Abstract
Selinexor, a selective inhibitor of nuclear export, has demonstrated promising activity in patients with acute myeloid leukemia (AML). This randomized, phase II study evaluated selinexor 60 mg twice weekly (n = 118) vs. physician’s choice (PC) treatment (n = 57) in patients aged ≥60 years with relapsed/refractory (R/R) AML. The primary outcome was overall survival (OS). Median OS did not differ significantly for selinexor vs. PC (3.2 vs. 5.6 months; HR = 1.18 [95% CI: 0.79–1.75]; p = 0.422). Complete remission (CR) plus CR with incomplete hematologic recovery trending in favor of selinexor occurred in a minority of patients. Selinexor treated patients had an increased incidence of adverse events. The most common grade ≥3 adverse events were thrombocytopenia, febrile neutropenia, anemia, hyponatremia. Despite well-balanced baseline characteristics, there were numerically higher rates of TP53 mutations, prior myelodysplastic syndrome, and lower absolute neutrophil counts in the selinexor group; warranting further investigation of selinexor in more carefully stratified R/R AML patients.
Registered trial: NCT02088541.
Keywords:
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults, and the incidence increases with age from ∼1.3 per 100,000 in patients aged <65 years to 12.2 per 100,000 in those over 65 years [Citation1]. Treatment of AML in older patients often requires the use of less intensive options, and the prognosis is affected by a higher frequency of comorbid conditions, unfavorable cytogenetic and molecular characteristics, and antecedent hematologic disorders [Citation2–4].
Until recently, treatment options for older patients with AML were limited. DNA methyltransferase inhibitors (DNMTi) and low-dose cytosine arabinoside (LDAC) were reserved for less fit patients, and alternative cytotoxic chemotherapy regimens could be offered only to more fit, elderly patients [Citation2,Citation5]. Nonetheless, most older patients will relapse even after achieving the first remission and subsequently have a poor prognosis: 5-year survival rates remain <10% in patients aged more than 60 years [Citation6,Citation7]. While several new drugs have been approved by the FDA for use in combination with prior standard of care agents for the management of untreated AML [Citation8–14], novel therapies for R/R AML have also been approved including ivosidenib [Citation15], enasidenib [Citation16], gilteritinib [Citation17], and gemtuzumab ozogamicin [Citation18]. Despite the dramatic expansion of new therapy options, improvement in overall survival (OS) is modest.
The continued development of new therapies addressing novel molecular targets in AML and other hematologic malignancies is critical. The nuclear export protein 1 (exportin 1 or XPO1, also known as chromosome region maintenance 1) is the lone exporter of nearly all tumor suppressor proteins from the nucleus to the cytoplasm and is overexpressed in a range of malignancies, including AML [Citation19,Citation20]. Recently, the combination of the selective inhibitor of nuclear export (SINE) compound selinexor and low-dose dexamethasone (Sel-dex) was approved by the US Food and Drug Administration (FDA) for patients with penta-refractory multiple myeloma who received at least four prior therapies, and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody [Citation21]. Selinexor was also recently approved for patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma [Citation22]. Selinexor is also currently being evaluated in a variety of hematologic and solid malignancies, including studies combining selinexor with other agents in patients with R/R AML (NCT02249091, NCT03071276, and NCT02416908).
Several orally bioavailable, small-molecule XPO1-inhibitor agents, termed SINE compounds, are in development and have demonstrated promising activity in AML cell lines, animal models, and patient samples [Citation20,Citation23–25]. Inhibition of XPO1 has been shown to have antileukemic activity in patient samples by, among other mechanisms, selectively killing neoplastic blast cells with minimal effects on normal cells [Citation25]. XPO1 inhibition also decreases cell levels of Mcl-1, an antiapoptotic protein in the Bcl-2 family, and thereby induces apoptosis [Citation26].
Previously, a phase I dose-escalation study of selinexor in patients with advanced hematologic malignancies showed an overall response rate (ORR) of 14% among 81 evaluable patients with R/R AML. Median progression-free survival (PFS) with selinexor was 5.1 months in responders, compared with 1.3 months in non-responders (p = 0.008, hazard ratio [HR] = 3.1), and corresponding median overall survival (OS) durations were 9.7 and 2.7 months, respectively (p = 0.01, HR = 3.1) [Citation27]. Furthermore, 31% of AML patients from this phase I study had at least a 50% decrease in bone marrow blasts from baseline. In light of these results, a randomized phase II trial was designed to compare selinexor with the treatment of physician’s choice (PC) in patients aged more than 60 years with R/R AML who were ineligible for intensive chemotherapy and/or stem cell transplantation.
