Abstract
Navitoclax, a novel BCL-2 and BCL-XL inhibitor, demonstrated promising antitumor activity in the dose-escalation part of a phase 1/2a study (NCT00406809) in lymphoid tumors. Herein, we report the continued safety and efficacy results of the phase 2a portion. Twenty-six adult patients with relapsed/refractory follicular lymphoma (n = 11, Arm A) and other relapsed/refractory lymphoid malignancies (n = 15, Arm B) were enrolled. Navitoclax administration schedule consisted of a 150-mg 7-day lead-in dose followed by 250-mg daily dosing with the option to further increase to 325 mg after 14 days if the 250-mg dose was tolerated. All patients experienced at least 1 treatment-related adverse event (TRAE). Seventeen (65.4%) patients reported grade 3/4 TRAEs; thrombocytopenia (38.5%) and neutropenia (30.8%) were the most common. Two patients reported serious AEs; none were fatal (no deaths occurred within 30 days of last dose of study drug). The objective response rate (complete and partial) was 23.1% (6/26; Arm A: 9.1%, Arm B: 33.3%). Median progression-free survival and time to progression were identical: 4.9 months (95% CI: 3.0, 8.2); median overall survival: 24.8 months (95% CI could not be computed). Navitoclax monotherapy has an acceptable safety profile and meaningful clinical activity in a minority of patients with relapsed/refractory lymphoid malignancies.
Introduction
Apoptosis is determined by prosurvival proteins and proapoptosis proteins of the B-cell lymphoma 2 (BCL-2) family. The antiapoptotic proteins of the BCL-2 family such as BCL-2 and BCL-XL prevent the activation of proapoptotic proteins BAX/BAK that are necessary to initiate the mitochondrial apoptosis pathway, subsequently leading to enhanced cancer cell survival [Citation1–4]. Overexpression of antiapoptotic BCL-2 family members frequently occurs in lymphoid malignancies via a variety of mechanisms [Citation5–8] and is associated with chemotherapy resistance and disease progression [Citation9–12]. Several lymphoid malignancies such as chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) are highly dependent on BCL-2 family members for cancer cell survival [Citation10,Citation13,Citation14]. Therefore, targeting antiapoptotic BCL-2 family proteins represents a rational therapeutic approach in patients with lymphoid malignancies.
Navitoclax is an orally bioavailable, novel small-molecule inhibitor that targets and binds with high affinity (Ki ≤1 nM) to multiple antiapoptotic BCL-2 family proteins including BCL-XL, BCL-2, BCL-w, and BCL-B, with the exceptions of MCL-1 or BFL-1, resulting in cancer cell death via apoptosis [Citation15]. Preclinical studies have demonstrated potent cytotoxic activity (half-maximal effective concentration ≤1 μM) of navitoclax in human tumor cell lines derived from T- and B-cell lymphoid malignancies that overexpress BCL-2, and showed rapid and complete tumor regression in xenograft models, with durable responses lasting for several weeks in some models [Citation15]. Clinical antitumor activity of navitoclax has been observed in lymphoid malignancies believed to be dependent on BCL-2 for survival [Citation16,Citation17].
The phase 1 results of this study in 55 patients with lymphoid malignancies have been previously reported [Citation16]. With the intermittent 14/21-day dosing schedule, the dose-limiting toxicities (DLTs) were hospital admissions for bronchitis (n = 1) and pleural effusion (n = 1), grade 3 increase in aminotransferases (n = 1), grade 4 thrombocytopenia (n = 1), and grade 3 cardiac arrhythmia (n = 1). Clinical responses were seen across the range of doses and histologies. Ten of 46 patients with assessable disease had a partial response (PR), with median progression-free survival (PFS) of 15.0 months. A 150-mg 7-day lead-in dose followed by a 325-mg dose administered on a continuous 21/21-day dosing schedule was selected for the phase 2a study. Herein, we report the continued safety and efficacy results of the phase 2a portion of this study.
