 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Vertebrate-bitten coprolites are seemingly rare; nonetheless, within the past dozen years, a handful of these composite trace fossils have been found and described. Here, we describe a single crocodile coprolite from the Lower Miocene Calvert Formation in New Kent County, Virginia, USA, showing bite marks. The size and morphology of the coprolite is consistent with a crocodilian origin. Seven parallel, gently curving gouges, of biogenic origin, disrupt the surface of the coprolite. As it is a medium preserving bite marks, this coprolite qualifies as a morderolite. Furthermore, because of the presence of larger/deeper primary, and finer secondary gouges, which we interpreted as individual tooth marks, the identity of the vertebrate that bit the coprolite is most likely gar (Lepisosteidae). Because other comparable coprolites preserving similar sets of primary and secondary gouges are known, this unique trace fossil is given a new ichnotaxonomic name, Machichnus dimorphodon isp. nov. Many more much smaller markings, interpreted as feeding traces by smaller organisms (possibly invertebrates) also ornament the surface of the coprolite.
Introduction
Of all the vertebrate coprolites known from the fossil record, very few have been recognized as preserving vertebrate tooth impressions or bite marks (Godfrey & Smith, Citation2010; Godfrey & Palmer, Citation2015; Godfrey & Frandsen, Citation2016; Dentzien-Dias et al., Citation2018a, Citation2018b; Collareta et al., Citation2019; Frandsen & Godfrey, Citation2019). These are currently regarded as composite trace fossils (Bertling et al., Citation2006). Over the past 13 years, increasing numbers of bitten coprolites have been described. These bitten coprolites can be referred to as morderolites – any bitten medium that has become fossilized (Godfrey & Collareta, Citation2022b). In this case study, we describe another bitten coprolite from the Lower Miocene Calvert Formation along the Pamunkey River, New Kent County, Virginia, USA, preserving vertebrate bite marks (sensu Zonneveld et al., Citation2022) over its surface (). These bite marks are referred to Machichnus, but because they are unique and easily distinguishable from the seven ichnospecies already assigned to this ichnogenus, they are described, figured, and given a new ichnospecific name.
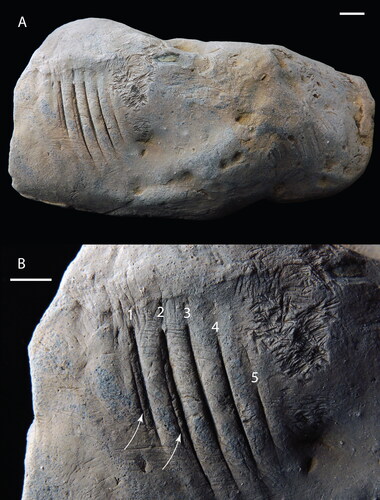
Figure 1. CMM-V-8998, holotype of Machichnus dimorphodon isp. nov., a gar-bitten crocodile coprolite. (a) Full view of the coprolite showing the location of the bite marks towards one end of the specimen. (b) Close-up view of the gar bite marks showing the two orders of tooth gouge marks (primary and secondary). The white numbers 1–5 demark the primary gouges. The white arrows point to two of the secondary gouges that lie parallel and adjacent to the primary gouges. To the right of numbers 4 and 5, notice the tight clustering of numerous short surface feeding traces, thought to be invertebrate grazing in origin. Specimen lightly dusted with sublimed ammonium chloride. Scale bars equal 10 mm.
Materials and methods
To highlight surface detail, the coprolites figured herein were lightly dusted with sublimed ammonium chloride, a whitening technique described by Cooper (Citation1935) and Feldman (Citation1989). After the specimens were photographed with a Nikon Coolpix P510 camera on black velvet under fluorescent light, the ammonium chloride was removed by holding the specimen under running water (Shelburne & Thompson, Citation2016). As a cautionary note, specimens coated with ammonium chloride should be stable enough to withstand a fresh water rinse because there would be the possibility of a residue of hydrochloric acid (HCl) left on the specimens if they are not washed thoroughly (Godfrey et al., Citation2022a). The images were edited in Adobe Photoshop® and compiled in Adobe Illustrator®.
Specimen repository
All specimens described or figured herein are housed in the Modern Osteology or Vertebrate Paleontology Collections at the Calvert Marine Museum, Solomons, Maryland, USA.
Abbreviations
CMM-O-: Calvert Marine Museum, osteology collection; CMM-V-: Calvert Marine Museum, vertebrate paleontology collection.
Location and geology
CMM-V-8998 was collected by author LWW from along the Pamunkey River at Horseshoe Bend (right bank), New Kent County, Virginia, USA. The coprolite was found in situ at the base of the Calvert Formation (approximately 4.5 m above the contact with the underlying rocks). Approximate GPS coordinates are: N 37°39’50.27”, W 77°09’05.68”. At this location in Virginia, the Miocene deposition of the Calvert strata occurred in a marine environment during multiple transgressions in what is known in geologic literature as the Salisbury Embayment.
