Abstract
Asparaginase is a key component of therapy for acute lymphoblastic leukemia (ALL). Traditionally, asparaginase was administered intramuscularly but is now commonly given intravenously. Although intravenous administration is less painful, it can be challenging to differentiate hypersensitivity versus infusion-related reactions. The ability to distinguish between asparaginase-mediated reactions is critical to ensure optimal asparaginase treatment. In this paper, we will review the differences in pharmacokinetics and toxicities, when asparaginase is administered intravenously versus intramuscularly in pediatric patients with ALL. Differences between antibody-mediated hypersensitivity events and nonantibody-mediated infusion reactions will be addressed to assist practitioners in distinguishing between these clinically similar asparaginase-associated toxicities.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children aged 15 years or younger, accounting for 25% of cancer diagnoses.[Citation1] Outcomes for children with ALL have improved greatly over the past decades with overall survival now approaching 90%.[Citation2–5] These gains in survival are partly due to the use of intensified multiagent chemotherapy regimens that include asparaginase.[Citation6,Citation7]
Traditionally, asparaginase was administered as a series of intramuscular (IM) injections, but is now approved for both IM and intravenous (IV) administration. IM injections can be painful and are commonly reported as a source of anxiety.[Citation8] Although IV administration offers a less painful option, concerns exist for infusion-related reactions that can be common with protein-based therapies such as asparaginase. Additionally, the pharmacokinetics and time course of serum asparaginase activity differ between IV and IM administration. These differences can have important consequences on observed toxicity and determining optimal asparaginase dosing schedules. Understanding these differences allows clinicians to optimize their patient’s treatment and more effectively identify and manage infusion-related adverse events. The goal of this review is to provide an overview of the differences in pharmacokinetics and toxicities when asparaginase is administered IV versus IM in pediatric patients with ALL. Differences between antibody-mediated hypersensitivity events and nonantibody-mediated infusion reactions will be addressed to assist practitioners in distinguishing between these two clinically similar asparaginase-associated toxicities.
Asparaginase background
Asparaginase is a bacterial-derived enzyme that was first identified as an anticancer agent in the 1960s.[Citation9] Many lymphoblastic leukemia cells show little or no expression of the enzyme asparagine synthetase and are unable to synthesize sufficient concentrations of asparagine de novo.[Citation10] Asparaginase depletes serum levels of asparagine, effectively depriving leukemia cells of this critical amino acid.[Citation10] Although the relationship between asparagine–synthetase expression and outcomes remains controversial,[Citation11–13] it has been shown that continuous and prolonged asparagine depletion is associated with improved outcomes.[Citation6] In a study of 377 pediatric patients treated on a Dana-Farber Cancer Institute (DFCI) protocol, investigators found superior outcomes for patients who completed ≥26 consecutive weeks of asparaginase treatment compared with patients who received <26 weeks (90 ± 2% vs. 73 ± 7%; p < .01).[Citation6]
There are currently three formulations of asparaginase approved by the U.S Food and Drug Administration for use in patients with ALL. Native E. coli asparaginase and pegylated (PEG)-asparaginase are derived from the same bacterial source, E. coli. PEG-asparaginase is a form of E. coli asparaginase that has been covalently linked to polyethylene glycol to increase circulation time and reduce its immunogenicity. The third formulation, asparaginase Erwinia chrysanthemi, is derived from an alternate bacterial source, Erwinia chrysanthemi, and thus carries a distinct immunologic profile. Given this immunologic difference, asparaginase Erwinia chrysanthemi is the preferred alternative for patients who develop clinical hypersensitivity to E. coli–derived asparaginases. Patients switched to asparaginase Erwinia chrysanthemi have outcomes similar to patients who never experience a hypersensitivity reaction.[Citation14] In the United States, the supply of native E. coli asparaginase has been discontinued by the manufacturer and PEG-asparaginase is now the first-line asparaginase for patients with ALL as part of a multiagent chemotherapy regimen.[Citation15] In Europe, native E. coli asparaginase remains commercially available.
Asparaginase pharmacokinetics
Asparaginase pharmacokinetics differ greatly between the two routes of administration and the various formulations.[Citation16–19] The half-life of plasma activity of native E. coli asparaginase following IV versus IM administration is 18.3 ± 2.8 h versus 41.7 ± 4.3 h, respectively.[Citation16] The mean half-life of IV asparaginase Erwinia chrysanthemi is estimated at 7.51 h,[Citation20] substantially shorter than the half-life following IM administration of asparaginase Erwinia chrysanthemi of 15.6 h().[Citation20]
Figure 1. Simulation of serum asparaginase activity following repeated administration of intravenous (IV) and intramuscular (IM) asparaginase Erwinia chrysanthemi in two example patients.[Citation17] Reproduced with permission from Albertsen et al. [Citation17].
