Abstract
ENESTnext (NCT01227577) was a single-arm, multicenter trial evaluating the rate of deep molecular response by 2 years in patients with newly diagnosed (within 6 months) chronic myeloid leukemia in chronic phase (CML-CP) treated with nilotinib 300 mg twice daily. Among 128 enrolled patients, 94 (73%) achieved major molecular response (MMR; BCR-ABL1 ≤ 0.1% on the International Scale [BCR-ABL1IS]) and 34 (27%) achieved confirmed MR4.5 (BCR-ABL1IS ≤0.0032% detectable or undetectable; primary endpoint) by 2 years. Three-month BCR-ABL1 levels were predictive of later responses. In exploratory analyses, digital polymerase chain reaction (PCR) detected BCR-ABL1 in 39.4% of samples from patients with confirmed MR4.5 and identified further decreases in BCR-ABL1 with continued nilotinib. Safety results, including cardiovascular events, were consistent with those in other nilotinib trials. These results further substantiate the molecular response rates associated with frontline nilotinib therapy and demonstrate the feasibility of monitoring very low BCR-ABL1 transcript levels using digital PCR.
Introduction
Tyrosine kinase inhibitors (TKIs) are the standard-of-care treatment for newly diagnosed Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP) [Citation1,Citation2]. Regular molecular monitoring of BCR-ABL1 transcript levels is recommended by the National Comprehensive Cancer Network (NCCN) and European LeukemiaNet (ELN) to assess responses to frontline TKI therapy and inform treatment decisions [Citation1,Citation2]. According to NCCN and ELN recommendations, favorable treatment responses include achievement of early molecular response (EMR; BCR-ABL1 ≤ 10% on the International Scale [BCR-ABL1IS] at 3 or 6 months) [Citation1,Citation2] and major molecular response (MMR; BCR-ABL1IS ≤0.1%) at 12 months [Citation2]. With continued TKI treatment, some patients may achieve deep molecular response (DMR; i.e. MR4 [BCR-ABL1IS ≤0.01%] or MR4.5 [BCR-ABL1IS ≤0.0032%]) [Citation3–5]. An association between deeper molecular responses and improved clinical outcomes has been suggested [Citation5–7]. Additionally, achievement of sustained DMR is required for suspending TKI therapy in clinical trials of treatment-free remission (TFR; i.e. cessation of TKI treatment with maintenance of molecular response) [Citation8–17], an increasingly relevant goal of TKI therapy [Citation1,Citation18].
Assessment of DMR requires precise, highly sensitive, and reproducible monitoring techniques; the European Treatment and Outcome Study for CML group has developed guidelines for monitoring molecular response using real-time quantitative polymerase chain reaction (RQ-PCR) [Citation19]. Considering the sensitivity limits of RQ-PCR, there is evolving interest in establishing even more sensitive monitoring methods, such as digital PCR [Citation20], that may help better predict sustained response after treatment cessation. Digital PCR provides improved detection of BCR-ABL1 versus conventional RQ-PCR by distributing the PCR reaction into smaller volumes (e.g. 8 μL distributed into 765 wells with digital PCR versus 10 μL into a single well with RQ-PCR), thereby reducing background and increasing the likelihood of detecting an event (i.e. amplification). Thus, digital PCR can detect lower-level, increasing BCR-ABL1 transcript levels earlier than RQ-PCR and thus track measurable residual disease at levels not assessable by conventional RQ-PCR [Citation20].
In the phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, frontline nilotinib provided high rates of MR4 and MR4.5 in patients with Ph + CML-CP [Citation3]. The ENESTnext study was designed to assess rates of DMR in patients with CML-CP treated with frontline nilotinib for up to 2 years. In addition, patients with confirmed MR4.5 were evaluated using a novel, microfluidic digital PCR assay that is >1 log more sensitive than conventional RQ-PCR. Herein, we present final results of ENESTnext, with up to 2 years’ follow-up.