Results
Specimen description
The term “gouge” was preferentially used to describe the linear indentations/troughs in CMM-V-8998, because the word implies a blow to a surface. CMM-V-8998 () has the overall appearance of a tapering, irregular cylinder, 110 mm long and 58 mm wide at its point of maximum girth. The specimen is subcircular across its diameter and slightly flattened on one side. This is a fairly common coprolite morphology, the flattened side being interpreted as that which came to rest on the substrate (Hunt et al., Citation1994; Godfrey & Smith, Citation2010; Collareta et al., Citation2019). The specimen is pale brown in colour, with darker patches throughout (not shown in the accompanying figures, because the specimen was temporarily whitened with ammonium chloride to improve the contrast/visibility of the specimen’s surface features). At its broadest terminal face, the specimen appears to have broken, providing a view into its internal structure, which, on that homogeneous surface is devoid of any obvious inclusions.
CMM-V-8998 bears a series of seven parallel gouges, reaching a maximum length of 21.5 mm and oriented nearly perpendicular to the longitudinal axis of the coprolite (). These gouges may be divided into a primary set of five gently curved incisions (numbers 1–5 in ), spaced approximately 4 mm apart (on average), and two associated finer/shallower gouges (as indicated by the two white arrows in ), spaced only 1 mm adjacent to two larger gouges (i.e., numbers 1 and 2 in ) proximal to the specimen’s wider terminal face.
Much of the surface of the coprolite is marked by fine, possibly deliberate feeding marks by other organisms. The most conspicuous patch of these markings occurs close to the aforementioned primary and secondary gouges (i.e., the traces next to numbers 4 and 5 in ). These many fine striations occupy an area of about 10 mm × 25 mm, located 5 mm from the aforementioned series of longer gouges towards the tapering end of the coprolite. These presumed feeding marks may have been paired, with approximately 39 total pairs, all approximately 1.5 mm long, and 0.8 mm apart. Some of these tiny striations deviate from the main cluster extending into a large divot in the specimen, 12 mm in diameter (not seen in ). Additionally, several isolated pairs of striations are scattered across the surface of the convex face of the specimen. Comparable surface striations are occasionally found on the surface of other Miocene coprolites from Calvert Cliffs (Godfrey & Palmer, Citation2015), and especially on heavily burrowed specimens (Godfrey & Collareta, Citation2022a; Godfrey et al., Citation2022b).
Systematic ichnology
Ichnogenus Machichnus Mikuláš et al. (Citation2006).
Emended diagnosis: Groups of shallow, linear or regularly arcuate, parallel to subparallel grooves on firm or hard biogenic substrates (commonly bone, though also reported on coprolites); flat-bottomed to U-shaped in transverse section; trace-parallel striae or grooves may also be present.
Type ichnospecies: Machichnus regularis Mikuláš et al. (Citation2006).
Other included ichnospecies: Machichnus bohemicus Mikuláš et al. (Citation2006); M. multilineatus Mikuláš et al. (Citation2006); M. normani Chumakov et al. (Citation2013); M. harlandi Chumakov et al. (Citation2013); M. jeansi Chumakov et al. (Citation2013); M. fatimae de Araújo-Júnior et al. (Citation2017); M. dimorphodon isp. nov.
Machichnus dimorphodon isp. nov.
Etymology
“dimorphodon” from a combination of “di” (Greek, “two,” “twice,” or “double”), “morph” (Greek, “morphē” = “form,” “shape,” or “outward appearance”), and “odon” (Greek for “tooth”). This ichnospecific name was chosen because of the two sizes (orders or ranks) of teeth (or denticles or cusps) that are prerequisite in the formation of this trace fossil.
Holotype
A set of seven parallel, gently curving gouges, including five larger/deeper (primary) gouges and two finer (secondary) gouges (), preserved on the external surface of CMM-V-8998, a vertebrate (probably crocodilian) coprolite ().
Type locality and type horizon
Horseshoe, right bank of the Pamunkey River, New Kent County, Virginia, USA. The coprolite was found in situ at the base of the Miocene Calvert Formation (see the “Location and geology” paragraph for more details).
Paratype
A set of several primary and secondary gouges on the external surface of CMM-V-4480 (), a vertebrate (probably crocodilian) coprolite. The latter was found along an underwater bank of Clapp Creek, a tributary of the Black River, within the city limits of Kingstree, Williamsburg County, South Carolina (Godfrey & Palmer, Citation2015). It was recovered from a thick lag deposit of unconsolidated sediment, predominantly phosphatic quartz sands, comprising a bone-bed that includes a temporally mixed/time-averaged vertebrate assemblage of Late Cretaceous, Early Paleocene, and Plio-Pleistocene taxa (Cicimurri, Citation2010; Soehner, Citation2012). The paleoenvironment in the Kingstree area was a nearshore coastal environment, with the coprolite-rich bone-bed probably deposited in an estuary (Weems & Bybell, Citation1998; Soehner, Citation2012).
