Abstract
α-Hydroxyphosphonates have been in the focus of interest for many years due to their potential in biological activity. Not only α-hydroxyphosphonates are of general importance, but also their derivatives. α-Aminophosphonates and α-hydroxyphosphonic acids have already been recognized for their biological effects, while phosphoryloxyphosphonates represent a new family of compounds. In this paper we present alternative syntheses towards α-hydroxyphosphonates and their derivatives.
Graphical Abstract

Introduction
α-Hydroxyphosphonates form a class of important organophosphorus compounds. The representatives of this family may have enzyme inhibitory, herbicidal, antibacterial, antibiotic, antifungal or antioxidant effect.[Citation1–7] The cytotoxic activity of some α-hydroxyphosphonates was also studied.[Citation8] Besides their bioactivity, α-hydroxyphosphonates also represent a privileged class in synthetic organic chemistry as starting materials for a series of related derivatives.[Citation9] The derivatives obtained by different transformations of α-hydroxyphosphonates may also include biologically active species.[Citation10–13] O-phosphorylation, nucleophilic substitution and catalytic debenzylation of α-hydroxyphosphonates result in valuable derivatives. Among those, α-aminophosphonates are of special importance due to their analogy to α-amino acid derivatives.[Citation14,Citation15]
Results and discussion
Green synthesis of α-hydroxyphosphonates
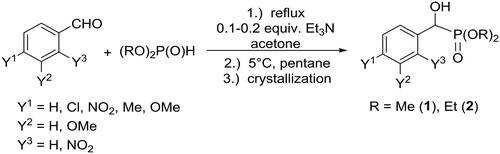
α-Hydroxyphosphonates are generally synthesized by the Pudovik reaction involving the nucleophilic addition of a dialkyl phosphite to a carbonyl compound. Although many base- or acid-catalyzed procedures are known for the addition, these methods often suffer of certain limitations regarding the work-up that usually requires extraction, crystallization and chromatography.[Citation16]
In contrast to the literature methods, we developed a procedure involving a robust work-up. According to our method, the mixture of benzaldehyde derivative and dialkyl phosphite is heated in the presence of 10% of triethylamine as the catalyst in a minimum quantity of acetone as the solvent (Scheme 1, ).[Citation17]
Table 1. Experimental details for the preparation of α-hydroxyphosphonates (1 and 2).
After completion of the reaction, the work-up comprised the addition of a small amount of n-pentane, and crystallization on cooling to room temperature. Filtration of the precipitate provided the desired α-hydroxyphosphonates in yields of 78–95%. The presence of different substituents in the aromatic ring had an impact on the reactivity: while the electron-withdrawing nitro group promoted the reaction (/Entries 3 and 4), electron-donating groups (such as alkyl and alkoxy) resulted in slower reactions and lower yields. In the latter cases, a higher amount (20 to 30%) of triethylamine was applied (/Entries 5 and 6).
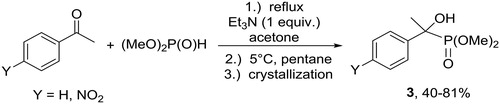
The addition of dimethyl phosphite to acetophenones afforded sterically hindered α-hydroxyphosphonates 3. Due to the decreased reactivity of the ketones, 1 equivalent of the catalyst had to be applied, and the yields were somewhat lower (Scheme 2).