Materials and methods
Study design
Selinexor in Older Patients with Relapsed AML (SOPRA; NCT02088541) was a phase II, randomized, open-label, multicenter study conducted in 11 countries across North America and Europe. The protocol was approved by the institutional review board or independent ethics committee at each participating center, and in accordance with the Declaration of Helsinki, the International Conference on Harmonization (ICH), Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), and local laws. Written informed consent was obtained from all patients before study participation.
Treatment
Patients were randomized in a 2:1 allocation, within each of the 2 × 2 × 2 stratification levels to receive selinexor or PC treatment. Patients were stratified for randomization according to the duration of their first complete remission (CR) on prior therapy (>1 vs. ≤1 year or never achieved), and age (<70 vs. ≥70 years). Following an amendment mandating a reduction of the selinexor dose, stratification was revised to include three criteria: duration of patients’ first CR on prior therapy (>6 vs. ≤6 months or never achieved); the number of prior therapies (1 vs. >1); and peripheral leukemic blast counts (PLBC; ≥10,000 vs. <10,000/μL). In the original study protocol, selinexor was administered orally twice weekly at a dose of ∼55 mg/m2 (60–120 mg, depending on body surface area). Given concern for sepsis-related serious adverse events (SAEs), with an observed increase in neutropenic fever in the selinexor arm compared with PC arms (eight vs. two events, respectively, during the first ∼13 months of the study), the Drug Safety Monitoring Board and sponsor elected to reduce selinexor to a fixed dose of 60 mg (equivalent to ∼35 mg/m2) twice weekly. Thus, any patients enrolled from July 2015 onwards were started on selinexor 60 mg, and all patients previously randomized to selinexor 55 mg/m2 were switched to 60 mg. Treatment of PC included one of three treatment regimens: best supportive care (BSC), BSC plus LDAC, or BSC plus DNMTi (Supplementary Methods).
Inclusion and exclusion criteria
Patients ≥60 years of age with R/R AML (≥20% blasts), defined as either: recurrence of disease after a CR, or failure to achieve CR with initial therapy, of any type except acute promyelocytic leukemia and with Eastern Cooperative Oncology Group performance status of ≤2 were enrolled. Patients were required to have undergone at least one prior therapy at standard doses for AML, including at least one regimen including Ara-C chemotherapy and at least one regimen including DNMTi for at least two cycles and had an objective, documented evidence of disease progression or failure to respond to a reasonable trial of their most recent previous therapy before study entry. At least two weeks must have elapsed since the last treatment (with the exception of hydroxyurea) before the first dose. Patients had to show creatinine clearance >30 cc/min calculated using the Cockcroft and Gault [Citation28] formula or measured; total bilirubin ≤2 × upper limit of normal (ULN); transaminases (alanine transaminase [ALT] and aspartate transaminase [AST]) ≤2.5 × ULN or ≤5 × ULN if the patient had known liver involvement; and coagulation time ≤1.5 × ULN. Patients were ineligible if they had central nervous system (CNS) leukemia, AML M3, blast transformation of chronic myeloid leukemia, AML was classified as favorable according to the European LeukemiaNet (ELN) disease risk assessment, concurrent active malignancy, or uncontrolled infection. For full inclusion and exclusion criteria, see the Supplementary Methods.
Outcome measures
The primary efficacy endpoint was OS from date of randomization to date of death. Primary efficacy analyses were undertaken in all patients randomized to study treatment who received 60 mg selinexor or PC (intent-to-treat population). Secondary efficacy variables were OS, CR rate, CR rate plus CR with incomplete hematologic recovery, ORR, DOR, and DCR, assessed in a hierarchical fashion and defined in the Supplementary Methods. Patients randomized before the protocol amendment (exploratory efficacy population) were included in the safety population, but not primary or secondary efficacy analyses. The safety and tolerability of selinexor and PC were assessed in all patients who received any study treatment (safety population). Treatment-emergent adverse events (TEAEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) V4.03.
Predictive biomarkers for selinexor response
RNA sequencing was performed on AML samples from 23 patients enrolled in the selinexor arm, 9 of whom achieved CR or CR with incomplete hematologic recovery (CRi) and were classified as responders, and 14 of whom had progressive disease or stable disease with a minimum treatment duration of 30 days, who were classified as non-responders.
Protein activity measurements for 6213 regulatory proteins were inferred using gene expression profiles of pretreatment tumor samples using the VIPER algorithm [Citation29] (Supplementary Methods).
