ABSTRACT
The consumption of culinary herbs and spices in our daily dietary intake has been known to reduce the risk of developing chronic diseases. The efficacy of these seasoning agents towards preventing or alleviating chronic diseases is facilitated through the synergistic effect of their secondary metabolites. A majority of the scientific reviews which have been conducted till present emphasise that the positive effects of culinary herbs and spices on human health is predominantly due to the presence of dietary polyphenols. This review however will focus on the therapeutic benefits of essential oils of culinary herbs and spices from the genera Curcuma and Zingiber in the management of Type 2 Diabetes Mellitus and Alzheimer’s Disease.
Introduction
According to the World Health Organization (WHO), non-communicable diseases (NCDs) have been reported to be responsible for the death of more than 38 million people every year, among which 70% of these fatalities occur in middle-income countries (Citation1).
T2DM and AD are the two most prevalent NCDs worldwide (Citation2–4). The number of people suffering from T2DM and AD and deaths attributed to T2DM and AD is increasing at an alarming rate (Citation5–14).
The inhibition of the carbohydrate-hydrolyzing enzymes (alpha-amylase and alpha-glucosidase) in the human digestive organs and the cholinesterase enzymes (AChE and BChE) in the brain are crucial in controlling blood glucose and acetylcholine levels, thus making them effective therapeutic targets in the management of T2DM and AD (Citation15–18). Currently approved oral synthetic anti-diabetic and anti-Alzheimer’s drugs are expensive and have shown serious side effects (Citation19–26).
As a result, the resurgence of interest in the ethnopharmacological use of medicinal food plants as part of our diet to treat and prevent T2DM and AD has emerged in recent years. Conventional dietary methods include the use of culinary herbs, spices, fruits and vegetables (Citation12,Citation27–31). Globally, people are resorting to natural and herbal sources for this purpose as they are affordable, easily available and have fewer side effects compared to modern drug therapy.
Spices and herbs exhibit a broad spectrum of health benefits (Citation32–37). Therefore, the consumption of food and drinks which have been seasoned with these spices and herbs will indirectly protect the human body against the development of chronic diseases (Citation38,Citation39). These health promoting effects of spices and herbs are attributed to the presence of bioactive secondary metabolites; predominantly the phenolic acids and flavonoids (Citation33,Citation35,Citation36,Citation38,Citation40). Essential oils (EOs) which are also present in most spices and herbs are also partly responsible for many of their functional properties (Citation37).
Curcuma and Zingiber are economically important genera of the Zingiberaceae, having both food and medicinal values. Their rhizomes are used as spices and flavoring agents due to their aromatic odour as well as their pungent and spicy taste. Additionally, they are also used as preservatives to maintain the quality of food and as household remedies for the treatment of many ailments (Citation41–43).
Plants of the genera Curcuma and Zingiber produce EOs which have demonstrated various health-related biological activities. The current paper reviews the published scientific literature related to the enzyme inhibitory activities of the EOs from the Curcuma and Zingiber species which are used as culinary herbs and spices, with the emphasis being on the carbohydrate hydrolysing and the cholinesterase enzymes; the key enzymes which play essential catalytic roles in the development and progression of T2DM and AD, respectively. Therefore, this review will validate the potential of these food plants in the management of T2DM and AD.
Methodology
Search strategy
The methodological framework for conducting a scoping review described by Arksey and O’Malley (2005) was followed (Citation44). This scoping review was also written based on the guideline requirements of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (Citation45).
After identifying the research objectives, a search was conducted across three electronic databases to identify the relevant studies. The three electronic databases selected were SciFinder, Web of Science and Embase via Ovid. Keywords related to (‘essential oil’ or ‘aromatic oil’ or ‘aromatherapy oil’ or ‘scented oil’ or ‘essence’ or ‘volatile oil’) and (‘Zingiberaceae’ or ‘Curcuma’ or ‘Zingiber’) were searched using these databases. The search was limited to articles published in English between January 2000 to April 2022.
Article eligibility criteria
Following the inclusion and exclusion criteria listed in ; abstract, title screening and full-text screening were performed independently by two reviewers (A.P.-T.G. and K.-M. T.), using the Covidence software (https://www.covidence.org/). Instances of discordance were resolved by a third senior reviewer (Y.S) to prevent biases.
Table 1. Inclusion and Exclusion Criteria.
Data extraction and charting
Data extraction was conducted independently by two reviewers (K.-J. V. and A.P.-T.G.). The data gathered was then cross-checked between the reviewers to ensure accuracy and consistency throughout the data extraction process.
Results
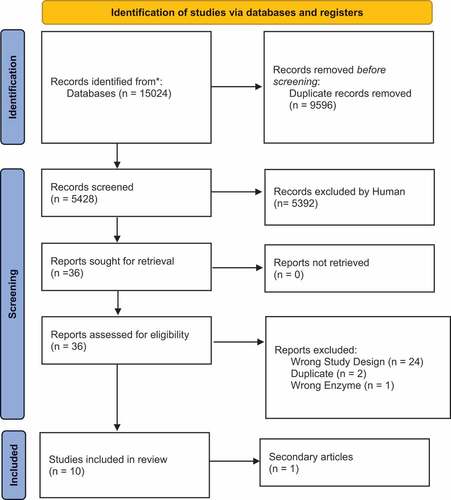
The search yielded a total of 15,024 articles from all three electronic databases. A total of 5428 articles remained after the duplicates were removed from Endnote and Covidence. Thirty-six articles remained after the title and abstract screening was conducted. Full text screening was subsequently carried out. The search strategy, the process of article selection and the reasons for the exclusion of certain articles are depicted in the schematic PRISMA flow diagram ().
