Abstract
Lenalidomide and hypomethylating agents (HMAs) azacitidine and decitabine are approved for treating myelodysplastic syndromes (MDS), but optimal sequencing is unclear. Adults with MDS were identified from a US payer claims database (Inovalon MORE2 Registry) to compare outcomes with lenalidomide followed by HMA (LEN-HMA) or HMA followed by lenalidomide (HMA-LEN). There were 96 patients who received LEN-HMA and 89 who received HMA-LEN. LEN-HMA-treated patients had a longer time to second treatment discontinuation (29.0 vs. 19.0 months, p=.009; adjusted hazard ratio [HR] 0.52, 95% confidence interval [CI] 0.29–0.91, p=.023). LEN-HMA-treated patients had a longer median time to insurance disenrollment (22.4 vs. 16.1 months, p<.001; adjusted HR 0.64, 95% CI: 0.44–0.92, p=.017), used as a proxy for survival. Longer treatment duration and survival with LEN-HMA support first-line use of lenalidomide in MDS in sequence with HMAs.
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of hematologic malignancies, with 20,000 cases diagnosed in the USA annually; the highest rate of incidence is among patients older than 70 years of age [Citation1,Citation2]. The variable presentation of MDS, as well as the high median age at diagnosis of ∼70–75 years [Citation3], complicates clinical management. Although erythropoiesis-stimulating agents (ESAs) are often used as supportive care in lower-risk (LR) disease, three agents are approved for the treatment of MDS in the USA: two hypomethylating agents (HMAs) (azacitidine and decitabine) and thalidomide analog lenalidomide. The indications for these agents differ. Decitabine is indicated across the various MDS subtypes and for Intermediate-1-, Intermediate-2-, and High-risk disease and azacitidine for all MDS subtypes, whereas lenalidomide is approved for the treatment of transfusion-dependent anemia due to low- or Intermediate-1-risk MDS associated with the del(5q) chromosomal abnormality (alone or with other cytogenetic abnormalities). Although not approved, lenalidomide has also demonstrated clinically meaningful activity in non-del(5q) MDS [Citation4,Citation5] and is listed in the National Comprehensive Cancer Network guidelines as an option in the setting of ESA failure for patients with red blood cell (RBC) transfusion-dependent anemia associated with non-del(5q) LR-MDS both as monotherapy and in combination with ESAs [Citation6]. Real-world use of lenalidomide for non-del(5q) MDS, ranging from low- to high-risk disease, has been described in the literature [Citation7].
Durable responses to MDS treatment, whether to lenalidomide or HMAs, are limited, resulting in their frequent use in sequence [Citation8]. However, despite being available for more than a decade, there is a paucity of data regarding the impact of the sequencing of HMAs and lenalidomide on clinical outcomes. A single-center retrospective study sought to determine the optimal sequencing of lenalidomide and azacitidine in 63 patients with non-del(5q) LR-MDS [Citation9]. While the erythroid hematologic improvement (HI-E) rate with lenalidomide was higher when used as first-line vs. second-line therapy (conversely, the HI-E rate with azacitidine was unaffected by line of therapy), the sequence had no apparent impact on the rates of overall survival (OS) or leukemic progression to acute myeloid leukemia (AML). Although limited by their retrospective nature and the small sample size, these results suggested that lenalidomide is optimally positioned before azacitidine when the drugs are used in sequence for non-del(5q) MDS.
The current study aimed to address outstanding questions regarding the sequencing of HMAs and lenalidomide using a real-world MDS patient data set not restricted to non-del(5q) LR-MDS patients, and to compare clinical outcomes between the following two cohorts of MDS patients: patients treated with lenalidomide followed by an HMA (LEN-HMA), and patients treated with an HMA followed by lenalidomide (HMA-LEN).
Methods
Study design
This study is a retrospective observational analysis of patient-level payer claims data. Patients with a diagnosis of MDS were indexed at the initiation of first-line therapy with azacitidine, decitabine, or lenalidomide. The index date was defined as the date of the first claim for lenalidomide or an HMA as first-line therapy. The index period spanned 1 July, 2008–30 September 2015, with the study period including data through 30 September 2016. A minimum of 6 months pre-index and 12 months post-index continuous health insurance plan enrollment was required to allow for sufficient baseline and follow-up periods.
