Abstract
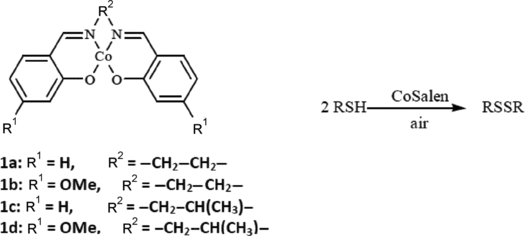
A convenient and facile catalytic oxidation of thiols to the corresponding disulfides is described using CoSalen as the catalyst and air as the oxidizing agent. This new approach provides an efficient method for the preparation of symmetrical disulfides in high yields and under mild conditions.
[Supplemental materials are available for this article. Go to the publisher's online edition of Phosphorus, Sulfur, and Silicon and the Related Elements for the following free supplemental resource: Spectroscopic Identification of Products 3a–3l.]
GRAPHICAL ABSTRACT
INTRODUCTION
Catalytic oxidation is a reaction of high interest for both industrial and academic research.Citation 1 There is a strong need for studying this powerful reaction, particularly toward the finding of catalysts that are more efficient, selective, and eco-friendly. Enzymes are natural catalysts, well known for their high selectivity, operating at mild temperatures. However, their use in an industrial environment is expensive and their handling is rather difficult.Citation 2 The desire to mimic enzymatic system has created an extensive area of research into synthetic porphyrin and Schiff base models of enzyme's active sites, especially for monooxygenase enzymes.Citation 3 Among them, N,N′-bis(salicylidene)ethylenediiminocobalt(II) (CoSalen 1a) is the initially and most extensively investigated oxygen carrier due to its structural similarity to the cytochrome P450 family found in biological systems.Citation 4 The properties of CoSalen complexes have received considerable attention in the past several yearsCitation 5 due to their oxygen-binding activity.
Although thiols can be easily oxidized to disulfides, they also suffer from overoxidation. The oxidation does not stop at the disulfide formation and proceeds further to sulfoxides and sulfones.Citation 6 Therefore, the careful choice of a proper oxidant and catalyst is important. Thus, there have been extensive studies carried out to elaborate controlled conditions such as halogens,Citation 7 Ph3PO/BTC,Citation 8 K+/CH3CN,Citation 9 iodine/hydrogen iodide,Citation 10 bromine,Citation 11 potassium dichromate,Citation 12 potassium permanganate/copper(II) sulfate,Citation 13 hydrogen peroxide in trifluoroethanol,Citation 14 dimethyl sulfoxide,Citation 15 cerium(IV) salts,Citation 16 sulfuryl chloride,Citation 17 cesium fluoride on celite,Citation 18 N-phenyltriazolinedione,Citation 19 silica-supported cobalt(II) tetrasulfophthalocyanine,Citation 20 monochloro poly(styrenehydantoin) beads,Citation 21 calcium hypochlorite,Citation 22 1,3-dibromo-5,5-dimethylhydantoin,Citation 23 potassium phosphate,Citation 24 enzymes,Citation 25 and electrochemical techniques.Citation 26
Although these previously reported methods are very effective, most if not all of them suffer from drawbacks including the use of expensive, rare, or toxic reagents and metal oxidants, low yields, long reaction times, high reaction temperatures, or the risk of overoxidation of the disulfides to sulfoxides. Mn(III)SalenCitation 27 offered the oxidation of thiols to disulfides using hydrogen peroxide as the oxidant.
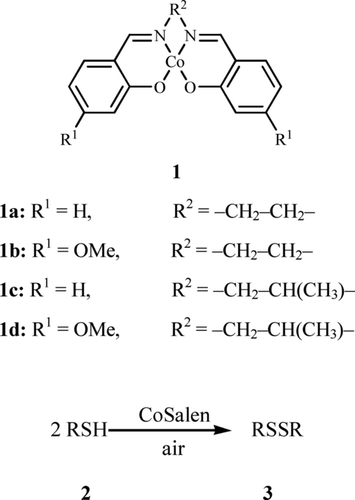
Herein, we report a facile, convenient, and efficient catalytic oxidation of thiols to the corresponding disulfides using N,N′-bis(salicylidene)ethylenediiminocobalt(II) (CoSalen 1a) and its derivatives (1b–d) using air as the oxidant ().
RESULTS AND DISCUSSION
Initially, a test reaction using 2-thiobenzothiazole as reagent was performed. Among the solvents tested, ethanol gave the best results. The temperature of 50 °C gave the highest yield of 93% (entries 1–4, ). Subsequently, the effect of the amount of CoSalen on the reaction efficiency was studied. It was observed that 2.5% (mol/eq.) of CoSalen was optimum for this reaction. The oxidation did not occur in the absence of CoSalen (entry 5, ).
Table 1 Oxidative coupling of 2-thiobenzothiazole under different conditionsFootnote a
CoSalens 1b–d were also tested in the oxidation of thiols to disulfides under the same reaction conditions. The results are shown in .
