Abstract
The single-arm, multicenter, phase 2 GIBB study (NCT02320487) investigated bendamustine plus obinutuzumab (BG) in previously untreated CLL. Patients (N = 102) received six cycles of intravenous obinutuzumab (cycle [C] 1: 100 mg day 1/900 mg day 2, and 1000 mg days 8/15; C2–6 1000 mg day 1) plus bendamustine (C1 90 mg/m2 days 2/3; C2–6 days 1/2). Complete response (CR), the primary endpoint, was 50%, overall response 89%. Estimated 2-year progression-free survival (PFS) and overall survival (OS) were 86% and 97%, respectively. Following initial minimal residual disease (MRD) negativity, median MRD negativity duration was 28.9 months. Undetectable MRD (<10−4) was observed in up to 83% of evaluable patients in peripheral blood (any time) and 59% in bone marrow at response evaluation. Most common grade 3/4 adverse events (AEs) were neutropenia (25%; 5% febrile) and infusion-related reactions (9%). BG proved clinically active in CLL with high response, MRD negativity, and survival rates, consistent with other first-line studies of anti-CD20 antibody/bendamustine combinations.
Introduction
Chronic lymphocytic leukemia (CLL) is the most frequently occurring leukemia in the western world, accounting for 34% of all adult leukemias in the United States [Citation1]. Standard first-line treatment for patients with CLL has evolved to include chemoimmunotherapy, Bruton tyrosine kinase (BTK) inhibitors, and BCL-2 inhibitor venetoclax, with the goals of prolonging progression-free survival (PFS) and eradicating minimal residual disease (MRD) [Citation2–6]. Upon relapse, CLL becomes more difficult to treat, and for some patients, chemoimmunotherapy regimens remain an effective, fixed-treatment duration option.
The combination of bendamustine plus rituximab (BR) was initially established in previously untreated CLL patients based on findings from the German CLL study group’s single-arm, phase 2 study, which showed high efficacy and manageable safety with BR [Citation7]. These results paved the way for subsequent expanded study and widespread adoption of the BR regimen for the treatment of CLL. Soon after, obinutuzumab (G), a glycoengineered anti-CD20 monoclonal antibody, was approved for the CLL treatment [Citation8,Citation9]. The phase 3b GREEN trial was designed to characterize the safety profile of obinutuzumab combination regimens in CLL, and an exploratory analysis of this trial included an analysis of the bendamustine plus obinutuzumab (BG) regimen [Citation10]. The GREEN study identified the most common grade ≥3 adverse events (AEs) as neutropenia (49%), thrombocytopenia (12%), and febrile neutropenia (11%); grade ≥3 infections were 20%. Overall response rate (ORR) was 81%, including a complete response (CR) rate of 35%, and 2-year PFS was 82%.
Treatments for CLL patients have undergone tremendous change with the introduction of BTK inhibitors (e.g. ibrutinib and acalabrutinib) and the BCL-2 inhibitor venetoclax [Citation3,Citation4,Citation11–14]. Both types of molecules are highly effective in prolonging PFS, although as a single agent, a BTK inhibitor’s ability to eradicate MRD is limited. Moreover, BTK inhibitor treatment is continuous to progression or intolerance, and is associated with toxicities including atrial fibrillation [Citation4]. Venetoclax (BCL-2 inhibitor) has a fixed duration of treatment of one year when used in combination with obinutuzumab [Citation13]. Safety precautions are needed to reduce the risk of tumor lysis syndrome (TLS) with venetoclax. Despite declining use, chemoimmunotherapy may still play an important role in specific patient populations, especially in patients for whom ibrutinib, acalabrutinib, and venetoclax are not appropriate or are not easily available.
Here, we report the final results of the GIBB study, which was designed to prospectively evaluate the efficacy and safety of the BG regimen, including its effects on eradicating MRD in the peripheral blood and bone marrow. This study is one of few with the BG regimen that measured MRD status serially along with the duration of MRD negativity in peripheral blood, as well as the relationship between loss of MRD negativity and clinical progression.
