibiotic age (Davies & Davies, 2010). The growing threat of antimicrobial resistance is fueled by complex factors with biological, behavioral, and societal aspects. This primer provides an overview of antibiotic resistance and its growing burden on public health, the biological and behavioral mechanisms that increase antibiotic resistance, and examples of where health communication scholars can contribute to efforts to make our current antibiotic drugs last as long as possible. In addition, we identify compelling challenges for current communication theories and practices.
WHAT IS AN ANTIBIOTIC DRUG?
Antibiotics are chemical substances that kill bacteria or slow bacterial growth; they are naturally produced by fungi and other microorganisms. An antibiotic drug refers to the synthesized medicine that is used to treat bacterial infections. In any given bacterial infection, there is a large population (e.g., millions or more) of bacteria in the human body. The term “antimicrobial” broadly refers to drugs that are used to treat infections caused by a variety of microorganisms (e.g., bacteria, viruses, fungi, parasites such as malaria).
WHEN WERE ANTIBIOTICS FIRST USED?
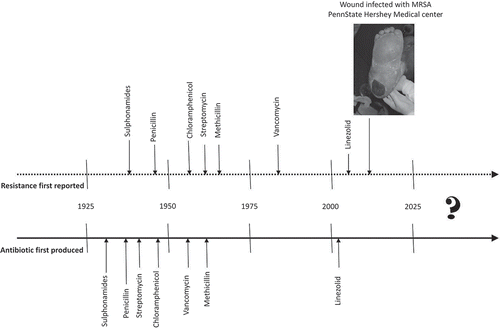
During the past century, scientific discoveries of microorganisms as causes of infections and antibiotics as effective therapeutic agents have contributed to enormous gains in public health (Davies & Davies, Citation2010). The first antibiotic widely distributed in the United States was penicillin in the mid-1940s, and a clinical bacterium isolate resistant to penicillin was documented shortly after (Miller, Citation1947). From the 1940s to the 1960s, 20 new classes of antibiotics were created (Coates, Halls, & Hu, Citation2011), and resistant strains appeared within 5 years of each (Centers for Disease Control and Prevention [CDC], Citation2013; see ). In the early 1960s, research and development of antibiotics by pharmaceutical companies stalled (Davies & Davies, Citation2010); since 1962, only two new classes have reached the market (Coates et al., Citation2011). Use and distribution of antibiotics, however, increased.
In the past 60 years, millions of metric tons of antibiotics have been produced and distributed worldwide in various products (e.g., toiletries, cleaning products) for many purposes (Davies & Davies, Citation2010), including veterinary use (75% of the antibiotic market in the United States; Fauci & Marston, Citation2014). People are exposed to antibiotics through their water (Venkatesan & Halden, Citation2014) and toiletries, in addition to what they take as prescriptions. In 2010, 258 million courses of oral antibiotics were dispensed (833 prescriptions per 1000 persons; Hicks & Taylor, Citation2013). Importantly, 50% of prescribed antibiotics are unnecessary (CDC, Citation2013). For example, although antibiotics are ineffective against viruses, nearly 75% of U.S. adults seeking treatment for acute bronchitis, generally caused by a virus, are prescribed antibiotics (Fauci & Marston, Citation2014).
WHAT DOES ANTIBIOTIC RESISTANCE MEAN?
Antibiotic resistance refers to genetic changes in bacteria that reduce or eliminate an antibiotic’s ability to destroy it (CDC, Citation2013). Drug resistance happens for almost every antimicrobial drug, not just antibiotics, and in almost all pathogens and parasites, not just bacteria. Whenever drugs are used against viruses, fungi, single-celled animals (e.g., malaria parasites), and multicelled animals (e.g., lice, parasitic worms), drug resistance almost always evolves and undermines drug efficacy (zur Wiesch, Kouyos, Engelstadter, Regoes, & Bohnoeffer, Citation2011); this is referred to as antimicrobial resistance.
HOW WIDESPREAD IS ANTIBIOTIC RESISTANCE?
