Abstract
The advent of novel B-cell receptor pathway targeting agents like ibrutinib dramatically changed management of B-cell malignancies. However, with concomitant anticoagulation (AC) and antiplatelet (AP) therapy, ibrutinib is associated with increased bleeding. This post hoc analysis aimed to determine the role of AC/AP therapy in patients with idelalisib-treated B-cell malignancies and to establish if it contributes to increased bleeding events. Data from two idelalisib trials (rituximab ± idelalisib in chronic lymphocytic leukemia [CLL] and idelalisib monotherapy in indolent non-Hodgkin lymphoma [iNHL]) were analyzed. Antithrombotic therapy was common (36%–63%), with comparable bleeding incidence across treatment groups (14%–19%; p = 0.56). Bleeding events of grade ≥3 occurred in 0.9% and 3.2% of the idelalisib-treated CLL and iNHL cohorts, respectively. Our findings demonstrate no increase in bleeding events with simultaneous AC/AP treatment and idelalisib use. Hemorrhagic risk is prevalent in these patients and an important consideration when evaluating available treatment options.
ClinicalTrials.gov identifiers: NCT01539512 and NCT01282424
Introduction
During the last decade, the treatment of B-cell malignancies, mainly chronic lymphocytic leukemia (CLL) and indolent non-Hodgkin lymphoma (iNHL), was revolutionized with the clinical development of targeted therapies. Several agents targeting the B-cell receptor signaling pathway have been approved for first-line or later-line use in the treatment of patients with CLL and iNHL. Though these agents are highly efficacious and well-tolerated, selecting the optimal therapeutic strategy remains a challenge when dealing with a patient whose comorbid condition may preclude the use of a particular agent. The Bruton’s tyrosine kinase inhibitor, ibrutinib, is routinely selected for management of both CLL and iNHL. Compared to the registrational trials, ibrutinib use in real-world studies has been associated with an increased risk of bleeding (up to a 20-fold increased risk of major bleeding) in patients taking concomitant anticoagulation and antiplatelet therapy [Citation1], with a median time to ibrutinib treatment discontinuation of 8 months due to bleeding events [Citation2]. The UK ibrutinib real-world study of 315 patients reported that 44% of the patients had a dose reduction, treatment interruption, or complete cessation in the first 12 months compared with the 4% reported in the registration trial [Citation3]. Although the development of this agent addresses the unmet need for effective therapy for the majority of patients with CLL requiring treatment, long-term exposure may not be an option for a select group of patients.
Both CLL and iNHL have a median age of onset of ≥65 years [Citation4,Citation5]. At diagnosis, up to 89% of these patients have comorbidities [Citation4,Citation6], the most prevalent being cardiovascular comorbidities [Citation4,Citation6–9]. Incidences of hypertension, hyperlipidemia, coronary artery disease, and atrial fibrillation (AF) in elderly patients with CLL are estimated to be 53%, 38%, 24%, and 20%, respectively [Citation8]. Older, less-fit patients with CLL or iNHL may have an increased thromboembolic risk due to cardiovascular comorbidities [Citation10] leading to the use of antiplatelet and/or anticoagulant therapy for thromboprophylaxis or management of thromboembolism [Citation11–13]. Serious hemorrhage may become a concern in these patients given the following risk factors: baseline thrombocytopenia from bone marrow infiltration, poor marrow reserve from the use of a prior myelosuppressive regimen, and the possibility of coexisting autoimmune cytopenias [Citation14,Citation15].
Idelalisib, a first-in-class, oral phosphatidylinositol 3-kinase δ (PI3Kδ) inhibitor [Citation16], selectively targets B lymphocytes with minimal direct toxicity to other hematopoietic cell types, including platelets [Citation17,Citation18]. Unlike targeting other PI3K isoforms, PI3Kδ inhibition does not affect platelet aggregation or signaling [Citation17]. However, we wanted to understand if concomitant antiplatelet and/or anticoagulant therapy use in patients with a diagnosis of CLL or iNHL in idelalisib registrational trials lead to higher rates of bleeding. Therefore, a post hoc analysis of the two idelalisib registrational trials was conducted to characterize the use of concomitant anticoagulant and/or antiplatelet therapy and bleeding events. Patients were treated with idelalisib monotherapy or in combination with rituximab in the setting of relapsed/refractory iNHL or CLL, respectively, [Citation19,Citation20]. The use of anticoagulant and/or antiplatelet agents or the presence of baseline bleeding diathesis or severe thrombocytopenia were not considered exclusions for participation in the trials. Participants in these studies included patients with a high comorbidity burden. The goals of this study were to: (1) determine the degree of comorbidity burden and the impact of idelalisib on bleeding events and (2) describe the concomitant use of antithrombotic therapy and bleeding incidence across treatment groups, and characterize the timing and severity of bleeding events in order to understand the potential for hemorrhage after idelalisib exposure.