Statistical analyses
The study sample size was designed to have an 80% power to detect a median OS of ∼5.2 months with selinexor and ∼3 months with PC, allowing for two interim analyses. Statistical significance of a difference in OS was based on the stratified log-rank test, using the randomization strata. A Cox proportional hazards model stratified by the randomization factors estimated the HR and 95% confidence interval (CI) for treatment arm difference. The Kaplan–Meier method was used to analyze OS. All statistical tests were performed at the one-sided 0.025 alpha level (Supplementary Methods).
Results
Patients
Between 23 June 2014 and 8 January 2018, a total of 317 patients were enrolled and randomized at 95 sites in Canada, Europe, and the US. Before the protocol amendment, 98 patients were randomized to treatment with selinexor (71 patients received 55 mg/m2 and 27 received 60 mg), and 44 to the PC arm. The intent-to-treat (ITT) population, enrolled after the amendment, included 118 patients randomized to selinexor 60 mg and 57 patients randomized to PC. One patient randomized to selinexor 60 mg received PC and was counted under selinexor 60 mg for the ITT population. Details of patient enrollment and disposition are shown in Supplementary Figure S1.
In the ITT population, patient disposition was well-balanced with respect to the three stratification factors of duration of patients’ first CR on prior therapy (>1 year vs. ≤1 year or never achieved), the number of prior therapies (1 vs. >1), and peripheral leukemic blast count (PLBC; ≥10,000/μL vs. <10,000/μL). However, patients in the PC arm were more likely than those in the selinexor arm to withdraw consent after randomization but before initiation of study treatment (19.3 vs. 0.8%, respectively). Baseline demographics and characteristics in the ITT population were generally well-balanced between the treatment groups (). However, ad hoc analyses revealed a greater proportion of patients in selinexor group with low baseline absolute neutrophil count (ANC), TP53 abnormalities, or prior MDS (Supplementary Table S1). Baseline demographics for the safety population are shown in Supplementary Table S1.
Table 1. Baseline demographics in the intent-to-treat population.
Efficacy
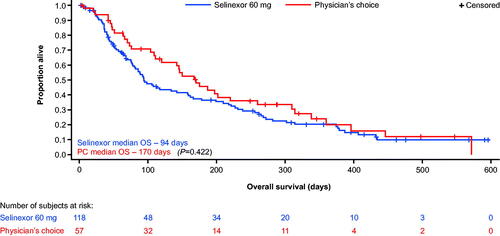
With respect to the primary endpoint in the ITT analysis, the median OS for selinexor vs. PC was 3.2 vs. 5.6 months, respectively (HR = 1.18 [95% CI: 0.79–1.15]; p = 0.422; ). An ad hoc subgroup analysis that excluded patients with TP53 mutations (selinexor, n = 70; PC, n = 32) also showed that median OS was similar in both treatment arms (median 3.6 and 4.7 months, respectively; HR = 0.89 [95% CI: 0.53–1.51]; p = 0.674). Kaplan–Meier curves for OS in patients without TP53 mutations are shown in Supplementary Figure S2. Analysis of responses to prior DNMTi (hypomethylating agent) therapy in the ITT population showed that 18 of the 118 (15.4%) selinexor-treated patients and 12 of the 57 (21.8%) PC-treated patients achieved CR on prior DNMTi therapy. Amongst the selinexor-treated patients, 34 (29.1%) patients had stable disease (SD) on prior DNMTi therapy (n = 117); in PC-treated patients (n = 55), SD occurred in 14 (25.5%) patients.
Results for the secondary efficacy variables are summarized in . CR was observed in six patients (5.1%) treated with selinexor and zero (0%) patients in the PC arm (p = 0.099). Rates of CR plus CRi were 11.9 and 3.5% in the selinexor and PC arms, respectively (p = 0.084). The ORRs (any CR plus partial remission [PR]) were similar between the selinexor and PC arm (13.6 and 8.8%, respectively [p = 0.384]). Disease control rates (DCRs), i.e. the proportion of patients who had CR, CRi, CR with incomplete platelet recovery (CRp), morphologic leukemia-free state, PR, or SD for at least 4 weeks, were numerically greater with selinexor, although this did not reach statistical significance (50.8 and 40.4%, respectively [p = 0.176]). Forty-two (35.6%) patients in the selinexor arm and 26 (45.6%) patients in the PC arm had no disease response assessments (no blasts from bone marrow aspirate or bone marrow biopsy reported beyond screening), largely due to death before day 30.
Table 2. Secondary efficacy variables in the intent-to-treat population.
