Abstract
The regimen of carfilzomib, daratumumab, and dexamethasone (KdD) shows activity in patients with relapsed/refractory multiple myeloma. KdD at the twice-weekly 56 mg/m2 carfilzomib dose (KdD56) was used in the randomized phase 3 CANDOR study (NCT03158688), whereas KdD at the once-weekly 70 mg/m2 carfilzomib dose (KdD70) was used in the phase 1 b EQUULEUS study (NCT01998971). We analyzed efficacy data from comparable CANDOR and EQUULEUS patients using inverse probability of treatment weighting (IPTW)–adjusted models. These weights were calculated from propensity scores derived to balance prespecified baseline covariates. The side-by-side and adjusted comparisons showed similar efficacy for overall response rates and progression-free survival in the two groups, with a series of sensitivity analyses showing consistent findings. Safety data were generally consistent with the known safety profiles of each individual drug. Once-weekly KdD70 is comparable to twice-weekly KdD56 in terms of efficacy and safety while being a more convenient dosing option.
Introduction
Regimens containing immunomodulatory imide drugs (IMiDs) are standard of care for the treatment of newly diagnosed multiple myeloma [Citation1–5]. With the adoption of lenalidomide maintenance therapy until disease progression, there is a further need for effective and tolerable IMiD-free regimens for patients who progress on lenalidomide. Worse clinical outcomes are seen in patients with relapse with IMiD refractoriness when compared with the overall population [Citation6–9], with relapse due to various mechanisms of drug resistance and alterations in the bone marrow microenvironment [Citation10]. The second-generation proteasome inhibitor (PI) carfilzomib and the anti-CD38 monoclonal antibody daratumumab are potent agents in their respective classes [Citation11–15]. In the phase 3 ENDEAVOR study, carfilzomib plus dexamethasone (Kd) showed superiority over the first-generation PI bortezomib plus dexamethasone (Vd) in relapsed and/or refractory multiple myeloma [Citation11,Citation16]. Likewise, the phase 3 CASTOR study demonstrated improved clinical outcomes for daratumumab with bortezomib and dexamethasone compared with bortezomib and dexamethasone alone [Citation17]. The regimen including carfilzomib and daratumumab is of high interest to clinicians for relapsed and/or refractory multiple myeloma.
The randomized phase 3 CANDOR study demonstrated a statistically significant improvement in progression-free survival (PFS) for carfilzomib, dexamethasone, and daratumumab at the twice-weekly 56 mg/m2 carfilzomib dose (KdD56), with a 37% reduction in the risk of progression or death versus carfilzomib and dexamethasone alone (Kd) [Citation18]. Additionally, patients treated with KdD56 achieved deep responses, with an overall response rate (ORR) of 84% and a nearly 10 times higher minimal residual disease (MRD)–negative complete response (CR) rate compared with Kd-treated patients. Overall, KdD56 was associated with a favorable benefit-risk profile. The promising activity of the KdD regimen is further supported by the non-randomized phase 1 b EQUULEUS study, in which KdD at the once-weekly 70 mg/m2 carfilzomib dose (KdD70) showed tolerability and efficacy, with an ORR of 84% and median PFS of nearly 26 months in patients who were almost all exposed to lenalidomide and were mostly lenalidomide refractory [Citation19].
Previously, the phase 3 A.R.R.O.W. study demonstrated that once-weekly Kd (Kd70) was effective and safe [Citation20]; this regimen achieved regulatory approval for the treatment of relapsed and/or refractory multiple myeloma in the United States and elsewhere. Patient-reported outcomes from the A.R.R.O.W. study showed delayed disease symptom worsening as well as greater convenience and satisfaction with once-weekly Kd70 as compared with twice-weekly Kd27. Improved clinical outcomes for the once-weekly Kd regimen translated into prolonged health-related quality of life in a cost-effective manner [Citation21,Citation22]. Thus, as observed with the Kd regimen, once-weekly carfilzomib dosing with KdD is expected to improve patients’ quality of life by allowing patients to maintain a social role and other meaningful activities, which is key from a patient’s perspective [Citation23].