Among these, ten articles specifically focusing on the isolation, chemical composition analysis and in vitro and in vivo enzyme inhibiting potentials of the EOs from the rhizomes of Curcuma caesia Roxb., Curcuma longa L., Zingiber cassumunar Roxb. (syn. Zingiber montanum (J. Konig) Link ex A. Dietr.) and Zingiber officinale Roscoe against the activities of the alpha-amylase enzyme, alpha-glucosidase enzyme, AChE and BChE were considered relevant for inclusion in this review, and were accordingly subjected to a thorough analysis. The results of the search are summarized in Tables S1-S3 which highlight the main findings obtained from our analyses of the ten articles.
Discussion
Chemical composition of the EOs of C. longa, Z. cassumunar and Z. officinale
There were five studies which documented the chemical composition of the EOs of the rhizomes of C. longa, Z. cassumunar and Z. officinale. Table S1 summarises the methods which were used to isolate, analyse and identify the chemical composition of each EO, the total number of constituents which were identified in each EO and the major constituents of each EO in relation to the different Curcuma and Zingiber species collected from different countries.
The EOs were isolated from either fresh or dried rhizomes of C. longa, Z. cassumunar and Z. officinale by hydrodistillation (3 hours or 5 hours) using a Clevenger type apparatus. Their respective composition was examined by either GCMS or a combination of GC-FID and GCMS using either a capillary column of intermediate polarity (HP-5 MS or DB-5 MS or Elite-5 MS) or a polar column (HP-Innowax) and temperature programmed conditions. The constituents of the respective EOs were identified either on the basis of their retention time and/or upon comparison of their mass spectra with those of the reference spectra in the computer library (NIST, Wiley, and Nbs databases) and further confirmed by comparison of the retention indices with those of authentic compounds or with the data in the literature (Table S1).
Monoterpenoids, sesquiterpenoids and their oxygenated derivatives were the important groups of chemical entities in the EOs of Nigerian, Mauritian and Indian C. longa; Thai and Indian Z. cassumunar and Nigerian Z. officinale. Phenylpropanoids, were also present in the EO of Indian Z. cassumunar, but in lower quantities (Table S1).
Variations were observed in the volatile profiles of the EOs isolated from C. longa. Akinyemi and Adeniyi in 2018 examined the EO composition of fresh Nigerian turmeric (Table S1). Their analysis revealed that the rhizome oil was monoterpenic in nature (86.12%) and this figure was largely due to 1,8-cineole (76.46%) (Citation46). Jugreet et al. found distinct differences in the oil composition of fresh turmeric from Mauritius harvested at a post-flowering stage compared to the composition of the Nigerian turmeric oil reported by Akinyemi and Adeniyi (Table S1) (Citation46). Interestingly, the Mauritian turmeric yielded an oil rich in sesquiterpenoids (79.7%), with a higher proportion of oxygen-containing compounds (62.6%) among which turmerone (31.4%), ar-turmerone (16.1%) and turmerol (14.6%) were the three most abundant constituents. On the other hand, terpinolene (11.0%), α-zingiberene (5.2%), β-sesquiphellandrene (4.8%) and β-caryophyllene (3.5%) which were also identified at significant concentrations in the Mauritian turmeric oil were however absent from the Nigerian turmeric oil (Citation47).
Earlier this year, Gururani and her co-workers conducted a comparative analysis of the variation in the chemical composition of the EOs isolated from dried turmeric collected from four different sites in Uttarakhand, India which varied in their altitudes; Tulyara (648 m), Chinyali (909 m), Bharsar (1806 m), Champawat (1615 m) (Citation48) (Table S1). The Tulyara and Bharsar turmeric EOs were principally sesquiterpenic in nature (84.9% and 89.6%, respectively) with their volatile profiles being characterised by high levels of tumerone (26.9% and 28.3%, respectively), α-zingiberene (12.9% and 16.6%, respectively), β-sesquiphellandrene (12.1% and 13.5%, respectively), β-caryophyllene (8.8% and 8.0%, respectively), curlone (7.8% and 8.2%, respectively) and α-curcumene (5.0% and 6.8%, respectively) (Table S1) (Citation48). Sesquiterpenoids were also significant in number in the Chinyali turmeric EO, totalling 71.09%. This figure was largely due to the presence of α-zingiberene (13.5%), α-santalene (13.5%), tumerone (10.7%), β-sesquiphellandrene (8.5%), α-humulene (7.4%) and β-bisabolene (6.8%) (Table S1). In contrast, monoterpenoids (56.0%) dominated the composition of the Champawat turmeric EO, comprising mainly 1.8-cineole (26.4%), terpinen-4-ol (13.8%) and sabinene (9.0%) (Table S1). It was clear from their analysis that there was no linear correlation between the chemical composition of the four EOs with the altitude from which their rhizomes were collected from (Citation48).
Marked differences were also evident in the chemical composition of the EOs of Z. cassumunar. Monoterpenoids (93.71%) predominated in the EO of fresh cassumunar ginger collected from Chiang Mai, Thailand. The two most abundant components were terpinen-4-ol and γ-terpinene, constituting 67.06% and 13.26% of the total sample, respectively (Table S1) (Citation49). In a separate investigation, Verma and his group of researchers carried out a comparative study on the EO composition of fresh cassumunar ginger rhizomes collected from three different towns in India; Khatima (Uttarakhand), Pantnagar (Uttarakhand) and Itanagar (Arunachal Pradesh) (Table S1). Based on their findings, it can be concluded that the rhizomes from Khatima, Pantnagar and Itanagar yielded oils which had similar volatile profiles to one another but differed considerably with the volatile profile of that of Thai casummunar. The chemical composition of the EOs of the rhizomes of Khatima, Pantnagar and Itanagar casummunar gingers were dominated by monoterpenoids (54.9%, 54.6% and 66.7%, respectively) and phenylbutanoids (39.9%, 41.2% and 27.5%, respectively). The major components in all three EOs were essentially similar being sabinene (38.0%, 20.8% and 13.5%, respectively), terpinen-4-ol (9.0%, 16.4% and 31.3%, respectively) and (E)-1-(3’,4’-dimethoxy phenyl)buta-1,3-diene (35.3%, 33.6% and 20.6%, respectively) (Table S1) (Citation50).