Patients and methods
Patients
Patients were enrolled across multiple sites between 01 November 2006 and 25 October 2010. Patients (≥18 years of age) were eligible if they met the following criteria: relapsed/refractory follicular lymphoma (FL; Arm A) or mantle cell lymphoma, peripheral T-cell lymphoma, cutaneous T-cell lymphoma including mycosis fungoides or Sézary syndrome, and other indolent B-cell lymphomas such as marginal zone lymphoma (Arm B); measurable disease per International Working Group (IWG) criteria (patients with CLL and/or small lymphocytic lymphoma [SLL] had measurable disease by the 1996 National Cancer Institute Working Group [NCI-WG] criteria with computed tomography imaging); Eastern Cooperative Oncology Group (ECOG) performance status ≤1; adequate coagulation, hepatic, and renal function, and adequate bone marrow function (absolute neutrophil count [ANC] ≥ 1000/μL; platelets ≥100,000/mm3 [entry platelet count independent of transfusion within 14 days of screening]; hemoglobin ≥9.0 g/dL) independent of any growth factor support (except for patients with heavily infiltrated bone marrow [80% or more], who could use growth factor support to achieve ANC eligibility criteria). Patients who had a history of autologous stem cell transplant had to be >6 months post-transplant with adequate bone marrow independent of growth factor support at screening (ANC ≥1500/μL; platelets ≥125,000/mm3 [entry platelet count independent of transfusion within 14 days of screening]; hemoglobin ≥10.0 g/dL). Patients were excluded if they had a history of or were clinically suspicious for central nervous system disease (lymphoid or nonlymphoid) or had undergone an allogeneic stem cell transplantation.
Study design
The trial was registered with ClinicalTrials.gov registry (NCT00406809) and was approved by an independent ethics committee/institutional review board prior to initiation. The study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki; written informed consent was obtained from all patients prior to any study-related procedures.
The objectives of this open-label study were to assess the safety and efficacy of navitoclax in the phase 2a portion of the phase 1/2a trial. The data cutoff was May 2018.
The navitoclax recommended phase 2 dose and schedule determined in phase 1 was used [Citation16].
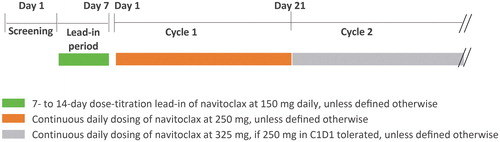
The previous intermittent 14/21-day schedule had been found to be associated with fluctuations in platelet counts, and therefore navitoclax was administered orally on a continuous dosing schedule with an intermediate step-up dosing regimen. A 7-day lead-in navitoclax dose-titration period was implemented to mitigate the severity of navitoclax-induced thrombocytopenia. Patients started navitoclax dosing at 150 mg orally daily on lead-in days 1 through 7, and proceeded to 250 mg daily on cycle 1, day 1 if the pre-dose platelet count was 50,000/mm3 (50 × 109/L) and was stable or increasing. If the 250-mg dose level was tolerated through cycle 1, the dose was titrated to the 325-mg dose level at the discretion of the investigator in consultation with the sponsor. A cycle was defined as 21 days. The study schematic is shown in .
Safety and efficacy assessments
Safety evaluations were performed throughout the study and included adverse event (AE) monitoring, physical examination, and laboratory assessments including complete blood counts.
Efficacy endpoints included tumor response, PFS, time to tumor progression (TTP), overall survival (OS), and duration of overall response (DOR). Tumor responses were determined using IWG criteria [Citation18] and NCI-WG criteria [Citation19].
Statistical analyses
Descriptive statistics were computed for categoric variables and used to summarize numeric variables. The number and percentage of patients experiencing treatment-emergent AEs (TEAEs) were tabulated by Medical Dictionary for Regulatory Activities system organ class and preferred term. TEAEs were graded according to NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
The proportion of patients with a complete response (CR), unconfirmed CR (CRu), or PR on the basis of IWG criteria was estimated and the corresponding 95% CI for the proportion was calculated using the exact binomial distribution. The distribution of PFS, TTP, OS, and DOR was estimated using the Kaplan–Meier method; median time and the corresponding 95% CI were estimated for each endpoint.
The sample size was primarily based on clinical convention for a safety expansion, and designed to provide a certain degree of precision around the estimate for the true PR rate; the sample size of 20 patients provides 90% confidence that the true PR rate at 3 months is within ∼20% of the observed PR rate.