Methods
The analysis included data from 345 patients participating in the two registrational trials of idelalisib. These studies were selected for analysis to permit consistency with the approved indications of idelalisib for CLL and follicular lymphoma (FL) and to minimize the risk of adding confounders from earlier trials that explored the use of idelalisib with different dosages or with other combination strategies. Study 312-116 (NCT01539512) was a phase 3, randomized, double-blind, placebo-controlled trial conducted at 90 sites in the United States and Europe that enrolled patients (N = 220) with relapsed/refractory CLL who were poor candidates to receive cytotoxic agents [Citation19]. Study 101-09 (NCT01282424) was a phase 2 single-group, open-label study conducted at 41 sites in the United States and Europe (N = 125) [Citation20]. Patients ≥18 years of age with a confirmed diagnosis of iNHL (i.e. FL, small lymphocytic lymphoma [SLL], lymphoplasmacytic lymphoma with/without Waldenström [WM], and marginal zone lymphoma [MZL]) treated with ≥2 prior systemic therapies and refractory to both rituximab and an alkylating agent, absolute neutrophil count ≥1.0 × 109/L, and platelet count ≥50 × 109/L were eligible for the phase 2 study [Citation20]. Additional details regarding eligibility criteria are included in the Supplementary Materials.
Patients with CLL were randomized to receive a combination of idelalisib 150 mg twice daily (BID) or placebo plus 8 doses of rituximab; following unblinding, patients in the placebo arm had the option to receive idelalisib. In the iNHL trial, all patients received idelalisib 150 mg BID. All idelalisib-treated patients received study drug until disease progression or unacceptable toxicity, with the option for dose reductions to 100 or 75 mg BID in the CLL trial [Citation19,Citation20]. Concomitant anticoagulant and/or antiplatelet therapy, including warfarin use, was permitted in both trials. Data cutoffs were 11 June 2014 (iNHL), and 15 October 2014 (CLL), which reflect the final data cuts available for both registrational trials.
Anticoagulant/antiplatelet medications were identified using World Health Organization drug coding (World Health Organization Anatomical Therapeutic Chemical antithrombotic agents and drugs containing acetylsalicylic acid). Comorbidities at randomization were determined based on patient medical history and coded using the Medical Dictionary for Regulatory Activities version 17.0 (MedDRA 17.0). For the CLL study, medical history terms were identified for comorbid conditions associated with increased bleeding risk. All bleeding events occurring during the studies were recorded and classified by investigators according to MedDRA 17.0 preferred terms and graded using Common Terminology Criteria for Adverse Events version 4.0.3 (CLL study) and 3.0 (iNHL study). Adverse events and concomitant medications were assessed through 30 days after the last exposure.
For the CLL study, baseline characteristics were analyzed in the intent-to-treat population consisting of all randomized patients; all other analyses were performed in patients who received ≥1 dose of study medication. Comorbidity analyses were performed in patient subgroups based on the presence/absence of treatment-emergent bleeding events. Descriptive statistics are reported. Given the small sample sizes and the post hoc nature of this analysis, no comparative statistical analyses were performed.
Results
Patients
A total of 345 patients (CLL, n = 220; iNHL, n = 125) were enrolled in the two trials. Median (range) duration of therapeutic exposure was 8.1 (0.3–19.5), 4.6 (0.1–14.6), and 6.6 (0.6–35.4) months in the idelalisib + rituximab, placebo + rituximab, and idelalisib monotherapy groups, respectively. Demographics and baseline disease characteristics in patients with CLL and iNHL are presented in Supplemental Tables 1 and 2, respectively.
Baseline cytopenias were common in both studies (). In the CLL study, baseline median × 109/L platelet (range) levels were 108 (3-284) for patients receiving idelalisib + rituximab and 104 (4-439) for patients receiving placebo + rituximab, and, in the iNHL study, were 178 (15-535) for patients receiving idelalisib monotherapy.