Methods
Patients and treatment
ENESTnext was a phase 4, single-arm, open-label, multicenter trial of adults with Ph + CML-CP diagnosed ≤6 months before study entry. Eligible patients had an Eastern Cooperative Oncology Group performance status of ≤2, electrolyte levels within normal limits, and adequate organ function. Patients with acute or chronic liver, renal, or pancreatic disease, impaired cardiac function (details in Supplementary material), a clinically significant malignancy other than CML, or another severe or uncontrolled medical condition were ineligible. Additional exclusion criteria included documented T315I mutation, treatment with medications that prolong the QT interval, pregnancy, or nursing. Up to 6 months of prior therapy with interferon (with or without cytarabine), hydroxyurea, or anagrelide was permitted; prior therapy with nilotinib (≤6 months), imatinib (≤1 month), and dasatinib (≤14 days) was also allowed.
During the study, all patients received nilotinib 300 mg twice daily for up to 2 years after enrollment. Dose escalation to 400 mg twice daily was allowed for suboptimal response or treatment failure per 2009 ELN guidelines [Citation21]. Dose reductions to 400 mg once daily were recommended for patients with recurrent grade 3/4 hematologic adverse events (AEs) or grade ≥2 nonhematologic AEs (details in Supplementary material). Patients with nilotinib-related toxicities that did not resolve within 28 days were discontinued from the study.
Study assessments
RQ-PCR monitoring of BCR-ABL1 transcript levels was conducted in an IS-standardized central laboratory (MolecularMD, Portland, OR) using peripheral blood samples collected monthly for the first 3 months and every 3 months thereafter. Results were reported as the ratio of BCR-ABL1 to ABL1, standardized to the IS. For assessment of MR4.5, ≥25,614 ABL1 copies were required. The limit of detection for the RQ-PCR assay was MR4.5. Fluidigm digital PCR was performed at the Fred Hutchinson Cancer Research Center. Briefly, RNA was reverse transcribed to complementary DNA. Then, following a 14-cycle pre-amplification stage, samples were partitioned into 3 separate 765-well panels. After quantitative PCR amplification, BCR-ABL1 copy number was determined based on Poisson distribution. The limit of detection for the digital PCR assay was calculated by diluting IS calibrators into HL60 RNA and shown to be 0.0001% on the IS. Dilution experiments of IS standards (from 100% to 0.0001%) were performed to generate standard curves (Supplemental Table 1, Supplemental Figure 1). Each Fluidigm panel contained positive and ‘blank’ controls. The tempo of disease was displayed by plotting the number of BCR-ABL1 copies (summed over 3 panels) for each time point for each patient. For log10 transformation, values were transformed after adding 1 to each value (to make 0 values a valid integer). A restricted cubic spline with 5 knots (at the 5th, 25th, 50th, 75th, and 95th percentiles) was then fitted through the raw and log-transformed data points, as was a least squares regression line.
Study endpoints
The primary endpoint was the rate of confirmed MR4.5 (in 2 consecutive samples analyzed 3 months apart by RQ-PCR) with up to 2 years of on-study nilotinib treatment. Secondary efficacy endpoints included rates of MMR, loss of MMR, and loss of confirmed MR4.5 by 24 months, and median time to and duration of MMR and confirmed MR4.5 (details in Supplementary material); complete cytogenetic response (CCyR) rates were also assessed. In an exploratory analysis in patients with confirmed MR4.5, patient samples were analyzed for detectability of BCR-ABL1 transcripts with the Fluidigm digital PCR assay. Safety endpoints included AEs and laboratory abnormalities.
Statistical analyses
A sample size of ≥100 patients would allow estimation of the 2-year MR4.5 rate within 8.5% of the assumed true MR4.5 rate (25% based on 24-month follow-up in ENESTnd [Citation22]) with 95% confidence. Unless otherwise indicated, all efficacy assessments were conducted in patients who received ≥1 dose of study treatment. Only descriptive statistics were performed. The primary endpoint (confirmed MR4.5 with up to 2 years of on-study nilotinib treatment) and other response rates were summarized. Safety analyses were performed in all patients who received ≥1 dose of study treatment and had ≥1 postbaseline safety assessment.