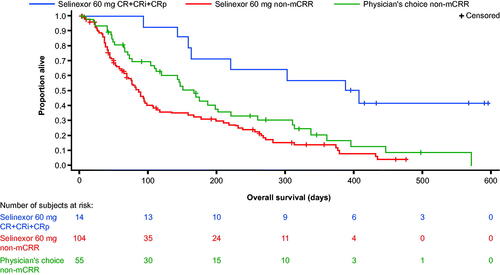
The median disease-free survival (DFS) was 175.0 days (95% CI: 61.0–288.0) for the 14 patients with CR or CRi in the selinexor arm. OS was longer in the 14 selinexor-treated patients who achieved CR, CRi, or CRp compared with those who did not achieve any type of CR regardless of the study arm ().
Figure 2. Overall survival in patients with and without CR plus CRi/CRp in the intent-to-treat population. mCRR = CR + CRi + CRp. CR: complete remission; CRi: complete remission with incomplete recovery; CRp: complete remission with incomplete platelet recovery; mCRR: modified complete remission rate; PC: treatment of physician’s choice.
Safety
The safety population included all patients who had at least one dose of study drug and comprised 297 patients (selinexor 55 mg/m2, n = 71; selinexor 60 mg, n = 142; PC n = 84). Patients received a median of two 28-day treatment cycles with selinexor 60 mg (range = 1–21), and the median duration of exposure for the selinexor and PC arms was 45 and 55 days, respectively. Selinexor 60 mg was better tolerated than selinexor 55 mg/m2: dose reductions were required in 33.1% of patients treated with selinexor 60 mg, compared with 42.3% on selinexor 55 mg/m2. Dose interruptions were required in 89.4 and 94.4% of patients in each group, respectively.
One or more TEAEs occurred in all selinexor-treated patients and in 95.2% of patients in the PC population (). At least one TEAE leading to treatment withdrawal occurred in 49.3% of patients on selinexor 55 mg/m2, 42.3% on selinexor 60 mg, and 23.8% on PC. One or more TEAEs leading to death were reported in 33.8, 21.8, and 19.0% of patients in each group, respectively. There were 64 deaths on treatment or within 30 days of the last dose, including 49 in the selinexor arm (22 on 55 mg/m2 and 27 on 60 mg) and 15 in the PC arm. Of the 49 deaths in the selinexor groups, 13 were due to disease progression. Other reasons included pneumonia (n = 12), sepsis (n = 5), septic shock (n = 4), respiratory failure (n = 2), and febrile neutropenia (n = 2).
Table 3. Summary of TEAEs occurring in the safety population.
Among patients treated with selinexor 55 mg/m2 and 60 mg, the most common non-hematologic TEAEs included nausea, decreased appetite, fatigue, hyponatremia, vomiting, constipation, asthenia, and diarrhea (). Among the hematologic TEAEs, thrombocytopenia was the most common overall in both the selinexor and PC groups (). TEAEs that were reduced in frequency after the selinexor dose was changed from 55 mg/m2 to the 60 mg fixed-dose included febrile neutropenia, hypokalemia, hyponatremia, hypophosphatemia, decreased weight, dehydration, acute kidney injury, sepsis, stomatitis, and blurred vision ().
Table 4. Most common TEAEs occurring in ≥10% of patients in any treatment group in the safety population.
The majority of TEAEs reported in the selinexor groups were grade ≤2 in intensity. The most frequently reported grade ≥3 TEAEs (occurring in ≥10.0% of patients) in the selinexor groups were thrombocytopenia, febrile neutropenia, anemia, hyponatremia, pneumonia, fatigue, and decreased appetite, generally occurring at a higher frequency in the selinexor 55 mg/m2 group, except for neutropenia (Supplementary Table S2). In the PC groups, the most frequently reported grade ≥3 TEAEs (occurring in ≥10.0% of patients) were thrombocytopenia, anemia, febrile neutropenia, and neutropenia.
At least one serious adverse event (SAE) was experienced by 81.7% of patients receiving selinexor 55 mg/m2, 73.2% receiving selinexor 60 mg, and 65.5% receiving PC (). The most common SAEs in the selinexor and PC arms were febrile neutropenia and pneumonia (Supplementary Table S3).
The most common TEAEs leading to discontinuation in the selinexor groups were febrile neutropenia (9.9% with selinexor 55 mg/m2 and 3.5% with selinexor 60 mg), pneumonia (5.6 and 3.5%, respectively), and fatigue (1.4 and 3.5%, respectively). In the PC patients, they were febrile neutropenia (4.8%), sepsis (2.4%), lung infection (2.4%), and pneumonia (2.4%).