Table 1. Baseline hemogram data in the CLL and iNHL trials.
Comorbidities
In the CLL patients, the most common comorbidities were hypertension (52%), chronic kidney disease (34%), hyperlipidemia (34%), osteoarthritis (20%), and diabetes (19%). In the iNHL patients, the most common were hypertension (35%), coronary artery disease (5%), cardiac arrhythmia (4% not AF; 3% AF), and venous thromboembolism/pulmonary embolism (3%). Analysis of comorbidities and their relationship to the presence/absence of treatment-emergent bleeding events showed no distinct pattern (data not shown) across the three treatment groups.
Anticoagulant/antiplatelet therapy
Concomitant anticoagulant/antiplatelet use at any time on study was frequent in all treatment groups (idelalisib + rituximab, 63%; placebo + rituximab, 36%; idelalisib monotherapy, 47%; ). The most commonly used medications were aspirin, enoxaparin, heparin/heparinoids, and warfarin. When aspirin was excluded from the analysis, the use of anticoagulant/antiplatelet therapy was reduced to 36%, 21%, and 34% in the idelalisib + rituximab, placebo + rituximab, and idelalisib monotherapy groups, respectively. Summary data reflecting duration of anticoagulant/antiplatelet therapy were not available.
Table 2. Anticoagulant and antiplatelet therapya in the CLLb and iNHLc trials.
Bleeding events
In the idelalisib + rituximab group, the median (range) duration of follow-up for safety assessment was 8.9 (0.9–20.5) and 9.4 (2.9–17.4) months for patients without or with treatment-emergent bleeding events, respectively; in the placebo + rituximab group, it was 5.6 (0.2–15.5) and 4.7 (0.4–13.2) months for patients without or with bleeding events, respectively; and in the idelalisib monotherapy group, it was 7.4 (1.5–35.4) months for patients without and 11.8 (1.2–26.1) months for patients with bleeding events.
Overall, proportions of patients experiencing bleeding events () were similar across the treatment groups. The exposure-adjusted incidence of bleeding events was 7.26/10,000 person-days (95% confidence interval [CI], 4.43–11.21) in the idelalisib + rituximab treatment group, 12.05/10,000 person-days (95% CI, 7.46–18.42) in the placebo + rituximab group, and 4.49/10,000 person days (95% CI, 2.66–7.10) in the monotherapy group. The majority of bleeding events occurred within the first 3 months of study treatment (). Median (range) times to onset of first bleeding event (any grade) were 37 (9–423), 29 (1–137), and 189 (7–519) days in the idelalisib + rituximab, placebo + rituximab, and idelalisib monotherapy groups, respectively.
Figure 1. Incidence of bleeding events (A) overall and by grade 1/2 or ≥3 and (B) by 3-month increments after initiation of study treatment. CLL: chronic lymphocytic leukemia; iNHL: indolent non-Hodgkin lymphoma. *Percentage values based on original patient sample size.
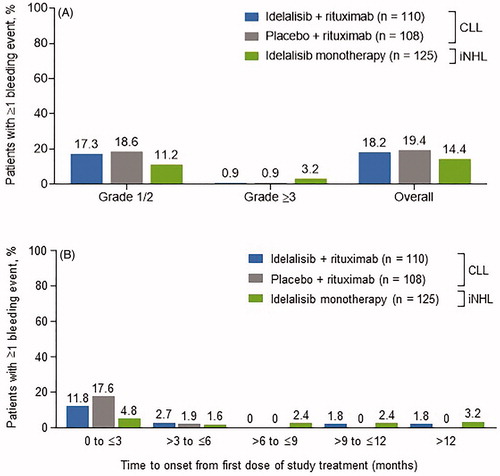
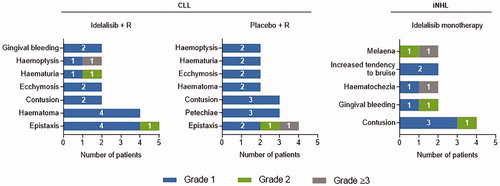
Of 59 patients with bleeding events, 40 (68%), 13 (22%), and 6 (10%) experienced highest grade 1, highest grade 2, and grade ≥3 events, respectively. No intracranial or intraspinal hemorrhage was reported. The distribution of patients by grade was similar for the idelalisib + rituximab and placebo + rituximab groups; most patients experienced grade 1 events (idelalisib + rituximab, 75%; placebo + rituximab, 86%). In patients receiving idelalisib monotherapy, the distribution trended toward a more balanced profile across grades. Most frequently reported grade 1 or 2 bleeding events included epistaxis and contusion (). Across all treatment groups, only a single patient who was receiving idelalisib + rituximab as well as anticoagulant and antiplatelet therapy discontinued due to a bleeding event of grade 4 hemoptysis (coughing up blood).