While we expect that the once-weekly KdD70 regimen would provide patients a more convenient treatment option and thus better adherence, the two regimens of twice-weekly KdD56 and once-weekly KdD70 have not been directly compared in a randomized trial. Therefore, we performed a robust cross-study comparison that demonstrated similar efficacy and safety, which was confirmed with sensitivity analyses.
Methods
Study design
CANDOR is a phase 3, open-label, multicenter, randomized study in patients with relapsed and/or refractory multiple myeloma who received 1–3 prior lines of therapy (NCT03158688). Patients were randomized 2:1 to receive KdD or Kd in 28-day cycles until disease progression (Figure S1A, Table S1). Treatment continued until confirmed disease progression, unacceptable toxicity, consent withdrawal, or death (whichever occurred first), and lasted up to 48 months. The primary endpoint was PFS; secondary endpoints were ORR, MRD-negative CR rate at 12 months, overall survival (OS), time to response, and safety. Data are through July 14, 2019 (primary analysis date).
Figure 1. Overall response rate (ORR) and progression-free survival (PFS) analyses. (A) Unadjusted and adjusted ORR and PFS. The ORR and PFS and associated 95% confidence intervals (CIs) are shown for twice-weekly (BIW) KdD56 and once-weekly (QW) KdD70. Also shown are the odds ratios (ORs; for ORR) and hazard ratios (HRs; for PFS) and associated 95% CIs. Sensitivity analyses for ORR (B) and PFS (C), with corresponding OR for ORR and HR for PFS, and associated 95% CIs are shown for BIW KdD56 and QW KdD70. Progression-free survival (PFS) for unadjusted primary analysis (D) and IPTW-IRC analysis (E).
BIW: twice-weekly; CI: confidence interval; HR: hazard ratio; IRC: Independent Review Committee; IPTW: inverse probability of treatment weighting; KdD: carfilzomib, dexamethasone, and daratumumab; NE: not estimable; QW: once weekly.
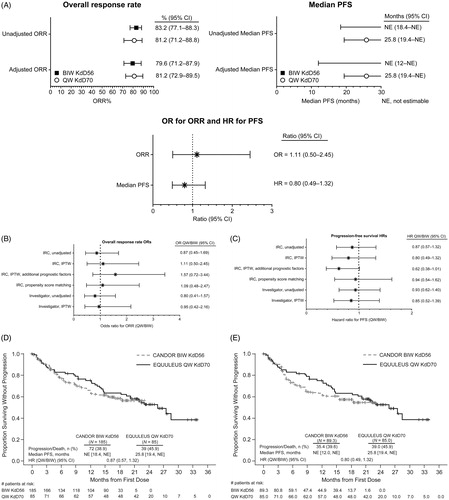
Table 1. Unadjusted baseline demographics and disease characteristics.
EQUULEUS is a phase 1 b, multicenter, open-label, multiarm, non-randomized study of daratumumab in combination with other agents in patients with relapsed and/or refractory multiple myeloma (NCT01998971) (Figure S1B, Table S1). One cohort combined daratumumab with carfilzomib and dexamethasone (i.e. KdD), which is the dataset described here and elsewhere [Citation19]. Patients received 1–3 prior lines of therapy, including bortezomib and an IMiD. Treatment continued until confirmed disease progression, unacceptable toxicity, withdrawal of consent, death, or end of study, which was approximately 25 months after the last patient received the first dose of daratumumab or when 38 PFS events were reached. The primary endpoints were safety and tolerability. Secondary endpoints included ORR and OS; PFS was an exploratory endpoint. Data are through January 31, 2019 (final analysis date for this cohort). In the original report, the number of patients evaluable for response per study criteria was 82 of 85; all 85 patients are included in our analyses.
Statistical analysis
This cross-study comparison was a prespecified meta-analysis exploring the similarity between the efficacy and safety outcomes for patients treated with the triplet combination KdD in two different studies. The efficacy data were analyzed both unadjusted and with propensity score adjustment, while safety data were summarized in side-by-side tables.