Figure 2. (a) CMM-V-4480, a gar-bitten coprolite featuring the paratype of Machichnus dimorphodon isp. nov. White arrows point to two of the secondary gouges that lie parallel and adjacent to the primary gouges. Modified from Godfrey and Palmer (Citation2015) (b) CMM-V-6615, a vertebrate-bitten coprolite featuring the referred specimen of M. dimorphodon isp. nov. Black arrows point to two of the secondary gouges that lie parallel and adjacent to the primary ones. Both specimens lightly dusted with sublimed ammonium chloride. Scale bars equal 10 mm.

Additional specimen
A set of primary and secondary gouges on the external surface of CMM-V-6615 (), a vertebrate (probably crocodilian) coprolite that was found near Summerville, South Carolina. Summerville is situated mostly in Dorchester County with small portions in the Berkeley and Charleston counties (Godfrey & Frandsen, Citation2016). The coprolite was acquired from an online vendor by George Frandsen and donated to the Calvert Marine Museum. Unfortunately, the vendor was unwilling to provide exact collecting locality information (other than to say that it was removed from a local sand pit), substantially diminishing the scientific value of this otherwise important specimen! Sand pits in the Summerville area remove sand down to the top of the Early Oligocene Givhans Ferry Member of the Ashley Formation (Robert Weems, pers. comm.). In so doing, Oligocene, Miocene, Pliocene, and Pleistocene fossils are also unearthed. Based on this information, the coprolite is likely not older than Early Oligocene. During the Oligocene, the area around Summerville was a nearshore coastal environment (Weems & Sanders, Citation2014).
Distribution
Verified distribution based on CMM-V-8993 () and CMM-V-6615 () includes the Miocene. Due to the broad uncertainties about the stratigraphic provenance of CMM-V-4480, the latter could possibly have originated from the Cretaceous (Maastrichtian stage) through to the Plio-Pleistocene.
Diagnosis
Parallel linear gouges of any length in nearly straight or variably curved lines, preserved together in two forms: 1) primary bold gouges and 2) fine secondary gouges, the latter being adjacent, variably interspersed, and parallel to the primary gouges.
Comparisons and remarks
Machichnus dimorphodon isp. nov. is distinct from the seven previously described ichnospecies included in Machichnus Mikuláš et al. (Citation2006). Machichnus dimorphodon isp. nov. differs from the type ichnospecies of Machichnus, M. regularis, as it consists of several discrete scratches that modify a smaller fraction of the affected surface. It differs from M. multilineatus in that it contains a smaller number of scratches (not the dozens characterizing M. multilineatus) which, again, only cover a small portion of the affected surface. Machichnus dimorphodon isp. nov. differs from M. bohemicus as it consists of non-overlapping, non-crossing scratches that co-occur with smaller accessory scratches. In addition, in each set of M. bohemicus, the individual traces resemble each other in terms of width and length, which is not the case in M. dimorphodon isp. nov. The latter differs from M. normani in that it is comprised of shorter groups of scratches that are not homogeneous in terms of dimensions (both width and length) and do not cross or overlap each other. Machichnus harlandi does possess accessory grooves parallel to larger, primary grooves, similar to M. dimorphodon isp. nov., but in M. harlandi the accessory grooves are found on both sides of a central primary groove that is characteristically striated longitudinally (grooves therefore occurring in a “1-3-1” pattern or alternating between “simple” and “W-shaped” scratch-makers). Machichnus jeansi involves multiple scratches overlapping along the same trajectory, and as such, is markedly different from M. dimorophodon isp. nov. Finally, M. fatimae is diagnosed by arcuate grooves that occasionally branch/combine, again a markedly different condition from those of M. dimorphodon isp. nov.
Discussion
Identity of the faecal producer
Given its large size and Lower Miocene age, relatively few organisms could have produced CMM-V-8998, the primary candidates being crocodiles and large chondrichthyans (Weems, Citation2018). Given aspects of its morphology and internal composition, it is most likely that CMM-V-8998 falls into the former category. Many fishes typically produce spiral faeces (Milàn et al., Citation2012) due to faecal matter being passed through the spiral valve in the colon prior to ejection (Williams, Citation1972; McAllister, Citation1985). Sharks and their relatives more specifically produce heteropolar spiralled forms, where the characteristic spiral groove is essentially continuous across the length of the faeces, rather than being concentrated at one end (Hunt et al., Citation1994). While not all chondrichthyan coprolites necessarily possess this exact morphology (Godfrey & Smith, Citation2010), their absence in CMM-V-8998 is more suggestive of a non-chondrichthyan origin for the specimen. Additionally, the overall shape of CMM-V-8998 is inconsistent with the typical expectation for chondrichthyan faeces, which is often pupate rather than elongated and cylindrical (Hunt & Lucas, Citation2010; Milàn, Citation2012; Weems, Citation2018).