Since the method of isolation, analysis and identification of the EOs were similar in each of the above investigation (Table S1), the variations in the chemical profiles of the EOs may have been due to the following factors: characteristics of the samples (fresh or dried rhizomes), collection site (different country or town), altitude, climate (humidity, rainfall, temperature), season, soil pH, plant maturity, genotype, variety, harvesting time, postharvest handling conditions and storage (Citation41,Citation51–54).
Cholinesterase enzymes and carbohydrate hydrolysing enzymes inhibitory activities of the EOs of C. caesia, C. longa, Z. cassumunar and Z. officinale
Altogether, there were seven studies which reported on the in vitro cholinesterase and carbohydrate-hydrolysing enzymes inhibition assays of the EOs of C. caesia, C. longa, Z. cassumunar and Z. officinale (Table S2). The AChE and BChE which were used in majority of the investigations were isolated from electric eel and horse serum, respectively. As for the alpha-amylase and alpha-glucosidase enzymes, they were obtained from porcine pancreas and Saccharomyces cerevisiae, respectively.
On the other hand, there were four studies which emphasised on the in vivo enzyme inhibiting potentials of the EOs of C. caesia, C. longa, Z. cassumunar and Z. officinale with the focus being on their ability to inhibit the activity of the AChE (Table S3). The test subjects for the in vivo assays were either female Wistar albino rats, adult male albino rats or male Swiss albino mice.
EOs of C. caesia and its cholinesterase enzymes inhibitory activities
There were only two studies which reported on the AChE inhibitory potential of the EO of fresh C. caesia rhizomes, with both of them being conducted recently by the same group of researchers (Citation55,Citation56). In their first investigation, Borah and co-workers evaluated the in vitro AChE inhibitory activity of the EO at 1 mg/mL and found that it was a potential inhibitor (74.27 ± 0.72%) though its activity was slightly weaker than that of the FDA approved synthetic standard drug, Rivastigmine (92.88 ± 0.92%) (Table S2) (Citation55).
In the following year, the same group of researchers conducted a study on the in vivo effect of C. caesia rhizome EO on the level of AChE in the brains of female Wistar Albino hypoxic and amnesic rats (Citation56) (Table S3). Sodium nitrite induced hypoxic conditions and scopolamine induced amnesic conditions resulted in a two-fold increase in the concentration of the AChE in the cerebral cortex region of the brain of the hypoxic rats (26.48 ± 0.57 units/mg of protein) and the amnesic rats (31.5 ± 1.57 units/mg of protein) compared to the corresponding rats in the control group (13.89 ± 0.23 units/mg of protein and 15.19 ± 0.10 units/mg of protein, respectively). Treatment of the hypoxic rats with C. caseia rhizome oil at 200 mg/kg body weight and 400 mg/kg body weight for 15 days was found to be effective in lowering the AChE concentration to 21.11 ± 1.3 units/mg of protein and 17.80 ± 0.39 units/mg of protein, respectively. Similarly, when the amnesic rats were administered with the same dosages of C. caseia rhizome oil, the level of AChE in their cerebral cortex region of the brain decreased to 25.21 ± 1.14 units/mg of protein and 21.15 ± 0.16 units/mg of protein, respectively (Citation56).
EOs of C. longa and its cholinesterase enzymes and carbohydrate-hydrolysing enzymes inhibitory activities
The cholinesterase and carbohydrate-hydrolysing enzymes inhibitory activities of the EOs of the C. longa rhizomes were the subject of four separate investigations. Four years ago, Akinyemi and Adeniyi evaluated the in vivo effect of fresh turmeric oil from Nigeria on the activity of the AChE in the hippocampus and pre-frontal cortex parts of the brain in cadmium-induced neurotoxic adult male albino rats (Table S3). Cadmium build up in the human body has been reported to induce neurotoxicity due to its high blood-brain barrier permeability. Akinyemi and Adeniyi observed that the rats which were administered with 2.5 mg Cadmium/kg body weight for 14 days exhibited a two-fold increase in the AChE activity in their brains compared to the corresponding activity in the brains of the rats in the control group and those only receiving saline and turmeric oil (50 mg/kg body weight). The simultaneous treatment of rats with 2.5 mg Cadmium/kg body weight and 50 mg turmeric oil/kg body weight for 14 days effectively maintained the AChE activity at normal or near normal levels. They concluded that the turmeric oil significantly (P < 0.05) inhibited the activity of the hippocampus and pre-frontal cortex AChE (Citation46).
The in vitro enzyme inhibitory potential of fresh Mauritian turmeric oil in relation to the AChE, BChE, alpha-amylase and alpha-glucosidase enzymes was assessed by Jugreet et al., 2020 (Table S2). Jugreet and co-workers identified turmeric oil to be a dual AChE and alpha-amylase enzymes inhibitor (4.74 ± 0.27 mg Galantamine equivalent/g EO and 0.93 ± 0.02 mM Acarbose equivalent/g EO, respectively). Molecular docking analysis revealed that turmerone, the major component in the Mauritian turmeric EO, demonstrated high binding affinities and strong interactions (i.e hydrogen bonds and pi-pi interactions) with the active site residues of both enzymes. This finding of Jugreet et al., therefore, provides a reasonable rationale for the Mauritian turmeric EO’s dual enzyme inhibitory activities (Citation47).