Treatment-related TEAEs occurred in 95.8% of patients treated with selinexor 55 mg/m2, 93.0% treated with selinexor 60 mg, and 78.6% of PC-treated patients (). The most common treatment-related TEAEs observed in selinexor-treated patients included decreased appetite, nausea, fatigue, thrombocytopenia, and hyponatremia (Supplementary Table S4). Fatal events considered to be possibly or probably related to selinexor occurred in 10 (4.7%) patients (three in the 55 mg/m2 group and seven in the 60 mg groups): pneumonia (n = 3), septic shock (n = 2), pleural effusion (n = 1), fall (n = 1), sepsis (n = 1), multiorgan failure (n = 1), and febrile neutropenia (n = 1).
There was no evidence of cardiac toxicity, pulmonary toxicity, neuropathy, or liver toxicity following treatment with selinexor.
Predictive biomarkers for selinexor response
The VIPER algorithm [Citation29] was used to infer protein activity from gene expression profiles of mononuclear cells from patients enrolled on the selinexor arm before they started treatment with selinexor. This was followed by the identification of master regulators of drug sensitivity using a ridge regression classifier trained on 23 patients, including nine responders and 14 non-responders. Six master regulator proteins were identified, with five proteins having higher activity in responders (PKIA, ZDBF2, BCL11B, FHIT, and CAMK4), and one protein having lower activity in responders (MGST2) (Supplementary Table S5 and Supplementary Figure S3). These were identified as the consensus from a three-fold cross-validation procedure, where the most differentially active proteins between each responder and the pool of non-responders were selected. The model achieved a strong predictive ability (area under the receiver operating characteristic curve [AUC-ROC] = 0.821) and reached statistical significance (p = 0.02 by permutation testing) (Supplementary Figure S4A). Using this model, six of eight responders and 12 of 14 non-responders to selinexor were correctly identified (Supplementary Figure S4B). Training the classifier using differential gene expression data alone produced no statically significant classification.
Discussion
The SOPRA study was designed to assess a potential survival benefit with selinexor over PC treatment in patients who had already experienced therapy with a hypomethylating agent and cytarabine. The promising preclinical and phase 1 results previously observed with selinexor treatment did not translate to efficacy in a randomized setting here, however, selinexor 60 mg was better tolerated than selinexor 55 mg/m2, and CR/CRi was achieved in a minority of patients. While the ORR and DCR were numerically higher in the selinexor arm than the PC arm, the differences lacked statistical significance. The results presented here set the stage for the possibility of using selinexor in combination with chemotherapy. Furthermore, as the heterogeneity of AML presentation requires different approaches, the data obtained in this study may also suggest that SINE compounds will need to be used in combination with other regimens.
SOPRA remains an important trial as there are currently few randomized studies of interventions in older patients with AML. Effective therapeutic options for patients with R/R AML remain limited. Recently, the approval of venetoclax combination therapy in untreated AML patients who are unsuitable for intensive chemotherapy has changed how these patients are treated [Citation31]. However, the use of these combinations in R/R disease has been met with less enthusiasm [Citation32]. Targeted therapy for subpopulations of R/R AML, specifically IDH1-, IDH2-, or FLT3-mutated patients, has provided some room for optimism [Citation15,Citation17,Citation33–35]; however, patients without these selected mutations have limited effective treatment options.
Following the protocol change to reduce the selinexor dose to 60 mg, there was no difference between the selinexor and PC groups in terms of the rate of sepsis (3 vs. 6%, respectively). Low-grade fatigue and gastrointestinal events, such as nausea, decreased appetite, vomiting, and diarrhea, were among the most common TEAEs observed with selinexor during this study. A similar TEAE profile was also reported in the phase I study in AML [Citation27]. Most of these were reversible with standard supportive care or dose adjustment. Increased appreciation and appropriate management of such toxicities are key to the future use of selinexor in AML and other malignancies and are indeed an intrinsic part of growing experience with any new anticancer agent [Citation36].