Results
Patient characteristics and study drug exposure
Twenty-six patients were enrolled in the phase 2a portion of the phase 1/2a study: 11 patients in Arm A had FL and 15 patients in Arm B had CLL (n = 7), SLL (n = 2), lymphoplasmacytic lymphoma (n = 2), mantle cell lymphoma (n = 2), low-grade B-cell lymphoma (n = 1), or marginal zone B-cell lymphoma (n = 1). Key patient demographics and clinical characteristics are shown in . The median age was 62 years (range, 42–77) in Arm A and 62 years (range, 44–86) in Arm B. Demographics were generally comparable to the previously reported phase 1 portion of the study [Citation16]. The median and mean time on treatment was 2.9 and 9.7 months (range, 0.4–71.8); in Arm A, 2.1 and 7.4 months (range, 1.2–52.8); and in Arm B, 4.9 and 11.4 months (range, 0.4–71.8). The mean dose of navitoclax administered during the first year of treatment ranged from 263 mg to 325 mg.
Table 1. Patient demographics and baseline characteristics.
Safety
All patients have discontinued navitoclax (Supplemental Table S1). The most common reasons for discontinuation were AEs and radiologic progressive disease (n = 10 [38.5% each]).
All-grade TEAEs and serious TEAEs that led to navitoclax dose interruption, delayed dosing, dose reduction, or discontinuation are summarized in . AEs leading to navitoclax discontinuation were reported for 2 (18.2%) patients in Arm A and 8 (53.3%) patients in Arm B.
Table 2. Summary of treatment-emergent adverse events related to navitoclax.
TEAEs related to navitoclax are summarized in . All patients experienced at least 1 navitoclax-related TEAE, mainly of low-grade gastrointestinal or hematologic type; most common were diarrhea (88.5%), nausea (61.5%), and thrombocytopenia (53.8%). Seventeen patients (65.4%) experienced grade 3/4 AEs related to navitoclax, with thrombocytopenia (38.5%) and neutropenia (30.8%) being most common. Serious related AEs were reported in 2 patients (bacterial sepsis and increased white blood cell count [n = 1 each]); none were fatal. Overall, no deaths occurred within 30 days of the last dose of study drug.
Hematologic AEs of NCI CTCAE grade 3/4 during the study recovered to grade 2 or better by the final visit, with the exception of 3 patients (11.5%) who did not have recovery of grade 4 thrombocytopenia to grade 2 or better.
Efficacy
The objective response rate (ORR) was 23.1% (95% CI: 9.0, 43.6) in all treated patients: 9.1% (95% CI: 0.2, 41.3) in Arm A, and 33.3% (95% CI: 11.8, 61.6) in Arm B (). One patient with FL achieved a CR (Supplemental Figure S1). Five patients achieved a PR: 3/7 patients with CLL, 1/2 patients with lymphoplasmacytic lymphoma, and 1/1 patients with low-grade B-cell lymphoma (). Best percentage change in tumor size from baseline in all patients is illustrated in Supplemental Figure S2.
Table 3. Tumor response rate.
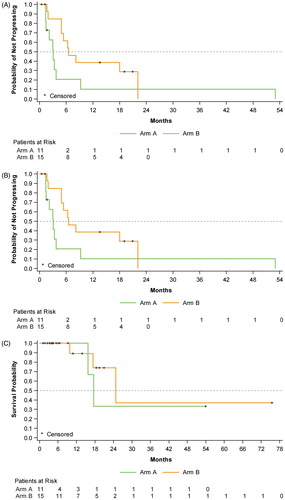
The median PFS and TTP were identical at 4.9 months (95% CI: 3.0, 8.2). The median PFS and TTP for Arm A was 3.1 months (95% CI: 1.4, 3.7), and 6.5 months (95% CI: 4.9, 22.1) for Arm B. In Arm B, 5 patients were observed to have a PFS duration beyond 1 year. Two of those patients eventually had events: 1 CLL patient had PD at month 18 and 1 low-grade B-cell lymphoma patient had PD at month 22. The other 3 patients (CLL, n = 1; lymphoplasmacytic lymphoma, n = 2) concluded the study prior to PD or death. The median OS was 24.8 months (95% CI could not be computed): 17.6 months (95% CI could not be computed) for Arm A and 24.8 months (95% CI could not be computed) for Arm B (). The median DOR was 20.5 months (95% CI: 4.9, 50.7) on the basis of 6 responders, 4 of whom experienced progressive disease or death events; median DOR was 50.7 months for Arm A and 20.5 months for Arm B.