Back in 2012, Lekshimi et al. investigated the antidiabetic capacity of the EOs isolated from fresh and dried Indian turmeric and their major component, ar-turmerone, in terms of their ability in inhibiting the activities of the alpha-glucosidase and alpha-amylase enzymes in vitro (Citation57) (Table S2). The EOs from fresh and dried turmeric rhizomes were found to be significantly (p < 0.05) more effective alpha-glucosidase enzyme (IC50 = 1.32 ± 0.08 µg/mL and IC50 = 0.38 ± 0.09 µg/mL, respectively) and alpha-amylase enzyme (IC50 = 64.7 ± 5.9 µg/mL and IC50 = 34.3 ± 6.2 µg/mL, respectively) inhibitors compared to the positive control, acarbose (IC50 = 18.12 ± 1.23 µg/mL and IC50 = 296.3 ± 12.7 µg/mL). Lekshimi and co-workers concluded that the presence of ar-turmerone in both oils could have been responsible for their potencies as this sesquiterpene was identified to be an active dual inhibitor of the alpha-glucosidase (IC50 = 0.28 ± 0.05 µg/mL) and alpha-amylase (IC50 = 24.5 ± 4.8 µg/mL) enzymes. Their study also revealed that the drying of the turmeric rhizomes notably (P < 0.05) increased their carbohydrate hydrolysing enzymes inhibitory potentials by two to four folds (Citation57).
Lately, Gururani and her co-workers conducted a comparative analysis of the variation in the alpha-amylase enzyme inhibitory activity of the EOs isolated from dried turmeric collected from four different sites in Uttarakhand, India which varied in their altitudes; Tulyara (648 m), Chinyali (909 m), Bharsar (1806 m), Champawat (1615 m) (Citation48) (Table S2). Gururani et al. discovered that all four of the turmeric EOs were able to inhibit the activity of the alpha-amylase enzyme. There was however a slight difference in the enzyme inhibiting potential of each EO. The Chinyali and Bharsar turmeric EOs (IA50 = 11.95 ± 0.11 μL and 17.19 ± 0.07 μL, respectively) were stronger alpha-amylase enzyme inhibitors compared to the Tulyara and Champawat turmeric EOs (IA50 = 22.29 ± 0.11 μL and 23.06 ± 0.08 μL, respectively) (Table S2). In general, the order of the alpha-amylase inhibitory activity of the turmeric EOs are Chinyali > Bharsar > Tulyara > Champawat. It was evident from their analysis that there was no linear correlation between the alpha-amylase enzyme inhibitory activities of the four EOs with the altitude from which their rhizomes were collected from (Citation48).
EOs of Z. cassumunar (syn. Z. montanum) and its cholinesterase enzymes inhibitory activities
Till present, there has only been three scientific reports on the in vitro and in vivo cholinesterase enzymes inhibitory activities of the EOs of the rhizomes of cassumunar ginger. In 2010, Chaiyana et al. reported that the EO isolated from the fresh rhizomes of cassumunar ginger collected from Chiang Mai, Thailand inhibited the activities of both the AChE and BChE, with the oil demonstrating a greater inhibitory activity against the BChE (47.5 ± 5.6%) compared to the AChE (28.4 ± 4.4%) (Citation58) (Table S2).
Subsequently, in 2012, the same group of (Citation59) researchers further characterised the rhizome oil of fresh cassumunar ginger collected from the same location as a moderate BChE and a weak AChE inhibitor based on the calculated IC50 values (IC50 BChE = 0.355 ± 0.137 mg/mL and IC50 AChE 5.573 ± 0.176 mg/mL) (Table S2). Okonogi and Chaiyana next investigated the enhancement of the cholinesterase enzymes inhibitory activities of the rhizome oil using a microemulsion technique. Their findings established that the formulation of the rhizome EO as a microemulsion composed of Triton X-114 combined with propylene glycol significantly increased the BChE and AChE inhibitory activities by twenty-five and twenty folds (IC50 BChE = 0.014 ± 0.002 mg/mL and IC50 AChE 0.252 ± 0.096 mg/mL), respectively, in comparison to the EO itself (Citation49). Okonogi and Chaiyana suggested that the casummunar ginger EO loaded microemulsion could be an attractive formulation for additional characterization and in vivo studies in animal models with AD.
In 2018, Verma et al. investigated the in vivo potential of the EO isolated from Pantnagar cassumunar ginger in inhibiting the activity of the AChE in the brain homogenate obtained from male Swiss albino mice. The AChE activity in the brain homogenate was induced by acetylthiocholine iodide (Table S3). According to their findings, the rhizome oil did not inhibit the activity of the AChE in the brain homogenate at all tested concentrations (0.001 mg/mL-10 mg/mL) (Citation50).
EOs of Z. officinale and its cholinesterase enzymes inhibitory activities
There were only two reports that discussed the cholinesterase enzymes inhibitory activities of ginger EO. In 2018, Akinyemi and Adeniyi assessed the in vivo effect of fresh Nigerian ginger oil on the activity of the AChE in the hippocampus and pre-frontal cortex parts of the brain in cadmium-induced neurotoxic adult male albino rats (Table S3). The rats which were administered with 2.5 mg Cadmium/kg body weight for 14 days exhibited a two-fold increase or more in the AChE activity in their brains compared to the corresponding activity in the brains of the rats in the control group and those only receiving saline and ginger oil (50 mg/kg body weight). Interestingly, the simultaneous treatment of rats with 2.5 mg Cadmium/kg body weight and 50 mg ginger oil/kg body weight for 14 days effectively lowered the AChE activity to below normal levels. Akinyemi and Adeniyi concluded that the ginger oil significantly (P < 0.05) inhibited the activity of the hippocampus and pre-frontal cortex AChE (Citation46).