Most characteristic of crocodilian faeces, however, is the specimen’s lack of any bony inclusions visible over its surface. Crocodilians possess large stomachs relative to their other internal organs, which not only enable them to consume large prey items, but also allows them to produce a large volume of stomach acid, fifty times more than humans in the case of Alligator mississippiensis (Coulson et al., Citation1989). Though of typical pH for stomach acids, the quantity crocodilians produce makes them extremely efficient in digestion (Coulson et al., Citation1989). This efficiency is most evident with respect to calcified tissues, which are demineralized in the stomach, converting bones to an “unrecognizable” organic matrix, and characteristically removing enamel from ingested teeth. Typically, the organic matrix decays within two days of faecal egestion (Fisher, Citation1981), meaning it would be completely absent by the time of fossilization, leading to a coprolite with a “clay-like,” homogeneous internal structure (Milàn, Citation2012). However, crocodilians cannot digest keratin or chitin and will egest structures like hair or feathers without significant alteration, typically in the outermost layers of the concentrically-structured crocodilian faecal package (Fisher, Citation1981; Milàn, Citation2012). Indeed, at least four examples of feathers preserved in crocodilian coprolites are known from along Calvert Cliffs (Wetmore, Citation1943; Mehling, Citation2010; Godfrey, pers. obs.). CMM-V-8998 bears no evidence of included keratinous or chitinous material, though this obviously does not preclude the specimen from being crocodilian in origin (e.g., Milàn et al., Citation2018).
In the Middle Miocene deposits of the Eastern Mid-Atlantic region of the United States, two species of crocodilians are known, both tomistomines belonging to the genus Thecachampsa: T. antiquus and T. sericodon. Weems (Citation2018) differentiated the two based on size and tooth morphology, T. antiquus being slightly larger (some individuals exceeding 4 m in body length) and possessing stockier teeth, interpreted as evidence of partitioning of food resources between these congeneric species, with T. sericodon targeting almost exclusively fish prey and T. antiquus being better equipped to hunt turtles. Though both postulated diets are conducive to a homogeneous coprolite without inclusions like CMM-V-8998, the slight difference in size could be diagnostic based on the diameter of the coprolite. Both Farlow et al. (Citation2010) and Milàn (Citation2012) noted a strong correlation between the diameter of a crocodilian coprolite and the length of the animal that produced it, the latter study giving the equation
where DS (in cm) is the diameter of the scat and TL (also in cm) is the total length of the producer. Given the irregular shape of CMM-V-8998 due to its aforementioned ventral flattening, diameter was taken as the average of the short and long diameters of the broken terminal face, i.e., 45 mm and 59 mm, respectively, yielding an average of 52 mm. By the above equation, this correlates with an animal 3.42 m in length. This value falls within the size range of both T. antiquus and T. sericodon, Weems (Citation2018) stating that only coprolites of 6 cm or more in diameter (4.1 m or more in animal length) could be confidently attributed to T. antiquus. Therefore, the producer of CMM-V-8998 can only be identified as Thecachampsa sp.
Identity of the biter
The seven long gouges on CMM-V-8998 () are interpreted as tooth marks, given their similarity to other such traces in fossilized faecal material described in the last 13 years (Godfrey & Smith, Citation2010; Godfrey & Palmer, Citation2015; Godfrey & Frandsen, Citation2016; Dentzien-Dias et al., Citation2018a, Citation2018b; Collareta et al., Citation2019; Godfrey et al., Citation2020). More specifically, the bite marks on CMM-V-8998 bear a strong resemblance to those on CMM-V-4480 () (Godfrey & Palmer, Citation2015). In both specimens, the gouges lie along a single plane, suggesting the organism responsible either had its teeth arranged in lateral/medial rows or in a row along an elongated jaw. Also like those on CMM-V-4480, the gouges on CMM-V-8998 are gently curved, suggesting a glancing bite from the side of the mouth, and have two different sizes (small or secondary gouges occurring in between some of the larger primary gouges), suggesting an organism with teeth of two different size classes. As in Godfrey and Palmer (Citation2015), the biter in question is concluded to be a gar (Lepisosteus sp.), which has elongated jaws with rows of teeth that regularly alternate in size, small teeth taking their place immediately beside larger teeth (). The prey capture habits of gars additionally reinforce this conclusion: modern gars (belonging either to Lepisosteus sp. or Atractosteus sp.) are ram feeders that hunt by slowly stalking a prey item until their jaws are aligned with the direction the prey is facing (Pavlov & Kasumyan, Citation2002; Porter & Motta, Citation2004; Herke, Citation2015). At this point, the gar rapidly moves its head laterally to grab its prey, such that it is now orthogonal to the length of the gar, and then uses suction to draw the prey into the buccal cavity, where it is ingested (Porter & Motta, Citation2004; Lemberg et al., Citation2019). The curvature of the gouges suggests such a capture style, the gar having turned its head to grab the faeces obliquely.
