Myelofibrosis (MF) is a progressive myeloproliferative neoplasm characterized by bone marrow fibrosis, cytopenia, constitutional symptoms, splenomegaly, and shortened survival. Approximately 60% of patients with MF are anemic and 45% are red blood cell (RBC) transfusion-dependent within 1 year of diagnosis [Citation1], with most progressing to transfusion-dependency over time. Anemia and transfusion-dependency are among the most critical negative prognostic factors in MF [Citation2,Citation3], where patients who have severe anemia and transfusion-dependency experience a shortened median survival of 2.1 years [Citation4]. Moreover, transfusions themselves are burdensome to both patients and healthcare systems, pose significant risk of complications such as alloimmunization and iron toxicity [Citation5], and can negatively impact quality of life [Citation6,Citation7].
Agents currently recommended to treat MF-associated anemia (i.e. erythropoietin-stimulating agents, immunomodulatory drugs, and androgens such as danazol) demonstrate modest clinical activity that is especially poor in transfusion-dependent patients [Citation8–10]. Given the near ubiquity of anemia in patients with advanced MF, and the absence of effective therapies including those that directly address the underlying iron-restricted anemia commonly observed in MF patients and caused by chronic inflammation and elevated hepcidin levels, there remains a significant unmet medical need in anemic patients with MF.
Momelotinib is a potent Janus kinase (JAK) 1, JAK2, and activin A receptor type 1 (ACVR1, also known as activin receptor-like kinase-2 [ALK2]) inhibitor with a differentiated clinical profile that has been investigated in >820 patients with MF, including in two phase 3 studies in JAK inhibitor–naïve and previously ruxolitinib-treated patients [Citation11,Citation12]. Data from these and earlier studies indicate that momelotinib elicits a clinically significant anemia benefit with low hematological toxicity, accompanied by symptom control and reduction in splenic volume [Citation11,Citation12]. Preclinical and clinical studies demonstrate that the mechanistic basis of the anemia benefit occurs via ACVR1/ALK2 inhibition, which leads to suppression of elevated hepcidin levels and correction of iron sequestration associated with anemia of inflammation [Citation13,Citation14]. Specifically, in a rodent anemia of chronic disease model, momelotinib inhibited not only the interleukin-6 (IL-6)-JAK/STAT inflammatory pathway, but also the bone morphogenic protein 6 (BMP6)-ACVR1/SMAD1/5/8 iron-sensing pathway, resulting in decreased liver Hamp mRNA expression (encoding hepcidin), reduced serum hepcidin, increased serum iron, increased hemoglobin, and increased RBC production [Citation13]. Consistent with preclinical findings, daily momelotinib treatment in transfusion-dependent patients with MF in a phase 2 translational biology study led to an acute and persistent decrease in circulating hepcidin, increased iron availability, and improved erythropoiesis [Citation14].
Here, we report novel findings from retrospective, dynamic, and time-to-event analyses from the phase 3, double-blind, ruxolitinib-controlled SIMPLIFY-1 trial in JAK inhibitor–naïve MF subjects (n = 432; NCT01969838) that more completely characterize momelotinib’s differentiated clinical profile.
Analysis of SIMPLIFY-1 patient hemoglobin concentrations over time showed that treatment with momelotinib resulted in a rapid, <1 g/dL increase in mean hemoglobin, which was significantly higher at week 24 compared to baseline (p < 0.001; ) and compared to the mean hemoglobin in patients treated with ruxolitinib (p < 0.001), which decreased significantly by week 24 compared to baseline (p < 0.001). Hemoglobin levels were maintained over time in momelotinib-randomized patients in the open-label phase. In the 91.2% of ruxolitinib-randomized patients who crossed over to momelotinib at week 24, a rapid and sustained increase in mean hemoglobin was also observed, with levels approaching those in patients originally randomized to momelotinib ().
Figure 1. Momelotinib-treated patients experienced increased hemoglobin levels and reduced transfusion requirements over those seen in ruxolitinib-treated patients in SIMPLIFY-1. (A) Mean hemoglobin concentrations over time for patients receiving momelotinib or ruxolitinib. In the 24-week double-blind period a statistically significant increase in mean hemoglobin concentration was observed in patients receiving momelotinib (p < 0.001), in contrast to a statistically significant decrease in patients receiving ruxolitinib (p < 0.001). Patients crossing over from ruxolitinib to momelotinib at the end of randomized treatment experienced a rapid and sustained increase in hemoglobin, with levels approaching those in patients originally randomized to momelotinib. (B–D) KM survival function estimates of (B) time-to-first, (C) time-to-third, and (D) time-to-fifth RBC transfusions for patients receiving momelotinib versus ruxolitinib, with 95% CIs shaded. (B) 73% of patients on momelotinib compared to 46% on ruxolitinib did not receive a transfusion unit by week 24 (log-rank p < 0.0001). (C) Odds of receiving <3 transfusion units were 3.6 times higher on momelotinib (81%) compared to ruxolitinib (54%, log-rank p < 0.0001). (D) Odds of receiving <5 transfusion units were 3.0 times higher on momelotinib (83%) compared to ruxolitinib (62%, log-rank p < 0.0001). (E) Cumulative transfusion burden for patients receiving momelotinib versus ruxolitinib, with 95% CIs shaded. The HR for a RBC unit transfused for patients receiving momelotinib was approximately one-half that for patients receiving ruxolitinib (HR 0.522; p < 0.0001), suggesting that at any time point, an average patient on ruxolitinib would receive twice as many RBC transfusions compared with an average patient on momelotinib. (F) KM survival function estimate of the durability of transfusion-independence for patients randomized to momelotinib. Time from first transfusion-independence rolling 12-week response (week 0 to week 24) to first failure (any time; event = any transfusion OR hemoglobin level <8 g/dL) is shown. The median time-to-loss of transfusion-independence was not reached for randomized momelotinib-treated patients, with a follow-up period exceeding 3 years. CI: confidence interval; HR: hazard ratio; KM: Kaplan-Meier; RBC: red blood cell.