Discussion
These results confirm the preliminary efficacy initially observed during the phase 1b portion of the study and provide extended follow-up with continuing acceptable toxicity data for navitoclax in patients with relapsed/refractory CLL and NHL. Navitoclax was well tolerated for up to 6 years of continuous dosing, one of the longest continuous exposures studied of BCL-2 family inhibitors to date. The most common related AEs were diarrhea, nausea, and thrombocytopenia. Treatment-related lymphopenia and thrombocytopenia were on-target BCL-2 and BCL-XL effects, respectively. The DLT of navitoclax was thrombocytopenia [Citation16,Citation17] due to inhibition of BCL-XL, the key prosurvival protein sustaining circulating platelet survival [Citation20,Citation21]. We again observed grade 3 and 4 neutropenia in 30.8% of patients, responsive to dose interruptions and growth factor support, a side effect possibly due to an on-target effect on myeloid progenitors [Citation16,Citation22].
Navitoclax had meaningful clinical activity in patients with relapsed/refractory indolent NHL and CLL/SLL. The ORR was 23.1%, in line with the ORR of 21.7% in the phase 1 trial (Supplemental Table S2) [Citation16], where all patients who responded had PRs [Citation16]. In this phase 2a part of the trial, 1 patient in Arm A achieved a CR (FL) and 5 patients in Arm B had PRs, including 3 patients with CLL, 1 with lymphoplasmacytic lymphoma, and 1 with indolent NHL. These response rates were mainly driven by CLL patients (42.9% ORR), consistent with phase 1 study results of navitoclax in patients with relapsed/refractory CLL demonstrating an ORR of 35% [Citation17]. Comparable response (38% ORR) was also reported in a multicenter international phase 2 study of navitoclax in patients with relapsed/refractory CLL [Citation23].
New effective therapies with favorable safety profiles are needed to improve outcomes for patients with relapsed/refractory lymphoid malignancies. Contrary to the dominant dependency of CLL cells on BCL-2 for survival, pro- and antiapoptotic proteins are expressed at varying levels in other lymphoid malignancies. It is not surprising that single agents with a limited on-target profile demonstrate varying responses in these histologies. Therefore, navitoclax combinations with potentially synergistic agents hold promise to improve the response rates when targeting NHL. Preclinical models have demonstrated synergistic activity of navitoclax with gemcitabine, vincristine, docetaxel, and bendamustine plus rituximab [Citation15,Citation24–26]. The clinical activity of navitoclax in a chemotherapy-free setting in combination with rituximab was investigated in patients with relapsed or refractory CD20-positive lymphoid malignancies and patients with previously untreated B-cell CLL, demonstrating good tolerability and promising synergistic antitumor activity [Citation27,Citation28]. Navitoclax was also tested in combination with bendamustine and rituximab in patients with relapsed diffuse large B-cell lymphoma as part of the NAVIGATE study, but recruitment was terminated in 2011 due to non–safety-related reasons (NCT01423539).
Due to the BCL-XL–mediated DLT of thrombocytopenia, a next-generation highly potent, orally bioavailable, BCL-XL–sparing and BCL-2–selective inhibitor, venetoclax, was developed [Citation29]. On the basis of significant activity as monotherapy in CLL, the US Food and Drug Administration (FDA) approved venetoclax for CLL with 17p deletion (April 2016) [Citation30], venetoclax plus rituximab for previously treated CLL (June 2018) [Citation31], and as combination therapy with obinutuzumab for treatment-naive CLL (May 2019) [Citation32]. Further, venetoclax received accelerated approval in combination with either azacitidine, decitabine, or low-dose cytarabine by the FDA for newly diagnosed acute myeloid leukemia patients ineligible for intensive induction chemotherapy (November 2018) [Citation33].