In order to evaluate efficacy in comparable populations, as all patients from the EQUULEUS KdD cohort were required to have prior therapy with bortezomib and an IMiD, similar patients (i.e. those with prior bortezomib and IMiD exposure) were selected from the CANDOR KdD arm; 185 of the 312 patients in the CANDOR study receiving KdD met this requirement. All these patients had at least one study treatment dose and were included in the safety summaries. The efficacy endpoints were derived based on the response and disease progression from both studies assessed post hoc by the same blinded Independent Review Committee (IRC). The PFS endpoint was analyzed using Cox models, while the Kaplan-Meier methodology was used to summarize its distribution, including the point estimates and 95% confidence intervals (CIs) for medians [Citation24]. ORRs were calculated as the rate of responders who achieved a partial response (PR) or better and associated 95% CIs were estimated with the Clopper-Pearson method. Odds ratios (ORs) were evaluated with logistic models, while 95% CIs were constructed with robust variance estimates.
For efficacy comparisons, results were adjusted with inverse probability of treatment weighting (IPTW). The weights were calculated using the propensity score that a patient received once-weekly KdD70 by balancing the baseline covariates that had been preselected as prognostic for multiple myeloma. Selection of these covariates was independent of prior knowledge of efficacy outcomes and was based on subject matter expert guidance and literature review. The covariates included baseline demographics (age), disease characteristics (prior treatment exposure/refractoriness and time from initial diagnosis to relapse), and other characteristics (creatinine clearance, Eastern Cooperative Oncology Group [ECOG] performance status), many of which have been evaluated in previous carfilzomib analyses [Citation25]. We did not include all components of the revised International Staging System (R-ISS) as data were not available for all patients for fluorescence in situ hybridization (FISH) and as β2 microglobulin data were not collected in the EQUULEUS study.
The appropriateness of the model was assessed by boxplots and absolute standardized difference, evaluating the balance reached between the two groups after weighting adjustment. Propensity score matching was also used as a sensitivity analysis by sequentially matching patients based on greedy nearest neighbor matching of the propensity scores derived for the IPTW method. Propensity score matching creates mutually exclusive sets of observations that have similar propensity scores. Greedy nearest neighbor matching was used to sequentially match each patient in the KdD70 cohort with one patient in the KdD56 external control group if the difference in the logits of the propensity score for pairs of patients from the two groups was less than or equal to 0.1 times the pooled estimate of the standard deviation [Citation26,Citation27]. Efficacy endpoints derived based on unblinded evaluations by the investigators were included as a sensitivity analysis. In addition, PFS and ORR were analyzed within prespecified subgroups, including by the number of prior regimens, prior transplant, refractoriness to PIs or IMiDs, age, ECOG performance status, and baseline creatinine clearance. The hazard ratios (HRs; with 95% CIs) for PFS and ORs (with 95% CIs) for ORR between treatment groups within each subgroup were estimated using the same methodology as for the overall analysis.
Results
Demographic and baseline characteristics
Baseline demographic and disease characteristics were generally comparable between the two groups in this analysis: twice-weekly KdD56 CANDOR arm (i.e. CANDOR subset with prior bortezomib and IMiD exposure) and once-weekly KdD70 EQUULEUS cohort. However, some baseline characteristics were imbalanced across the studies (). Specifically, in the KdD70 cohort, more patients had a higher ECOG performance status, at least two prior treatments, and prior exposure to lenalidomide, and disease duration was longer than in the KdD56 arm. Following IPTW adjustment, baseline and disease characteristics considered prognostic were balanced for the two groups ().
Table 2. Baseline demographics and disease characteristics of adjusted BIW KdD56 and QW KdD70 groups after using propensity score.
At the time of this analysis, 48% of CANDOR patients and 41% of EQUULEUS patients were continuing treatment. Of patients who discontinued treatment, the most common reasons for discontinuation were disease progression (CANDOR: 27%, EQUULEUS: 42%), adverse events (AEs, 10%, 6%), and per patient request (7%, 7%) ().