![Figure 1. Simulation of serum asparaginase activity following repeated administration of intravenous (IV) and intramuscular (IM) asparaginase Erwinia chrysanthemi in two example patients.[Citation17] Reproduced with permission from Albertsen et al. [Citation17].](/cms/asset/5fd01aec-0501-4418-9e21-c7d65eac7072/ilal_a_1213826_f0001_b.jpg)
The half-life of PEG-asparaginase is markedly longer than native E. coli asparaginase and asparaginase Erwinia chrysanthemi, with a half-life following IM administration of 5.73 ± 3.24 days.[Citation16] Recent pharmacokinetic reports of IV PEG-asparaginase in 43 newly diagnosed patients with high-risk B-ALL identified a mean half-life of 5.29 days (standard deviation, 1.13 days) during induction therapy, nearly identical to IM PEG-asparaginase.[Citation21] Similarly, Silverman et al. [Citation22] reported no discernable differences in the time course of serum enzymatic activity after IV administration of PEG-asparaginase when compared with previous reports of IM administration.[Citation19,Citation23]
The longer half-life of PEG-asparaginase allows for patients to maintain therapeutic levels of asparaginase activity (> 0.1 IU/mL) for a greater duration following a single dose. In one study, 88% of patients were found to have asparaginase activity levels >0.1 IU/mL at 18 days following a single dose of IV PEG-asparaginase (2500 IU/m2).[Citation22] In studies of asparaginase Erwinia chrysanthemi (25,000 IU/m2), 96% and 83% of patients showed asparaginase activity levels >0.1 IU/mL 48 h following IM or IV administration, respectively.[Citation24,Citation25] When the shorter-acting asparaginase Erwinia chrysanthemi is used to replace PEG-asparaginase due to hypersensitivity, the enzyme is given as six consecutive 25,000 IU/m2 doses for each dose of PEG-asparaginase, typically on a Monday/Wednesday/Friday schedule.[Citation20]
Asparaginase-associated hypersensitivity
One of the most common asparaginase-associated toxicities reported in pediatric and adult patients is clinical hypersensitivity or allergy.[Citation26] As a bacterial-derived enzyme, asparaginase has the potential to illicit a strong immune response manifested as hypersensitivity.
Common symptoms associated with clinical hypersensitivity include urticaria, dizziness, and respiratory symptoms ().[Citation27] In some cases, potentially life-threatening anaphylaxis may occur. Anaphylaxis requires immediate intervention and can be characterized by face and tongue swelling, angioedema, bronchospasm, respiratory distress, hypotension, shock, and organ-system failure.[Citation28,Citation29] Anaphylactic reactions typically occur within the first few minutes of an IV asparaginase infusion and later when given IM; therefore, close monitoring of patients and accurate documentation of symptom-onset times is critical.[Citation27]
Table 1. Common symptoms of hypersensitivity and infusion reactions by body system.[Citation27]
The prevalence of clinical hypersensitivity with asparaginase therapy depends on a number of factors including the type of asparaginase used, treatment intensity and history, concurrent corticosteroid use, and patient age and genetics.[Citation26,Citation30–32] Hypersensitivity reactions may be less common in adults compared with pediatric patients. Studies of adults with ALL treated on pediatric-inspired regimens have reported hypersensitivity rates as low as 1% in patients receiving PEG-asparaginase, but there is a higher rate of pre-medication.[Citation32–34] Recent evidence also supports the hypothesis that patient genetics can predispose patients to hypersensitivity reaction. Using a genomewide association approach, Fernandez et al. identified a specific gene variant involved in the regulation and function of T cells that were associated with hypersensitivity to asparaginase in 3308 patients with ALL.[Citation35]
Treatment-related factors also play an important role in determining a patient’s risk of hypersensitivity. As mentioned earlier, premedication with antihistamines and corticosteroids has been a common practice among adult providers and is associated with a reduced incidence in hypersensitivity reactions.[Citation4,Citation36] While premedication may reduce the risk of clinical symptoms, it is unknown whether the development of antiasparaginase antibodies is similarly reduced. The use of therapeutic drug monitoring (TDM) has thus been recommended in patients receiving premedication to identify patients who might develop subclinical hypersensitivity.[Citation37]
The intensity of treatment and a patient’s treatment history also impact the risk of developing hypersensitivity to asparaginase. Reactions are less prevalent in patients treated with high-intensity chemotherapy with little or no breaks in asparaginase treatment.[Citation38–40] In addition, previous exposure to an immunologically similar asparaginase can increase the risk of a subsequent hypersensitivity reaction. A number of studies report hypersensitivity rates to PEG-asparaginase in patients with previous exposure to native E. coli asparaginase.[Citation41,Citation42] These two formulations show considerable cross reactivity, and the incidence of hypersensitivity during PEG-asparaginase therapy is greater in patients with a history of native E. coli asparaginase therapy.[Citation43]
Early clinical reports suggested that IV administration of asparaginase was associated with an increased incidence of anaphylactic reactions.[Citation44,Citation45] Within the last 15 years, reported rates of hypersensitivity in clinical trials have varied across studies using different routes of administration and asparaginases ().[Citation14,Citation23–25,Citation34,Citation38,Citation42,Citation46–60] Recently, a number of retrospective reports have compared the incidence of hypersensitivity between IV and IM PEG-asparaginase ().[Citation8,Citation34,Citation60–64] In a pediatric study of 318 patients, Petersen et al. reported a 9% greater incidence of hypersensitivity following IV administration of PEG-asparaginase compared with IM PEG-asparaginase (p = .028).[Citation62] In addition, the DFCI identified an 11% overall incidence of hypersensitivity with IV PEG-asparaginase in 232 children with ALL during consolidation.[Citation59] Of note, this incidence was identical to what Petersen et al. [Citation62] reported for patients receiving IM PEG-asparaginase. Additionally, two recent reports of children treated in Children’s Oncology Group (COG) trials reported differing results regarding the prevalence of hypersensitivity between IV and IM asparaginase in high-risk (HR) and standard-risk (SR) patients with B-ALL.[Citation34,Citation60] In the AALL1131 trial for patients with HR B-ALL, similar rates of grade 3/4 hypersensitivity/anaphylaxis were observed between IV and IM administration during all treatment phases.[Citation60] However, in SR patients treated on COG AALL0932, a greater incidence of grade 3/4 hypersensitivity/anaphylaxis was seen during the delayed intensification phase in patients receiving IV PEG-asparaginase compared with IM administration, 1.8% versus 0.5%, respectively, p = .007.[Citation34] Given the variability of reported hypersensitivity rates and study reporting leading to ascertainment bias, additional studies are needed to better characterize the differences in the prevalence of clinical hypersensitivity between IV and IM asparaginase. It is also unclear to what degree infusion-related reactions in patients receiving IV asparaginase have been misinterpreted as hypersensitivity reactions, possibly accounting for the difference in rates of ‘allergy’ between the two routes of administration.