While an increased incidence of TEAEs was observed in selinexor treated patients leading to higher rates of selinexor withdrawal due to grade 3 TEAEs and SAEs, the SOPRA study demonstrates the importance of appropriate toxicity management, as the incidence of most of TEAEs was reduced when the selinexor dose was lowered from 55 mg/m2 to a fixed dose of 60 mg. The majority TEAEs observed with selinexor were grade 1 and 2 and were reversible with dose modification and/or standard supportive care. Use of selinexor combination therapies in the R/R AML setting support further investigation; combining selinexor with cytarabine and mitoxantrone, for which most patients with newly diagnosed or R/R AML received the target dose of 80 mg/day twice-weekly (n = 17/20), resulted in half of the patients achieving CR with no dose-limiting toxicities [Citation37]. Doses of selinexor up to 55 mg/m2 in combination with fludarabine and cytarabine were found to be efficacious and tolerable in pediatric patients (≤24 years) with relapsed acute leukemias, including AML [Citation37]. In a study including older patients (≥60 years) with untreated AML, selinexor 60 mg given twice weekly in combination with decitabine resulted in a 40% ORR (n = 25) without protocol-defined dose-limiting toxicities [Citation38]. Similarly, in patients with newly diagnosed AML and poor-risk cytogenetics, selinexor 80 mg twice weekly in combination with daunorubicin and cytarabine was tolerated and induced CR/CRi in 53% (n = 19) of evaluable patients [Citation39]. As we gain experience with selinexor in specific patient populations, it should be possible to identify patients at higher risk of toxicities and manage them accordingly with dose modification and/or suitable supportive treatment.
The results of the SOPRA trial were likely influenced by certain aspects of the study design. The study protocol allowed the inclusion of patients with only two prior cycles of a hypomethylating agent; this may have benefited PC patients, who could then go on to receive three or more cycles. Response to DNMTi may occur as late as 6 or more months from initiation of treatment, with a median time to response of ∼3 months [Citation40], therefore, at least some patients in the PC arm may not have had AML refractory to DNMTi at study entry.
It is also possible that the censoring of patients disproportionately lost to follow-up from the PC treatment arm may have biased results in favor of PC. Furthermore, the observed baseline imbalances between the two treatment arms may have influenced outcomes, despite the lack of statistically significant differences. The higher percentages of patients in the selinexor vs. the PC group with ANC <0.5 × 109/L (42.4 vs. 21.1%) and 0.1 × 109/L (13.6 vs. 5.3%), TP53 mutations (11.9 vs. 5.3%), and prior MDS (11.0 vs. 5.3%) suggest that their AML was generally higher risk compared with those in the PC group, with each of these characteristics conferring poor prognosis [Citation41–44].
Older patients with AML represent a challenging population because of the added difficulties of treating more frail patients with comorbid conditions, and a higher frequency of multidrug resistance [Citation45]. For elderly patients with AML whose disease progresses after DNMTi therapy, survival is typically <4–6 months [Citation46–48]. The availability of three new small-molecule inhibitors (ivosidenib, enasidenib, and gilteritinib), approved by the US FDA since the SOPRA study was conducted, is likely to improve outcomes in subgroups of patients with R/R AML. All three agents exhibited a relatively low frequency of grade ≥3 AEs in these studies; the most common included thrombocytopenia (all three agents), anemia (ivosidenib and gilteritinib), febrile neutropenia (gilteritinib), hyperbilirubinemia (enasidenib), QT interval prolongation (ivosidenib), and IDH differentiation syndrome (ivosidenib and enasidenib) [Citation15,Citation17,Citation33].
A potential limitation of the SOPRA study is that a large number of patients in the PC arm withdrew early from the study, before receiving any treatment. This may have been due to the open-label study design, which could have encouraged patients to withdraw when they learned that they were to receive standard therapy rather than the investigational study drug. Another limitation is the high proportion of deaths or withdrawals occurring before initiation of the study treatment; these patients had no disease response assessments, with no blasts from bone marrow aspirate or bone marrow biopsy results reported beyond initial screening.
In conclusion, whilst an improvement in OS was not demonstrated for selinexor when compared with PC, there was a slight trend toward increased ORR and DCR and future studies should aim to translate these increases into OS benefits. The observation that selinexor-treated patients who achieved CR/CRi had longer OS than those on either arm without CR/CRi supports further investigation of oral selinexor in AML, with careful selection of targeted populations. A search for biomarkers of response to selinexor is ongoing and may help to improve patient selection for future studies. Published and ongoing studies of selinexor as a monotherapy or in combination with other therapies in the R/R AML setting have shown promising response rates and may be key to providing a tolerable combination strategy for older patients [Citation27,Citation36–38,Citation49].