Efficacy
In a side-by-side comparison, after a median follow-up of 16.8 months, median PFS for the KdD56 group was not reached (not estimable [NE]; 95% CI, 18.4–NE), whereas for the KdD70 cohort, median PFS was 25.8 months (95% CI, 19.4–NE, median follow-up of 23.5 months) (, ). PFS event-free rates at 12 months were 64.9% (95% CI: 57.3%–71.4%) for KdD56 and 75.2% (95% CI: 64.2%–83.2%) for KdD70 (graph in ). ORR was 83.2% (95% CI, 77.1%–88.3%) for KdD56 and 81.2% (95% CI, 71.2%–88.8%) for KdD70. After IPTW adjustment, the 95% CI for the HR for PFS and the OR for ORR included 1, showing similar efficacy for the KdD56 and KdD70 groups (median PFS, NE and 25.8 months, respectively [HR, 0.80; 95% CI, 0.49–1.32]; ORR, 79.6% and 81.2%, respectively [OR, 1.11; 95% CI, 0.50–2.45]).
Table 3. Unadjusted and adjusted ORR and PFS.
Additional sensitivity analyses were performed to support the robustness of the main comparison results. One sensitivity analysis for efficacy results adjusted for IPTW using an extended list of covariates, specifically laboratory values for albumin, hemoglobin, lactate dehydrogenase (LDH), and platelet count, and the presence of plasmacytoma, in addition to the covariates included in the main analysis, yielded consistent results. Efficacy results adjusted by propensity score matching showed similar results as well. In another sensitivity analysis, the adjusted and unadjusted comparisons performed on investigator-assessed ORR and PFS yielded similar findings to those performed on IRC-assessed ORR and PFS (, Tables SIII, SIV). Prespecified subgroup analyses (e.g. number of prior regimens, prior transplant, refractoriness to PIs or IMiDs, age, ECOG performance status, baseline creatinine levels) were consistent with the results from the main analysis for PFS and ORR outcomes, including in the lenalidomide-refractory subgroup and by prior transplant status ().
Table 4. Efficacy results for selected subgroups.
Exposure and safety
The median duration of carfilzomib treatment was shorter for the twice-weekly KdD56 group (54.3 weeks) than for the once-weekly KdD70 cohort (66.0 weeks) and the median relative dose intensity was similar for both groups at approximately 90%. Safety analyses were performed side-by-side for all patients with no adjustment. All-grade treatment-emergent adverse events (TEAEs) occurred in 100% of patients in both groups (). Over the course of the studies (i.e. not duration-adjusted), the unadjusted frequency of grade ≥3 TEAEs was similar in the KdD56 and KdD70 groups (84.3% and 82.4%, respectively), while the incidence of serious (58.9% and 48.2%) and fatal (10.8% and 3.5%) TEAEs was higher in the KdD56 group. For fatal AEs, of the 20 in the KdD56 group, 10 were due to infections (five septic shock, four pneumonia, one respiratory tract infection), whereas for the three fatal AEs in the KdD70 cohort, two were due to general physical health and one due to multiple organ dysfunction. The proportions of patients with AEs leading to carfilzomib discontinuation (21.1% for KdD56 and 18.8% for KdD70) and daratumumab discontinuation (8.1% and 8.2%) were comparable between the two treatment groups. For AEs of interest, the rates of grade ≥3 AEs in the KdD56 and KdD70 groups, respectively, were 1.1% and 2.4% for cardiac failure, 0.5% and 3.5% for acute renal failure, 18.4% and 20.0% for hypertension, and 2.7% and 0.0% for respiratory tract infections. For the category of grade ≥3 infections/infestations, the rate was higher with KdD56 than with KdD70 (36.8% and 21.2%, respectively). These same general trends were seen in exposure-adjusted rates of AEs; however, conclusions are limited as there were relatively few events for several AEs of interest in both studies.
Table 5. Safety.