Table 2. Prevalence of hypersensitivity reactions (any grade) across trials by the method of administration and type of asparaginase.
Table 3. Summary of retrospective comparisons of the prevalence of hypersensitivity reactions with IV and IM PEG-asparaginase.
As expected, hypersensitivity reactions have been shown to occur considerably earlier following IV infusions compared with IM injection.[Citation47,Citation62] Petersen et al. [Citation62] reported that 26 of 27 reactions (96%) occurred within 30 minutes of IV administration compared with only two of 11 (18%) reactions in patients receiving IM asparaginase. Henriksen et al. [Citation47] identified that in the subgroup of patients who experienced clinical hypersensitivity with IM asparaginase, 58% exhibited symptoms within two hours of administration. Anaphylactic reactions to asparaginase, however, have a rapid onset that is independent of the route of administration.[Citation27] Henriksen et al. [Citation47] reported nine patients who experienced anaphylaxis after IM administration, with all events occurring <2-h postinjection. Given the possibility of a delayed reaction following IM administration of asparaginase, patients should be monitored for at least two hours following injection for the symptoms of a serious reaction.
Clinical hypersensitivity reactions have been associated with several negative effects on treatment efficacy and outcomes.[Citation54,Citation65] Patients who experience a grade ≥2 hypersensitivity reaction to asparaginase may be forced to discontinue asparaginase therapy, and failure to receive the complete course of asparaginase treatment has been associated with poor outcomes.[Citation6] In addition, patients who continue the same asparaginase therapy following a clinical hypersensitivity event may have altered pharmacokinetics that can negatively impact clinical outcomes.[Citation38,Citation54,Citation65]
Given the high degree of immunogenicity of asparaginase, antibody development leading to anaphylaxis and the neutralization of asparaginase via antibody-mediated inactivation is a concern.[Citation54] True antibody-mediated hypersensitivity reactions may require patients to be switched to an alternate asparaginase formulation; however, those who experience a nonantibody-mediated infusion reaction may be rechallenged with the same asparaginase and are often able to complete therapy. Thus, the ability to distinguish between these distinct asparaginase-associated adverse reactions and provide the appropriate response is critical to ensure optimal asparaginase therapy.
Patients who have exhibited hypersensitivity to E. coli-derived asparaginase show increased levels of antiasparaginase antibodies, decreased asparaginase half-life, and an overall reduction in asparaginase activity, compared with patients who did not experience hypersensitivity.[Citation19,Citation38,Citation42,Citation66] Tong et al. [Citation42] evaluated prospective drug monitoring of asparaginase activity in 89 patients with ALL treated on the Dutch Childhood Oncology Group (DCOG) protocol. All patients were treated with native E. coli asparaginase during induction followed by 15 doses of PEG-asparaginase IV (2500 IU/m2 every 2 weeks) during Intensification. The investigators reported that 20 patients (22%) experienced a clinical hypersensitivity reaction, and 18 (90%) of these patients reacted to the second dose of PEG-asparaginase. Antiasparaginase antibodies and reduced asparaginase activity levels were found in all patients who experienced clinical hypersensitivity. The development of antiasparaginase antibodies is commonly associated with reduced asparaginase activity.[Citation14,Citation38,Citation54] Additionally, patients who experience a hypersensitivity reaction are more likely to display a second immune reaction if rechallenged with the same asparaginase.[Citation14,Citation42]
With the discontinuation of E. coli asparaginase in the United States, PEG-asparaginase is now first-line therapy for patients with ALL. The covalent attachment of polyethylene glycol to therapeutic proteins allows for a significant extension of half-life and is generally believed to reduce immunogenicity.[Citation67] However, a number of studies have reported the presence of antibodies specific for the PEG conjugate in patients and healthy individuals.[Citation68–71] Recently, Armstrong et al. reported anti-PEG antibodies in 22–25% of healthy blood donors.[Citation70] In at least two cases, anti-PEG antibodies were associated with the rapid clearance of the PEGylated drug, potentially impacting outcomes.[Citation68,Citation71]
Asparaginase Erwinia chrysanthemi has shown limited cross reactivity with antibodies formed in patients treated with E. coli-derived asparaginase and can therefore successfully achieve therapeutic activity levels and maintain clinical outcomes in patients with hypersensitivity to PEG-asparaginase.[Citation24,Citation43,Citation50,Citation72] In clinical trials, 76–78% of patients who experienced previous hypersensitivity (grade ≥2) to an E. coli-derived asparaginase were able to complete their full asparaginase treatment regimen with asparaginase Erwinia chrysanthemi.[Citation24,Citation30] Due to differences in pharmacokinetics, the dosing frequency must be increased in patients who switch to asparaginase Erwinia chrysanthemi to achieve appropriate therapeutic activity.[Citation24,Citation30,Citation49]
If no alternate asparaginase formulations are available for a patient who has suffered a mild/moderate hypersensitivity reaction, further premedication with steroids and antihistamines or desensitization protocols may be considered.[Citation73] Additionally, real-time TDM can now be used to verify adequate asparaginase activity levels throughout a patient’s treatment. Several examples of desensitization protocols for asparaginase exist in the literature for patients with clinical hypersensitivity.[Citation74–76] We suggest that desensitization protocols be reserved as a last option for patients who have exhausted all available alternative asparaginases. Furthermore, clinicians should consider using TDM in patients undergoing desensitization to confirm adequate asparaginase activity.