Ethics
This study was designed in accordance with the ICH Harmonized Tripartite Guideline for Good Clinical Practice and the ethical principles established in the Declaration of Helsinki. The study protocol and informed consent form were reviewed and approved by the independent review board/independent ethics committee/research ethics board at each study center. Patients provided written informed consent before enrollment. The study was registered at ClinicalTrials.gov (NCT01227577).
Results
Patients
In total, 128 patients were enrolled; median age was 56.5 years, 50% were male, and 80.5% were white (). Median time from CML diagnosis to screening was 14 days (range, 1–183 days), and 16 patients (12.5%) had prior TKI treatment (nilotinib, n = 10 [median duration, 24 days; range, 3–151 days]; imatinib, n = 4 [median duration, 9 days; range, 5–27 days]; dasatinib n = 2 [14 and 30 days, respectively]). Median duration of on-study nilotinib exposure was 659 days (range, 4–714 days) and median nilotinib dose intensity was 598.9 mg/day (range, 300–763 mg/day); median duration of follow-up was 778 days (range, 9–1473 days). Ninety-three patients (72.7%) completed study treatment per protocol (Supplemental Table 2). The most common reason for early discontinuation was AEs (n = 17 [13.3%]); 5 patients (3.9%) discontinued due to unsatisfactory therapeutic effect.
Table 1. Baseline characteristics.
At study entry, 1 patient previously treated with nilotinib for 126 days had CCyR, 1 patient previously treated with nilotinib for 151 days had partial cytogenetic response, and 2 patients without prior antineoplastic medication had low levels of cytogenetically detected disease (equivalent to partial cytogenetic response). The median BCR-ABL1IS value at screening was 70.9% (range, 0.0018%–290.4756%); 5 patients (3.9%) had BCR-ABL1IS ≤10% at study entry, including 1 (0.8%) with MMR but not MR4.5 and 3 (2.3%) with MR4.5. The 3 patients with MR4.5 at screening were diagnosed 38, 31, and 14 days before screening and had received prior treatment with hydroxyurea (39 days), imatinib (5 days), and hydroxyurea (25 days), respectively. The patient with MMR but not MR4.5 at screening was diagnosed 146 days before screening and had previously received nilotinib (126 days).
Efficacy
By 24 months, the cumulative rate of MMR was 73.4% (94/128), including 26.6% (34/128) of patients who also achieved confirmed MR4.5 (). Excluding patients with MMR at baseline, the rate of MMR by 24 months was 72.6% (90/124), and in the subsets of patients with or without prior TKI treatment (excluding those with MMR at baseline), the cumulative rates of MMR by 24 months were 78.6% (11/14) and 71.8% (79/110), respectively. Excluding patients with MR4.5 at baseline, the rate of confirmed MR4.5 by 24 months was 25.6% (32/125), and in the subsets of patients with or without prior TKI treatment (excluding those with MR4.5 at baseline), the cumulative rates of confirmed MR4.5 by 24 months were 26.7% (4/15) and 25.5% (28/110), respectively. The CCyR rate by 24 months was 72.7% among all patients and 86.9% among the 107 patients in whom cytogenetic response was assessed.
Table 2. Molecular responses with up to 24 months of on-study treatment.
Among all patients who achieved MMR, 66% did so by 6 months on study; among patients who achieved confirmed MR4.5, 74% did so by 12 months on study. Among patients who achieved MMR, median time to MMR was 5.6 months and median duration of MMR was 16.5 months (for patients who did not lose response, the date of last dose of study drug was used as the end date); among patients who achieved confirmed MR4.5, median time to confirmed MR4.5 was 8.3 months and median duration of confirmed MR4.5 was 13.9 months. Kaplan–Meier curves of time to MMR and confirmed MR4.5 are shown in Supplemental Figure 2. Among patients with low, intermediate, and high Sokal risk at baseline, 47/58 (81.0%), 32/43 (74.4%), and 12/24 (50.0%) patients, respectively, achieved MMR by 24 months, and 13/58 (22.4%), 14/43 (32.6%), and 6/24 (25.0%) patients, respectively, achieved confirmed MR4.5 by 24 months. Among patients achieving MMR and confirmed MR4.5, 13.8% (13/94) lost MMR and 17.6% (6/34) lost confirmed MR4.5 on study, respectively (). Of the 13 patients who lost MMR, none discontinued study treatment due to lack of efficacy, had BCR-ABL1 mutations detected on study, or progressed to accelerated phase (AP)/blast crisis (BC). Eight of the 13 subsequently spontaneously regained MMR without a change in nilotinib dose. Of the 6 patients who lost confirmed MR4.5, 3 spontaneously regained the response with continuation of nilotinib treatment of the same dose. When comparing patients who lost MMR or confirmed MR4.5 with those who did not lose these responses, no notable differences were observed in nilotinib dose intensity or total exposure, nilotinib treatment duration, or Sokal risk category (Supplemental Table 3).