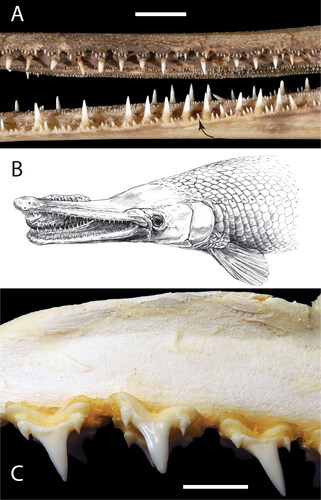
Figure 3. (a) Left lateral view of the mid-section of the rostrum of an extant gar (Lepisosteus osseus, CMM-O-33) showing the presence of small peripheral teeth (one of which is highlighted by a black arrow along the lower jaw) adjacent to the fewer larger fangs in both the upper and lower jaws. (b) Life drawing by Tim Scheirer of the way in which the type specimen might have been bitten. Reproduced from Godfrey and Palmer (Citation2015). (c) CMM-0-0007, labial view of a short section of the upper left lateral dentition of the extant porbeagle shark, Lamna nasus, showing teeth with a large main cusp and smaller bilateral cusplets. Scale bars equal 10 mm.
Given the exquisite preservation of the bite marks as well as its flattened ventral morphology, it is likely that CMM-V-8998 was not ingested, but rather that the gar’s attempted coprophagy was abortive in nature. It was probably more of a glancing bite. Though feeding style among fishes is highly variable across families and even between growth stages of an individual (Pavlov & Kasumyan, Citation2002), and though the diets of modern lepisosteids are diverse (Goodyear, Citation1967) and differ enough interspecifically to partition food sources (Walker et al., Citation2013), lepisosteids are not known to be coprophagous, meaning the bite marks likely do not record an attempt to consume the faeces or to assess their edibility (Godfrey & Palmer, Citation2015). Instead, the traces likely reflect abortive accidental coprophagy, supported by the tendency of gars to increase their hunting speed in response to prey motion (Porter & Motta, Citation2004). Therefore, the Miocene gar in question likely registered the motion of the crocodilian faeces as it sank through the water column, misidentified it as prey, and then immediately released it upon attempted “predation”.
Lepisosteus is known from the Miocene deposits of Southern Maryland, but the only known occurrences of this genus are from the St. Marys Formation (Carnevale & Godfrey, Citation2018), which postdates this specimen’s occurrence by more than 10 million years. While this does not negate that a lepisosteid is responsible for the bite marks on CMM-V-8998, regional occurrences of the family above and below the Calvert Formation suggesting its continuity through the Maryland Miocene, it does preclude any more detailed identification of the traces’ producer.
Although we focus on lepisosteids as the likely producers of M. dimorphodon isp. nov., we think it possible that traces consistent with this new ichnospecies could also be produced by at least sand tiger (Carchariidae or Odontaspididae), porbeagle () and salmon sharks (both Lamnidae). These sharks possess teeth with a large central cusp, usually bracketed by at least one much smaller cusplet laterally. Several Miocene carchariid/odontaspidid species are known locally (Kent, Citation2018), and could fit the bill.
In light of previous works, such as those by Eriksson et al. (Citation2011), Godfrey and Palmer (Citation2015) and Collareta et al. (Citation2022), the tiny incisions that locally ornament the coprolite’s outer surface were likely made by presently unknown invertebrates. They are reminiscent of the markings that characterize the walls of Transexcrementum cuniculus (Godfrey & Collareta, Citation2022a). The same kind of linear markings also variously ornament the surface of other local Miocene coprolites, both burrowed or not.
Additional remarks on Machichnus dimorphodon isp. nov.