Some life scientists believe that we are approaching the post-antibiotic age (Davies & Davies, Citation2010). Approximately 50% of tested infections for Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus showed resistance to commonly used antibiotics (World Health Organization [WHO], Citation2014). In the United States alone, more than 2 million people acquire antibiotic-resistant infections and at least 23,000 people die as a direct result (CDC, Citation2013). The agencies producing these estimates acknowledge that they underestimate the problem. Resistant infections can be difficult to detect in clinical settings (CDC, Citation2013; WHO, Citation2014). Still, in 2011, one in every 25 inpatients in U.S. acute care hospitals had a hospital-acquired infection, and 9700 of these infections were methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (Magill et al., Citation2014). Of particular concern are gram-negative pathogens, such as Pseudomonas aeruginosa (associated with diseases such as pneumonia; Peleg & Hooper, Citation2010), because “they are becoming resistant to nearly all drugs that would be considered for treatment” (CDC, Citation2013, p. 22). Health agencies around the globe are galvanizing attention toward antibiotic resistance, because it is considered a major threat to public health (CDC, Citation2013; WHO, Citation2014).
WHAT CAUSES ANTIBIOTIC RESISTANCE?
Several factors determine the useful lifetime of an antibiotic drug. The first is the time until resistance initially arises. Bacteria with genes providing antibiotic resistance are found naturally in microbial populations: they can arise de novo because of random mutations in a bacterium or as a method of surviving antibiotics produced by competing bacteria (D’Costa, McGrann, Hughes, & Wright, Citation2006).
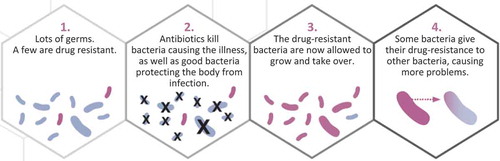
Once the gene is present in a population, by mutational accident or by virtue of history, bacteria can acquire the resistance genes by descent, as we inherit our genes from our parents or, unlike us, from nonrelatives (Step 4 in ). This phenomenon of lateral gene transfer—the acquisition of a gene from another unrelated bacterium—often involves plasmids or other mobile genetic elements that can rapidly be exchanged between bacteria (Davies & Davies, Citation2010). Importantly, these genetic elements can be exchanged between members of different species of bacteria. This phenomenon has profound implications for antibiotic preservation. To see why, consider the following illustration.
FIGURE 2 How exposure to antibiotics creates resistance (CDC, Citation2013, p. 14). © CDC. Reproduced by permission of the CDC. Permission to reuse must be obtained from the rightsholder.
Imagine Susan has been infected with Bacteria X, which lives in the human gut and is targeted by Drug Y. Six months earlier Susan took Drug Y for a different infection, which may or may not have been due to Bacteria X. Bacteria X is not the only bacteria in Susan’s system. Millions of bacteria thrive in healthy humans, performing many helpful functions; many of these “helpful” bacteria were also killed when she took Drug Y, but the ones that were resistant to Drug Y survived. During Susan’s new infection, the Bacteria X multiplying in Susan’s system laterally transferred genes with the Drug-Y-resistant helpful bacteria nearby; thus, Susan’s Bacteria X infection becomes resistant to Drug Y.
Although antibiotic resistance is a natural adaptation, human use of antibiotic drugs exacerbates its appearance and spread (Fauci & Marston, Citation2014) through evolution by natural selection. Several steps are involved (). Although individual bacteria in a bacterial infection may vary from each other in many ways, the evolution of drug resistance begins when at least one bacterium is resistant to an antibiotic drug (i.e., drug-resistant), such as Drug Y. When Drug Y enters the system (e.g., after taking an antibiotic), the drug acts disproportionately against the bacteria without resistance (i.e., drug-sensitive bacteria, Step 2 in ). The drug-sensitive bacteria die, while some drug-resistant bacteria continue to replicate. This replication advantage can be substantial: resistant bacteria not only survive the drug treatment, but also have fewer competitors for resources, because the drug-sensitive bacteria have been removed (Step 3 in ). Thus, drug-resistant bacteria can dominate the bacterial population in an infection, causing drug treatments to fail.
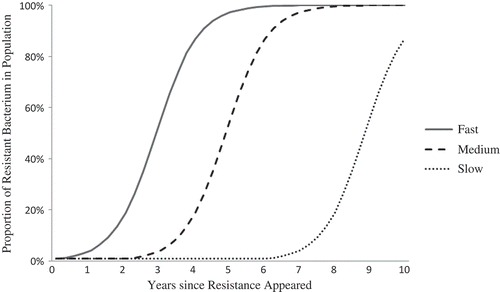
If Drug-Y-resistant Bacteria X are transmitted to another person, then they may spread through the entire host population. If the Drug-Y-resistant bacteria are transmitted to a susceptible host taking Drug Y, the resistant bacteria gain the advantage again, and may continue to spread, thus becoming more common in the overall Bacteria X population in humans. In the simplest case, the spreading resistance looks like the curve shown in . The curve’s shape depends on the proportion of infected people who get treated with Drug Y, and the dose and length of exposure to the drug in treated people. All else equal, the more people who are treated with Drug Y and the more aggressive the treatment (e.g., longer courses at high concentrations), the faster resistance spreads, because the drug treatment itself confers the selective advantage on the resistant bacteria.