Data source
Data were derived from the Inovalon MORE2 Registry (Inovalon, Bowie, MD, USA), a payer claims database that compiles longitudinal claims data from more than 100 US health plans with 137 million unique covered patient lives since 2001. The data represent ∼95% of the US counties, and the data come from more than 804,000 physicians and 306,000 clinical facilities in the USA. The database is updated quarterly. The mix of patients by payer type is about 49% commercial, 46% Medicare and Medicaid, and about 5% other. The patients are linked with pharmacy, medical, and laboratory claims across health plans. Thirty percent of the patient population is 50 years of age and older. These adjudicated payer claims contain longitudinal records representing both medical and pharmacy benefits for commercial, Medicare, Medicare Advantage, and Medicaid patients and include small, medium, and large commercially based health plans. They include information on patient demographics, primary and secondary diagnoses, payer information, medication utilization, refill history for all drugs and dosage forms including intravenous and oral drugs, and information on place of service including hospitalizations and emergency department visits. All patient data are de-identified to protect personal health information in accordance with the Health Insurance Portability and Accountability Act.
Cohort selection
Adult patients with a diagnosis of MDS were identified from the Inovalon database. Of these patients, those who initiated first-line HMA (defined as ≥2 claims of HMA) followed by second- line lenalidomide were classified as one treatment group and those who initiated first-line lenalidomide (defined as ≥2 claims of LEN) followed by second-line HMA as a second treatment group. Study inclusion and exclusion criteria are summarized in .
Table 1. Patient selection criteria.
Analyses
Patient demographics and clinical characteristics were summarized using descriptive statistics (mean and standard deviation, median and interquartile range, and counts and proportions). Univariate analyses of baseline characteristics were conducted to compare means (or medians) and proportions across treatment groups using t-tests (Wilcoxon where appropriate) and chi-square (Fisher exact test where the patient count was fewer than five).
Outcomes of interest included time to treatment discontinuation and OS. A treatment discontinuation was defined as any gap of ≥90 days between a patient’s last claim for a given line of therapy and the end of follow-up or study period. Date of death was unavailable from the claims record. As such, survival time and OS were defined as the time from the index date to the date of insurance disenrollment as a proxy. A gap of ≥90 days from the last observed claim for any healthcare resource to the end of the study period was considered disenrollment from health insurance [Citation10].
Descriptive statistics were calculated for each treatment group and statistical tests were performed to assess differences in outcomes across both treatment groups (LEN-HMA vs. HMA-LEN). Time to discontinuation was evaluated using the Kaplan–Meier method, with log-rank tests applied to the differences in median values. We conducted multivariate analyses with the time from index date to discontinuation and disenrollment adjusted for potential confounders at the start of first-line therapy. Covariates used in Cox proportional hazard models included gender, age, payer, region, Charlson Comorbidity Index (CCI), cytopenias, and the SEER-Medicare MDS Risk Score (SMMRS) [Citation11], a claims-based risk score, in lieu of the International Prognostic Scoring System (IPSS), which is unavailable and cannot be calculated from administrative claims data.
Results
Of 6009 patients with an MDS diagnosis in the database, 530 patients had ≥1 claim for lenalidomide or an HMA (); 56% of these patients went on to the other regimen during the study period. We identified 96 patients treated with LEN-HMA and 89 patients treated with HMA-LEN for inclusion in the final study cohort (). Both groups were statistically similar with respect to demographic characteristics. For the overall population, median age was 74 years for both groups and more than three-quarters were covered by Medicare insurance (). The LEN-HMA-treated patients had a lower rate of cytopenias at baseline (12.5% vs. 29.2%; p=.005), but similar distributions of SMMRS categories (p=.161) and CCI scores (p=.098) () compared with HMA-LEN-treated patients. Use of concomitant medications was similar between the LEN-HMA and HMA-LEN groups overall, including the use of hematopoietic growth factors (36.5% vs. 38.2%), ESAs (69.8% vs. 62.9%), platelet transfusions for the management of thrombocytopenia (53.1% vs. 62.9%), and deferasirox (19.8% vs. 23.6%) and deferiprone (1.0% and 0%) for the management of iron overload (data not shown).
Table 2. Patient attrition.
Table 3. Patient demographic characteristics at baseline and clinical characteristics in the 6 months prior to the initiation of first-line therapy.