In 2021, Mahnashi et al. investigated the in vitro potential of the EO of fresh ginger rhizomes from Pakistan in inhibiting the activities of the cholinesterase enzymes (Table S2). Preliminary screening of the ginger oil at various concentrations (62.5 µg/mL-1000 µg/mL) proved it to be a potential AChE and BChE inhibitor although its percentage inhibition was lower than those of the positive control, Galantamine, for the corresponding concentrations. Based on the IC50 values which were subsequently determined along with the results obtained from the Lineweaver-Burk plots, they concluded that ginger oil was a dual AChE (IC50 = 88 μg/mL) and BChE (IC50 = 101 μg/mL) inhibitor with a higher binding affinity to the AChE (Km = 38.88 μg/mL) compared to the BChE (Km = 47.48 μg/mL) (Citation59).
Conclusion
In addition to the role that the rhizomes of C. caesia, C. longa, Z. cassumunar and Z. officinale play in imparting organoleptic properties to the food we eat while preserving their quality and nutritional values, it is evident from this review that the moderate in vitro and in vivo cholinesterase and carbohydrate-hydrolysing enzymes inhibiting abilities of their essential oils provides scientific justification that the consumption of C. caesia, C. longa, Z. cassumunar and Z. officinale in small quantities in our daily diet could play a vital role in the management of T2DM and AD.
Research gaps and future research
The majority of the constituents which make up the composition of the rhizome oils are monoterpenoids and sesquiterpenoids. Since these compounds have chiral centers, they are optically active and could be present either in pure dextrorotatory (+) form, or pure levorotatory (-) form, or a mixture of both. Therefore, enantiomeric analysis via chiral GC and GCMS analyses should be conducted in order to determine the enantiomeric distribution for each monoterpenoid and sesquiterpenoid in each rhizome oil.
The major constituents of the EOs of the rhizomes of C. caesia, C. longa, Z. cassumunar and Z. officinale were unlikely to be the only constituents accountable for either their cholinesterase or carbohydrate-hydrolysing enzymes inhibitory activities although in certain cases they might have contributed actively or insignificantly. On that account, a synergistic, additive or antagonistic interaction among the different major and minor constituents present in each EO instead, could have been responsible for their bioactivities (Citation47). There is also a possibility that the constituents which were unable to be identified using the techniques employed by the researchers listed in Table S1 could have also given rise to the bioactivities of the various rhizome oils.
Therefore, attempt should be made to identify each and every constituent which is present in each of the rhizome oils. On this basis, research needs to be carried out on a large scale of the rhizomes of each species in order to isolate and characterise a sufficient amount of all of the constituents with the intention of identifying the constituents of each rhizome oil which were responsible in giving rise to the enzyme inhibitory activities. Since the constituents of the rhizome oils are volatile, they can be isolated using preparative gas chromatography and characterised via spectroscopic techniques such infrared spectroscopy, nuclear magnetic spectroscopy and mass spectrometry.
Subsequently, these compounds should be screened for their inhibitory activities against the AChE, BChE, alpha glucosidase and alpha-amylase enzymes in order to identify the constituents which were responsible in giving rise to the inhibitory activities of the rhizome oils. Kinetic studies and molecular docking studies should also be carried out on the constituents which actively inhibited the AChE, BChE, alpha-glucosidase and alpha-amylase enzymes in order to determine their mode of inhibition and to investigate the site at which the active constituents bind to the enzymes.
For the monoterpenoids and sesquiterpenoids which are present as a mixture of their dextrorotatory and levorotatory forms, each optical isomer should be isolated, characterised and evaluated for their enzyme inhibitory activities in order to determine which of the optical isomers could be responsible for the rhizome oils being able to inhibit the activity of the AChE, BChE, alpha-glucosidase and alpha-amylase enzymes.
The Curcuma and Zingiber are two very large genera of the Zingiberaceae. They consist of 120 and 141 species, respectively. Although many of these species are reportedly being used as culinary herbs and spices, eaten either raw or cooked as vegetables and used as ingredients in the preparation of salads and pickles, however, till present, there is very limited information available on the ability of their rhizome oils in inhibiting the activities of the cholinesterase and carbohydrate-hydrolysing enzymes except for those of C. caesia, C. longa, Z. cassumunar and Z. officinale which were discussed in the present review. On that account it would be worthy to investigate the in vitro and in vivo AChE, BChE, alpha-glucosidase and alpha-amylase enzymes inhibiting activities of the EOs of other edible species in both of these genera in order to provide valuable insight to their potential as functional food in the treatment and prevention of degenerative diseases.
List of abbreviations
Author’s contribution
Conceptualization, A.P.-T.G., K.-M. T., U.S., Y.S.; methodology, A.P.-T.G., K.-M. T., U.S,; software, A.P.-T.G., K.-M. T., U.S.,; validation, A.P.-T.G., K.-M. T; formal analysis, A.P.-T.G., K.-M. T., K.-J. V., U.S.; investigation, A.P.-T.G., K.-M. T., K.-J. V., Y.S.; resources, A.P.-T.G., K.-M. T., K.-J. V.,; data curation, A.P.-T.G., K.-M. T., K.-J. V.; writing – review and editing, Y.S.; visualization, U.S., Y.S.; supervision, Y.S.; project administration, Y.S. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (29 KB)Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10412905.2023.2196521.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Z. Aumeeruddy-Elalfi, N. Lall, B. Fibrich, A.B. van Staden, M. Hosenally and M.F. Mahomoodally, Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. Journal of Food and Drug Analysis, 2018, 26(1), 232–243. doi: 10.1016/j.jfda.2017.03.002.
- V. Boccardi, I. Murasecco and P. Mecocci, Diabetes drugs in the fight against Alzheimer’s disease. Ageing Research Reviews, 2019, 54, 100936. doi: 10.1016/j.arr.2019.100936.
- A.C. Silveira, J.P. Dias, V.M. Santos, P.F. Oliveira, M.G. Alves, L. Rato and B.M. Silva, The action of polyphenols in diabetes Mellitus and Alzheimer’s Disease: a common agent for overlapping pathologies. Current Neuropharmacology, 2019, 17(7), 590–613. doi: 10.2174/1570159X16666180803162059.
