Abstract
Entospletinib (GS-9973), an oral, selective inhibitor of spleen tyrosine kinase (SYK), was evaluated as monotherapy in this multicenter, phase 2 study (NCT01799889) of 49 patients with relapsed or refractory chronic lymphocytic leukemia (CLL), including those with Richter’s transformation (RT), who had received prior therapy with a B-cell receptor (BCR) inhibitor. Patients were treated with entospletinib 400 mg BID as the starting dose. Sixteen patients achieved partial response and 21 had stable disease. The overall response rate was 32.7% (95% confidence interval [CI]: 21.7–45.3%). The median progression-free survival (PFS) was 5.6 (95% CI: 3.7–8.3) months. Twenty-one (of 43) patients (48.8%) experienced nodal response. Adverse events (AEs) occurred in all patients; most commonly fatigue, diarrhea, and anemia. Entospletinib monotherapy has clinical activity for patients with CLL and RT who have relapsed following therapy with BCR inhibitors.
Introduction
The therapeutic landscape for relapsed and refractory chronic lymphocytic leukemia (R/R CLL) is constantly evolving, with multiple targeted agents recently approved. Of these, the Bruton’s tyrosine kinase inhibitor (BTKi) ibrutinib, the phosphoinositide 3-kinase inhibitor (PI3Kδi) idelalisib, and the bcl-2 inhibitor venetoclax have demonstrated efficacy and safety in these patients [Citation1–7]. In particular, patients with CLL with high-risk features, including unmutated immunoglobulin heavy chain variable region (IGHV), del17p with or without TP53 mutation, or del11q have benefited from these new agents [Citation2,Citation8–11]. However, many patients are intolerant of or develop resistance to these therapies, and additional treatment options are needed [Citation12,Citation13]. A potential therapeutic target upstream of both BTK and PI3Kδ in the B-cell receptor (BCR) signaling pathway is spleen tyrosine kinase (SYK), which mediates signaling, proliferation, migration, and survival of CLL cells [Citation14–16]. Because of its upstream position, inhibition of SYK signaling may overcome drug resistance to BTKi or PI3Kδi [Citation17].
Entospletinib is a small molecule inhibitor of SYK that has demonstrated clinical efficacy and acceptable tolerability as monotherapy in a cohort of patients with R/R CLL in an open-label, phase 2 trial [Citation18]. The objective response rate (ORR) was 61.0% (95% confidence interval [CI], 44.5–75.8%) for patients without previous exposure to either BTKi or PI3Kδi, including 7.3% of patients who achieved nodal response with persistent lymphocytosis. This report provides safety and efficacy data from an additional cohort of patients with CLL who developed progressive disease during treatment with either a BTKi or a PI3Kδi and subsequently were treated with entospletinib.
Methods
The multi-cohort, phase 2, open-label study (NCT01799889) enrolled a cohort of patients with R/R CLL previously treated with either a BTKi or a PI3Kδi. Patients with R/R CLL with Richter’s transformation (RT) were also eligible. Patients received 400 mg BID entospletinib orally (1 h before or 2 h after meals); the dose of entospletinib could be reduced or interrupted as needed due to toxicities. Treatment continued until progression of disease (PD), unacceptable toxicity, or withdrawal of consent. This study adhered to the Declaration of Helsinki and was approved by the relevant institutional review boards. A more detailed description of the methods has been previously published [Citation18].
Computed tomography or magnetic resonance imaging was performed every 8 weeks through the first 24 weeks and then every 12 weeks. Determination of response and progression was based on modified International Workshop on CLL criteria [Citation19]. The primary endpoint was PFS rate at 16 weeks. Secondary endpoints included evaluation of safety, ORR, duration of response (DoR), and time to response (TTR). Primary and secondary efficacy endpoints were assessed by an independent review committee (IRC). Four groups of patients with CLL were evaluated: non-RT with prior BTKi therapy, non-RT with prior PI3Kδi therapy, RT with prior BTKi therapy, and RT with prior PI3Kδi therapy. Given the small numbers of patients within each subgroup, no formal statistical analysis was performed among the subgroups for efficacy.