Figure 2. Most frequent bleeding events* by type and severity. CLL: chronic lymphocytic leukemia; iNHL: indolent non-Hodgkin lymphoma; R: rituximab. *Bleeding events reported in >1 patient shown.
Among patients receiving anticoagulant/antiplatelet therapy, the incidence of bleeding events was similar across treatment groups, irrespective of whether aspirin was excluded or if anticoagulant/antiplatelet use occurred within 30 days of the bleeding event (). Among idelalisib-treated patients (idelalisib + rituximab or idelalisib monotherapy), fewer patients experienced bleeding events while receiving antiplatelet therapy than while receiving anticoagulant therapy (). As detailed in , of the 30 patients receiving concomitant warfarin, seven experienced bleeding events while on trial; all were grade 1 with the exception of one case of grade 2 observed in the iNHL cohort.
Figure 3. Incidence of bleeding events (A) in patients receiving anticoagulant and/or antiplatelet therapy, (B) while on anticoagulant therapy alone or antiplatelet therapy alone, and (C) while on warfarin therapy*. CLL: chronic lymphocytic leukemia; iNHL: indolent non-Hodgkin lymphoma. *Within 30 days of the event.
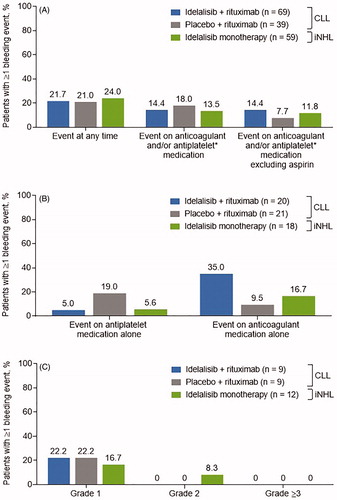
When comparing the incidence of bleeding events between groups which did or did not receive anticoagulant/antiplatelet treatment, the proportion of patients experiencing bleeding events was similar (18.8%–24.0%) across treatment arms and conditions, with one exception: Lack of concomitant administration of anticoagulant/antiplatelet therapy correlated with fewer bleeding events (idelalisib + rituximab, with vs without anticoagulant/antiplatelet treatment: 21.7% vs 12.2%; idelalisib monotherapy: 24.0% vs 6.1%). Twice as many patients who experienced treatment-emergent bleeding events (n = 15/59 [25.4%]) as patients who did not (n = 36/284 [12.7%]) had grade ≥3 thrombocytopenia at baseline. Platelet counts remained stable or improved while on treatment with idelalisib, and improvements tended to be more substantial in patients with baseline thrombocytopenia below 100 K/μL compared with patients with platelet counts above 100 K/μL (Supplemental Figure 1A and 1B).
Severe (grade ≥3) bleeding events occurred in 1 patient (0.9%) treated with idelalisib + rituximab (hemoptysis), 1 (0.9%) treated with placebo + rituximab (epistaxis), and 4 (3.2%) treated with idelalisib monotherapy (melena, hematochezia, hematuria, and upper gastrointestinal hemorrhage; each n = 1). The patient treated with idelalisib + rituximab who experienced hemoptysis was receiving both anticoagulant and antiplatelet therapy. The patient treated with placebo + rituximab had grade 4 thrombocytopenia both at baseline and at the time of the event and received 1 day of anticoagulant therapy 7 days before the bleeding event. Of the 4 patients receiving idelalisib monotherapy that sustained a severe bleeding event, 2 were taking antiplatelet therapy and the other 2 were not receiving any anticoagulant or antiplatelet therapy.