While antibodies to asparaginase are often accompanied by overt clinical symptoms, studies have shown that patients can develop anti-asparaginase antibodies in the absence of clinical symptoms.[Citation14,Citation38,Citation77] This condition, referred to as subclinical hypersensitivity or ‘silent inactivation,’ is associated with reduced asparaginase activity, and linked to poor outcomes if not rapidly identified and treated.[Citation7,Citation26,Citation78] Due to the lack of clinical symptoms, the prevalence of subclinical hypersensitivity can be difficult to estimate; however, studies of E. coli asparaginase have reported rates from 8–44%.[Citation7,Citation14,Citation42,Citation77] As patients with subclinical hypersensitivity exhibit no apparent symptoms, treatment with the same asparaginase formulation is often continued, possibly with insufficient asparaginase activity and no therapeutic benefit.[Citation42,Citation79]
Infusion-related adverse events
True clinical hypersensitivity reactions are antibody mediated, while infusion reactions do not require antibody development. The mechanisms by which chemotherapeutic drugs elicit an infusion reaction are not fully known and may include direct release of cytokines or histamine, direct activation of the complement system, and sharp elevation of ammonia levels.[Citation27,Citation80] Cytokine release syndrome is a type of infusion reaction commonly associated with monoclonal antibody therapy and other immunotherapies. Cellular destruction leads to an increase in the release of cytokines and can result in a number of clinical symptoms resembling true antibody-mediated hypersensitivity ().[Citation27] Symptoms of complement activation-related pseudoallergy are similar to those reported in patients with antibody-mediated clinical hypersensitivity. Activation of the complement system, through both classical and alternative pathways, can increase levels of C3a and C5a anaphylatoxins and trigger mast cell and basophil activation.[Citation81] Patients with solid tumors treated with PEG liposomal doxorubicin who experienced symptoms of a hypersensitivity reaction showed a greater proportion of elevated C-terminal complex (SC5b-9) levels compared with patients who did not exhibit signs of a reaction (92% vs. 56%, respectively).[Citation82]
Asparaginase therapy has also been associated with nonantibody-mediated infusion reactions that may result from spikes in serum ammonia levels.[Citation83–86] Ammonia accumulation occurs as asparaginase facilitates the rapid conversion of asparagine and glutamine to aspartic acid and glutamic acid, with ammonia as the shared by-product of both reactions.[Citation10] Symptoms of hyperammonemia are often transient in nature and can include nausea, vomiting, headache, dizziness, and rash.[Citation83,Citation87,Citation88] In patients receiving IV asparaginase, serum levels of ammonia have been shown to rise sharply following drug administration. Steiner et al. [Citation89] reported a significant elevation in ammonia levels in 10 patients 1 day after IV administration of native E. coli asparaginase (5000 IU/m2 every 3 days), with recorded peak ammonia levels up to sevenfold the normal value. Paulides et al. [Citation90] assessed the change in ammonia levels every two hours in six patients following infusions of native E. coli asparaginase (10,000 IU/m2 every 3 days). The results showed a consistent peak in ammonia in patients 2-h postinfusion, with a median peak value of 144 μM (normal range, 15–55 μM).[Citation90] In a pig model, asparaginase was found to be associated with a rapid increase in serum ammonia.[Citation88] Interestingly, the overall increase in ammonia levels was greater following subsequent doses of asparaginase, and all animals eventually developed symptoms of an infusion reaction along with ammonia toxicity.[Citation88] Regardless of the underlying cause, the prevalence of nonantibody-mediated infusion reactions can be difficult to estimate, as symptoms often overlap with what is seen in clinical hypersensitivity and have traditionally been lumped together with allergic reactions.