Materials and methods
Study design and treatment
GIBB was a single-arm, open-label, multicenter, phase 2 study (NCT02320487). The primary objective was to determine the efficacy of BG in previously untreated patients with CLL (). Obinutuzumab was administered intravenously over six 28-d treatment cycles. During cycle 1, the first 1000 mg was given over the first 2 d of treatment: 100 mg on day 1 and 900 mg on day 2, followed by 1000 mg on days 8 and 15 (total of 3000 mg in cycle 1). During cycles 2-6, doses of 1000 mg obinutuzumab were given on day 1 of each cycle (in accordance with pivotal CLL11 study dosing [Citation8]). Bendamustine was administered intravenously at a dose of 90 mg/m2 on days 2 and 3 in cycle 1 and days 1 and 2 of cycles 2–6 for six total cycles.
Figure 1. GIBB study design for previously untreated patients with chronic lymphocytic leukemia receiving obinutuzumab/bendamustine (NCT02320487). BM: bone marrow; CLL: chronic lymphocytic leukemia; d: day; iwCLL: International Working Group guidelines for CLL; mo: month; MRD: minimal residual disease; PB: peripheral blood; y: year.
Prior to obinutuzumab infusion, patients were premedicated with glucocorticoids (dexamethasone 20 mg or methylprednisolone 90 mg), antihistamines (e.g. diphenhydramine 50 mg), and acetaminophen (650–1000 mg) to reduce the risk of infusion-related reactions. For patients with a high tumor burden, prophylaxis for TLS was required prior to treatment initiation, including appropriate hydration (3 L/d beginning 1–2 d prior to the first dose of obinutuzumab) and allopurinol or rasburicase prior to the first obinutuzumab infusion. Patients remaining at high risk for TLS due to persistently high tumor burden continued the above preventive measures until the risk was abated per the investigator’s judgment. It was also recommended that patients receive prophylaxis for Pneumocystis carinii pneumonia infections with sulfamethoxazole 800 mg/trimethoprim 160 mg twice daily (BID; or suitable alternative) and antimicrobials for patients with neutropenia. Growth factor prophylaxis and use were encouraged, but not required, to ensure necessary supportive measures per American Society of Clinical Oncology guidelines [Citation15] and antiemetics were used when clinically indicated.
The GIBB study was conducted in accordance with the Declaration of Helsinki; Good Clinical Practice and Institutional Review Board/Ethics Committee (IRB/EC) guidelines; and in accordance with institutional, local, and country regulations. Patients provided written informed consent prior to study initiation.
Patients
Previously untreated, CD20+ CLL patients ≥18 years of age were eligible if they had no evidence of aggressive CLL (i.e. Richter transformation), were in need of treatment per 2008 International Workshop on CLL (iwCLL) Working Group guidelines [Citation11], had Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2, absolute neutrophil counts of ≥1.5 × 109/L and platelets ≥75 × 109/L (unless cytopenia was caused by underlying disease), adequate creatinine clearance (≥40 mL/min) and liver function, and agreed to appropriate abstinence and contraception to prevent pregnancy.
Study endpoints
The primary endpoint of CR, which included CR or incomplete CR (CRi), at the end of treatment was assessed by investigator assessment per iwCLL guidelines [Citation11], and required bone marrow confirmation. Patients with no post-baseline or end of response assessment were considered non-responders.
Secondary endpoints were ORR (CR, CRi, partial response [PR], nodular PR [nPR]), duration of response (DOR), PFS, and overall survival (OS) by investigator assessment. Response evaluations were performed according to iwCLL criteria [Citation11]. Computed tomography (CT) scans were performed at the response evaluation visit (∼2 months after last study treatment) and to confirm suspected progression thereafter.