Discussion
The development of highly effective therapies that target dysregulated pathways in CLL and iNHL has transformed the treatment landscape with several novel oral agents recently approved and adopted into routine clinical practice [Citation21,Citation22]. Although highly effective and well-tolerated treatment options for relapsed or refractory CLL and iNHL are available, certain risk factors and comorbidities may preclude the use of specific therapies. Longer follow-up on these novel agents demonstrates new challenges including the emergence of tolerability issues depending on the patient’s comorbidities and the selected drug. BTK inhibitor use carries the risk for increased bleeding events [Citation1], which could lead to treatment discontinuation. Our goal was to study and describe the rate of bleeding for patients treated with idelalisib in the setting of antiplatelet medications and/or anticoagulant use and with baseline thrombocytopenia. These novel agents can put the patients at risk for bleeding, and the underlying malignancy can also predispose to adverse effects that may require the initiation of antiplatelet/anticoagulant therapy. Thus, there is the need to carefully evaluate baseline bleeding risk, comorbidities, and concurrent medications, particularly in patients with hematologic malignancies who have a prior medical history of a bleeding disorder or who require the use of anticoagulant/antiplatelet agents. To our knowledge, no prior data on bleeding events has been reported for any drug targeting the PI3Kδ signaling pathway.
In this post hoc analysis of idelalisib registrational trials, we evaluated whether idelalisib use impacts the risk of bleeding events in patients at high risk for bleeding (due to comorbidities or baseline thrombocytopenia) and described the impact of the concomitant use of antiplatelet and anticoagulant agents in idelalisib-treated patients. The focus of this analysis was to explore the quality, timing, and outcomes of bleeding events in patients treated on idelalisib registrational trials. Overall, we found a similar low grade of severity of bleeding events related to the use of anticoagulant/antiplatelet agents in patients with iNHL and CLL, as well as in patients treated with idelalisib (monotherapy or in combination with the monoclonal antibody rituximab) when compared with rituximab alone.
Cardiovascular comorbidities were prevalent in patients participating in the idelalisib trials (85% and 41% in the CLL and iNHL cohorts, respectively), which appeared to be similar to that seen in the real-world setting for these types of patients [Citation23], resulting in a considerable rate of anticoagulant/antiplatelet use amongst trial participants. The majority of bleeding events were grade ≤2, with no reports of central nervous system hemorrhage. These events occurred predominantly during the first 3 months on trial, with similar frequency in patients treated with idelalisib + rituximab, placebo + rituximab, and idelalisib monotherapy, irrespective of concomitant antiplatelet/anticoagulant therapy including warfarin, suggesting the possibility that the bleeding was more a result from baseline thrombocytopenia rather than drug exposure. Warfarin use was not an exclusion criterion to participate in any of the idelalisib clinical trials. Data regarding dose intensity and therapeutic ranges of the anticoagulant/antiplatelet agents at the time of the bleeding events were not collected; thus, any contribution of supratherapeutic levels at the time of the events to the severity of the events is not known.
Concomitant use of anticoagulant/antiplatelet drugs in these studies was comparable to that reported for studies with ibrutinib. An integrated analysis of incidence and risk factors for major hemorrhage in 15 ibrutinib clinical studies revealed common anticoagulant/antiplatelet use in approximately 50% of patients [Citation24]. In the phase3 RESONATE trial of ibrutinib in patients with previously-treated CLL/SLL (NCT01578707), of four ibrutinib-treated patients who experienced major hemorrhage, two used concomitant anticoagulant/antiplatelet medications [Citation25]. Anticoagulant/antiplatelet use in the current analysis was also comparable to clinical practice use in similarly age-matched patients with CLL and iNHL in a prescription and medical claims database analysis in which an estimated 15% of patients with CLL and 14% with iNHL were taking anticoagulant/antiplatelet medications (excluding aspirin) in 2014 [Citation11]. Since our study was not randomized based on use of anticoagulant/antiplatelet agents, there is a higher incidence of concomitant anticoagulant/antiplatelet use in the idelalisib + rituximab cohort compared with the placebo + rituximab cohort, without apparent clinical consequence. The overall incidence of bleeding events was similar in these two groups. The exposure-adjusted incidence rate of bleeding events was actually lower in the idelalisib group than in the rituximab-treated group and lowest in the iNHL patients treated with idelalisib monotherapy. Interestingly, the iNHL cohort had a longer median time to bleeding event (∼6 months compared to ∼1 month). Moreover, the frequency of events did not increase with idelalisib exposure despite differences in the disease biology and clinical behavior of patients with a CLL vs iNHL diagnosis. This may be a consequence of improvement of the thrombocytopenia in the idelalisib treatment arms, particularly in patients with severe baseline thrombocytopenia.