Landmark analyses were performed based on achievement of BCR-ABL1IS ≤10% at 3 months from the start of study treatment among patients who had not received any TKI therapy prior to the study (). For assessment of MMR, 87 patients with BCR-ABL1IS ≤10% at 3 months were included (patients with MMR at baseline and/or prior TKI therapy were excluded); similarly, for assessment of confirmed MR4.5, 87 patients with BCR-ABL1IS ≤10% at 3 months were included (patients with MR4.5 at baseline and/or prior TKI therapy were excluded). MMR was achieved by 75/87 patients (86.2%) with BCR-ABL1IS ≤10% at 3 months compared with 2/10 patients (20.0%) without this response at 3 months. Confirmed MR4.5 was achieved by 28/87 patients (32.2%) with BCR-ABL1IS ≤10% at 3 months compared with none of the 10 patients without this response at 3 months (median follow-up, 766.5 days [range, 609–1338 days]). Overall, patients with BCR-ABL1IS ≤10% at 3 months maintained a lower average BCR-ABL1IS transcript level throughout the study than patients without this response at 3 months ().
Figure 1. Change in BCR-ABL1IS over time based on achievement of BCR-ABL1IS ≤10% at 3 months (patients who had MMR or MR4.5 at baseline were excluded from this analysis. The number of evaluable patients at each time point is indicated). IS: International Scale; MMR: major molecular response (BCR-ABL1IS ≤0.1%); MR4.5: BCR-ABL1IS ≤0.0032%; SD: standard deviation.
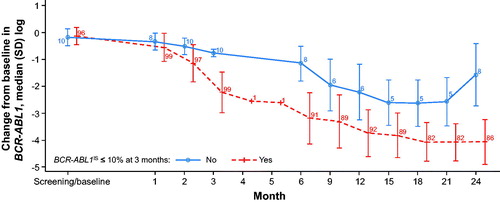
Table 3. Landmark analysis of molecular response according to BCR-ABL1IS at 3 months on study.
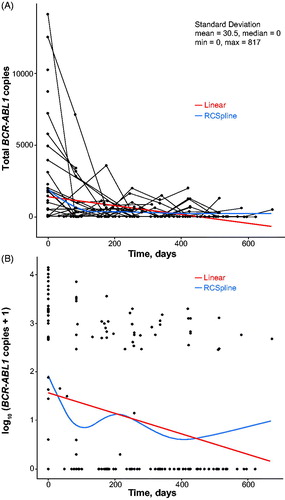
Digital PCR testing was conducted on 195 samples from 33 patients who achieved confirmed MR4.5, which corresponded to the detection limit of the conventional RQ-PCR assay used in the central laboratory. Overall, 39.4% of 195 samples assessed by digital PCR had detectable BCR-ABL1. To evaluate the impact of nilotinib treatment duration on BCR-ABL1 detectability by digital PCR, results were also analyzed based on the time point for sample collection—i.e. the first sample (taken at the same time as the first peripheral blood sample confirming MR4.5) versus final sample from each patient assessed by digital PCR. When the first samples were assessed, BCR-ABL1 was detected by digital PCR in 18/33 patients (54.5%). With continued nilotinib treatment, 10 of these 18 patients had undetectable BCR-ABL1 in the final samples assessed. Of the remaining 15 patients (45.5%) with undetectable BCR-ABL1 by digital PCR in their first samples assessed, 12 had undetectable BCR-ABL1 by digital PCR in their final samples (Supplemental Table 4; as an example of digital PCR detection, the kinetics of BCR-ABL1 disappearance in 8 cases with MR4.5 at diagnosis and beyond but with BCR-ABL1 positivity by digital PCR throughout follow-up are shown in Supplemental Figure 3, with the Poisson BCR-ABL1 copy estimates standardized by total RNA input). The change in the Poisson estimates of BCR-ABL1 copy number is shown in (raw number) and (log transformed). The average decrease in BCR-ABL1 level, based on the slope of the linear regression line of BCR-ABL1 over time, was 315 copies/100 days (≈100 copies/month). Digital PCR results were also analyzed according to baseline Sokal risk category. Of the patients with high Sokal risk at baseline who had digital PCR testing, 6/24 (25%) achieved undetectable BCR-ABL1 on ≥1 digital PCR test. For patients with low and intermediate Sokal risk at baseline, 16/58 (28%) and 13/43 (30%) achieved undetectable BCR-ABL1 on ≥1 digital PCR test, respectively.