Discussion
The KdD regimen is recommended by the National Comprehensive Cancer Network (NCCN 2020, version 3) guidelines for patients with previously treated multiple myeloma [Citation4]. As weekly carfilzomib dosing can be more convenient and improve the quality of life [Citation21,Citation22], we undertook rigorous efficacy analyses in this cross-study comparison to support the comparability of twice-weekly KdD56 to once-weekly KdD70. We found that the corresponding groups of the CANDOR and EQUULEUS studies showed similar efficacy in terms of ORR and PFS, both before and after IPTW adjustment, including in patients who were refractory to lenalidomide and independent of past stem cell transplant status. We also examined safety in side-by-side unadjusted analyses and found that the safety profiles of KdD56 and KdD70 were generally consistent with the known safety profiles of the individual agents. The proportions of patients with grade ≥3 AEs were similar in the KdD56 and KdD70 groups and the incidences of grade ≥3 cardiac AEs, renal failure, hypertension, and respiratory tract infections did not differ appreciably. However, there were more grade ≥3 infectious AEs and fatal infections with KdD56 than with KdD70. Although the benefit-risk profile was similar for both regimens, the once-weekly carfilzomib regimen may be more convenient for patients and healthcare providers. The more convenient regimen may improve adherence to the on-label carfilzomib dosing and frequency, and thus optimize carfilzomib dose intensity and outcomes in the real-world setting, as suboptimal carfilzomib dose intensity has been shown to be associated with poorer outcomes [Citation28].
Direct comparisons across studies can be confounded by differences in study design, including eligibility criteria that result in patient populations with different characteristics, such as prior treatment history, leading to biased estimates of treatment effects [Citation29–33]. To account for this, the propensity score method was used to balance prespecified baseline covariates selected to be prognostic for multiple myeloma, with the goals of controlling for potential confounding factors of cross-trial comparison and minimizing selection bias. The IPTW/propensity score approach is a well-established method intended to mirror the effects of randomization [Citation34,Citation35]. Specifically, adjustment for selection bias was done by weighting the data based on the propensity score that a patient received once-weekly KdD70. As IPTW is based on covariates selected as prognostic for multiple myeloma outcomes, the methodology was applied to efficacy data, but not to safety events. Other studies evaluating treatment outcomes among patients with hematologic malignancies have also used propensity score analysis to compare two distinct study populations and achieve balance in baseline factors [Citation36,Citation37]. Besides possible confounding due to the nature of cross-study comparisons, another limitation of this analysis was that baseline covariates in this analysis did not include R-ISS as β2 microglobulin data were not collected in the EQUULEUS study and as FISH data were not available for many patients in both studies. However, LDH and albumin levels, two components of R-ISS, were used as additional variables, along with platelet count, hemoglobin, and plasmacytoma presence, in a sensitivity analysis. In this sensitivity analysis, there appears to be a larger effect size associated with KdD70 versus KdD56 for both ORR and PFS. This sensitivity analysis may have reduced residual confounding; however, the 95% CIs still included 1.
Previously, once-weekly carfilzomib dosing with Kd70 was shown to have improved patients’ quality of life with improved clinical outcomes relative to twice-weekly carfilzomib dosing [Citation21–23]. In line with these results for the once-weekly Kd70 doublet regimen, we expect that the once-weekly KdD70 triplet regimen will provide patients a more convenient lenalidomide-free treatment option, thus fostering adherence to the treatment schedule, dose, and treatment duration. Future analyses could confirm whether a weekly regimen is associated with better adherence and improved responses and survival. In addition, in the era of COVID-19, guidelines have recommended switching patients from twice-weekly to weekly carfilzomib at an appropriate dose [Citation38,Citation39], thus decreasing patients’ potential exposure to coronavirus as well as enabling adherence and reducing strain on the healthcare system during a time when non-emergent visits to healthcare facilities should be limited. As the recently approved subcutaneous daratumumab [Citation40] becomes more widely available, the treatment option of weekly KdD may result in further improvements in patient satisfaction, since use of subcutaneous daratumumab has been associated with higher patient satisfaction versus intravenous daratumumab [Citation41], as would be expected with reduced infusion chair time and decreased infusion-related reactions.