Additional secondary endpoints included central review assessment of MRD status in peripheral blood and bone marrow, time to and duration of MRD negativity and patient subgroup analyses of response rates, and MRD status. MRD was assessed by 4-color flow cytometry using iwCLL criteria [Citation11] for MRD negativity (<1 CLL cell detected in 10,000 leukocytes [sensitivity <10−4]) in peripheral blood mononuclear cells and bone marrow. MRD was assessed at baseline, treatment completion (28 d after cycle 6), response evaluation visit, and every 6 months thereafter for ≤ 2 years. The first MRD assessment was scheduled at baseline. Time to MRD-negative status in the peripheral blood was defined as the time between MRD positivity to the first achievement of MRD negativity (at the 10−4 sensitivity level) in the peripheral blood. Patients who did not achieve peripheral blood MRD-negative status were censored at the time of the last available MRD assessment. Among patients who achieved MRD negativity in the study, duration of MRD negativity was defined as the period between the first occurrence of MRD-negative status and a subsequent MRD-positive status. Patients who did not revert to MRD-positive status after achieving MRD negativity were censored at the time of the last MRD assessment.
Safety
Safety and tolerability of the combination regimen were assessed based on AEs, serious AEs (SAEs), incidence of AEs leading to dose modifications or discontinuations, AEs of special interest, clinical laboratory abnormalities, and death. AEs were graded according to National Cancer Institute Common Terminology Criteria for AEs version 4.0 (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm).
Statistical methodology
The intent-to-treat (ITT) population was defined as patients who enrolled in the study, regardless of whether they received study drug. Efficacy was assessed in the modified ITT (mITT) population, which included all enrolled patients who received any amount of study treatment. Patients in the safety population were those who were enrolled and received any amount of either study drug. Assuming a 10% rate of patients being unavailable for evaluation, a total of 100 enrolled patients would provide 90 patients for the primary analysis. If the expected true CR rate is 40%, the 2-sided 95% confidence intervals on the true CR rate would extend ±10.1% from the observed response rate.
Primary efficacy analyses were performed after all patients completed the response evaluation visit (∼2 months after last study treatment [∼3 months for CR patients to include bone marrow confirmation]). Final analyses were performed after all patients completed 36 months of study treatment or discontinued prematurely from the study.
DOR was defined as the time from first documented response (CR, CRi, PR, or nPR) to the time of disease progression, relapse, or death, whichever occurred first. PFS was defined as the time from initiation of study treatment to disease progression, relapse, or death, whichever occurred first. OS was defined as the time from initiation of study treatment to death from any cause. Time-to-event data were estimated by Kaplan–Meier method and included 95% CI.
Results
Patients, treatment exposure, and observation time
From 16 March 2015 until 15 February 2016, a total of 102 patients were enrolled and the data cutoff for final analysis was 3 June 2019. Patients had a median age of 61 years (range, 35–90) and 97% had an ECOG PS of 0–1 ().
Table 1. Baseline patient demographics and disease characteristics in patients receiving obinutuzumab/bendamustine in the GIBB study (intent-to-treat population).
The median time from initial diagnosis to first dose of study treatment was 2.1 years. The mean number of full treatment cycles for both study treatments was 5.4 (standard deviation 1.4) and median number of treatment cycles was 6; 79% of patients completed 6 treatment cycles of obinutuzumab and bendamustine. The median follow-up time for the study was 34.3 months (range, 0.5–43.1).
Response and survival assessments
CR and CRi were achieved in 51 (50%) patients (95% CI, 40–60%), which contributed to an ORR of 89% (95% CI, 82–95%; ). The median DOR for responders was not reached (95% CI, 27.2 months to not reached; ). Estimated 2-year PFS and OS were 86% and 97%, respectively (). The estimated 30-month PFS was 72%. Median OS was not reached.
Figure 2. Time-to-event results for patients receiving obinutuzumab/bendamustine in the GIBB study for (A) duration of response, (B) progression-free survival, and (C) overall survival (modified intent-to-treat population). Data cutoff: 3 June 2019.