Figure 2. Change in detectable BCR-ABL1 transcript levels over time by Fluidigm digital PCR after achievement of confirmed MR4.5. Data are shown with linear regression and cubic spline fits as raw copy numbers (panel A) and after log transformation (panel B). The reproducibility of the estimated BCR-ABL1 level of 3 panels per sample (see Methods) was good (standard deviation, mean, 30.5 copies; median, 0 copies). MR4.5: BCR-ABL1 ≤0.0032% on the International Scale; PCR: polymerase chain reaction; RCSpline: restricted cubic spline.
Safety
The most common grade 3/4 AEs were increased lipase (n = 16 [12.5%]), thrombocytopenia (n = 11 [8.6%]), and neutropenia (n = 8 [6.3%]) (Supplemental Table 5). Cardiac AEs (all-grade) were reported in 23 patients (18%) and most commonly included palpitations (n = 8 [6.3%]), atrial fibrillation, myocardial infarction, and tachycardia (n = 3 [2.3%] each). Ischemic cardiovascular events included cerebrovascular accident and transient ischemic attack (n = 1 [0.8%] each). No incidences of peripheral arterial occlusive disease were reported.
One patient, who had high Sokal risk at baseline, progressed to AP/BC at 2.76 months on study and discontinued treatment on the same day due to unsatisfactory therapeutic effect. Following discontinuation, this patient was treated with ponatinib and was alive at last contact (40.5 months’ follow-up). Six patients died during the study. Two patients died during study treatment (due to malignant lung neoplasm and pneumonia, respectively), and 1 patient died after the 30-day follow-up period (due to an unknown cause suspected to be related to study treatment). Three patients died during the posttreatment survival follow-up period: 1 completed study treatment and died due to cardiac arrest 133 days after study completion, 1 discontinued treatment due to an AE of electrocardiogram change and died due to cardiac arrest and gastrointestinal bleed 738 days after study discontinuation, and 1 discontinued treatment due to a serious AE of anaplastic astrocytoma and died due to anaplastic astrocytoma 217 days after study discontinuation.
Discussion
Consistent with other studies of frontline nilotinib [Citation3,Citation23,Citation24], a substantial proportion of patients in ENESTnext achieved early and deep molecular responses with nilotinib. The majority of patients (73%) achieved MMR and 27% achieved confirmed MR4.5 with up to 2 years of follow-up. These MMR and confirmed MR4.5 rates through 2 years were similar to those observed in other studies of frontline nilotinib that did not require confirmation of MR4.5 [Citation22–24]. For further context, key studies that assessed MR4.5 with the approved imatinib dose of 400 mg once daily showed 2-year rates ranging from 8% and 9% to 21% (calculated based on competing risks analysis) [Citation5,Citation22,Citation25]. In ENESTnext, higher rates of MMR and confirmed MR4.5 by 24 months were observed among patients who achieved BCR-ABL1IS ≤10% at 3 months than among those without this response, confirming the importance of achieving BCR-ABL1IS ≤10% at 3 months [Citation1,Citation2]. Confirmed MR4.5 was achieved by patients across Sokal risk categories, although the study was not powered to statistically compare response rates between Sokal risk categories, and response rates at long-term time points were not captured. The safety profile of nilotinib was consistent with prior reports of frontline nilotinib [Citation3,Citation23], including observation of cardiovascular AEs. Although no cardiovascular-related deaths were reported during study treatment, 2 patients died due to cardiac arrest after completing the study or discontinuing study treatment, including 1 patient who discontinued due to an AE of electrocardiogram change.