There are limitations to this study, and these data should be interpreted with caution. These clinical trials were not designed to assess bleeding events with idelalisib. In particular, hemorrhage was not identified as a toxicity of special interest, so under-reporting of bleeding events by the investigator may have occurred. Due to the post hoc nature of this analysis and the small patient numbers, substantive statistical comparisons between treatment groups were not feasible. Additionally, the follow-up time was relatively short, and longer studies may be needed to elucidate whether prolonged idelalisib exposure affects the outcomes. Despite these limitations, the analyses were warranted, as there is a paucity of data on bleeding events with the use of PI3Kδ inhibitors. A major limitation to the present study is the known toxicity profile of idelalisib [Citation19,Citation20], which may have reduced overall dose intensity and adherence based on the need to withhold the drug to permit toxicity resolution. However, the exposure-adjusted bleeding rates would normalize for this potential bias. On the other hand, a drug-related toxicity such as colitis could further increase an inherent risk to the development of gastrointestinal bleeding.
Importantly, the incidence of bleeding events may also have been confounded by patients’ baseline thrombocytopenic status. Twice as many patients with grade ≥3 thrombocytopenia at baseline experienced bleeding events compared to those that did not have severe thrombocytopenia. We found that platelet counts remained stable or improved while on treatment with idelalisib, with more pronounced improvements in patients with baseline thrombocytopenia <100 K/μL. Our findings suggest that idelalisib use did not increase bleeding events even in the presence of antiplatelet/anticoagulant use; rather, bleeding events were associated with underlying disease states (i.e. thrombocytopenia from bone marrow infiltration or myelosuppression from recent cytotoxic chemotherapy) or the concomitant use of warfarin.
To conclude, this study shows no difference in the frequency of bleeding events related to the use of anticoagulant and/or antiplatelet agents in patients with CLL and iNHL treated with idelalisib. Although idelalisib is well known to cause other serious or even fatal toxicities (colitis, pneumonitis, transaminitis, and infections), our data show no increase in bleeding events in patients with relapsed/refractory CLL or iNHL treated with idelalisib. The modest rate of bleeding events in patients treated with idelalisib (± rituximab) was similar when compared to rituximab monotherapy use. Although the data presented do not change the known risk-benefit profile for idelalisib use, it may be informative when considering next-line therapy for patients with relapsed/refractory CLL or iNHL at high risk for major bleeding events.
Author contributions
JCB designed the analysis, analyzed and interpreted data, and wrote the manuscript; PH, GS, JS, SS, PG, and JMP provided patients, collected data, and critically reviewed the manuscript at each draft; OG, GX, and SS designed the analysis, analyzed and interpreted data, and critically reviewed the manuscript at each draft. BR interpreted data, wrote and edited sections of the manuscript, and critically reviewed the manuscript. PB interpreted data and critically reviewed and edited the manuscript. All authors approved the manuscript for submission.
GLAL-2020-1306-File007.docx
Download MS Word (157.3 KB)Acknowledgments
We thank the patients who participated in these studies, their family members, the investigators, and the clinical research staff from the study centers. Medical writing and editorial assistance for the development of this manuscript was provided by C4 MedSolutions, LLC (Yardley, PA), a CHC Group company; Percolation Communications LLC (Annandale, NJ); and AlphaScientia (San Francisco, CA).
Disclosure statement
Jacqueline C. Barrientos: Institutional research funding from AbbVie and Acerta; advisory board consulting from AbbVie, Acerta, Genentech, Gilead Sciences, and Sandoz; and medical education honoraria from Janssen.
Peter Hillmen: Honoraria and institutional research funding from AbbVie, Gilead Sciences, Inc, Janssen, Novartis, Pharmacyclics, and Roche.
Gilles Salles: Research funding from Roche/Genentech; consultant or advisory role with Celgene, Gilead Sciences, Inc, Janssen Pharmaceuticals, Novartis, Amgen, AbbVie, Autolus, Epizyme, Karyopharm, Morphosys, Servier, Takeda, Genmab, and Roche/Genentech; honoraria from Amgen and Roche/Genentech; reimbursement of travel, accommodations, and expenses from Roche Genentech.