In conclusion, this cross-study analysis showed that once-weekly KdD70 in the EQUULEUS study is comparable to the twice-weekly KdD56 regimen used in the pivotal phase 3 CANDOR trial in terms of efficacy and safety. The weekly KdD70 dosing option addresses an important need for patients by providing a more convenient lenalidomide-free regimen that may encourage adherence and potentially lead to improved outcomes for patients.
Author contributions
Monica Khurana, Mihaela Obreja, and Jianqi Zhang contributed to the conception and design of the analyses. All authors had a role in collection of data, analyzing and interpreting data, and reviewing the manuscript.
GLAL-2020-1052-File007.docx
Download MS Word (112.2 KB)Acknowledgments
Amgen conducted the CANDOR study; Janssen conducted the EQUULEUS study. Amgen funded the analyses for this publication. Medical writing assistance was provided by Susanna Mac and Jacqueline Sayyah, both of Amgen.
Disclosure statement
Xavier Leleu reports honoraria from Janssen, Celgene, Amgen, Takeda, Merck, GlaxoSmithKline, Sanofi, CARsgen, AbbVie, Incyte, and Novartis.
Meral Beksac reports consulting/advising for Celgene, Takeda, Amgen, Sanofi, and Janssen, and being a speaker for Celgene, Amgen, Sanofi, and Janssen.
Takaaki Chou reports receiving honoraria from Janssen, Takeda, Celgene, and Ono, and consulting for Takeda and Ono.
Meletios Dimopoulos reports consulting fees, lecture fees, and honoraria from Janssen, Amgen, Celgene, and Takeda; research funding from Janssen, Amgen, Takeda, and Genesis Pharma; and consulting fees from Bristol-Myers Squibb.
Sung-Soo Yoon reports consulting/advising for Janssen, Takeda, Amgen, Celgene, and Novartis, and receiving research funding from Kyowa Kirin and Roche-Genentech.
Henry Miles Prince reports honoraria from Amgen, Novartis, and Takeda; consulting for Takeda; and research funding from Allergan.
Ludek Pour and Tatiana Shelekhova report no disclosures.
Ajai Chari reports research funding from Amgen, Celgene, Janssen, Millennium/Takeda, Novartis, Pharmacyclics, and Seattle Genetics; has served in an advisory role for Amgen, Celgene, Janssen, Karyopharm, Novartis, Oncopeptides, Sanofi, and Seattle Genetics; and consulting fees from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Millennium/Takeda, and Novartis.
Monica Khurana, Jianqi Zhang, and Mihaela Obreja are employees and stockholders of Amgen.
Ming Qi reports being an employee and stockholder of Janssen R&D LLC.
Albert Oriol reports consulting fees from Celgene and Janssen, and has been a speaker for Celgene and Amgen.
David Siegel reports honoraria and speakers’ bureau fees from Amgen, Celgene, Takeda, Novartis, Bristol-Myers Squibb, and Janssen, and honoraria from Karyopharm.
References
- Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131(3):301–310.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J Clin Oncol. 2017;35(29):3279–3289.
- Moreau P, San Miguel J, Sonneveld P, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv52–iv61.
- NCCN. NCCN clinical practice guidelines in oncology (NCCN guidelines®): multiple myeloma version 3.2020; 2020 [cited 2020 Mar 3]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf.
- Pulte ED, Dmytrijuk A, Nie L, et al. FDA approval summary: lenalidomide as maintenance therapy after autologous stem cell transplant in newly diagnosed multiple myeloma. Oncologist. 2018;23(6):734–739.
- Moreau P, Zamagni E, Mateos M-V. Treatment of patients with multiple myeloma progressing on frontline-therapy with lenalidomide. Blood Cancer J. 2019;9(4):38
- Cornell RF, Kassim AA. Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity. Bone Marrow Transplant. 2016;51(4):479–491.