ilal_a_1950706_sm2478.docx
Download MS Word (523.6 KB)Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in this study. The medical writing support was provided by Minal Kotecha, Ph.D., and Liz Anfield, and editorial support by Bethany King, BSc, all of Core Medica, UK, supported by Karyopharm according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, the ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Disclosure statement
KS: advisory board honorarium from Astellas, Bristol-Myers Squib, Novartis, and Takeda. Consulting fees from Stemline Therapeutics. Research support from Incyte. HL: research support from Karyopharm Therapeutics Inc and Bristol-Myers Squibb advisory board honorarium from Agios Pharmaceuticals. VK: advisory board honorarium from Pfizer Inc., Novartis, Incyte. Research funding from Amgen. SSJ: advisory board honorarium from AbbVie Inc, Astellas Pharma Inc, Incyte, Jazz Pharmaceuticals, KiTE Pharma, Kura Oncology, Novartis, and Pfizer. Research support from Sunesis Pharmaceuticals. TJU, SS, MGK, ST, YL, and JS: employees and stockholders of Karyopharm Therapeutics Inc. MC: former employee and stockholder of Karyopharm Therapeutics Inc. AJ: consultant to Karyopharm Therapeutics Inc. GR: research support from Cellectis. Consultancy, advisory board or data and safety monitoring committee membership of AbbVie Inc, Actinium Pharmaceuticals Inc, Agios Pharmaceuticals, Amphivena Therapeutics, Aargenx, Array BioPharma, Astex Therapeutics, Astellas Pharma Inc, AstraZeneca, Bayer, Celgene, Celltrion, Daiichi Sankyo, Eisai Co Ltd, Epizyme, Helsinn, Janssen Pharmaceutica, Jasper Therapeutics, Jazz Pharmaceuticals, MEI Pharma, Novartis, Orsenix, Otsuka Pharmaceutical, Pfizer, Roche, Genentech, Sandoz, Takeda, and TrovaGene. MRS: research support from Astex, Incyte, Takeda, TG Therapeutics. Stockholder of Karyopharm Therapeutics Inc. Consulting or advisory board honoraria from Abbvie, BMS, Celgene, Karyopharm Therapeutics Inc, Ryvu, Sierra Oncology, Takeda, TG Therapeutics. MJA: full-time employee and equity holder at DarwinHealth, Inc. AC: personal fees and other from DarwinHealth Inc., outside the submitted work. Patent applications pending, patents 62/211,373 and 62/211,562 filed on 8/28/15 pending to Columbia University, and a patent 62/253,342 provisional filed on 11/10/15 pending to Columbia University. BB: research support from Karyopharm Therapeutics Inc and Cell Therapeutics Inc. Advisory board honoraria from Cell Therapeutics Inc, Novartis, Astellas, and Kite Pharma. WD: research support from AbbVie, Aileron Therapeutics, Astex Pharmaceuticals, AstraZeneca, Bellicum Pharmaceuticals, Bristol-Myers Squibb, Cantex Pharmaceutics, Celgene, Celularity, CTI Biopharma, Forma Therapeutics, Forty Seven Inc, Genentech, H3 Biomedicine, Incyte, Janssen, Karyopharm Therapeutics Inc, Kite Pharma, MedImmune, Onconova Therapeutics, Pfizer, PTC Therapeutics, Seattle Genetics, Stemline Therapeutics, Takeda Pharmaceuticals, and Ryvu Therapeutics. Consulting fees from AbbVie, Amgen, Seattle Genetics, and PTC Therapeutics. OF: personal fees from AbbVie, Agios, Celgene, Jazz Pharmaceuticals, Bristol-Myers Squibb, and Pfizer. HD: personal fees from AbbVie, Agios, Amgen, Astellas, Astex Pharmaceuticals, Celgene, Janssen, Jazz Pharmaceuticals, Helsinn Healthcare, Novartis, Oxford Biomedica, and Roche. Research support from Amgen, Celgene, Jazz Pharmaceuticals, Novartis, AROG Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Sunesis Pharmaceuticals. MH: honoraria from Novartis, Pfizer, prIME Oncology Inc. Consulting fees from Novartis, Pfizer, AbbVie, Bayer, Daiichi Sankyo. Research support from Novartis, Pfizer, Bayer, Daiichi Sankyo, Astellas, BerGenBio, and Roche. CR: research support from AbbVie, Amgen, Bayer, Celgene, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, and Roche. Personal fees from AbbVie, Amgen, Astellas, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, and Roche. SK: advisory board honoraria from AbbVie, Celgene, Janssen, Takeda, Adaptive Biotechnologies, Kite Pharma, and MedImmune/AstraZeneca. Research support from AbbVie, Celgene, Janssen, Merck, Novartis, Roche, Sanofi, and Takeda. ER, MMS, SV, and PM: no conflict of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary material.