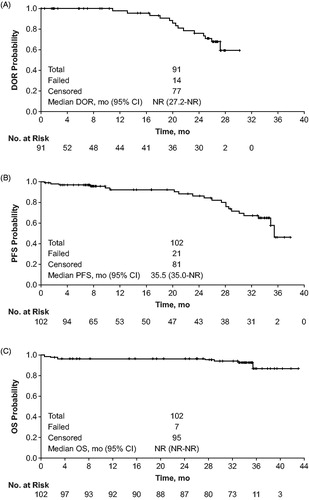
Table 2. Response at treatment completion in patients receiving obinutuzumab/bendamustine in the GIBB study (modified intent-to-treat population).
MRD analysis
Peripheral blood samples were obtained for MRD testing from 102 patients throughout specified timepoints in the protocol. At the response evaluation timepoint, samples from 74 patients were successfully analyzed with the appropriate assay sensitivity (10−4). Ninety-five patients had at least one successful peripheral blood MRD assessment at any timepoint during the study. Bone marrow samples for MRD assessments were taken at the response evaluation timepoint; samples for 51 patients were successfully analyzed with the appropriate assay sensitivity.
Undetectable MRD in peripheral blood from evaluable patient samples at the sensitivity of <10−4 level was shown in 45% (33/74; 95% CI, 33–57%; 32% [33/102] in all patients) at the response evaluation timepoint and 59% (30/51; 95% CI, 44–72%; 29% [30/102] in all patients) in the bone marrow. At any time during the study, 83% of the 95 evaluable patients (77% [79/102] in all patients) achieved MRD negativity (<10−4) in the peripheral blood. In 51 patients who achieved a CR/CRi, MRD negativity was achieved by 88% of patients in the peripheral blood and 43% in the bone marrow, and 43% in both the blood and bone marrow at any time after treatment. The median time to MRD-negative status, which was defined as the time between MRD positivity to the first achievement of MRD negativity in the peripheral blood was 8.2 months (95% CI, 7.1–10.3; ). In the mITT population, the estimated 6- and 12-month landmark MRD-positive rates were 77% and 22%, respectively.
Figure 3. Time to and duration of MRD negativity (<10−4) status in patients receiving obinutuzumab/bendamustine in the GIBB study in peripheral blood: (A) time to MRD negativity (<10−4) following initial positive MRD assessment and (B) duration of MRD-negativity defined as the time from first occurrence of MRD-negative status to subsequent MRD-positive status. Data cutoff: 3 June 2019.
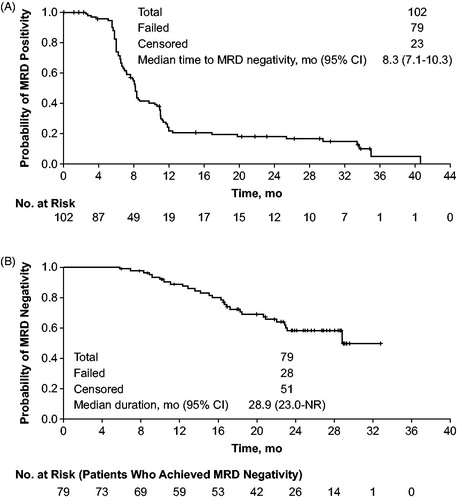
Among the 79 patients who achieved MRD negativity in the peripheral blood at any time during the study, the median duration of MRD negativity from the first occurrence of MRD-negative status to subsequent MRD-positive status was 28.9 months (95% CI, 23.0 months to not reached; ). Of these 79 patients achieving MRD negativity, 28 patients became MRD positive by their final analysis; the median time from identification of MRD positive status to progressive disease/death was 10.5 mo (95% CI, 6.3–17.9).
Safety
All patients had at least one treatment-emergent adverse event (TEAE), with 70% reporting a grade 3 or above TEAE (). The most common any-grade TEAEs were infusion-related reactions (71%), nausea (53%), pyrexia (36%), fatigue (35%), and neutropenia (33%). The most common grade 3/4 TEAEs were neutropenia (25%; 5% febrile neutropenia), infusion-related reactions (9%), anemia (8%), thrombocytopenia (8%), pneumonia (6%), and TLS (6%). All other grade ≥ 3 TEAEs occurred in fewer than 5% of patients.