- Nijhof IS, van de Donk NWCJ, Zweegman S, et al. Current and new therapeutic strategies for relapsed and refractory multiple myeloma: an update. Drugs. 2018;78(1):19–37.
- Sonneveld P, De Wit E, Moreau P. How have evolutions in strategies for the treatment of relapsed/refractory multiple myeloma translated into improved outcomes for patients? Crit Rev Oncol Hematol. 2017;112:153–170.
- Nass J, Efferth T. Drug targets and resistance mechanisms in multiple myeloma. Cancer Drug Resist. 2018;1:87–117.
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152.
- Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621–1634.
- Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(10):1327–1337.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766.
- Dimopoulos M, Quach H, Mateos M-V, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186–197.
- Chari A, Martinez-Lopez J, Mateos M-V, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019;134(5):421–431.
- Moreau P, Mateos M-V, Berenson JR, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.). Interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–964.
- Moreau P, Kumar S, Boccia R, et al. Convenience, satisfaction, health-related quality of life of once-weekly 70 mg/m2 vs. twice-weekly 27 mg/m2 carfilzomib (randomized A.R.R.O.W. study). Leukemia. 2019;33(12):2934–2946.
- Kumar SK, Majer I, Panjabi S, et al. Cost-effectiveness of once weekly carfilzomib 70 mg/m2 plus dexamethasone in patients with relapsed and refractory multiple myeloma in the United States. Expert Rev Hematol. 2020;13(6):687–696.
- Mortenson GL, Salomo M. Quality of life in patients with multiple myeloma: a qualitative study. J Cancer Sci Ther. 2016;8:289–293.
- Klein J, Moeschberger M. Survival analysis: techniques for censored and truncated data. New York, NY: Springer; 1997.
- Dimopoulos MA, Niesvizky R, Weisel K, et al. Once- versus twice-weekly carfilzomib in relapsed and refractory multiple myeloma by select patient characteristics: phase 3 A.R.R.O.W. study subgroup analysis. Blood Cancer J. 2020;10(3):35.
- Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849.
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38.
- Rifkin RM, et al. Carfilzomib dosing patterns and time to next treatment among adult patients with multiple myeloma treated in the US community oncology setting. Blood. 2017;130(Supplement 1):3433.
- Laubach JP, Faber EA, Voorhees P, et al. The challenge of cross-trial comparisons using limited data. Haematologica. 2014;99(8):e145–e146.
- Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109
- Greipp PR, San Miguel J, Durie BGM, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420.
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128.
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Cin Oncol. 2015;33(26):2863–2869.
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679.
- Kumar S, Durie B, Nahi H, et al. Propensity score matching analysis to evaluate the comparative effectiveness of daratumumab versus real-world standard of care therapies for patients with heavily pretreated and refractory multiple myeloma. Leuk Lymphoma. 2019;60(1):163–171.
- Rambaldi A, Ribera J-M, Kantarjian HM, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. 2020;126(2):304–310.
- Malard F, Mohty M. Management of patients with multiple myeloma during the COVID-19 pandemic. Lancet Haematol. 2020;7(6):e435–e437.
- ESMO. European Society for Medical Oncology management and treatment adapted recommendations in the COVID-19 era: Multiple myeloma; 2020 [cited 2020 May 14]. Available from: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/haematological-malignancies-multiple-myeloma-in-the-covid-19-era.
- Genmab. Genmab announces U.S. FDA approval of subcutaneous formulation of daratumumab, DARZALEX FASPRO™ (daratumumab and hyaluronidase-fihj), for the treatment of patients with multiple myeloma; 2020. Available from: https://ir.genmab.com/news-releases/news-release-details/genmab-announces-us-fda-approval-subcutaneous-formulation.
- Moench S. Subcutaneous daratumumab associated with higher patient satisfaction, drastically fewer infusion reactions than intravenous formulations; 2019. Available from: https://www.cancertherapyadvisor.com/home/news/conference-coverage/american-society-of-clinical-oncology-asco/asco-2019/multiple-myeloma-subcutaneous-daratumumab-higher-patient-satisfaction-risk/.