HOW DO WE SLOW THE EVOLUTION OF ANTIBIOTIC RESISTANCE?
To slow the evolution of antibiotic resistance, we must reduce the two determinants of the speed of the evolutionary process: (a) the probability a resistance gene will first appear in a disease-causing organism (by natural mutation or lateral gene transfer), and (b) the strength of the reproductive advantage conferred on that resistance by drug use.
One method to reduce the probability that resistance will first arise in an otherwise drug-sensitive pathogen population is antibiotic stewardship. Every administered dose of antibiotic drugs selects for resistance in the harmless bacteria that naturally occur on or in us, which can be a potential source for the lateral transfer of resistance genes to bacterial infections. The lower our antibiotic use, the fewer of those genes will reside in us, or in our environment, thereby reducing the likelihood that pathogens acquire resistance from them. Thus, it is critical that people take antibiotics only when necessary. Ideally, people would take only narrow-spectrum antibiotics, which precisely target problematic bacteria with little collateral effect on harmless or useful bacteria that reside on or in us. The first rule of resistance management is to take antibiotics only when absolutely necessary. The second rule is to choose those that cause the smallest amount of selection for resistance in nontarget organisms.
Two major methods by which to slow spread are to reduce the number of infections and to reduce antibiotic use in community and health care settings (CDC, Citation2013). Whenever an infection is prevented, one less person may be prescribed drugs. Infections can be prevented by vaccines, hygiene, and sanitation, as well as by isolation during infectivity. These steps can be challenging to implement; even in hospitals, it is a challenge to implement routine hand washing.
Reducing antibiotic use slows the spread of resistance genes once they emerge in pathogen populations. Prescribing antibiotic drugs at all sets up an interesting conundrum. If we can, in fact, destroy every bacterium X in a person with a treatment of drug Y (i.e., all are drug sensitive), then we could prevent any bacterium from acquiring resistance in the first place (dead bacteria cannot mutate or receive resistance genes by lateral transfer). However, if resistant bacteria are already present, the more aggressively we kill the drug-sensitive bacteria, the stronger is the evolutionary advantage we confer on any existing, drug-resistant bacteria. When full eradication is not guaranteed, then biologically we can reduce the selection pressure to evolve resistance by reducing antibiotic use (e.g., treating for fewer days; Huijben et al., Citation2013). Hence the conundrum: Aggressive treatment may help to prevent resistance from appearing in the first place, but at the cost of maximizing selection in favor of any existing resistance (Read et al., Citation2011). Balancing these opposing forces for resistance management is an area of controversy in the life sciences (e.g., Ankomah & Levin, Citation2014). This biological uncertainty presents a nontrivial conundrum for influencing prescription behavior, because it may create mixed messages about how long to take antibiotic drugs. The best resistance management solution will likely vary with the details of the bacteria, drug, and clinical and epidemiological circumstances (Read et al., Citation2011). As the science advances, the complexities in designing health messages and conveying them to clinicians, patients, and the general public will increase.
WHAT IS THE ROLE OF HEALTH COMMUNICATION IN ANTIBIOTIC RESISTANCE?
Antibiotic use and misuse are present globally, which increases the likelihood of evolution and spread of antibiotic resistance (Fauci & Marston, Citation2014). Multiple actions and actors worldwide are needed to slow it. The CDC (Citation2013) has outlined four strategies to slow antibiotic resistance: (a) prevent infections, (b) increase surveillance, (c) improve antibiotic stewardship, and (d) develop new drugs and diagnostic tests. Health communication scholars can assist with the behavioral goals for preventing infections, such as promoting vaccination for seasonal influenza and proper hand hygiene in public and private settings (Barnett & Linder, Citation2014). Equally, the suboptimal adherence to hand hygiene among health care providers (Pittet & Boyce, Citation2001) demands attention.