Differentiation of hypersensitivity and infusion reactions
There are currently no accepted guidelines differentiating the two types of asparaginase-associated toxicities: antibody-mediated hypersensitivity reactions and nonantibody-mediated infusion reactions. The Common Terminology Criteria for Adverse Events (version 4.03) includes distinct entries for ‘allergic reaction’ and ‘infusion-related reaction’; however, grading criteria heavily overlap and cannot be used to reliably distinguish between these two reactions in practice.[Citation91] Therefore, clinicians must decide how, if, and when to intervene based on the available clinical and biochemical evidence. Although clinical symptoms alone cannot be used to differentiate hypersensitivity and infusion reactions, some symptoms are more commonly observed with one type of reaction and may help inform a physician’s treatment decision (). The timing of symptom onset can be an important distinguishing factor to differentiate clinical hypersensitivity from acute infusion reactions. True hypersensitivity reactions are antibody mediated and require previous exposure to asparaginase to promote antibody development. As such, these reactions rarely occur during the initial treatment cycle, and when a reaction does occur on the initial exposure, strong consideration should be given to an infusion-related event rather than allergy. The risk of antibody development increases with subsequent exposure to an antigen, and several studies report clinical hypersensitivity to asparaginase exclusively after multiple doses have been administered.[Citation14,Citation38,Citation41,Citation92] In contrast, infusion reactions do not require prior exposure or antibody development and are more likely to occur with the initial infusion. As an example, rituximab, an anti-CD20 monoclonal antibody used in the treatment of leukemias/lymphomas, has been associated with infusion reactions in up to 77% of patients following the initial infusion.[Citation93,Citation94] The prevalence of infusion reactions with rituximab decrease significantly during subsequent stages of therapy, with only 30% and 14% of patients experiencing an infusion reaction after the fourth and eighth infusion, respectively.[Citation93]
Management of asparaginase-associated toxicities
provides an algorithm for the specific management of asparaginase-related toxicity. In the event, an adverse reaction occurs during the administration of asparaginase, the infusion should be immediately paused, and all relevant clinical symptoms documented. The appropriate management pathway for each reaction should be based on the individual patient’s symptoms and clinical status. If signs are suggestive of an anaphylactic reaction, clinicians should treat immediately with antihistamines, corticosteroids, and epinephrine.[Citation27] For less severe reactions, patients may continue the infusion after symptoms have resolved postintervention. Continued antihistamine or corticosteroid use may be considered, dependent upon individual symptoms, and the infusion speed can be restarted at 50% of the target infusion rate and titrated to tolerance.[Citation27,Citation95–97]
Figure 2. Suggested clinical pathway to classification and management of adverse reactions to asparaginase. E coli: Escherichia coli; LLQ: lower limit of quantification; M/W/F: Monday/Wednesday/Friday; PEG: pegylated.
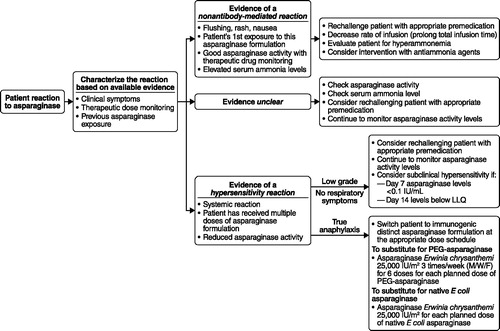
When the symptoms do not point clearly to an allergic reaction, a measurement of ammonia levels can be obtained at the time of the reaction or 10–20 minutes into the subsequent infusion. Nausea/vomiting and occasionally mild neurologic complaints, such as headache, are the primary symptoms patients manifest with hyperammonemia. Slowing the infusion to ≥2 hours can sometimes improve symptomatology by attenuating the rise in ammonia levels associated with asparaginase. An alternative measure would be to give asparaginase IM, which is associated with slower absorption and should reduce the resultant ammonia spike.[Citation17] Howard et al.[Citation88] using pig models, reported that the initial administration of asparaginase at very slow infusion rates is associated with a leveling off of ammonia production and the absence of high serum ammonia spikes that result in hyperammonemia. Of note, patients with metabolic disorders who experience hyperammonemia due to defects in their ammonia metabolism, are treated with agents such as L-arginine, metformin, lactulose, or sodium phenylacetate/sodium benzoate to lower elevated ammonia levels.[Citation85,Citation98] Consideration can be given to using the same agents in patients with evidence of encephalopathic changes due to hyperammonemia.
Therapeutic drug monitoring
The clinical availability of TDM provides practitioners with an additional tool to distinguish between true clinical hypersensitivity, subclinical hypersensitivity, and nonantibody-mediated infusion reactions. In cases where timing or symptoms alone may be insufficient to distinguish reaction type, patients’ asparaginase activity levels can be measured to assist in identifying the underlying cause. TDM measures serum asparaginase activity in patients and can be used as a proxy indicator for asparagine concentrations and the presence of neutralizing antiasparaginase antibodies.[Citation36] The occurrence of clinical symptoms without a measurable decrease in asparaginase activity could be considered evidence toward an acute infusion reaction in patients rather than true allergy. Recently, published recommendations for drug monitoring of PEG-asparaginase suggest sampling asparaginase activity 3–5 days postdose if the patient was able to receive 10–50% of the infusion and after 4–7 days postdose if the patient received >50% of the dose.[Citation99] If the infusion was stopped prior to a patient receiving at least 10% of the dose, asparaginase activity will inevitably be low, and it is recommended that TDM be assessed on a subsequent dose.[Citation99]
In the DCOG protocol, subclinical hypersensitivity was defined as serum asparaginase activity <0.1 U/mL on day 7 ± 1 or <0.02 U/mL on day 14 ± 1 postinfusion in a patient without clinical symptoms.[Citation42] In the DFCI ALL protocol, patients were classified with subclinical hypersensitivity if they showed trough asparaginase activity of <0.1 IU/mL on two successive measurements.[Citation7]
Bleyer et al. recommend considering subclinical hypersensitivity if a patient exhibits asparaginase activity of <0.05 IU/mL 4–7 days after receiving a full dose of PEG-asparaginase.[Citation99] An activity threshold of 0.05 IU/mL for asparaginase is lower than the commonly accepted 0.1 IU/mL, and debate exists over what the appropriate cutoff point should be.[Citation37,Citation100] One recent study measuring asparaginase activity levels and plasma asparagine concentrations found that asparagine was completely depleted at higher asparaginase activity levels, but began to rebound once plasma asparaginase activity declined to <0.4 IU/mL.[Citation21] Another modeling study in adult ALL patients found that PEG-asparaginase activity levels of 0.2 IU/mL were required to achieve 90% asparagine depletion.[Citation101] Since suboptimal depletion has been associated with poor outcomes, caution should be exercised in continuing patients on a treatment that does not sufficiently deplete their asparagine. If subclinical hypersensitivity is identified, it is recommended that patients be switched to the alternate formulation asparaginase Erwinia chrysanthemi.[Citation7,Citation42,Citation79,Citation99] Once a patient is switched, TDM can also be used to assess asparaginase activity related to the shorter half-life of asparaginase Erwinia chrysanthemi to determine whether patients are obtaining satisfactory asparagine depletion over the 72-h weekend with IV versus IM administration.