O-phosphorylation of α-hydroxyphosphonates
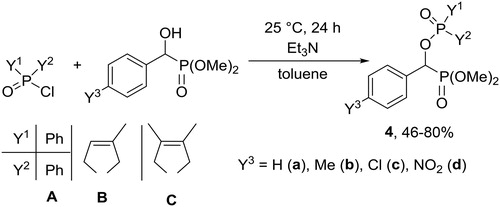
Surprisingly, while α-acyloxyphosphonates were already known as potential pharmaceutical and agricultural agents,[Citation18] the analogous α-phosphoryloxyphosphonates have been unknown. We reported the reaction of dimethyl 1-hydroxy-1-arylmethylphosphonates (1) with different P-chlorides to afford new compounds with > P(O)OCHP(O)< moiety.[Citation19]
1-Hydroxy-1-arylmethylphosphonates (1) prepared from the corresponding substituted benzaldehyde and dimethyl phosphite as described above, were reacted with phosphinic chlorides to afford 1-phosphinoyloxyphosphonates (4) (Scheme 3). Three phosphinic chlorides were chosen as phosphorylating agents: diphenylphosphinic chloride (A), 1-chloro-3-methyl-3-phospholene 1-oxide (B) and 1-chloro-3,4-dimethyl-3-phospholene 1-oxide (C). 1-Chloro-3-phospholene 1-oxides (B and C) were prepared from the corresponding cyclic phosphinic acids by reaction with thionyl chloride.
Phosphinoylation of 1 took place in the presence of 1.1 equivalents of phosphinic chloride and 1.2 equivalents of triethylamine in toluene (Scheme 3). The mixture was stirred at the temperatures and for the times given in . When diphenylphosphinic chloride (A) was used, the phosphinoylation required a somewhat longer reaction time of 48 h, as compared to 1-chloro-3-phospholene 1-oxides. Electron-withdrawing substituents facilitated the reaction, while electron-donating substituents resulted in somewhat lower (46–50%) yields (). The products so-obtained were purified by column chromatography to give the corresponding α-phosphoryloxyphosphonates in yields of 46–80%.
Table 2. Experimental details for the preparation of α-phosphoryloxyphosphonates (4).
The reaction of α-hydroxyphosphonates with primary amines to afford α-aminophosphonates
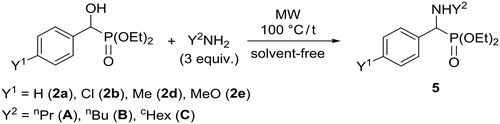
We aimed at the conversion of α-hydroxyphosphonates (1) prepared by us to the corresponding α-aminophosphonates (5) by reaction with primary amines (Scheme 4). The substitution reactions took place under MW irradiation at 100 °C, without the use of any solvent. It was previously found by us, that the substitution is facilitated by the beneficial neighboring group effect of the P = O function as was also suggested by high level DFT calculations.[Citation20]
It can be seen in that after a reaction time of 10–30 min, the yields after purification by column chromatography fell in the range of 50–79%.
Table 3. Experimental details for the preparation of α-aminophosphonates (5).
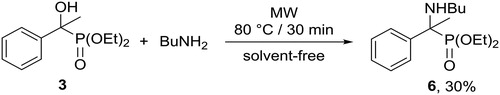
The sterically more hindered diethyl α-hydroxy-α-methyl-benzylphosphonate (3) was also reacted with n-butylamine under MW-assisted and solvent-free conditions to prepare α-aminophosphonate 6 (Scheme 5). However, in this case, the reaction was more reluctant, and product 6 showed sensitivity to thermal effects. Therefore, the substitution was carried out at a lower temperature of 80 °C to provide aminophosphonate 6 in a modest yield of 30%.[Citation15]
Catalytic debenzylation of dibenzyl α-hydroxyphosphonates 7
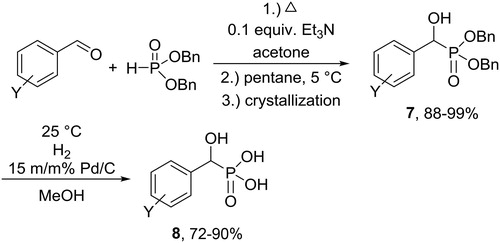
Extending our method to the synthesis of α-hydroxyphosphonates formed by the addition of dibenzyl phosphite to benzaldehydes, we synthesized a series of dibenzyl α-hydroxyphosphonates as precursors of the corresponding α-hydroxyphosphonic acids.