Mass media campaigns, such as the “Get Smart” campaign, have shown reductions in the unnecessary use of antibiotics, particularly in pediatrics (Gonzales et al., Citation2008), an important area because prescription rates in the United States are highest among children under 10 years (Hicks & Taylor, Citation2013). Yet persons 65 years or older have similarly high rates (Hicks & Taylor, Citation2013) and are the most likely to receive broad-spectrum agents (Gahbauer, Gonzales, & Guglielmo, Citation2014). Older adults have received little attention and may be a promising group for future interventions.
Clinicians and the public likely vary in their beliefs, attitudes, behaviors, and barriers to change. Clinicians, for example, vary in their knowledge of antibiotic resistance and perceived barriers to changing their prescription practices (Ackerman, Gonzales, Stahl, & Metlay, Citation2013). Members of the public may not know that nonessential use of antibiotics is harmful to themselves and others. Public knowledge about the antibiotic use in food-animal production and the need for antibiotic stewardship in this context may also be limited: A large survey showed that only 52% of the public were aware of antibiotic use in animal feed (M’ikanatha, Dewar, Rankin, & Lautenbach, Citation2007). Last, people exposed to germ-warfare metaphors describing the relationship between humans and bacteria (humans-good/microbes-bad; Lederberg, Citation2000) may have misperceptions about antibiotic resistance. We may need to segment target audiences, based on shared beliefs, attitudes, fears, behaviors, or barriers, to provide different, targeted interventions.
At the policy level, attempts around the world to ban nontherapeutic uses of antibiotics have been difficult to employ, much less the widespread, unregulated distribution of antibiotics in countries with bans in place (Davies & Davies, Citation2010). Researchers need theoretical guidance on how to prepare systems (communities, states, nations) for the uptake and diffusion of new or different beliefs and practices. Furthermore, researchers need theoretical guidance on which parts of health messages are retained and which parts change as these messages spread through communication networks. Historically, social science disciplines have never enjoyed the funding and resources of the life sciences, and in the life sciences, resources have been skewed toward new drugs and diagnostics, instead of how to evolution-proof existing drugs. We need theoretical guidance on how to make large-scale changes with limited resources and on how to monitor and adapt to changes created by communication shocks to the existing system.
CONCLUSION
Antibiotic resistance is a major public health concern, as drugs that were once highly efficacious no longer cure bacterial infections. In addition to greater morbidity, disability, and mortality, antibiotic-resistant infections contribute to other costs: They require prolonged and costlier treatments, extended hospital stays, and additional medical visits (WHO, Citation2014). The viability of many areas of medicine is threatened. Antibiotics are involved in cancer treatments, organ transplantation, general surgery, and a range of therapies against autoimmune diseases (CDC, Citation2013). The growing threat of antimicrobial resistance is fueled by complex biological, behavioral, and societal factors. Transdisciplinary collaborations (Parrott & Kreuter, Citation2011) involving social, biological, medical, and public health scholars are needed to address the growing health burdens imposed by drug-resistant infections.
ACKNOWLEDGEMENTS
We thank Amanda Applegate, Madisen Quesnell, and Lydia Glick for feedback on earlier versions of this primer; Bob Woods for discussion; and Sameh Boktor for assistance with figures.
FUNDING
This project was supported by Awards P50 DA010075 from the National Institute on Drug Abuse (NIDA; RS) and R01-GM089932 from the National Institute for General Medicine (NIGM; AR). The content is solely the responsibility of the authors and does not necessarily represent the views of NIDA, NIGM, or the National Institutes of Health.
Additional information
Funding
REFERENCES
- Ackerman, S. L., Gonzales, R., Stahl, M. S., & Metlay, J. P. (2013). One size does not fit all: Evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Services Research, 13, 1–9. doi:10.1186/1472-6963-13-462
- Ankomah, P., & Levin, B. R. (2014). Exploring the collaboration between antibiotics and the immune response in the treatment of acute self-limiting infections. Proceedings of the National Academy of Sciences USA. Advance online publication. doi:10.1073/pnas.1400352111
- Barnett, M. L., & Linder, J. A. (2014). Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. Journal of the American Medical Association. 311, 2020–2022.
- Centers for Disease Control and Prevention. (2013). Antibiotic resistance threats in the United States, 2013. Retrieved from http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
- Coates, A. R., Halls, G., & Hu, Y. (2011). Novel classes of antibiotics or more of the same? British Journal of Pharmacology, 163, 184–194. doi:10.1111/j.1476-5381.2011.01250.x
- D’Costa, V. M., McGrann, K. M., Hughes, D. W., & Wright, G. D. (2006). Sampling the antibiotic resistome. Science, 311, 374–377.
- Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews, 74, 417–433. doi:10.1128/MMBR.00016-10
- Fauci, A. S., & Marston, H. D. (2014). The perpetual challenge of antimicrobial resistance. Journal of the American Medical Association. Advance online publication. doi:10.1001/jama.2014.2465
- Gahbauer, A. M., Gonzales, M. L., & Guglielmo, B. J. (2014). Patterns of antibacterial use and impact of age, race/ethnicity, and geographic region on antibacterial use in an outpatient medicaid cohort. Pharmacotherapy. Advance online publication. doi:10.1002/phar.1425
- Gonzales, R., Corbett, K. K., Wong, S., Glazner, J. E., Deas, A., Leeman-Castillo, B., … Kafadar, K. (2008). “Get smart Colorado”: Impact of a mass media campaign to improve community antibiotic use. Medical Care, 46, 597–605. doi:10.1097/MLR.0b013e3181653d2e
- Hicks, L. A., & Taylor, T. H. (2013). U.S. outpatient antibiotic prescribing, 2010. New England Journal of Medicine, 368, 1461–1462. doi:10.1056/NEJMc1212055
- Hornik, R., & Woolf, K. D. (1999). Using cross-sectional surveys to plan message strategies. Social Marketing Quarterly, 5, 34–41. doi:10.1080/15245004.1999.9961044
- Huijben, S., Bell, A. S., Sim, D. G., Tomasello, D., Mideo, N., Day, T., & Read, A. (2013) Aggressive chemotherapy and the selection of drug resistant pathogens. PLoS Pathogens 9(9), e1003578. doi:10.1371/journal.ppat.1003578
- Lederberg, J. (2000). Infectious history. Science, 288, 287–293. doi:10.1126/science.288.5464.287
- Magill, S. S., Edwards, J. R., Bamberg, W., Beldavs, Z. G., Dumyati, G., Kainer, M. A., … Fridkin, M. D. (2014). Multistate point-prevalence survey of health care-associated infections. New England Journal of Medicine, 370, 1198–1208. doi:10.1056/NEJMoa1306801
- M’ikanatha, N., Dewar, R. F., Rankin, S. C., & Lautenbach, E. (2007 , October). Prevalence of antibiotic prescriptions and attitudes toward antibiotic-free food. Poster presented at the annual meeting of the Infectious Diseases Society of America, San Diego, CA.
- Miller, C. P. (1947). Development of bacterial resistance to antibiotics. Journal of the American Medical Association, 135, 749–751. PMID:18897582
- Parrott, R. L., & Kreuter, M. W. (2011). Multidisciplinary, interdisciplinary, and transdisciplinary approaches to health communication: Where do we draw the lines? In T. L. Thompson, R. Parrott, & J. F. Nussbaum ( Eds.), The Routledge handbook of health communication (2nd ed., pp. 3–17). New York, NY: Routledge.
- Peleg, A. Y., & Hooper, D. C. (2010). Hospital-acquired infections due to gram-negative bacteria. New England Journal of Medicine, 362, 1804–1813. doi:10.1056/NEJMra0904124
- Pittet, D., & Boyce, J. M. (2001). Hand hygiene and patient care: Pursuing the Semmelweis legacy. Lancet Infectious Diseases, 1, 9–20. doi:10.1016/S1473-3099(09)70295-6
- Read, A. F., Day, T., & Huijben, S. (2011) The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proceedings of the National Academy of Sciences USA, 108, 10871–10877.
- U.S. Census Bureau. (2012). American community survey, 2012. Retrieved from http://factfinder2.census.gov
- Venkatesan, A. K., & Halden, R. U. (2014). Wastewater treatment plants as chemical observatories to forecast ecological and human health risks of manmade chemicals. Scientific Reports, 4, 1–7. doi:10.1038/srep03731
- World Health Organization. (2014). Antimicrobial resistance: Global report on surveillance. Geneva, Switzerland: WHO.
- Zur Wiesch, P. A., Kouyos, R., Engelstadter, J., Regoes, R. R., & Bonhoeffer, S. (2011). Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infectious Diseases, 11, 236–247. doi:10.1016/S1473-3099(10)70264-4