Treatment discontinuation
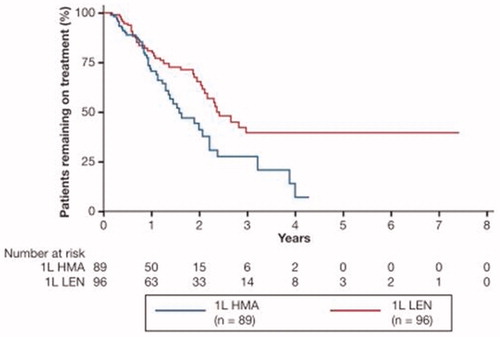
Patients treated with LEN-HMA had a significantly longer time to overall treatment discontinuation compared with HMA-LEN (29.0 vs. 19.0 months; log-rank p=.009; ), translating into a statistically significant 48% reduction in the risk of treatment discontinuation (adjusted hazard ratio [HR] 0.52; 95% confidence interval [CI] 0.29–0.91; p=.023).
Figure 1. Time to discontinuation. Median (95% CI) time to disenrollment, months: 19.0 (95% CI: 15.8–NR) among HMA-LEN patients and 29.0 (95% CI: 24.9–NR) among LEN-HMA patients (29.0 vs. 19.0 months; log-rank p = 009). LEN-HMA patients had a lower adjusted risk of discontinuation (HR: 0.52; 95% CI: 0.29–0.91; p=.023). 1L: first-line therapy; CI: confidence interval; HMA: hypomethylating agent; HR: hazard ratio; LEN: lenalidomide; NR: not reached.
Overall survival
Annual insurance disenrollment rates fell more sharply over time in the patients treated with HMA-LEN, as shown in . Median OS, using the time to disenrollment as a proxy, was longer in patients treated with LEN-HMA (22.4 vs. 16.1 months; log-rank p<.001; ). LEN-HMA-treated patients had a statistically significant 36% reduction in the risk of disenrollment compared with patients treated with HMA-LEN (adjusted HR: 0.64; 95% CI: 0.44–0.92; p=.017).
Figure 2. Approximate median OS using time to insurance disenrollment. Median (95% CI) time to disenrollment, months: 16.1 (95% CI: 13.6–18.9) among HMA-LEN patients and 22.4 (95% CI: 19.5–27.7) among LEN-HMA patients (22.4 vs. 16.1 months; log-rank p<.001). LEN-HMA patients had a lower adjusted risk of disenrollment (HR: 0.64; 95% CI: 0.44–0.92; p=.017). 1L: first-line therapy; CI: confidence interval; HMA: hypomethylating agent; HR: hazard ratio; LEN: lenalidomide; OS: overall survival.
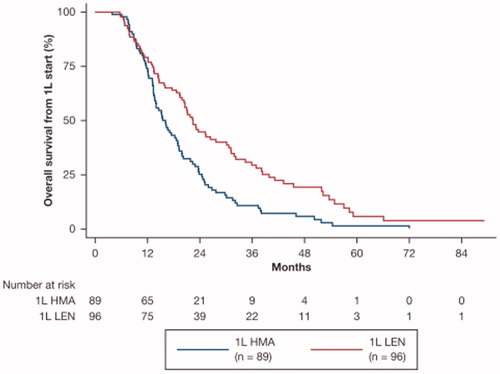
Table 4. Estimated yearly survival rate as approximated by annual insurance disenrollment rates.
A sensitivity analysis censored patients at the earliest date of claim with an AML diagnosis and found that the median OS remained significantly longer (p=.004) with LEN-HMA at 30.6 months (95% CI: 21.1–39.9) vs. 19.0 months (95% CI: 15.5–23.8) with HMA-LEN.
Discussion
Using a payer claims database, we addressed the uncertainty regarding treatment sequencing in MDS, finding that patients receiving LEN-HMA remained on treatment longer compared with those receiving the reversed sequence of HMA-LEN. Additionally, the LEN-HMA-treated patients derived additional clinical benefit from first-line use of lenalidomide through improvements in OS, as measured by the proxy of adjusted time to insurance disenrollment.