α-Hydroxyphosphonic acids are usually prepared from the corresponding α-hydroxyphosphonates by hydrolysis in the presence of hydrochloric acid used in an excess.[Citation21] In order to provide a greener approach, we aimed at the synthesis of α-hydroxyphosphonic acids (8) by catalytic debenzylation of the corresponding dibenzyl α-hydroxyphosphonates (7) (Scheme 6).
The hydrogenation reactions were performed using 10% Pd/C in methanol under mild conditions (25 °C, 10 bar). The reactions took place easily. After a short reaction time of 5–15 min, the α-hydroxyphosphonic acids were obtained in yields of 72–90% following purification ().[Citation22]
Table 4. Experimental details for the preparation of α-hydroxyphosphonic acids.
Conclusions
In this paper we presented alternative protocols for the synthesis of α-hydroxyphosphonates, 1-phosphinoyloxyphosphonates, α-aminophosphonates and α-hydroxyphosphonic acids. Among the compounds prepared, many representatives showed promising activity against human uterine sarcoma cell lines. These results will be published elsewhere.
Acknowledgments
This work was supported by National Research, Development and Innovation Office (NKFIH project TÉT_16-1-2016-0111).
References
- Tao, M.; Bihovsky, R.; Wells, G. J.; Mallamo, J. P. Novel Peptidyl Phosphorus Derivatives as Inhibitors of Human Calpain I. J. Med. Chem. 1998, 41, 3912–3916. DOI:10.1021/jm980325e.
- Dellaria, J. F.; Jr.; Maki, R. G.; Stein, H. H.; Cohen, J.; Whittern, D.; Marsh, K.; Hoffman, D. J.; Plattner, J. J.; Perun, T. J. Organocatalytic Enantioselective Synthesis of α-Hydroxy Phosphonates. J. Med. Chem. 1990, 33, 534–542. DOI:10.1021/ja062091r.
- Kafarski, P.; Lejczak, B. Review on Enzymatic Methods. J. Mol. Catal. B Enzym. 2004, 29, 99–104. DOI:10.1016/j.molcatb.2003.12.013.
- Fleisch, H. Bisphosphonates: mechanisms of Action. Endocr. Rev. 1998, 19, 80–100. DOI:10.1210/edrv.19.1.0325.
- Kategaonkar, A. H.; Pokalwar, R. U.; Sonar, S. S.; Gawali, V. U.; Shingate, B. B.; Shingare, M. S. Synthesis, in Vitro Antibacterial and Antifungal Evaluations of New Alpha-Hydroxyphosphonate and New Alpha-Acetoxyphosphonate Derivatives of Tetrazolo [1, 5-a] Quinoline. Eur. J. Med. Chem 2010, 45, 1128–1132. DOI:10.1016/j.ejmech.2009.12.013.
- Snoeck, R.; Holy, A.; Dewolf-Peeters, C.; Van Den Ord, J.; De Clercq, E.; Andrei, G. Antivaccinia Activities of Acyclic Nucleoside Phosphonate Derivatives in Epithelial Cells and Organotypic Cultures. Antimicrob. Agents Chemother. 2002, 46, 3356–3361. DOI:10.1128/AAC.46.11.3356-3361.2002.
- Pokalwar, R. U.; Hangarge, R. V.; Maske, P. V.; Shingare, M. S. Synthesis and Antibacterial Activities of α-Hydroxyphosphonates and α-Acetyloxyphosphonates Derived from 2-Chloroquinoline-3-Carbaldehyde. Arkivoc 2006, 11, 196–204. DOI:10.3998/ark.5550190.0007.b20.
- Kalla, R. M. N.; Lee, H. R.; Cao, J.; Yoo, J. W.; Kim, I. Phospho Sulfonic Acid: An Efficient and Recyclable Solid Acid Catalyst for the Solvent-Free Synthesis of α-Hydroxyphosphonates and Their Anticancer Properties. New J. Chem. 2015, 39, 3916–3922. DOI:10.1039/C5NJ00312A.
- Rádai, Z.; Keglevich, G. Synthesis and Reactions of α-Hydroxyphosphonates. Molecules 2018, 23, 1493–1522. DOI:10.3390/molecules23061493.