- S. Chatterjee and A. Mudher, Alzheimer’s disease and Type 2 Diabetes: a critical assessment of the shared pathological traits. Frontiers in Neuroscience, 2018, 12, 383. doi: 10.3389/fnins.2018.00383.
- K. Venkatakrishnan, H.F. Chiu and C.K. Wang, Popular functional foods and herbs for the management of type-2-diabetes mellitus: a comprehensive review with special reference to clinical trials and its proposed mechanism. Journal of Functional Foods, 2019, 57, 425–438. doi: 10.1016/j.jff.2019.04.039.
- E.A. Makinde, C. Ovatlarnporn, A.E. Adekoya, O.F. Nwabor and O.J. Olatunji, Antidiabetic, antioxidant and antimicrobial activity of the aerial part of tiliacora triandra. South African Journal of Botany, 2019, 125, 337–343. doi: 10.1016/j.sajb.2019.08.012.
- C. Peña-Bautista, M. Vento, M. Baquero and C. Cháfer-Pericás, Lipid peroxidation in neurodegeneration. Clinica Chimica Acta, 2019, 497, 178–188. doi: 10.1016/j.cca.2019.07.037.
- B. Salehi, A. Ata, N.V.A. Kumar, F. Sharopov, K. Ramírez-Alarcón, A. Ruiz-Ortega, S.A. Ayatollahi, P.V.T. Fokou, F. Kobarfard, Z.A. Zakaria, M. Iriti, Y. Taheri, M. Martorell, A. Sureda, W.N. Setzer, A. Durazzo, M. Lucarini, A. Santini, R. Capasso, E.A. Ostrander, C. Atta-Ur-Rahman, M.I. Cho and J. Shari?-Rad, Antidiabetic potential of medicinal plants and their active components. Biomolecules, 2019, 9(10), 551. doi: 10.3390/biom9100551.
- S. Sestito, S. Wang, Q. Chen, J. Lu, S. Bertini, C. Pomelli, G. Chiellini, X. He, R. Pi and S. Rapposelli, Multi-targetedChEI-copper chelating molecules as neuroprotective Agents. European Journal of Medicinal Chemistry, 2019, 174, 216–225. doi: 10.1016/j.ejmech.2019.04.060.
- J. Grizzanti, R. Corrigan, S. Servizi and G. Casadesus, Amylin signaling in diabetes and alzheimer’s disease: therapy or pathology?. Journal of Neurology & Neuromedicine, 2019, 4(1), 12–16. doi: 10.29245/2572.942X/2019/1.1212.
- S.E. Mabhida, P.V. Dludla, R. Johnson, M. Ndlovu, J. Louw, A.R. Opoku and R.A. Mosa. 2018. Protective effect of triterpenes against diabetes-induced β-cell damage: An overview of in vitro and in vivo studies. Pharmacological Research. 137 179–192.10.1016/j.phrs.2018.10.004.
- M.N. Beidokhti and A.K. Jäger, Review of antidiabetic fruits, vegetables, beverages, oils and spices commonly consumed in the diet. Journal of Ethnopharmacology, 2017, 201, 26–41. doi: 10.1016/j.jep.2017.02.031.
- O. Ojo and J. Brooke, Evaluating the association between diabetes, cognitive decline and dementia. International Journal of Environmental Research and Public Health, 2015, 12(7), 8281–8294. doi: 10.3390/ijerph120708281.
- N.B. Qin, C.C. Jia, J. Xu, D.H. Li, F.X. Xu, J. Bai, Z.L. Li and H.M. Hua, New amides from seeds of Silybum marianum with potential antioxidant and antidiabetic activities. Fitoterapia, 2017, 119, 83–89. doi: 10.1016/j.fitote.2017.04.008.
- D. Kumar, N. Gupta, R. Ghosh, R.H. Gaonkar and B.C. Pal, α-Glucosidase and α-amylase inhibitory constituent of Carex baccans: bio-assay guided isolation and quantification by validated RP-HPLC–dad. Journal of Functional Food, 2013, 5(1), 211–218. doi: 10.1016/j.jff.2012.10.007.
- B.P. Pandey, S.P. Pradhan, K. Adhikari and S. Nepal, Bergenia pacumbis from Nepal, an astonishing enzymes inhibitor. BMC Complementary Medicine and Therapies, 2020, 20(1), 198. doi: 10.1186/s12906-020-02989-2.
- M.A.T. Phan, J. Wang, J. Tang, Y.Z. Lee and K. Ng, Evaluation of α-glucosidase inhibition potential of some flavonoids from epimedium brevicornum. Lwt-Food Science and Technology, 2013, 53(2), 492–498. doi: 10.1016/j.lwt.2013.04.002.
- T. Zhao, K.M. Ding, L. Zhang, X.M. Cheng, C.H. Wang and Z.T. Wang, Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-Carboline and quinoline alkaloids derivatives from the plants of genus peganum. Journal of Chemistry, 2013, ID, 717232. doi: 10.1155/2013/717232.
- S. Agatonovic-Kustrin, E. Kustrin and D.W. Morton, Essential oils and functional herbs for healthy aging. Neural Regeneration Research, 2020, 14(3), 441–445. doi: 10.4103/1673-5374.245467.
- D. Islam, A. Akter, A. Huque, S. Akhter, D.C. Roy, C. Lyzu, M. Hakim, L.C. Mohanta, E.P. Lipy, M.A. Siddique, K.M.M.R. Linkon and M.N. Rahman, Hypoglycemic effect study of a combination of some stipulated spices in alloxan induced diabetic wistar albino rats along with nutritional value evaluation. Journal of Diabetes Mellitus, 2018, 8(02), 43–53. doi: 10.4236/jdm.2018.82005.