That all the specimens of M. dimorphodon isp. nov. known to date occur on vertebrate coprolites is not surprising in light of its likely producers, i.e., gars of the family Lepisosteidae. Indeed, lepisosteids are mostly known as largely piscivorous predators that feed on smaller fishes and, subordinately, on crustaceans and insects (Kammerer et al., Citation2006), none of these prey types being provided with skeletal elements that are remarkably large and robust (at least, not enough to likely record recognizable tooth marks like those described above). In the fossil record, fish bite marks usually occur in the form of incisions on relatively large and robust bones such as those of marine mammals and reptiles (e.g., Hunt & Lucas, Citation2021, and the many references therein). Although gars are not believed to engage in coprophagy, faeces appear to be well suited for recording lepisosteid bite marks! That said, the faecal substrate is not regarded as diagnostic of M. dimorphodon isp. nov., as new finds may demonstrate the occurrence of lepisosteid tooth marks on e.g. large crustacean remains, especially in those Mesozoic deposits where remains of large gar species are present (Grande, Citation2010; Brito et al., Citation2017). Again, though not considered diagnostic for M. dimorphodon isp. nov., the faecal substrate of the known examples also expands the known substrates on which Machichnus may be found. The seven previously described Machichnus ichnospecies are either grouped into predatory traces on osteic substrates (M. regularis, M. bohemicus, M. multilineatus and M. fatimae), or as grazing traces on lithic substrates (M. normani, M. harlandi and M. jeansi) (Wisshak et al., Citation2019).
Conclusions
CMM-V-8998 is probably a crocodilian coprolite that was bitten by a gar (Lepisosteidae). Composite trace fossils like this are exceedingly rare. The gar bite marks were either exploratory or accidental in origin, as those fish are not known to engage in coprophagy. Other smaller/shorter incisions that ornament the coprolite’s outer surface were likely made by presently unknown invertebrates.
Acknowledgements
We would like to thank the late Billy Palmer for having found and donated CMM-V-4480 to CMM. Furthermore, we are also indebted to George Frandsen for having purchased and donated CMM-V-6615 to CMM. CMM Paleontology Collections Manager, John Nance was ever helpful in accessing specimens in his care. We gratefully acknowledge Robert Weems for his helpful comments on the geology in the area around Summerville, South Carolina. We also sincerely thank Dirk Knaust and an anonymous referee for their skilful reviews. Murray Gingras and Max Wisshak edited this contribution for Ichnos; thank you.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Araújo-Júnior, H. D., de Souza Barbosa, F. H., & da Silva, L. H. M. (2017). Overlapping paleoichnology, paleoecology and taphonomy: Analysis of tooth traces in a Late Pleistocene-early Holocene megafaunal assemblage of Brazil and description of a new ichnotaxon in hard substrate. Palaeogeography, Palaeoclimatology, Palaeoecology, 468, 122–128.
- Bertling, M., Braddy, R., Bromley, R. G., Demathieu, G., Genise, J., Mikuláš, R., Nielsen, J., Nielsen, K., Rindsberg, A., Schlirf, M., & Uchman, A. (2006). Names for trace fossils: A uniform approach. Lethaia, 39(3), 265–286.
- Brito, P. M., Alvarado-Ortega, J., & Meunier, F. J. (2017). Earliest known lepisosteoid extends the range of anatomically modern gars to the Late Jurassic. Scientific Reports, 7(1), 17830.
- Carnevale, G., & Godfrey, S. J. (2018). Miocene bony fishes of the Calvert, Choptank, St. Marys, and Eastover Formations, Chesapeake Group, Maryland and Virginia. In Godfrey, S. J. (Ed.), The geology and vertebrate paleontology of Calvert Cliffs (Vol. 100, pp. 161–213). Smithsonian Contributions to Paleobiology.
- Chumakov, N. M., Dronov, A. V., & Mikuláš, R. (2013). New Ichnospecies of scratching traces from phosphatic nodules (Cenomanian, England). Stratigraphy and Geological Correlation, 21(3), 291–299.
- Cicimurri, D. J. (2010). Fossil Chimaeroid remains (Chondrichthyes: Holocephali) from Williamsburg County, South Carolina, USA. Paludicola, 8(1), 37–48.
- Collareta, A., Gemelli, M., Varola, A., & Bianucci, G. (2019). Trace fossils on a trace fossil: A vertebrate-bitten vertebrate coprolite from the Miocene of Italy. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 293(2), 117–126.
- Collareta, A., Peri, E., Godfrey, S. J., & Bianucci, G. (2022). Just a different place to graze? An unusual occurrence of the echinoid feeding trace Gnathichnus pentax on a marine vertebrate coprolite (Miocene, Italy) and its palaeoethological implications. Carnets de Géologie, 22(20), 847–855.
- Cooper, C. L. (1935). Ammonium chloride sublimate apparatus. Journal of Paleontology, 9(4), 357–359.
- Coulson, R. A., Herbert, J. D., & Coulson, T. D. (1989). Biochemistry and physiology of alligator metabolism in vivo. American Zoologist, 29(3), 921–934.
- Dentzien-Dias, P., Carrillo-Briceño, J. D., Francischini, H., & Sánchez, R. (2018a). Paleoecological and taphonomical aspects of the Late Miocene vertebrate coprolites (Urumaco Formation) of Venezuela. Palaeogeography, Palaeoclimatology, Palaeoecology, 490, 590–603.