Results
Patient characteristics and disposition
A total of 49 patients (41 without and 8 with RT) received entospletinib in this cohort. Demographics and baseline disease characteristics are shown in . Across subgroups, the median number of prior treatments was 3–6 and the range was 1–8. Of the 41 patients without RT, 33 were previously treated with a BTKi (32 ibrutinib, 1 spebrutinib [AVL-292/CC292]) and 8 with a PI3Kδi (7 idelalisib and 1 umbralisib). There were eight patients with RT: Five were previously treated with a BTKi (ibrutinib) and three with a PI3Kδi (idelalisib). A median of 1.6 (range 0.1–12.2) months had elapsed since discontinuation of the prior treatment to initiation of entospletinib. In the non-RT groups, 23 (56.1%) patients had PD on prior therapy, while 15 (36.6%) discontinued prior therapy due to intolerance and an additional 1 (2.4%) patient each in the non-RT group with prior PI3Kδi treatment experienced suboptimal response, drug-induced pneumonitis or completed the previous trial. Seven of 8 (87.5%) patients in the RT groups progressed on prior therapy, while one discontinued prior therapy due to intolerance.
Table 1. Patient demographics and baseline disease characteristics.
The median duration of exposure to entospletinib was 5.2 (range: 0.2–36.9) months. Thirteen (26.5%) patients required ≥1 dose reduction from the starting dose (12 reduced to 200 mg and 1–100 mg). Of the 49 treated patients, 37 (75.5%) discontinued the study, primarily due to PD (40.8%), adverse events (AEs) (12.2%), and physician discretion (10.2%); other reasons were withdrawal by subject (6.1%) and lack of efficacy and death (3% each). As of November 2017, 12 patients (1 RT and 11 non-RT) remained on entospletinib.
Efficacy
The median PFS rate at week 16 for the overall group was 65.7% (95% CI: 49.0–78.2%), and the median IRC-assessed PFS was 5.6 (95% CI: 3.7–8.3) months. PFS rate and median PFS for the four patient groups by reason for discontinuing prior BCR are shown in . The Kaplan–Meier estimates of PFS for the four patient groups are shown in . In the non-RT prior BTKi group, median PFS was 8.2 months in those who were intolerant of prior therapy and 3.7 months in those with PD on prior therapy. In the non-RT prior PI3Kδi group, median PFS was not reached in those who were intolerant and 11.1 months in those with PD on prior therapy. PFS in IGHV-mutated (n = 3) vs unmutated (n = 38) CLL was 5.6 months (95% CI 4.4–not reached) vs. 5.6 months (95% CI 3.7–8.3) and in TP53 mutation or del17p vs. neither mutation was 5.6 months (95% CI 3.5–13.5) vs. 5.6 months (95% CI 2.8–11.1).
Figure 1. Kaplan–Meier curves of progression-free survival by cohort. (A) Non-Richter’s with prior BTKi exposure. (B) Non-Richter’s with prior PI3Kδi exposure.
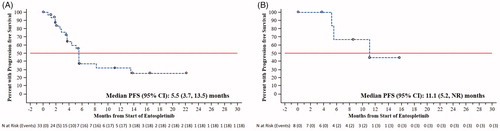
Table 2. ORR and PFS by reason for discontinuing prior BCR – IRC assessment.
Overall, 42 (85.7%) patients were evaluable for response (non-RT: 31 BTKi, 7 PI3Kδi, and RT: 3 BTKi and 1 PI3Kδi); one patient was not evaluable and six patients were not assessed. There were no complete responses. Sixteen patients (32.7%) achieved a partial response, of whom 11 were IGHV unmutated, four had either a TP53 mutation or del17p, and eight had PD on their prior therapy. Among the 16 responding patients, the median IRC-assessed DoR was 5.6 (95% CI: 2.4–9.9) months. The median TTR was 2.0 (range: 1.8–12.9) months. Stable disease was reported for an additional 21 (42.9%) patients (18 IGHV unmutated, 10 with either a TP53 mutation or del17p, and 13 with PD on their prior therapy).
The total ORR was 32.7% (90% CI: 21.7–45.3%), with a higher ORR of 75.0% (90% CI: 40.0–95.4%) for the non-RT group with prior PI3Kδi exposure compared to 24.2% (90% CI: 12.7–39.5%) for the non-RT group with prior BTKi exposure. In patients with known RT, two patients with prior BTKi exposure and no patients with prior PI3Kδi exposure had an objective response. ORR by reason for discontinuing prior BCR is outlined in . ORR in IGHV-mutated CLL vs. unmutated was 66.7% (90% CI 13.5–98.3%) vs. 28.9% (17.2–43.3%) and in TP53 mutation or del17p vs. neither mutation was 21.1% (7.5–41.9%) vs. 38.5% (22.6–56.4%).