These findings confirm the efficacy of frontline nilotinib in achieving DMR in patients with CML-CP. DMR has been associated with long-term clinical benefits, such as high rates of failure-free survival [Citation6], progression-free survival [Citation7], and overall survival [Citation5,Citation7]. Additionally, sustained DMR is a key requirement for attempting TFR [Citation1,Citation8–17]. Specifically, the NCCN designates ≥3 years of treatment with an approved TKI and achievement of stable MR4 for ≥2 years as prerequisites for attempting TFR [Citation1]. Clinical trials of TFR have shown that up to ≈60% of patients who achieve sustained DMR on long-term imatinib can remain treatment free with at least sustained MMR for multiple years after stopping imatinib [Citation8–10]; results in a generally similar range have been reported after nilotinib cessation [Citation12,Citation13,Citation26,Citation27].
Fluidigm digital PCR analysis, which has greater sensitivity than RQ-PCR [Citation20], was performed on samples from patients who achieved confirmed MR4.5. With DMR and TFR emerging as goals of treatment, more sensitive methods for molecular monitoring, such as digital PCR, are of increasing interest. Such methods may enable assessment of residual disease with greater precision, may help to inform decisions regarding cessation of therapy [Citation11,Citation28], and may allow evaluation of the dynamics of changes in BCR-ABL1 levels below the limit of detection of conventional RQ-PCR. As digital PCR is a more sensitive method for detecting increases in BCR-ABL1 levels than conventional RQ-PCR (Supplemental Figure 4), it may provide a means for earlier detection of potential relapse; although loss of MMR during TFR is a common criterion for treatment reinitiation [Citation1,Citation10–14,Citation16], digital PCR may identify patients with increasing BCR-ABL1 levels who may require more frequent monitoring. In ENESTnext, digital PCR detected BCR-ABL1 in ≈40% of samples from patients with confirmed MR4.5. Additionally, BCR-ABL1 levels were shown using digital PCR to decrease with continued nilotinib treatment, at a rate of ≈100 copies/month. Although the number of patients whose samples were evaluated using digital PCR was small, these findings demonstrate the utility of digital PCR for monitoring BCR-ABL1 levels below the sensitivity threshold of conventional RQ-PCR and support a potential role for digital PCR in providing a more sensitive and dynamic assessment of BCR-ABL1 levels in patients with DMR, including in TFR clinical trials. However, the current method of digital PCR is somewhat handicapped by the limited amount of partitions available for digital testing (up to 10,000 wells). This means, first, that the ABL1 signal always saturates the system and thus cannot be used to standardize the BCR-ABL1 amount, and, second, that both the upper and lower bounds of the dynamic range of the test are restricted. Thus, in the current form, digital PCR using the Fluidigm system is best considered a more sensitive qualitative (or perhaps semiquantitative) method to detect BCR-ABL1 levels below the threshold of conventional BCR-ABL1 testing. Newer methods that expand the number of potential digital assays should ameliorate these restrictions and make digital PCR more quantitative.
Interpretation of results from ENESTnext should be done in the context of understanding the limitations of the study. The relatively short duration of follow-up in this study was a limitation for assessing the rate of DMR as well as duration of response (i.e. most patients who achieved MMR or confirmed MR4.5 did not lose their response on study, and, for these patients, the calculation of duration of response was truncated at the date of last dose of study drug). In ENESTnd, the percentage of nilotinib-treated patients with MR4.5 continued to increase with each year of follow-up [Citation3]; thus, nilotinib treatment beyond 2 years in patients enrolled in ENESTnext could potentially have increased the rates of MR4.5. Furthermore, the high rates of spontaneously regaining MMR or MR4.5 among patients who lost either of these responses suggests that loss of response in some cases may have reflected simple fluctuations in BCR-ABL1 transcript levels rather than a true loss of response; future studies will be needed to further evaluate this possibility. Assessment of digital PCR was limited by the small subset of patients (i.e. those with confirmed MR4.5) who received digital PCR testing on study and a lack of IS standardization. Additionally, although ABL1 is a widely used and accepted control gene for monitoring BCR-ABL1 levels, it is known that its use can result in skewed results when BCR-ABL1 levels are high (such as at diagnosis); this must be taken into consideration when interpreting the results of our landmark analysis [Citation29].