- Chen, X. B.; Shi, D. Q. Synthesis and Biological Activity of Novel Phosphonate Derivatives Containing of Pyridyl and 1,2,3-Triazole Rings. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1134–1144. DOI:10.1080/10426500701578522.
- Chen, T.; Shen, P.; Li, Y.; He, H. Synthesis and Herbicidal Activity of O,O-Dialkyl Phenoxyacetoxyalkylphosphonates Containing Fluorine. J. Fluorine Chem. 2006, 127, 291–295. DOI:10.1016/j.jfluchem.2005.11.013.
- Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H. W.; Wan, J. Molecular Docking and Three-Dimensional Quantitative Structure-Activity Relationship Studied on the Binding Modes of Herbicidal 1-(Substituted Phenoxyacetoxy)Alkylphosphonates to the E1 Component of Pyruvate Dehydrogenase. J. Agric. Food Chem. 2007, 55, 1871–1880. DOI:10.1021/jf062730h.
- Wang, W.; Wang, L. P.; Ning, B. K.; Mao, M. Z.; Xue, C.; Wang, H. Y. Synthesis and Insecticidal Activities of O,O-Dialkyl-2-[3-Bromo-1-(3-Chloropyridin-2-yl)-1H-Pyrazole-5-Carbonyloxy] (Aryl) Methylphosphonates. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1362–1367. DOI:10.1080/10426507.2016.1206103.
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. DOI:10.1021/jm200587f.
- Kiss, N. Z.; Rádai, Z.; Mucsi, Z.; Keglevich, G. Synthesis of α‐Aminophosphonates from α‐Hydroxyphosphonates; a Theoretical Study. Heteroatom Chem. 2016, 27, 260–268. DOI:10.1002/hc.21324.
- Rádai, Z.; Kiss, N. Z.; Keglevich, G. Synthesis of α-Hydroxyphosphonates, an Important Class of Bioactive Compounds. 91–107. In Organophosphorus Chemistry: Novel Developments; Keglevich, G.; Ed..; Walter de Gruyter: Berlin, 2018.
- Keglevich, G.; Rádai, Z.; Kiss, N. Z. To Date the Greenest Method for the Preparation of α-Hydroxyphosphonates from Substituted Benzaldehydes and Dialkyl Phosphites. Green Proc. Synth. 2017, 6, 197–201. DOI:10.1515/gps-2016-0125.
- Chen, X.B.; Shi, D.Q. Synthesis and biological activity of novel phosphonate derivatives containing of pyridyl and 1,2,3-triazole rings. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1134–1144. DOI:10.1080/10426500701578522
- Rádai, Z.; Hodula, V.; Kiss, N. Z.; Kóti, J.; Keglevich, G. Phosphorylation of (1-Aryl-1-Hydroxymethyl)Phosphonates. Mend. Commun. 2019, 29, in press. DOI:10.1016/j.mencom.2019.03.
- Kiss, N. Z.; Kaszás, A.; Drahos, L.; Mucsi, Z.; Keglevich, G. A Neighbouring Group Effect Leading to Enhanced Nucleophilic Substitution of Amines at the Hindered α-Carbon Atom of an α-Hydroxyphosphonate. Tetrahedron Lett. 2012, 53, 207–209. DOI:10.1016/j.tetlet.2011.11.026.
- Desai, J.; Wang, Y.; Wang, K.; Malwal, S. R.; Oldfield, E. Isoprenoid Biosynthesis Inhibitors Targeting Bacterial Cell Growth. Chem. Med. Chem. 2016, 11, 2205–2215. DOI:10.1002/cmdc.201600343.
- Rádai, Z.; Szeles, P.; Kiss, N. Z.; Hegedűs, L.; Windt, T.; Nagy, V.; Keglevich, G. Green Synthesis and Cytotoxic Activity of Dibenzyl α‐Hydroxyphosphonates and α‐Hydroxyphosphonic Acids. Heteroatom Chem. 2018, 29, e21436. DOI:10.1002/hc.21436.