In all, 21 patients (48.8%; 90% CI, 35.5–62.3%) achieved a lymph node response (≥50% reduction from baseline in the sum of the products of the diameters of index lesions) (). Response varied across the groups (0–85.7%), with the one evaluable patient in the RT group with prior PI3Kδi exposure having no response.
Safety
All patients experienced a treatment-emergent AE (TEAE) (69.4% with AEs ≥ grade 3) and 42.9% experienced a serious AE (SAE) (). AEs most commonly considered related to entospletinib were diarrhea (30.6%), fatigue (24.5%), nausea (16.3%), anemia (14.3%), and neutropenia (12.2%). There were no events of pneumonitis. Alanine aminotransferase was increased in three patients (1 with grade ≥3), and two of these three also had aspartate aminotransferase increased. Infections occurred in 65.3% of patients (12.2% considered SAEs) and there were no opportunistic infections. AEs led to entospletinib discontinuation, dose reductions and dose interruptions in eight patients (16.3%), four patients (8.2%), and 23 patients (46.9%), respectively. There were five deaths within 30 d of last dose of entospletinib: four due to PD and one from cardiac arrest (cause unknown). Nine (50%) patients experienced serious TEAEs (three grade 3, five grade 4, and one grade 5) in the intolerant cohort (n = 18), while 12 (38.7%) experienced serious TEAEs (eight grade 3, three grade 4, and one grade 5) in the cohort with progressive disease (n = 31). Most of these events included sepsis, febrile neutropenia, and anemia.
Table 3. Treatment-emergent adverse events.
Discussion
Targeting the BCR signaling cascade has transformed the management of CLL. The BTK inhibitors ibrutinib and acalabrutinib have both led to very high ORR (albeit mostly partial responses) in R/R CLL; however, resistance and/or intolerance to these agents is not uncommon and the residual tumor cells provide an opportunity for the emergence of resistance [Citation20–22]. One single-institution study of ibrutinib reported that 31 (10.1%) patients discontinued for disease progression (RT or progressive CLL) [Citation23]. Notably, RT appeared to occur early and CLL progressions later, and median survival was only 3.5 months following RT and 17.6 months following CLL progression. Targeted deep sequencing was performed for patients who discontinued therapy because of disease progression, which found BTK mutations in two of the eight patients with RT and BTK or PLCG2 mutations in all 11 patients with CLL progression that were not evident at baseline. This study confirmed the poor prognosis after ibrutinib discontinuation, especially for those patients with RT. Another study analyzed long-term outcomes in 90 of 320 patients with CLL (28.1%; 80 R/R and 10 treatment-naïve) who discontinued ibrutinib most commonly for intolerance (32.2%), miscellaneous (31.1%), progression (21.1%), and RT (10.0%) [Citation24]. The median survival according to the reason for discontinuation was 33 months for ibrutinib intolerance, 11 months for miscellaneous causes, 16 months for progressive CLL, and 2 months for RT. Of the 19 patients who had progressive CLL, 42% responded to subsequent therapy. Acalabrutinib progression and resistance studies in CLL are pending, but it has been preliminarily shown to have activity in patients intolerant of ibrutinib [Citation25]. In patients who have failed both BTKi and other targeted agents, such as PI3Kδi, it is likely that the prognosis is worse. Venetoclax has been evaluated in patients with R/R CLL who progressed on or after ibrutinib [Citation6] and following idelalisib therapy [Citation7]. Both these studies report objective responses in ∼2/3 of patients and a 12-month PFS rate of ∼80%.