Table 3. Treatment-emergent adverse events (TEAEs; ≥ 5% any grade) in patients receiving obinutuzumab/bendamustine in the GIBB study (safety population; N = 102).
About 79% of patients experienced a TEAE that led to dose modification/interruption of any study treatment (67 patients [66%] with related infusion-related reactions during cycle 1). The primary reason for discontinuation of any study drug was due to AEs in 14 patients (14%), which was predominantly due to grade 3 neutropenia in 4 patients (4%). All other AEs leading to discontinuation occurred in a single patient. About 18% of patients had a treatment-related SAE, 63% grade 3–5 TEAEs, and 75% infusion-related reaction. About 8% of patients had a related TEAE leading to withdrawal of any study drug. Seven patients died during the study (three from cardiac events not attributed to treatment by investigators [congestive heart failure, myocardial infarction, and cardiac arrest]; four from other/unknown causes). Ninety-five of 102 (93%) patients have entered the post-treatment follow-up period of the study.
Discussion
Final results for the GIBB study showed that the BG combination was an effective regimen for previously untreated patients with CLL, with no unexpected safety signals. CRs were achieved in approximately half of all patients after treatment and persisted over time. Estimated 1- and 2-year PFS rates were 92% and 86%, respectively; estimated 1- and 2-year OS rates were 97% and 97%. These results were consistent with those reported in the cohort receiving first-line BG from the GREEN phase 3 b study (n = 158); patients showed an 81% ORR, 35% CR/CRi, and 2-year PFS of 82% (median not reached) [Citation10]. Although using a different anti-CD20 antibody, these results were generally consistent (albeit with slightly different endpoints) with results shown by the German CLL study group with BR treatment in previously untreated CLL patients, leading to an 88% ORR, 23% CR, and median event-free survival of 33.9 months [Citation7].
Importantly, the safety profile was consistent with that shown in the GREEN study of BG in a similar patient population [Citation10], as well as demonstrating lower rates of grade ≥3 myelosuppression when considered in context with separate studies of BR [Citation5,Citation16]. In this study, the most common grade 3/4 TEAEs were neutropenia and infusion-related reactions.
The planned follow-up period for the GIBB study was capped at 3 years, which resulted in a time-restricted estimation of PFS and censoring of events at the end of the PFS curve. Therefore, a reliable median could not be established.
A notable aspect of the GIBB trial is the evaluation of serial measurements of MRD status from the peripheral blood in patients who have received chemoimmunotherapy. As shown previously, 45% of the patients assessed for MRD status in peripheral blood at the response evaluation timepoint achieved MRD-negative status. In the 51 patients who were evaluated for bone marrow MRD status at the same timepoint, 59% were MRD-negative in the bone marrow.
Understanding the temporal dynamics of MRD status is a novel finding offered by this study. The median duration of MRD negativity measured from the first documentation of negative status in the peripheral blood was 28.9 months. By following patients serially, we show that loss of MRD-negative status in the peripheral blood precedes clinical progression by approximately 10.5 months. Understanding the longitudinal course of MRD status may help anticipate a future clinical course and develop treatment strategies where MRD negativity can be maintained, or where intervention can occur after the loss of MRD negativity to delay disease progression.
It is evident that the relationship between attaining MRD negativity and prolonging PFS seems to be applicable to treatment regimens that can induce deep responses, such as chemoimmunotherapy or venetoclax. This relationship is not observed with ibrutinib, where it is possible to achieve long duration of PFS without establishing MRD negativity (and often with low CR rate) [Citation4,Citation17]. How the suppression of disease progression, despite the inability to eradicate disease, will impact OS is an area of continued investigation. Several studies have shown the survival benefit associated with MRD-negative status regardless of the treatment used to attain it [Citation8,Citation18]. Most data were derived from studies of chemoimmunotherapy, including several large phase 3 randomized studies (CLL8, CLL10, CLL11) [Citation8,Citation16,Citation19–21], as well as smaller studies in both first-line and the relapsed settings [Citation22,Citation23].