Additional information
Funding
References
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Acute Myeloid Leukemia. Version 2 [Internet]. 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- Boddu PC, Kantarjian HM, Ravandi F, et al. Characteristics and outcomes of older patients with secondary acute myeloid leukemia according to treatment approach. Cancer. 2017;123(16):3050–3060.
- O'Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 3.2017: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(7):926–957.
- Heiblig M, Elhamri M, Tigaud I, et al. Treatment with low-dose cytarabine in elderly patients (age 70 years or older) with acute myeloid leukemia: a single institution experience. Mediterr J Hematol Infect Dis. 2016;8:2016009.
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924.
- Wang ES. Treating acute myeloid leukemia in older adults. Hematol Am Soc Hematol Educ Program. 2014;2014(1):14–20.
- Krauss AC, Gao X, Li L, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–2690.
- Yilmaz M, Wang F, Loghavi S, et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood Cancer J. 2019;9.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Wei AH, Strickland SA, Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284.
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389.
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464.
- Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract Res Clin Haematol. 2019;32(2):145–153.
- DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with Ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398.
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740.
- Bross PF, Beitz J, Chen G, et al. Approval summary gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496.
- Gravina GL, Senapedis W, McCauley D, et al. Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol. 2014;7:85.
- Ranganathan P, Yu X, Na C, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012;120(9):1765–1773.
- Chari A, Vogl DT, Gavriatopoulou M, et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med. 2019;381(8):727–738.
- Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7(7):e511–e522.
- Etchin J, Sanda T, Mansour MR, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013;161(1):117–127.
- Hing ZA, Fung HYJ, Ranganathan P, et al. Next-generation XPO1 inhibitor shows improved efficacy and in vivo tolerability in hematological malignancies. Leukemia. 2016;30(12):2364–2372.
- Etchin J, Sun Q, Kentsis A, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia. 2013;27(1):66–74.
- Luedtke DA, Su Y, Liu S, et al. Inhibition of XPO1 enhances cell death induced by ABT-199 in acute myeloid leukaemia via Mcl-1. J Cell Mol Med. 2018;22(12):6099–6111.
- Garzon R, Savona M, Baz R, et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017;129(24):3165–3174.
- Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- Alvarez MJ, Shen Y, Giorgi FM, et al. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat Genet. 2016;48(8):838–847.
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. Blood. 2010;115(3):453–474.
- Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood. 2017;130(23):2469–2474.
- DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401–407.
- Stein EM, DiNardo CD, Fathi AT, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–687.
- Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–1075.
- Cortes JE, Tallman MS, Schiller GJ, et al. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. 2018;132(6):598–607.
- Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020;34:2430–2440.
- Wang AY, Weiner H, Green M, et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia. J Hematol Oncol. 2018;11(1):4.
- Bhatnagar B, Zhao Q, Mims AS, et al. Selinexor in combination with decitabine in patients with acute myeloid leukemia: results from a phase 1 study. Leuk Lymphoma. 2020;61(2):387–396.
- Sweet K, Komrokji R, Padron E, et al. Phase I clinical trial of selinexor in combination with daunorubicin and cytarabine in previously untreated poor-risk acute myeloid leukemia. Clin Cancer Res. 2020;26(1):54–60.
- Al-Ali HK, Jaekel N, Niederwieser D. The role of hypomethylating agents in the treatment of elderly patients with AML. J Geriatr Oncol. 2014;5(1):89–105.
- Gupta A, Singh M, Singh H, et al. Infections in acute myeloid leukemia: an analysis of 382 febrile episodes. Med Oncol. 2010;27(4):1037–1045.
- Renaud L, Nibourel O, Berthon C, et al. De novo and secondary acute myeloid leukemia, real world data on outcomes from the French Nord-Pas-De-Calais Picardie acute myeloid leukemia observatory. Blood. 2016;128(22):4013–4013.
- Ali AM, Weisel D, Gao F, et al. Patterns of infectious complications in acute myeloid leukemia and myelodysplastic syndromes patients treated with 10-day decitabine regimen. Cancer Med. 2017;6(12):2814–2821.
- Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312–1323.
- Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485.
- Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923–932.
- Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894–898.
- Medeiros BC. Is there a standard of care for relapsed AML? Best Pract Res Clin Haematol. 2018;31(4):384–386.
- Alexander TB, Lacayo NJ, Choi JK, et al. Phase I study of selinexor, a selective inhibitor of nuclear export, in combination with fludarabine and cytarabine, in pediatric relapsed or refractory acute leukemia. J Clin Oncol. 2016;34(34):4094–4101.