Summary and conclusions
The ability to complete the prescribed asparaginase treatment schedule is associated with improved outcomes in patients with ALL. Additionally, hypersensitivity to asparaginase is often reported as the most common reason for the discontinuation of asparaginase treatment. Immune reactions to asparaginase are heterogeneous and not always accompanied by antibody development. Patients who experience nonantibody-mediated infusion reactions can often continue asparaginase treatment with premedication and a reduced rate of infusion, while patients who experience a true clinical or subclinical hypersensitivity reaction to E. coli-derived asparaginase should be immediately switched to asparaginase Erwinia chrysanthemi. True antibody-mediated immune reactions require previous exposure and are highly unlikely to occur on a patient’s first infusion. The rapid identification and classification of an immune reaction as a clinical allergy versus an infusion reaction using available TDM to identify insufficient asparaginase activity levels, can assist clinicians in determining the appropriate treatment response and ensure patients receive optimal asparaginase exposure.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at http://dx.doi.org/10.1080/10428194.2016.1213826.
ICMJE Forms for Disclosure of Potential Conflicts of Interest
Download Zip (2.2 MB)References
- Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995, NIH Pub. No. 99-4649. Bethesda (MD): National Cancer Institute, SEER Program; 1999.
- Hunger SP, Loh ML, Whitlock JA, et al. Children's oncology group's 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957–963.
- Moricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489.
- Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–249.
- Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia. 2010;24:320–334.
- Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218.
- Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210.
- August KJ, Miller WP, Dalton A, et al. Comparison of hypersensitivity reactions to PEG-asparaginase in children after intravenous and intramuscular administration. J Pediatr Hematol Oncol. 2013;35:e283–e286.
- Broome JD. Evidence that the L-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature. 1961;191:1114–1115.
- Muller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113.
- Stams WA, den Boer ML, Beverloo HB, et al. Sensitivity to L-asparaginase is not associated with expression levels of asparagine synthetase in t(12;21)+ pediatric ALL. Blood. 2003;101:2743–2747.
- Stams WA, den Boer ML, Holleman A, et al. Asparagine synthetase expression is linked with L-asparaginase resistance in TEL-AML1-negative but not TEL-AML1-positive pediatric acute lymphoblastic leukemia. Blood. 2005;105:4223–4225.
- Chien WW, Le Beux C, Rachinel N, et al. Differential mechanisms of asparaginase resistance in B-type acute lymphoblastic leukemia and malignant natural killer cell lines. Sci Rep. 2015;5:8068.
- Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18:1525–1532.
- Keding RRE. Discontinuation of Elspar®, (asparaginase for injection) 10,000 IU. Deerfield (IL): Lundbeck LLC. Published August 3, 2012. [cited 2016 Jan 30]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM321556.pdf.
- Ho DH, Yap HY, Brown N, et al. Clinical pharmacology of intramuscularly administered L-asparaginase. J Clin Pharmacol. 1981;21:72–78.
- Albertsen BK, Jakobsen P, Schroder H, et al. Pharmacokinetics of Erwinia asparaginase after intravenous and intramuscular administration. Cancer Chemother Pharmacol. 2001;48:77–82.
- Schrey D, Borghorst S, Lanvers-Kaminsky C, et al. Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 protocol between 2000 and 2007. Pediatr Blood Cancer. 2010;54:952–958.
- Asselin BL, Whitin JC, Coppola DJ, et al. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11:1780–1786.
- Erwinaze® (asparaginase Erwinia chrysanthemi) [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2014 Dec [cited 2016 Jan 30]. Available from: http://erwinaze.com/ERWINAZEPI.pdf.
- Angiolillo AL, Schore RJ, Devidas M, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children's Oncology Group Study AALL07P4. J Clin Oncol. 2014;32:3874–3882.
- Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115:1351–1353.
- Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99:1986–1994.
- Salzer WL, Asselin B, Supko JG, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children's Oncology Group. Blood. 2013;122:507–514.
- Vrooman LM, Kirov II, Dreyer ZE, et al. Activity and toxicity of intravenous Erwinia asparaginase following allergy to E. coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63:228–233.