Since the initiation of the GIBB trial, the use of ibrutinib has emerged as a preferred first-line treatment in older CLL patients. In the RESONATE-2 study, ibrutinib demonstrated significantly improved efficacy versus chlorambucil in first-line CLL, regardless of cytogenetic prognostic subgroups [Citation4]. A randomized Alliance study compared ibrutinib (±rituximab) versus BR in previously untreated CLL patients ≥65 years of age [Citation5]. Although PFS was improved with ibrutinib ± rituximab (2-year PFS: 88% ibrutinib + rituximab, 87% ibrutinib alone, and 74% with BR), there was no OS advantage between regimens after a median follow-up 38 mo. While the rate of hematologic AEs was higher with BR, non-hematologic AEs were higher in patients receiving ibrutinib. Since cardiac arrhythmias and hypertension are known complications of ibrutinib treatment [Citation24] for some patients (especially the elderly), ibrutinib may not be the optimal therapy. This same study showed higher rates of CR and MRD negativity with BR versus ibrutinib [Citation5].
Ibrutinib induces high PFS but lacks the ability to induce high rates of CR and MRD negativity when used as a continuously-administered treatment, contrasting the limited 6 cycles of chemoimmunotherapy (e.g. BR or BG) [Citation5]. Active CLL research is being conducted to improve upon the efficacy of ibrutinib and/or shorten the treatment duration, such as by combining ibrutinib with other agents like venetoclax [Citation25]. In the CLL14 study, venetoclax + obinutuzumab (VenG), a regimen with a limited duration of 1 year, resulted in significantly higher PFS compared to obinuzumab + chlorambucil (2-year PFS: 88% vs 64%, respectively) [Citation13]. In this study, 76% of the VenG patients achieved MRD negativity in the peripheral blood and 57% in the bone marrow three months post-treatment completion [Citation13].
In summary, the GIBB study showed that BG is a clinically active CLL regimen with a high CR rate, consistent with other first-line studies of anti-CD20 antibody and bendamustine combinations. This study also showed that the BG combination induced a high rate of MRD negativity in the peripheral blood and bone marrow, and is one of a few that evaluated MRD status over time. The GIBB study demonstrated that BG induced durable MRD negativity and showed a relationship between loss of MRD negativity and the development of disease progression. The relationship between MRD status and disease progression should be further characterized with other treatments to inform future treatment strategies. Although the use of chemoimmunotherapy has been diminished with the advent of more targeted therapeutic agents, chemoimmunotherapy such as BG remains a mainstay of CLL therapy [Citation26], providing high response and MRD-negative rates, and may still play an important and valuable role in the treatment of patients for whom oral, targeted therapies are not appropriate due to toxicities or other factors, or are not available.
Author contributions
JPS, JL, YM, AVD: Designed the study; JPS, JMB, HAY, MAB, SB, AVD: Performed the research; JPS, JL, YM, AVD: Analyzed the data and wrote the first version of the article. All authors revised the manuscript critically, and approved the submitted and final version of the article.
Prior presentation
This study was previously presented in part at the following meetings: European Hematology Association (EHA) (Haematologica. 2017;102(s2):abstract P249), American Society of Clinical Oncology (ASCO) (J Clin Oncol. 2017;35(suppl):abstract 7523), and American Society of Hematology (ASH) (Blood. 2017;130(suppl 1):abstract 683).
Acknowledgments
We would like to thank the patients, their families and caregivers, and the study investigators, coordinators, and nurses who participated in the GIBB clinical trial. Thank you to the data monitoring committee that served as an independent expert advisory group to evaluate safety and efficacy throughout the study. We would also like to thank Ken Wilhelm, MD for his work on this study. This study was sponsored by Genentech, South San Francisco, CA. The authors directed development of the manuscript and were fully responsible for all content and editorial decisions for this manuscript. The GIBB study was sponsored by Genentech, South San Francisco, CA.