- A. Mohammed and M.S. Islam, Spice-derived bioactive ingredients: potential agents or food adjuvant in the management of diabetes mellitus. Frontiers in Pharmacology, 2018, 9, 893. doi: 10.3389/fphar.2018.00893.
- K. Sharma, Cholinesterase inhibitors as Alzheimer’s therapeutics. Molecular Medicine Reports, 2019, 20, 1479–1487. doi: 10.3892/mmr.2019.10374.
- C. Sunil, V. Kumar and J. Van Staden, In vitro alpha-glucosidase inhibitory, total phenolic composition, antiradical and antioxidant potential of heteromorpha arborescens (Spreng) Cham. & Schltdl. leaf and bark extracts. South African Journal of Botany, 2019, 124, 380–386. doi: 10.1016/j.sajb.2019.05.017.
- S. Lordan, T.J. Smyth, A. Soler-Vila, C. Stanton and R.P. Ross, The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chemistry, 2013, 141(3), 2170–2176. doi: 10.1016/j.foodchem.2013.04.123.
- K.R.R. Rengasamy, M.A. Aderogba, S.O. Amoo, W.A. Stirk and J. Van Staden, Potential antiradical and alpha-glucosidase inhibitors from ecklonia maxima (Osbeck) Papenfuss. Food Chemistry, 2013, 141(2), 1412–1415. doi: 10.1016/j.foodchem.2013.04.019.
- A. Tabussum, N. Riaz, M. Saleem, M. Ashraf, M. Ahmad, U. Alam, B. Jabeen, A. Malik and A. Jabbar, α-Glucosidase inhibitory constituents from chrozophora plicata. Phytochemistry Letters, 2013, 6(4), 614–619. doi: 10.1016/j.phytol.2013.08.005.
- X. Bi, J. Lim and C.J. Henry, Spices in the management of diabetes mellitus. Food Chemistry, 2017, 217, 281–293. doi: 10.1016/j.foodchem.2016.08.111.
- J. Gregory, Y.V. Vengalasetti, D.E. Bredesen and R.V. Rao, Neuroprotective herbs for the management of Alzheimer’s Disease. Biomolecules, 2021, 11(4), 543. doi: 10.3390/biom11040543.
- M.R. Khazdair, A. Anaeigoudari, M. Hashemzehi and R. Mohebbati, Neuroprotective potency of some spice herbs, a literature review. Journal of Traditional and Complementary Medicine, 2019, 9(2), 98–105. doi: 10.1016/j.jtcme.2018.01.002.
- Y. Peng, H. Tao, S. Wang, J. Xiao, Y. Wang and H. Su, Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: a compendium of time-tested strategy. Journal of Functional Foods, 2021, 81, 104463. doi: 10.1016/j.jff.2021.104463.
- A.D. Seetaloo, M.Z. Aumeeruddy, R.R. Rengasamy Kannan and M.F. Mahomoodally, Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management — a systematic review. South African Journal of Botany, 2019, 120, 3–24. doi: 10.1016/j.sajb.2018.05.015.
- B. Guldiken, G. Ozkan, G. Catalkaya, F.D. Ceylan, I.E. Yalcinkaya and E. Capanoglu, Phytochemicals of herbs and spices: health versus toxicological effects. Food and Chemical Toxicology, 2018, 119, 37–49. doi: 10.1016/j.fct.2018.05.050.
- T.A. Jiang, Health benefits of culinary herbs and spices. Journal of AOAC International, 2019, 102(2), 395–411. doi: 10.5740/jaoacint.18-0418.
- N.K. Rakhi, R. Tuwani, J. Mukherjee and G. Bagler, Data-driven analysis of biomedical literature suggests broad-spectrum benefits of culinary herbs and spices. PLoS One, 2018, 13(5), e0198030. doi: 10.1371/journal.pone.0198030.
- A. Vallverdu-Queralt, J. Regueiro, M. Martinez-Huelamo, J.F.R. Alvarenga, L.N. Leal and R.M. Lamuela-Raventos, A comprehensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chemistry, 2014, 154, 299–307. doi: 10.1016/j.foodchem.2013.12.106.
- M. Viuda-Martos, Y. Ruiz-Navajas, J. Fernandez-Lopez and J.A. Perez-Alvarez, Spices as Functional Food. Critical Reviews in Food Science and Nutrition, 2011, 51(1), 13–28. doi: 10.1080/10408390903044271.
- A. Yashin, Y. Yashin, X. Xia and B. Nemzer, Antioxidant activity of spices and their impact on human health: a review. Antioxidants, 2017, 6(3), 70. doi: 10.3390/antiox6030070.
- A.B. Kunnumakkara, B.L. Sailo, K. Banik, C. Harsha, S. Prasad, S.C. Gupta, A.C. Bharti and B.B. Aggarwal, Chronic diseases, inflammation, and spices: how are they linked?. Journal of Translational Medicine, 2018, 16(1), 14. doi: 10.1186/s12967-018-1381-2.
- R. Vázquez-Fresno, A.R.R. Rosana, T. Sajed, T. Onookome-Okome, N.A. Wishart and D.S. Wishart, Herbs and spices- biomarkers of intake based on human intervention studies – a systematic review. Genes & Nutrition, 2019, 14(1), 18. doi: 10.1186/s12263-019-0636-8.
- E.I. Opara and M. Chohan, Culinary herbs and spices: their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. International Journal of Molecular Sciences, 2014, 15(10), 19183–19202. doi: 10.3390/ijms151019183.
- M. Ivanovic, K. Makoter and M.I. Razboršek, Comparative study of chemical composition and antioxidant activity of essential oils and crude extracts of four characteristic Zingiberaceae herbs. Plants, 2021, 10(3), 501. doi: 10.3390/plants10030501.