In a venetoclax phase 1 monotherapy study, the reported ORR varied across the subtypes of NHL, with an overall ORR of 44% (mantle cell lymphoma, 75%; FL, 38%; diffuse large B-cell lymphoma, 18%) [Citation34]. The lower-than-expected response rate in FL was surprising and exemplified the molecular complexity of this malignancy. Therefore, combination therapy and better understanding of mechanisms of primary and secondary resistance to BCL-2 inhibition are required to improve response rates and durations in lymphoma. Venetoclax has been safely combined with multiagent chemotherapy such as bendamustine-rituximab [Citation35], rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP; CAVALLI trial) [Citation36], or rituximab-etoposide-prednisone-vincristine-cyclophosphamide-doxorubicin (R-EPOCH; NCT03036904).
The role of navitoclax combinations in inducing cancer cell apoptosis is an area of active investigation. Multiple clinical trials using navitoclax are underway, including a study of navitoclax in combination with ruxolitinib for patients with myelofibrosis, and in combination with venetoclax and multiagent chemotherapy for patients with relapsed/refractory acute lymphocytic leukemia (ALL) and lymphoblastic lymphoma. In the pediatric and adult study of ALL evaluating weight-adjusted low doses of navitoclax (25 to 100 mg) with venetoclax and optional low-dose chemotherapy, preliminary data in 9 patients demonstrated 5 CRs and 1 PR, with a tolerable safety profile [Citation37]. Of interest is the observation that optimized dosing levels of navitoclax may provide efficacy without severe thrombocytopenia.
In a preclinical study, the potential role of navitoclax to counteract the upregulation of BCL-XL as a mechanism of resistance in mantle cell lymphoma to treatment with venetoclax (BCL-2–specific inhibitor) was demonstrated. Functional modeling in mantle cell lymphoma cell lines demonstrated that increased BCL-XL expression provides a dominant escape mechanism from BCL-2–specific inhibitors, and combination therapy with BCL-2 and BCL-XL inhibitors was able to overcome this resistance [Citation38]. While navitoclax may have a role in combination with venetoclax, neither drug targets MCL-1, a potential venetoclax-resistance mediator. However, MCL-1 may be targeted with specific MCL-1 inhibitors, phosphoinositide 3-kinase inhibitors, or CDK9 inhibitors that downregulate MCL-1 expression [Citation39]. BCL-2 homology domain 3 profiling [Citation40] may improve our ability to better understand the antiapoptotic makeup of lymphoid malignancies and to select the appropriate targeting agent in the future. Navitoclax could possibly have a role as an effective tool in such a personalized effort targeting the antiapoptotic defense of lymphoid and other hematologic malignancies.
GLAL-2020-0219-File006.docx
Download MS Word (1.8 MB)Acknowledgements
Medical writing support was provided by Mary L. Smith, PhD, CMPP, of Aptitude Health, Atlanta, GA, funded by AbbVie. This manuscript is original and not under consideration for publication elsewhere. The phase 1 portion of this study has been previously published in Wilson et al. [Citation16]. Results of this trial have partially been presented at the 16th Congress of the European Hematology Association (EHA).
Disclosure statement
Sven de Vos is in the advisory boards for Incyte, Bayer, and Genentech. John P. Leonard is a consultant for Sutro, Bayer, Gilead, AstraZeneca, Celgene, Roche/Genentech, ADC Therapeutics, Sandoz, Karyopharm, Miltenyi, Epizyme, MEI Pharma, BMS, Regeneron, Genmab, AbbVie, Incyte. Jonathan W. Friedberg is involved in the data and safety monitoring committee activities: Bayer and Ascerta; travel support from Roche. Jasmine Zain reports no conflict of interest. Kieron Dunleavy holds consultant/advisory roles for AbbVie, Adaptive, Amgen, Celgene, Janssen, Seattle Genetics, Pharmacyclics, Kite. John Hayslip and John Pesko are AbbVie employees and may own stock. Rod Humerickhouse is a former employee of AbbVie and may hold AbbVie stock or options. Wyndham Wilson reports no conflict of interest.
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g. protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Additional information
Funding
References
- Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–1399.
- Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26(1):61–66.
- Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730.