Disclosure statement
JPS reports consultancy, honoraria, and research funding from AbbVie, Acerta, AstraZeneca, Celgene, Genentech, Gilead, Pharmacyclics LLC (an AbbVie Company), and TG Therapeutics; consultancy and research funding from Janssen and Seattle Genetics; and employment with Willamette Valley Cancer Institute and Research Center.
JMB reports consultancy from AbbVie, AstraZeneca, Bayer, Celgene, Gilead, Juno, Kite, Roche/Genentech, Tempus Labs, and Verastem; consultancy and speakers’ bureau from Seattle Genetics; research funding from Janssen; and employment with Rocky Mountain Cancer Center.
HAY reports consultancy from Amgen; honoraria from Celgene and Seattle Genetics; speakers’ bureau from AstraZeneca and Janssen; and equity ownership of Clovis Oncology and Puma Biotechnology.
MAB reports consultancy from Best Doctors and Gerson Lerman; and honoraria and speakers’ bureau from AbbVie and Takeda.
SB reports consultancy and research funding from Bristol-Myers Squibb; research funding from AbbVie, Genentech, Incyte, Janssen, Novartis, Pfizer; honoraria from AstraZeneca; honoraria and research funding from Eli Lilly; honoraria, research funding and speakers’ bureau from Alexion; membership on an entity’s Board of Directors or advisory committee for Lutheran Hospital in Fort Wayne, Indiana; and equity ownership in and employment with Fort Wayne Medical Oncology & Hematology.
JL reports employment with Genentech and equity ownership with Roche.
YM reports employment with Genentech and equity ownership with Roche.
AVD reports consultancy and other travel reimbursement and research funding with Verastem Oncology; consultancy with Celgene, Curis, Janssen, Seattle Genetics, Teva Oncology, and TG Therapeutics; research funding with Aptose Biosciences, Bristol-Myers Squibb, and Takeda Oncology; and consultancy and research funding from AstraZeneca, Bayer Oncology, Genentech, and Gilead Sciences.
Data availability statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche's criteria for eligible studies are available here: (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34.
- Seymour EK, Ruterbusch JJ, Beebe-Dimmer JL, et al. Real-world testing and treatment patterns in chronic lymphocytic leukemia: a SEER patterns of care analysis. Cancer. 2019;125(1):135–143.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103(9):1502–1510.
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443.
- Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–3216.
- Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110.
- GAZYVA (obinutuzumab) injection prescribing information. South San Francisco (CA): Genentech, Inc. A Member of the Roche Group; 2020.
- Stilgenbauer S, Leblond V, Foa R, et al. Obinutuzumab plus bendamustine in previously untreated patients with CLL: a subgroup analysis of the GREEN study. Leukemia. 2018;32(8):1778–1786.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456.
- Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–778.
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236.
- Kater AP, Seymour JF, Hillmen P, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269–277.
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205.
- Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942.
- Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–2042.
- Molica S, Giannarelli D, Montserrat E. Minimal residual disease and survival outcomes in patients with chronic lymphocytic leukemia: a systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2019;19(7):423–430.
- Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–988.
- Kovacs G, Robrecht S, Fink AM, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL Study Group. J Clin Oncol. 2016;34(31):3758–3765.
- Dimier N, Delmar P, Ward C, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood. 2018;131(9):955–962.
- Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–3024.
- Thompson PA, Peterson CB, Strati P, et al. Serial minimal residual disease (MRD) monitoring during first-line FCR treatment for CLL may direct individualized therapeutic strategies. Leukemia. 2018;32(11):2388–2398.
- IMBRUVICA (ibrutinib) prescribing information. Sunnyvale (CA): Pharmacyclics LLC; 2020.
- Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–2103.
- Danilov AV, Pagel JM, Brown JR, et al. Chemo-immunotherapy for older patients with chronic lymphocytic leukemia - Passé Yet? HemaSphere. 2019;3(4):e275