- M. Sharifi-Rad, E.M. Varoni, B. Salehi, J. Sharifi-Rad, K.R. Matthews, S.A. Ayatollahi, F. Kobarfard, S.A. Ibrahim, D. Mnayer, Z.A. Zakaria, M. Sharifi-Rad, Z. Yousaf, M. Iriti, A. Basile and D. Rigano, Review plants of the genus Zingiber as a source of bioactive phytochemicals: from tradition to pharmacy. Molecules, 2017, 22(12), 2145. doi: 10.3390/molecules22122145.
- S. Rajkumari and K. Sanatombi, Nutritional value, phytochemical composition, and biological activities of edible curcuma species: a review. International Journal of Food Properties, 2017, 20(sup3), S2668–2687. doi: 10.1080/10942912.2017.1387556.
- H. Arksey and L. O’malley, Scoping studies: towards a methodological framework. International Journal of Social Research Methodology, 2005, 8(1), 19–32. doi: 10.1080/1364557032000119616.
- A.C. Tricco, E. Lillie, W. Zarin, K.K. O’brien, H. Colquhoun, D. Levac, D. Moher, M.D.J. Peters, T. Horsley, L. Weeks, S. Hempel, E.A. Akl, C. Chang, J. McGowan, L. Stewart, L. Hartling, A. Aldcroft, M.G. Wilson, C. Garritty, S. Lewin, C.M. Godfrey, M.T. Macdonald, E.V. Langlois, K. Soares-Weiser, J. Moriarty, T. Clifford and O. Tunçalp & S.E. Straus, PRISMA Extension for Scoping Reviews (PRISMA ScR): checklist and Explanation. In: Annals of Internal Medicine. Edit., G. R. Heudebert, Vol. 169, pp. 467–473, American College of Physicians, Philadelphia (2018).
- A.J. Akinyemi and P.A. Adeniyi, Effect of essential oils from ginger (Zingiber officinale) and Turmeric (curcuma longa) rhizomes on some inflammatory biomarkers in cadmium induced neurotoxicity in rats. Journal of Toxicology, 2018, ID, 4109491. doi: 10.1155/2018/4109491.
- B.S. Jugreet, M.F. Mahomoodally, K.I. Sinan, G. Zengin and H.H. Abdallah, Chemical variability, pharmacological potential, multivariate and molecular docking analyses of essential oils obtained from four medicinal plants. Industrial Crops & Products, 2020, 150, 112394. doi: 10.1016/j.indcrop.2020.112394.
- S. Gururani, K. Gairola, R. Kumar, O. Prakash and S.K. Dubey, Altitudinal and geographical variations in phytochemical composition and biological activities of curcuma longa accession from Uttarakhand, the Himalayan region. Food Processing and Preservation, 2022, 46(3), e16384. doi: 10.1111/jfpp.16384.
- S. Okonogi and W. Chaiyana, Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discoveries & Therapeutics, 2012, 6, 249–255. doi: 10.5582/ddt.2012.v6.5.249.
- R.S. Verma, N. Joshi, R.C. Padalia, V.R. Singh, P. Goswami, S.K. Verma, H. Iqbal, D. Chanda, R.K. Verma, M.P. Darokar, A. Chauhan and M.K. Kandwal, Chemical composition and antibacterial, antifungal, allelopathic and acetylcholinesterase inhibitory activities of cassumunar-ginger. Journal of the Science of Food and Agriculture, 2018, 98(1), 321–327. doi: 10.1002/jsfa.8474.
- N. Sabulal and S. Baby, Chemistry of amomum essential oils. Journal of Essential Oil Research, 2021, 33(5), 427–441. doi: 10.1080/10412905.2021.1899065.
- M.D. Ibáñez and M.A. Blázquez, Curcuma longa L. rhizome essential oil from extraction to its agri-food applications. A Review. Plants, 2021, 10(1), 44. doi: 10.3390/plants10010044.
- N.S. Dosoky and W.N. Setzer, Chemical composition and biological activities of essential oils of curcuma species. Nutrients, 2018, 10(9), 1196. doi: 10.3390/nu10091196.
- K. Sowndhararajan, P. Deepa, M. Kim, S.J. Park and S. Kim, A review of the composition of the essential oils and biological activities of angelica species. Scientia Pharmaceutica, 2017, 85(3), 33. doi: 10.3390/scipharm85030033.
- S. Borah, P. Sarkar and H.K. Sharma, Chemical profiling, free radical scavenging and anti‐acetylcholinesterase activities of essential oil from curcuma caesia of Arunachal Pradesh, India. Pharmacognosy Research, 2020, 12(1), 76–84. doi: 10.4103/pr.pr_84_19.
- S. Borah, P. Sarkar and H.K. Sharma, Analysing curcuma caesia fractions and essential oil for neuroprotective potential against anxiety, depression, and amnesia. 3 Biotech, 2021, 11(5), 240. doi: 10.1007/s13205-021-02793-w.
- P.C. Lekshmi, R. Arimboor, P.S. Indulekha and A.N. Menon, Turmeric (curcuma longa L.) volatile oil inhibits key enzymes linked to type 2 diabetes. International Journal of Food Sciences and Nutrition, 2012, 63(7), 832–834. doi: 10.3109/09637486.2011.607156.
- W. Chaiyana, K. Saeio, W.E. Hennink and S. Okonogi, Characterization of potent anticholinesterase plant oil based microemulsion. International Journal of Pharmaceutics, 2010, 401(1–2), 32–40. doi: 10.1016/j.ijpharm.2010.09.005.
- M.H. Mahnashi, B.A. Alyami, Y.S. Alqahtani, A.O. Alqarni, M.S. Jan, M. Ayaz, F. Ullah, M. Shahid, U. Rashid and A. Sadiq, Neuroprotective potentials of selected natural edible oils using enzyme inhibitory, kinetic and simulation approaches. BMC Complementary Medicine and Therapies, 2021, 21(1), 248. doi: 10.1186/s12906-021-03420-0.