- Dentzien-Dias, P., Hunt, A., Lucas, S., Francischini, H., & Gulotta, M. (2018b). Traces on the surface of coprolites from shallow marine deposits of the Nanjemoy Formation (Early Eocene), Virginia, USA. Abstract: IV Latin American Symposium on Ichnology (SLIC 2018) in Santa Marta, Colombia on October 8, 2018.
- Eriksson, M. E., Lindgren, J., Chin, K., & Månsby, U. (2011). Coprolite morphotypes from the Upper Cretaceous of Sweden: Novel views on an ancient ecosystem and implications for coprolite taphonomy. Lethaia, 44(4), 455–468.
- Farlow, J. O., Chin, K., Argast, A., & Poppy, S. (2010). Coprolites from the Pipe Creek Sinkhole (Late Neogene, Grant County, Indiana, U.S.A.). Journal of Vertebrate Paleontology, 30(3), 959–969.
- Feldman, R. M. (1989). Whitening fossils for photographic purposes. Paleotechniques, 4, 342–346.
- Fisher, D. C. (1981). Crocodilian scatology, microvertebrate concentrations, and enamel-less teeth. Paleobiology, 7(2), 262–275.
- Frandsen, G., & Godfrey, S. J. (2019). A gar-bitten coprolite from the Eocene Green River Formation near Kemmerer, Wyoming, U.S.A. The Ecphora, 34(1), 2–4.
- Godfrey, S. J., Alford, A., Collareta, A., & Weems, R. E. (2020). A Paleocene vertebrate-bitten crocodilian coprolite from Liverpool Point, Maryland, USA. Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen, 296(3), 237–244.
- Godfrey, S. J., Bohaska, D. J., & Maisey, J. (2022a). The report of a rare deformed eagle ray (Myliobatiformes: Myliobatidae) tooth plate from the Neogene of Calvert Cliffs, Maryland, U.S.A. Carnets de Géologie, 22(6), 161–169.
- Godfrey, S. J., & Collareta, A. (2022a). A new ichnotaxonomic name for burrows in vertebrate coprolites from the Miocene Chesapeake Group of Maryland, U.S.A. Swiss Journal of Palaeontology, 141(1), 9.
- Godfrey, S. J., & Collareta, A. (2022b). Suggested names for major classes of fossils. The Ecphora, 37(2), 25. https://www.calvertmarinemuseum.com/DocumentCenter/View/4310/Ecphora-June-2022
- Godfrey, S. J., Collareta, A., & Nance, J. R. (2022b). Coprolites from Calvert Cliffs: Miocene fecal pellets and burrowed crocodilian droppings from the Chesapeake Group of Maryland, U.S.A. Rivista Italiana DI Paleontologia E Stratigrafia, 128(1), 69–79.
- Godfrey, S. J., & Frandsen, G. (2016). Vertebrate-bitten coprolite from South Carolina. The Ecphora, 31(1), 12–14.
- Godfrey, S. J., & Palmer, B. T. (2015). Gar-bitten coprolite from South Carolina, USA. Ichnos, 22(2), 103–108.
- Godfrey, S. J., & Smith, J. (2010). Shark-bitten vertebrate coprolites from the Miocene of Maryland. Naturwissenschaften, 97(5), 461–467.
- Goodyear, C. P. (1967). Feeding habits of three species of gars, Lepisosteus, along the Mississippi Gulf Coast. Transactions of the American Fisheries Society, 96(3), 297–300.2.0.CO;2]
- Grande, L. (2010). An empirical synthetic pattern study of gars and closely related species (Lepisosteiformes) based mostly on skeletal anatomy: The resurrection of Holostei. American Society of Ichthyologists and Herpetologists, Special Publication, 7, 1–874.
- Herke, M. (2015). Functional morphology of the occipital and neck region and prey capture in gars (Lepisosteus sp.) [Master’s thesis]. University of Wien. https://utheses.univie.ac.at/detail/34655#.
- Hunt, A. P., Chin, K., & Lockley, M. G. (1994). The palaeobiology of vertebrate coprolites. In Donovan, S. K. (Ed.), The palaeobiology of trace fossils (1st ed., pp. 221–240). John Wiley & Sons.
- Hunt, A. P., & Lucas, S. G. (2010). Crocodylian coprolites and the identification of the producers of coprolites. New Mexico Museum of Natural History and Science Bulletin, 51, 219–226.
- Hunt, A. P., & Lucas, S. G. (2021). The ichnology of vertebrate consumption: Dentalites, gastroliths and bromalites. New Mexico Museum of Natural History and Science Bulletin, 87, 1–226.