- Degenhardt K, Sundararajan R, Lindsten T, et al. Bax and Bak independently promote cytochrome c release from mitochondria. J Biol Chem. 2002;277(16):14127–14134.
- Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228(4706):1440–1443.
- Pettersson M, Jernberg-Wiklund H, Larsson LG, et al. Expression of the bcl-2 gene in human multiple myeloma cell lines and normal plasma cells. Blood. 1992;79(2):495–502.
- Marschitz I, Tinhofer I, Hittmair A, et al. Analysis of Bcl-2 protein expression in chronic lymphocytic leukemia. A comparison of three semiquantitation techniques. Am J Clin Pathol. 2000;113(2):219–229.
- Agarwal B, Naresh KN. Bcl-2 family of proteins in indolent B-cell non-Hodgkin's lymphoma: study of 116 cases. Am J Hematol. 2002;70(4):278–282.
- Minn AJ, Rudin CM, Boise LH, et al. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86(5):1903–1910.
- Robertson LE, Plunkett W, McConnell K, et al. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. 1996;10:456–459.
- Amundson SA, Myers TG, Scudiero D, et al. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60(21):6101–6110.
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337.
- Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365.
- Ghielmini M. Follicular lymphoma. Ann Oncol. 2010;21:vii151–vii153.
- Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428.
- Wilson WH, O'Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–1159.
- Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–496.
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244.
- Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997.
- Zhang H, Nimmer PM, Tahir SK, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14(5):943–951.
- Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128(6):1173–1186.
- Chipuk JE, Fisher JC, Dillon CP, et al. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci U S A. 2008;105(51):20327–20332.
- Seymour F, Roberts A, Carney D, et al. Phase-II study of navitoclax (ABT-263) safety and efficacy in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): interim results. Haematologica. 2011;96(suppl 2:227.
- Ackler S, Mitten MJ, Foster K, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66(5):869–880.
- Chen J, Jin S, Abraham V, et al. The Bcl-2/Bcl-X(L)/Bcl-w inhibitor, navitoclax, enhances the activity of chemotherapeutic agents in vitro and in vivo. Mol Cancer Ther. 2011;10(12):2340–2349.
- Ackler S, Mitten MJ, Chen J, et al. Navitoclax (ABT-263) and bendamustine ± rituximab induce enhanced killing of non-Hodgkin’s lymphoma tumours in vivo. Br J Pharmacol. 2012;167(4):881–891.
- Kipps TJ, Eradat H, Grosicki S, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56(10):2826–2833.
- Roberts AW, Advani RH, Kahl BS, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170(5):669–678.
- Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208.
- U.S. Food and Drug Administration. News & Events. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. 2016. [cited 2019 Mar 28]. Available from: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm.
- Genentech. Genentech announces FDA approval for Venclexta plus Rituxan for people with previously treated chronic lymphocytic leukemia. 2018. [cited 2019 Mar 28]. Available from: https://www.gene.com/media/press-releases/14728/2018-06-08/genentech-announces-fda-approval-for-ven.
- U.S. Food and Drug Administration. Drugs. FDA approves venetoclax for CLL and SLL. 2019. [cited 2019 Nov 11]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll.
- U.S. Food and Drug Administration. Drugs. FDA approves venetoclax in combination for AML in adults. 2018. [cited 2019 Mar 28]. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626499.htm.
- Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–833.
- de Vos S, Swinnen LJ, Wang D, et al. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol. 2018;29(9):1932–1938.
- Morschhauser F, Feugier P, Flinn IW, et al. Venetoclax plus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) improves outcomes in BCL2-positive first-line diffuse large B-cell lymphoma (DLBCL): First safety, efficacy and biomarker analyses from the phase II CAVALLI study. Blood. 2018;132(Supplement 1):782–782.
- Alexander T, Lacayo NJ, Pullarkat VA, et al. Venetoclax and navitoclax in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Blood. 2018;132(Supplement 1):3966–3966.
- Agarwal R, Chan Y-C, Tam CS, et al. Dynamic molecular monitoring reveals that SWI-SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat Med. 2019;25(1):119–129.
- Choudhary GS, Al-Harbi S, Mazumder S, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6:e1593.
- Del Gaizo Moore V, Letai A. BH3 profiling–measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013;332(2):202–205.