Table 2 Oxidative coupling of 2-thiobenzothiazole catalyzed by different CoSalensFootnote a
As it can be seen from , CoSalens 1b and 1d showed higher oxidation efficiency than 1a and 1c, respectively. The results indicated that an electron-donating group such as methoxy may increase the oxygen-binding ability. In addition, the lower oxidation efficiency of 1a and 1b in comparison with that of 1c and 1d, respectively, possibly shows that iso-propyl groups may cause some steric hindrance and reduces the oxygen-binding ability ().
With optimized conditions in hand, we explored the scope of this process with respect to thiol structure (). The catalytic oxidation occurred smoothly with excellent selectivity and yield. No sulfoxides or sulfones were detected by gas chromatography (GC) in the reaction mixtures.
Table 3 Aerobic oxidative coupling of thiols to disulfides catalyzed by CoSalen Footnote a
CONCLUSIONS
In summary, we have developed an experimentally simple CoSalen-catalyzed aerobic oxidation of thiols to disulfides using air as the oxidant. Although the exact mechanism of this transformation is still unclear, this catalytic oxidation system is clean, selective, and very effective. Further oxidation reactions using this system are in progress in our laboratory.
EXPERIMENTAL
All chemicals were used as received from different commercial sources without further purification. The CoSalen complexes 1a–d were prepared by a procedure similar to that reported by R. H. Bailes.Citation 33 Melting points were determined on a digital melting point apparatus WRS-1B and are uncorrected. Infrared (IR) spectra were recorded as KBr pellets on a Nicolet Avatar-370 infrared spectrophotometer. 1H NMR spectra (500 MHz) were measured on a Varian 500 instrument using CDCl3 or DMSO-d6 as the solvent with tetramethylsilane as an internal standard. Mass spectra (MS) were recorded on a Finnigan Trace DSQ (EI, 70 eV) spectrometer.
General Procedure for the Oxidation of Thiols
To CoSalen 1a (8.1 mg, 0.025 mmol) in ethanol (10 mL), 2-thiobenzothiazole (167 mg, 1.00 mmol) was added and air was bubbled through the reaction mixture. The reaction mixture was stirred at 50 °C until thin layer chromatography indicated the disappearance of the starting material. Evaporation of the solvent left the crude product, which was purified by column chromatography over silica gel (hexane) to provide pure 2,2-dibenzothiazyl disulfide as a light yellow solid. All the isolated disulfide compounds are known and they were identified through comparison of IR, 1H NMR, MS, and melting points with literature data. All of the spectra are in excellent agreement with the literature. As the products have all been previously reported and referenced in , the 1H NMR data are presented in the Supplemental Materials.
gpss_a_568025_sup_22304528.docx
Download MS Word (27.5 KB)Acknowledgments
We are grateful to the financial support (grant no. Y307008) from Zhejiang Provincial Natural Science Foundation and for the review of the manuscript by Dr. Cassia S. Mizuno.
Notes
aAll reactions were carried out using 2-thiobenzothiazole (1 mmol), ethanol (10 mL), and 2.5% (mol/eq.) of CoSalen 1a.
bIsolated yields based on 2-thiobenzothiazole.
aAll reactions were carried out using 2-thiobenzothiazole (1 mmol), ethanol (10 mL), and CoSalen complex (0.025 mmol).
bIsolated yields based on 2-thiobenzothiazole.
aThe reactions were carried out using 1 mmol of substrate in 10 mL of ethanol at 50 °C with 0.025 mmol of CoSalen 1a and bubbling of air into ethanol.
bIsolated yields based on the thiols.
cThe reaction was carried out at room temperature.
REFERENCES
- Peyrovi , M. H. , Mahdavi , V. , Salehi , M. A. and Mahmoodian , R. 2005 . Catal. Commun. , 6 : 476 – 479 .
- Kaim , W. and Schwederski , B. 1994 . Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life , 113 – 115 . New York : Wiley .
- Haber , J. , Pamin , K. and Poltowicz , J. 2004 . J. Mol. Catal. Chem. , 224 : 153 – 159 .
- Monti , D. , Tagliatesta , P. , Mancini , G. and Boschi , T. 1998 . Angew. Chem. Int. Ed. , 37 : 1131 – 1133 .
- Birnhaum , E. R. , Labinger , J. A. , Bercaw , J. E. and Gray , H. B. 1998 . Inorg. Chem. , 270 : 433 – 439 .
- Tsumaki , T. 1938 . Bull. Chem. Soc. Jpn. , 13 : 252 – 260 .
- Wilmarth , W. K. , Aranoff , S. and Calvin , M. 1946 . J. Am. Chem. Soc. , 68 : 2263 – 2266 .
- Chen , D. , Martell , A. E. and Sun , Y. 1989 . Inorg. Chem. , 28 : 2647 – 2652 .
- Gunter , M. J. and Turner , P. 1991 . Coord. Chem. Rev. , 108 : 115 – 161 .