Sharman et al. [Citation18] first reported on this multi-cohort study of entospletinib therapy. In that cohort of patients with CLL, who had been previously treated with anti-CD20 antibodies or alkylating agents (median of two prior therapies), the PFS rate at 24 weeks was 70.1% with a median PFS of 13.8 months and the ORR was 61.0%. In this cohort, we report activity of entospletinib in patients with R/R CLL (with and without RT) and a significant prior treatment history including BTKi and/or PI3Kδi therapies (median of three prior therapies). Median PFS for the overall group was 5.6 (95% CI: 3.7–8.3) months. Partial responses and stable disease were experienced by 32.7% and 42.9% of patients, respectively, including those patients with RT and those with unfavorable cytogenetics. Patients who progressed on prior BCR treatment responded to entospletinib monotherapy at a rate that is clinically significant. Most of these responders did not have RT, although two patients with RT (one with PD and one who received prior BTKi therapy) responded. Interestingly, in patients with RT and prior BTKi exposure, therapy with entospletinib led to an ORR of 40.0% and median PFS of 2.9 months, while there were no responses to entospletinib in RT patients with prior PI3Kδi exposure and a median PFS of 4.6 months. Our study demonstrated encouraging responses to entospletinib in patients with R/R CLL who have failed BTK and/or PI3Kδ inhibitors, especially in patients with RT, a group that has historically demonstrated a very poor prognosis [Citation26,Citation27]. This provides rationale for the combination use of this agent and the possibility of using this for disease control while transitioning to more definitive treatment like chimeric antigen T-cell receptor therapy and/or allogeneic hematopoietic cell transplant. The AEs related to entospletinib are manageable.
Entospletinib may provide an option for patients with R/R CLL who cannot tolerate or who become resistant to BTK- and PI3Kδ-targeted therapies. Given the demonstrated efficacy of venetoclax in patients with CLL previously treated with ibrutinib or idelalisib [Citation6,Citation7], venetoclax may be the favored therapeutic option for these patients. As no patients in our trial had received venetoclax therapy, it is unknown whether entospletinib might be a salvage option after venetoclax. Future development of entospletinib will be focused on combination therapy.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2018.1562180.
Acknowledgments
We thank Beth Sesler, PhD, CMPP, for editorial assistance in preparing the manuscript and Steve Abella for his scientific contributions on the entospletinib development program.
Additional information
Funding
References
- Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007.
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322.
- Tsang M, Shanafelt TD, Call TG, et al. The efficacy of ibrutinib in the treatment of Richter syndrome. Blood. 2015;125:1676–1678.
- Giri S, Hahn A, Yaghmour G, et al. Ibrutinib has some activity in Richter’s syndrome. Blood Cancer J. 2015;5:e277.
- Jones JA, Mato AR, Wierda WG, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:65–75.
- Coutre S, Choi M, Furman RR, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131:1704–1711.
- Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223.
- O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood. 2015;126:2686–2694.
- Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397.
- Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91.
- Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128:2199–2205.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103:874–879.
- Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23:686–697.
- Kipps TJ. The B-cell receptor and ZAP-70 in chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007;20:415–424.
- Stevenson FK, Caligaris CF. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–4395.
- Sharman J, Di Paolo J. Targeting B-cell receptor signaling kinases in chronic lymphocytic leukemia: the promise of entospletinib. Ther Adv Hematol. 2016;7:157–170.
- Sharman J, Hawkins M, Kolibaba K, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015;125:2336–2343.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456.
- Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332.
- Burger JA, Tedeschi A, Barr PM, et al.; RESONATE-2 investigators. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437.
- Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817.
- Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87.
- Jain P, Thompson PA, Keating M, et al. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123:2268–2273.
- Awan FT, Schuh A, Brown JR, et al. Acalabrutinib monotherapy in patients with ibrutinib intolerance: results from the phase 1/2 ACE-CL-001 clinical study. Blood. 2016;128:638.
- Khan M, Siddiqi R, Thompson PA. Approach to Richter transformation of chronic lymphocytic leukemia in the era of novel therapies. Ann Hematol. 2018;97:1–15.
- Rogers KA, Huang Y, Ruppert AS, et al. A single-institution retrospective cohort study of first-line R-EPOCH chemoimmunotherapy for Richter syndrome demonstrating complex chronic lymphocytic leukaemia karyotype as an adverse prognostic factor. Br J Haematol. 2018;180:259–266.

![Figure 2. Best percent change from baseline in tumor size (sum of the product of the diameters [SPD] of nodal or tumor mass).](/cms/asset/7578715a-890b-4869-91bf-fa9cd17dc109/ilal_a_1562180_f0002_c.jpg)