- Raetz EA, Salzer WL. Tolerability and efficacy of L-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–563.
- Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14:E10–E21.
- Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy. 2011;66:1–14.
- Murphy K, Travers P, Walport M, editors. Janeway's immunobiology. New York, (NY): Garland Science, Taylor & Frances Group, LLC; 2008.
- Burke MJ. How to manage asparaginase hypersensitivity in acute lymphoblastic leukemia. Future Oncol. 2014;10:2615–2627.
- Shinnick SE, Browning ML, Koontz SE. Managing hypersensitivity to asparaginase in pediatrics, adolescents, and young adults. J Pediatr Oncol Nurs. 2013;30:63–77.
- Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52:2237–2253.
- Advani A, Earl M, Douer D, et al. Toxicities of intravenous pegasparaginase (Oncaspar) in adults with acute lymphoblastic leukemia. Blood. 2007;110:2811.
- Maloney KW, Angiolillo AL, Schore RJ, et al. Association of intravenous (IV) and intramuscular (IM) pegaspargase (PEG) administration with rate of adverse events (AE) in standard risk (SR) acute lymphoblastic leukemia (ALL) children’s oncology group (COG) trials. J Clin Oncol. 2015;33:10035.
- Fernandez CA, Smith C, Yang W, et al. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood. 2015;126:69–75.
- Asselin B, Rizzari C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk Lymphoma. 2015;56:2273–2280.
- van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101:279–285.
- Liu C, Kawedia JD, Cheng C, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–2309.
- Albertsen BK, Schroder H, Jakobsen P, et al. Monitoring of Erwinia asparaginase therapy in childhood ALL in the Nordic countries. Br J Clin Pharmacol. 2001;52:433–437.
- Asselin BL. The right dose for the right patient. Blood. 2012;119:1617–1618.
- Wang B, Relling MV, Storm MC, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003;17:1583–1588.
- Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123:2026–2033.
- Zalewska-Szewczyk B, Gach A, Wyka K, et al. The cross-reactivity of anti-asparaginase antibodies against different L-asparaginase preparations. Clin Exp Med. 2009;9:113–116.
- Evans WE, Tsiatis A, Rivera G, et al. Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer. 1982;49:1378–1383.
- Nesbit M, Chard R, Evans A, et al. Evaluation of intramuscular versus intravenous administration of L-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol. 1979;1:9–13.
- Aldoss I, Douer D, Behrendt CE, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol. 2016;96:375–380.
- Henriksen LT, Harila-Saari A, Ruud E, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr Blood Cancer. 2015;62:427–433.
- Fernandez CA, Smith C, Yang W, et al. HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood. 2014;124:1266–1276.
- Plourde PV, Jeha S, Hijiya N, et al. Safety profile of asparaginase Erwinia chrysanthemi in a large compassionate-use trial. Pediatr Blood Cancer. 2014;61:1232–1238.
- Vrooman LM, Supko JG, Neuberg DS, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:199–205.
- Chen SH, Pei D, Yang W, et al. Genetic variations in GRIA1 on chromosome 5q33 related to asparaginase hypersensitivity. Clin Pharmacol Ther. 2010;88:191–196.
- Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904.
- Wacker P, Land VJ, Camitta BM, et al. Allergic reactions to E. coli L-asparaginase do not affect outcome in childhood B-precursor acute lymphoblastic leukemia. A Children's Oncology Group Study. J Pediatr Hematol Oncol. 2007;29:627–632.
- Zalewska-Szewczyk B, Andrzejewski W, Mlynarski W, et al. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48:931–936.
- Albertsen BK, Schroder H, Jakobsen P, et al. Antibody formation during intravenous and intramuscular therapy with Erwinia asparaginase. Med Pediatr Oncol. 2002;38:310–316.
- Abshire TC, Pollock BH, Billett AL, et al. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a pediatric oncology group study. Blood. 2000;96:1709–1715.
- Muller HJ, Beier R, Loning L, et al. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol. 2001;114:794–799.
- Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–818.
- Silverman LB, Stevenson K, Vrooman LM, et al. Randomized comparison of IV PEG and IM E. coli sparaginase in children and adolescents with acute lymphoblastic leukemia: results of the DFCI ALL Consortium Protocol 05-01. Blood. 2011;118:874.
- Salzer W, Burke MJ, Larsen EC, et al. Incidence of allergic reactions to pegaspargase (PEG) administered intramuscularly versus intravenously (IM vs. IV) in children and young adults with high risk B-lymphoblastic leukemia (HR B-ALL): results of Children's Oncology Group (COG) studies AALL0232/AALL1131. Blood. 2015;126:1303.
- Abbott LS, Zakova M, Shaikh F, et al. Allergic reactions associated with intravenous versus intramuscular pegaspargase: a retrospective chart review. Paediatr Drugs. 2015;17:315–321.
- Petersen WC Jr., Clark D, Senn SL, et al. Comparison of allergic reactions to intravenous and intramuscular pegaspargase in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2014;31:311–317.
- Pidaparti M, Bostrom B. Comparison of allergic reactions to pegasparaginase given intravenously versus intramuscularly. Pediatr Blood Cancer. 2012;59:436–439.
- MacDonald TJ, Kulkarni K, Bernstein M, et al. Significantly higher incidence of allergic reactions for intravenous PEG-asparaginase as compared to intramuscular PEG-asparaginase in children with high risk acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61:016.
- Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119:1658–1664.
- Kurtzberg J, Asselin B, Bernstein M, et al. Polyethylene glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse. A Children's Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33:610–616.
- Morar AS, Schrimsher JL, Chavez MD. PEGylation of proteins: a structural approach. Biopharm Int. 2006;19:32–34.
- Armstrong JK, Hempel G, Koling S, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111.
- Richter AW, Akerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol. 1984;74:36–39.
- Armstrong JK, Leger R, Wenby RB, et al. Occurrence of an antibody to poly(ethylene glycol) in normal donors. Blood. 2003;102–556A.
- Ganson NJ, Kelly SJ, Scarlett E, et al. Control of hyperuricemia in subjects with refractory gout, and induction of an antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 2006;8:R12.
- Ko RH, Jones TL, Radvinsky D, et al. Allergic reactions and antiasparaginase antibodies in children with high-risk acute lymphoblastic leukemia: a children's oncology group report. Cancer. 2015;121:4205–4211.
- Joerger M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol. 2012;23:x313–x319.
- Akbayram S, Dogan M, Akgun C, et al. A desensitization protocol in children with L-asparaginase hypersensitivity. J Pediatr Hematol Oncol. 2010;32:e187–e191.
- Kawahara Y, Morimoto A, Hayase T, et al. Monitoring of anti-L-asparaginase antibody and L-asparaginase activity levels in a pediatric patient with acute lymphoblastic leukemia and hypersensitivity to native Escherichia coli L-asparaginase during desensitization courses. J Pediatr Hematol Oncol. 2014;36:e91–e93.
- Soyer OU, Aytac S, Tuncer A, et al. Alternative algorithm for L-asparaginase allergy in children with acute lymphoblastic leukemia. J Allergy Clin Immunol. 2009;123:895–899.
- Panosyan EH, Seibel NL, Martin-Aragon S, et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: children's cancer group study CCG-1961. J Pediatr Hematol Oncol. 2004;26:217–226.
- Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–629.
- Tong WH, Pieters R, Tissing WJ, et al. Desensitization protocol should not be used in acute lymphoblastic leukemia patients with silent inactivation of PEGasparaginase. Haematologica. 2014;99:e102–e104.
- Ring J, Brockow K, Behrendt H. History and classification of anaphylaxis. Novartis Found Symp. 2004;257:6–16.
- Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121.
- Chanan-Khan A, Szebeni J, Savay S, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430–1437.
- Heitink-Polle KM, Prinsen BH, de Koning TJ, et al. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving pegylated asparaginase. JIMD Rep. 2013;7:103–108.
- Jaing TH, Lin JL, Lin YP, et al. Hyperammonemic encephalopathy after induction chemotherapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:955–956.
- Nussbaum V, Lubcke N, Findlay R. Hyperammonemia secondary to asparaginase: a case series. J Oncol Pharm Pract. 2014;22:161–164.
- Tong WH, Pieters R, de Groot-Kruseman HA, et al. Toxicity of very prolonged PEGasparaginase and Erwinia asparaginase courses in relation to asparaginase activity levels with a special focus on dyslipidemia. Haematologica. 2014;99:1716–1721.
- Jorck C, Kiess W, Weigel JF, et al. Transient hyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Pediatr Hematol Oncol. 2011;28:3–9.
- Howard S, Szwiec K, Buddington K, et al. The weaned pig as a model for asparaginase infusion reactions. Pediatr Blood Cancer. 2015;62:698.
- Steiner M, Attarbaschi A, Kastner U, et al. Distinct fluctuations of ammonia levels during asparaginase therapy for childhood acute leukemia. Pediatr Blood Cancer. 2007;49:640–642.
- Paulides M, Jung R, Chada M, et al. Prospective longitudinal examination of hyperammonemia during L-asparaginase treatment within 24 hours after administration in childhood lymphoblastic malignancies. J Leuk. 2013;1:117. doi: 10.4172/2329-6917.1000117.
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. NIH Publication No. 09-5410; 2010 Jun 14 [cited 2016 May 1]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- Woo MH, Hak LJ, Storm MC, et al. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia. 1998;12:1527–1533.
- Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143.
- Kimby E. Tolerability and safety of rituximab (MabThera). Cancer Treat Rev. 2005;31:456–473.
- Breslin S. Cytokine-release syndrome: overview and nursing implications. Clin J Oncol Nurs. 2007;11:37–42.
- Rituxan® (rituximab) [prescribing information]. South San Francisco (CA): Genentech USA, Inc; 2014 [cited 2016 Jan 30]. Available from: http://www.gene.com/download/pdf/rituxan_prescribing.pdf.
- Erbitux® (cetuximab) [prescribing information]. Indianapolis (IN): Eli Lilly and Company; Oct 2015 [cited 2016 Jan 30]. Available from: http://uspl.lilly.com/erbitux/erbitux.html.
- Matoori S, Leroux J-C. Recent advances in the treatment of hyperammonemia. Adv Drug Deliv Rev. 2015;90:55–68.
- Bleyer A, Asselin BL, Koontz SE, et al. Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer. 2015;62:1102–1105.
- Benitez L, Perissinotti AJ, Santarossa M, et al. Pharmacokinetic and clinical considerations for monitoring asparaginase activity levels during pegaspargase therapy. Pediatr Blood Cancer. 2015;62:1115.
- Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109:2744–2750.