- Roman , I. C. 1984 . U.S. Patent, 4,451,270 . Chem. Abstr. , 100 : 158928b
- Adduci , A. J. 1976 . Chemtech. , 6 : 575 – 580 .
- Norman , J. A. T. , Pez , G. P. and Roberts , D. A. 1988 . Oxygen Complexes and Oxygen Activation by Transition Metals , Edited by: Martell , A. E. and Sawyer , D. T. 107 – 112 . New York : Plenum .
- Iranpoor , N. , Mohajer , D. and Rezaeifard , A. R. 2004 . Tetrahedron Lett. , 45 : 3811 – 3815 .
- Lazzaro , F. , Crucianelli , M. , De Angelis , F. , Neri , V. and Saladino , R. 2004 . Tetrahedron Lett. , 45 : 9237 – 9240 .
- Peskin , A. V. and Winterbourn , C. C. 2001 . Free Radical Biol. Med. , 30 : 572 – 579 .
- Li , Y.-S. , Liang , X.-R. and Su , W.-K. 2003 . Org. Prep. Proced. Int. , 35 : 613 – 616 .
- Dong , W.-L. , Huang , G.-Y. , Li , Z.-M. and Zhao , W.-G. 2009 . Phosphorus, Sulfur, Silicon Relat. Elem. , 184 : 2058 – 2065 .
- Lang , R. F. , Ju , T. D. , Kiss , G. , Hoff , C. D. and Bryan , J. C. 1997 . Inorg. Chim. Acta , 259 : 317 – 327 .
- Ali , M. H. and McDermott , M. 2002 . Tetrahedron Lett. , 43 : 6271 – 6273 .
- Patel , S. and Mishra , B. K. 2004 . Tetrahedron Lett. , 45 : 1371 – 1372 .
- Shaabani , A. and Lee , D. J. 2001 . Tetrahedron Lett. , 42 : 5833 – 5836 .
- Kesavan , V. , Bonnet-Delpon , D. and Begue , J.-P. 2000 . Synthesis , : 223 – 225 .
- Sanz , R. , Aguado , R. , Pedrosa , M. R. and Arnaiz , F. J. 2002 . Synthesis , : 856 – 858 .
- Karimi , B. , Hazatkhani , H. and Zareyee , D. 2002 . Synthesis , : 2513 – 2516 .
- Dhar , D. N. and Bag , A. K. 1984 . Ind. J. Chem. , 23 : 974
- Firouzabadi , H. , Iranpoor , N. and Parham , H. A. 1984 . Synth. Commun. , 14 : 717 – 723 .
- Leino , R. and Lonnqvist , J.-E. 2004 . Tetrahedron Lett. , 45 : 8489 – 8491 .
- Shah , S. T. A. , Khan , K. M. , Fecker , M. and Voelter , W. 2003 . Tetrahedron Lett. , 44 : 6789 – 6791 .
- Christoforou , A. , Nicolaou , G. and Elemes , Y. 2006 . Tetrahedron Lett. , 47 : 9211 – 9213 .
- Chauhan , S. M. S. , Kumar , A. and Srinivas , K. A. 2003 . Chem. Commun. , : 2348 – 2349 .
- Shaabani , A. , Safari , N. , Shoghpour , S. and Rezayan , A. H. 2008 . Monatsh Chem. , 139 : 613 – 615 .
- Akdag , A. , Webb , T. and Worley , S. D. 2006 . Tetrahedron Lett. , 47 : 3509 – 3510 .
- Hirano , M. , Yakabe , S. , Uraoka , N. and Marimoto , T. 1998 . Org. Prep. Proced. Int. , 30 : 360 – 363 .
- Khazaei , A. , Zolfigol , M. A. and Rostami , A. 2004 . Synthesis , : 2959 – 2961 .
- Joshi , A. V. , Bhusare , S. , Baidossi , M. , Qafisheh , N. and Sasson , Y. 2005 . Tetrahedron Lett. , 46 : 3583 – 3585 .
- Sridhar , M. , Vadivel , S. K. and Bhalerao , U. T. 1998 . Synth. Commun. , 28 : 1499 – 1502 .
- Leite , S. L. S. , Pardini , V. L. and Viertler , H. 1990 . Synth. Commun. , 20 : 393 – 397 .
- Hosseinpoor , F. and Golchoubian , H. 2006 . Catal. Lett. , 111 : 165 – 168 .
- Sharma , S. P. 2009 . Catal. Commun. , 10 : 905 – 912 .
- Golchoubian , H. and Hosseinpoor , F. 2007 . Catal. Commun. , 8 : 697 – 700 .
- Chen , F.-E. , Lu , Y.-W. , He , Y.-P. , Luo , Y.-F. and Yan , M.-G. 2002 . Synth. Commun. , 32 : 3487 – 3492 .
- Pilgram , K. 1965 . Tetrahedron , 21 : 1999 – 2013 .
- Cohen , V. I. 1976 . Helv. Chim. Acta , 59 : 840 – 844 .
- Bailes , R. H. and Calvin , M. 1947 . J. Am. Chem. Soc. , 69 : 1886 – 1893 .