Overall, final results of ENESTnext further substantiate the role of nilotinib in frontline therapy for CML-CP. Early nilotinib therapy is associated with more rapid DMR, which impacts longer-term clinical outcomes, offset by a modest and well-described safety profile. Additionally, deeper molecular responses may enable more patients to be eligible for TFR, especially in light of the addition of TFR to the US Food and Drug Administration–approved prescribing information for nilotinib [Citation30].
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2019.1590569.
Supplemental Appendix
Download MS Word (576.8 KB)Acknowledgments
The authors thank Ghulam Warsi, PhD, formerly of Novartis Pharmaceuticals Corporation, for statistical support of this study, Das Purkayastha, PhD, of Novartis Pharmaceuticals Corporation for statistical review of the manuscript, and Jenna Voutsinas, MPH, of the Fred Hutchinson Cancer Research Center for statistical support on the digital PCR analyses. The authors also thank Joy Loh, PhD, and Jonathan Morgan, PhD, formerly of ArticulateScience LLC for medical editorial assistance. Editorial assistance was funded by Novartis Pharmaceuticals Corporation.
Additional information
Funding
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Chronic Myeloid Leukemia [Internet]. V1. 2019. [cited 2018 Aug 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf.
- Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884.
- Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054.
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333–2340.
- Hehlmann R, Müller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol. 2014;32:415–423.
- Etienne G, Dulucq S, Nicolini FE, et al. Achieving deeper molecular response is associated with a better clinical outcome in chronic myeloid leukemia patients on imatinib frontline therapy. Haematologica. 2014;99:458–464.
- Falchi L, Kantarjian HM, Wang X, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88:1024–1029.
- Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) Study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305.
- Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER Study. Blood. 2013;122:515–522.
- Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–430.
- Lee SE, Choi SY, Song HY, et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101:717–723.
- Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31:1525–1531.
- Mahon FX, Boquimpani C, Kim DW, et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–470.
- Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–854.
- Mahon FX, Baccarani M, Mauro MJ, et al. Treatment-free remission (TFR) following nilotinib (NIL) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP): ENESTfreedom, ENESTop, ENESTgoal, and ENESTpath. J Clin Oncol. 2014;32:Abstract TPS7124.
- Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–757.
- Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2:e528–e535.
- Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23.
- Cross NCP, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular response following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003.
- Goh HG, Lin M, Fukushima T, et al. Sensitive quantitation of minimal residual disease in chronic myeloid leukemia using nanofluidic digital polymerase chain reaction assay. Leuk Lymphoma. 2011;52:896–904.
- Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051.
- Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–851.
- Hochhaus A, Rosti G, Cross NCP, et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia. 2016;30:57–64.
- O'Dwyer ME, Swords R, Nagler A, et al. Nilotinib 300 mg BID as frontline treatment of CML: prospective analysis of the Xpert BCR-ABL monitor system and significance of 3-month molecular response. Leuk Res. 2014;38:310–315.
- Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119:1123–1129.
- Takahashi N, Nishiwaki K, Nakaseko C, et al. Successful treatment free remission in CML after 2 year consolidation with nilotinib of an MR4.5 response level achieved originally with imatinib treatment: first report from STAT2 trial in Japan. Haematologica. 2016;101:Abstract P229.
- Ross DM, Masszi T, Gomez-Casares MT, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–954.
- Mori S, Vagge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90:910–914.
- Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
- Novartis Pharmaceuticals Corporation. Tasigna [package insert]. Novartis Pharmaceuticals Corporation. East Hanover, NJ, July 2018.