- Kammerer, C. F., Grande, L., & Westneat, M. W. (2006). Comparative and developmental functional morphology of the jaws of living and fossil gars (Actinopterygii: Lepisosteidae). Journal of Morphology, 267(9), 1017–1031.
- Kent, B. W. (2018). The cartilaginous fishes (chimaeras, sharks and rays) of Calvert Cliffs, Maryland, USA. In Godfrey, S. J. (Ed.). The geology and vertebrate paleontology of Calvert Cliffs, Maryland, USA (Vol. 100, pp. 45–160). Smithsonian Contributions to Paleobiology.
- Lemberg, J. B., Shubin, N. H., & Westneat, M. W. (2019). Feeding kinematics and morphology of the alligator gar (Atractosteus spatula, Lacépède, 1803). Journal of Morphology, 280(10), 1548–1570.
- McAllister, J. A. (1985). Reevaluation of the formation of spiral coprolites. The University of Kansas Paleontological Contributions, 114, 1–12.
- Mehling, C. (2010). Turds of a feather. Journal of Vertebrate Paleontology, 30(2), 133A.
- Mikuláš, R., Kadlecová, E., Fejfar, O., & Dvořák, Z. (2006). Three new Ichnogenera of biting and gnawing traces on reptilian and mammalian bones: A case study from the miocene of the Czech Republic. Ichnos, 13(3), 113–127.
- Milàn, J. (2012). Crocodylian scatology – A look into morphology, internal architecture, inter- and intraspecific variation and prey remains in extant crocodylian feces. New Mexico Museum of Natural History and Science Bulletin, 57, 65–72.
- Milàn, J., Rasmussen, B. W., & Bonde, N. (2012). Coprolites with prey remains and traces from coprophagous organisms from the Lower Cretaceous (Late Berriasian) Jydegaard Formation of Bornholm, Denmark. New Mexico Museum of Natural History and Science Bulletin, 57, 235–240.
- Milàn, J., Rasmussen, E. S., & Dybkjær, K. (2018). A crocodilian coprolite from the lower Oligocene Viborg Formation of Sofienlund Lergrav, Denmark. Bulletin of the Geological Society of Denmark, 66, 181–187.
- Pavlov, D. S., & Kasumyan, A. O. (2002). Feeding diversity in fishes. Journal of Ichthyology, 42(2), 5137–5159.
- Porter, H. T., & Motta, P. J. (2004). A comparison of strike and prey capture kinematics of three species of piscivorous fishes: Florida gar (Lepisosteus platyrhincus), redfin needlefish (Strongylura notata), and great barracuda (Sphyraena barracuda). Marine Biology, 145(5), 989–1000.
- Shelburne, E. C. H., & Thompson, A. C. (2016). Specimen whitening: An assessment of methods of ammonium chloride smoke removal. Collection Forum, 30(1–2), 63–72.
- Soehner, J. R. (2012). Why is there such a high concentration of vertebrate remains within a bone-bed along Clapp Creek, Williamsburg County, South Carolina? [Master’s thesis]. Department of Earth and Environmental Sciences, Wright State University.
- Walker, R. H., Kluender, E. R., Inebnit, T. E., & Adams, S. R. (2013). Differences in diet and feeding ecology of similar-sized spotted (Lepisosteus oculatus) and shortnose (Lepisosteus platostomus) gars during flooding of a south-eastern US river. Ecology of Freshwater Fish, 22(4), 617–625.
- Weems, R. E. (2018). Crocodilians of the Calvert Cliffs. In Godfrey, S. J. (Ed.). The geology and vertebrate paleontology of Calvert Cliffs, Maryland, USA (Vol. 100, pp. 213–241). Smithsonian Contributions to Paleobiology.
- Weems, R. E., & Bybell, L. M. (1998). Geology of the Black Mingo Group (Paleocene) in the Kingstree and St. Stephen areas of South Carolina. Transactions of the American Philosophical Society, 88(4), 9–27.
- Weems, R. E., & Sanders, A. E. (2014). Oligocene pancheloniid sea turtles from the vicinity of Charleston, South Carolina, U.S.A. Journal of Vertebrate Paleontology, 34(1), 80–99.
- Wetmore, A. (1943). The occurrence of feather impressions in the Miocene deposits of Maryland. The Auk, 60(3), 440–441.
- Williams, M. E. (1972). The origin of “spiral coprolites”. University of Kansas Paleontological Contributions, 59, 1–19.
- Wisshak, M., Knaust, D., & Bertling, M. (2019). Bioerosion ichnotaxa: Review and annotated list. Facies, 65(2), 1–39.
- Zonneveld, J.-P., Fiorillo, A. R., Hasiotis, S., & Gingras, M. K. (2022). Tooth marks, gnaw marks, claw-marks, bite marks, scratch marks, etc: Terminology in ichnology. Ichnos, 29(2), 93–101.