Using time to disenrollment as a proxy for OS, we found a significant 36% reduction in the risk of death with first-line use of lenalidomide in our broader population that included patients with del(5q) and non-del(5q) MDS, in which median survival was <2 years (22.4 months with LEN-HMA vs. 16.1 months with HMA-LEN). Comparisons of our real-world effectiveness data to prior clinical trial experiences are confounded by a paucity of clinical trial data for sequential use of lenalidomide and HMAs. Per preliminary results of a small phase 2 clinical trial of second-line lenalidomide after HMA failure (due to intolerance or disease progression) in adult patients with MDS (mostly Intermediate-1- and Intermediate-2-risk disease [n = 12 and n = 9, respectively]), median OS was 9.3 months, shorter than our reported durations but comparing favorably with historical estimates for patients failing HMAs (∼4–6 months) [Citation8].
Our study has important limitations. As in any claims database, data were collected for billing purposes rather than for conducting clinical research. It is well appreciated that the identification of diagnoses and treatments relies on the accuracy of claims coding. Additionally, the claims database included information on patients for only as long as they were covered by a health plan in the database. The database does not capture potential resource use and diagnoses before enrollment in a health plan in the database, as well as resources used and paid out of pocket or not billed. While adjusted HRs were estimated to address potential confounding, unobserved or residual confounding may exist, inherent in any retrospective data analysis. As the date of death was not available, time from index date to date of insurance disenrollment served as a proxy for survival with the assumption that older patients with chronic conditions are unlikely to change insurance provider and have a high healthcare burden. The extent to which this proxy was reflective of actual deaths cannot be determined. Lastly, analyses involving treatment response in terms of transfusion frequency and independence were precluded by the inclusion of platelet transfusion codes in the analytic sources, with no means of reliably differentiating claims for RBC vs. platelet transfusions based on how the data were generated [Citation12].
In conclusion, these observational data from a nationally representative database directionally confirm the benefits of the LEN-HMA treatment sequence with respect to prolonging treatment duration and possibly OS compared with the HMA-LEN treatment sequence. These data support the first-line use of lenalidomide in lieu of HMAs in patients with LR-MDS after the failure of (or among those unlikely to respond to) ESAs.
Potential conflict of interest:
Disclosure forms provided by the authors are available with the full text of this article online at http:\\10.1080/10428194.2018.1551538.
Additional information
Funding
References
- Clara JA, Sallman DA, Padron E. Clinical management of myelodysplastic syndrome/myeloproliferative neoplasm overlap syndromes. Cancer Biol Med. 2016;13:360–372.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER) cancer statistics review 1975-2010: Section 30 – Myelodysplastic syndromes (MDS), chronic myeloproliferative disorders (CMD), and chronic myelomonocytic leukemia (CMML). 2012 [cited 2017 Dec 21]. Available from: http://seer.cancer.gov/archive/csr/1975_2010/results_merged/sect_30_mds.pdf.
- Ma X, Does M, Raza A, et al. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542.
- List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557.
- Santini V, Almeida A, Giagounidis A, et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion-dependent patients with lower-risk non-del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis-stimulating agents. Jco. 2016;34:2988–2996.
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx
- Zeidan AM, Gore SD, McNally DL, et al. Lenalidomide performance in the real world: patterns of use and effectiveness in a Medicare population with myelodysplastic syndromes. Cancer. 2013;119:3870–3878.
- Kim H, Lee JH, Lee WS, et al. Lenalidomide as a second-line therapy after failure of hypomethylating agents in patients with myelodysplastic syndrome. Blood. 2015;126:1687.
- Zeidan AM, Al Ali NH, Padron E, et al. Lenalidomide treatment for lower risk nondeletion 5q myelodysplastic syndromes patients yields higher response rates when used before azacitidine. Clin Lymphoma Myeloma Leuk. 2015;15:705–710.
- Wade RL, Korytowsky B, Singh P, et al. The novel generation and validation of survival curves in oncology utilizing electronic medical records linked to point of service claims data. Poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) European Congress; 2017 November 4–8; Glasgow, Scotland, UK.
- Uno H, Cronin A, Wadleigh M, et al. Derivation and validation of the SEER-Medicare myelodysplastic syndromes risk score (SMMRS). Leuk Res. 2014;38:1420–1424.
- Carnahan RM. Mini-Sentinel’s systematic reviews of validated methods for identifying health outcomes using administrative data: summary of findings and suggestions for future research. Pharmacoepidemiol Drug Saf. 2012;21:90–99.