Jeff Sharman: Research funding from AbbVie, AstraZeneca, Genentech, Gilead Sciences, Inc, Pharmacyclics, TG Therapeutics; and consulting fees from AbbVie, AstraZeneca, Bayer, Genentech, Pharmacyclics, TG Therapeutics, and Verastem.
Stephan Stilgenbauer: Honoraria; consulting or advisory role; research funding; and travel, accommodations, and expenses from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Genentech, Genzyme, Gilead Sciences, Inc, GlaxoSmithKline, Janssen, Mundipharma, Novartis, Pharmacyclics, Roche, and Sanofi.
Paolo Ghia: Consulting or advisory role for AbbVie, Adaptive Biotechnologies, ArQule, BeiGene, Dynamo, Gilead Sciences, Inc, Janssen, MEI, Pharmacyclics, Roche, and Sunesis; speaker’s bureau for Gilead Sciences, Inc; and research funding from AbbVie, Celgene/Juno, Gilead Sciences, Inc, Janssen, and Novartis.
John M. Pagel: Institutional research funding and advisory board consulting from Pharmacyclics and Gilead Sciences, Inc.
Oksana Gurtovaya, Guan Xing, Bianca Ruzicka, Sanatan Shreay, and Pankaj Bhargava are employees of Gilead Sciences, Inc. (Foster City, CA, USA)
Additional information
Funding
References
- Mock J, Kunk PR, Palkimas S, et al. Risk of major bleeding with ibrutinib. Clin Lymphoma Myeloma Leuk. 2018;18(11):755–761.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879.
- Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–1572.
- Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49(1):49–56.
- Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105(11):1684–1692.
- Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (>/=80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012;156(2):196–204.
- Goede V, Cramer P, Busch R, on behalf of the German CLL Study Group, et al. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica. 2014;99(6):1095–1100.
- Satram-Hoang S, Reyes C, Hoang KQ, et al. The unmet need in chronic lymphoctytic leukemia: impact of comorbidity burden on treatment patterns and outcomes in elderly patients. JCT. 2013;04(08):1321–1329.
- Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80(7):1273–1283.
- Robert-Ebadi H, Le Gal G, Righini M. Use of anticoagulants in elderly patients: practical recommendations. Clin Interv Aging. 2009;4:165–177.
- IMS LifeLink. IMS oncology prescription and medical claims database (IMS Health Incorporated, University of Arkansas for Medical Sciences, all rights reserved).
- Jones JA, Hillmen P, Coutre S, et al. Pattern of use of anticoagulation and/or antiplatelet agents in patients with chronic lymphocytic leukemia (CLL) treated with single-agent ibrutinib therapy [abstract]. Blood. 2014;124(21):1990–1990.
- Jones JA, Hillmen P, Coutre S, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017;178(2):286–291.
- Hodgson K, Ferrer G, Montserrat E, et al. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96(5):752–761.
- Zelenetz AD, Gordon LI, Wierda WG, et al H. Non-Hodgkin's lymphomas, version 2.2014. J Natl Compr Canc Netw. 2014;12(6):916–946. Available from: http://williams.medicine.wisc.edu/nhl.pdf
- Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594.
- Eisenreich A, Rauch U. PI3K inhibitors in cardiovascular disease. Cardiovasc Ther. 2011;29(1):29–36.
- Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088.
- Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
- Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas version 1.2020. [cited 2020 Jun 20]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma version 4.2020. [cited 2020 Jun 20]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- Barrientos JC, Meyer N, Song X, et al. Characterization of atrial fibrillation and bleeding risk factors in patients with chronic lymphocytic leukemia (CLL): a population-based retrospective cohort study of administrative medical claims data in the United States (US). Blood. 2015;126(23):3301–3301.
- Brown JR, Moslehi J, Ewer MS, et al. Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: an integrated analysis. Br J Haematol. 2019; 184(4):558–569.
- Barrientos JC, O’Brien S, Brown JR, et al. Improvement in parameters of hematologic and immunologic function and patient well-being in the Phase III RESONATE study of ibrutinib versus ofatumumab in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Lymphoma Myeloma Leuk. 2018; 18(12):803–813 e7.