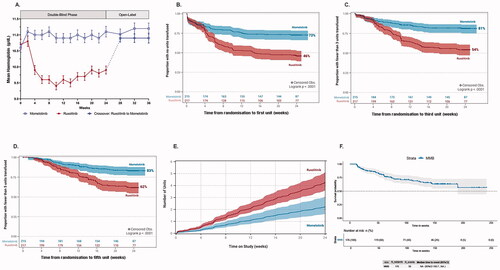
As previously reported [Citation11], in SIMPLIFY-1 more momelotinib-treated patients achieved RBC transfusion-independence (67%) at week 24 than did patients receiving ruxolitinib (49%; p < 0.001) and fewer were RBC transfusion-dependent (30% vs. 40%, respectively; p = 0.019) [Citation11]. Taking into account transfusion status at baseline, we found that patients who were initially transfusion-independent were significantly more likely to remain transfusion-independent at week 24 when treated with momelotinib compared to ruxolitinib (81% vs. 62%; p < 0.001). A significantly higher rate of conversion from transfusion-dependence to -independence for any 12-week period during the randomized phase was also observed during treatment with momelotinib compared to ruxolitinib (44% vs. 25%; p = 0.034).
For a chronic disease where survival is typically measured in years, the use of dichotomous endpoints at a single landmark timepoint provides a limited view of treatment response. To better contextualize the differences in transfusion burden between momelotinib and ruxolitinib in the SIMPLIFY-1 study, we employed the zero-inflated negative binominal (ZINB) model to compare the proportions of patients with zero transfusions (i.e. transfusion-free) for each treatment arm during the randomized treatment period. When baseline covariates of disease diagnosis (primary, post–polycythemia vera, or post–essential thrombocythemia MF), International Prognostic Scoring System (IPSS) status, bone marrow fibrosis grade, and number of RBC units transfused in the 8 weeks prior to randomization were included in the model, the odds of zero RBC units transfused in SIMPLIFY-1 were 9.69 times higher for patients receiving momelotinib than ruxolitinib (p < 0.0001), with the model predicting that a typical patient had an 83% chance of receiving no transfusions on momelotinib versus only a 34% chance when receiving ruxolitinib (). Further, the model predicted higher probability of zero transfusions for momelotinib compared to ruxolitinib within critical baseline groups, such as patients with baseline IPSS High and baseline bone marrow fibrosis grade 3 (). These data emphasize momelotinib’s ability to reduce transfusion burden in patients with MF compared to ruxolitinib.
Table 1. Probability of zero transfusions was greater for patients receiving momelotinib versus ruxolitinib in SIMPLIFY-1.
Consistent with the ZINB findings, Kaplan-Meier (KM) analyses of the time-to-first, time-to-third, and time-to-fifth RBC units transfused for momelotinib and ruxolitinib demonstrated a significant benefit of momelotinib therapy for patients not dependent on transfusions as well as for those who maintained a transfusion requirement. In these analyses, more patients randomized to momelotinib received zero transfusions (KM estimate: 73%) compared to patients randomized to ruxolitinib (46%) during the 24-week randomized treatment period (log-rank p < 0.0001; ). Similar findings were noted for the burden of <3 and <5 RBC units transfused by week 24, in which the proportion of patients with fewer transfusions was greater in the momelotinib arm than in the ruxolitinib arm. Specifically, the odds of receiving <3 transfusion units by 24 weeks were 3.6 times higher for patients taking momelotinib (81%) compared to ruxolitinib (54%, log rank p < 0.0001; ), and the odds of receiving <5 transfusion units were 3.0 times higher for patients taking momelotinib (83%) compared to ruxolitinib (62%, log rank p < 0.0001; ).
Cumulative transfusion burden analysis using recurrent events modeling indicated that the hazard ratio (HR) for an RBC unit transfused for patients receiving momelotinib was approximately one-half that for patients receiving ruxolitinib (HR 0.522; p < 0.0001; ). This suggests that an average patient on ruxolitinib would receive twice as many RBC transfusions at any time point in the SIMPLIFY-1 study than a patient on momelotinib would receive.
Finally, KM analysis of duration of transfusion-independence indicated that the median time-to-loss of transfusion-independence was not reached for momelotinib-randomized patients within a follow-up period exceeding 3 years (), suggesting that transfusion-independence from momelotinib is durable.
Taken together, these data further characterize momelotinib’s unique and clinically relevant anemia benefit observed in earlier clinical studies, with momelotinib-treated patients experiencing increased hemoglobin levels, a markedly greater chance of zero transfusions, extended duration of transfusion-independence, and administration of fewer transfusions among those who are transfusion-requiring. While use of a dichotomous, landmark endpoint of clinical response such as achieving transfusion independence at week 24 provides an informative data point in evaluating MF therapies, assessment of multiple anemia-related endpoints over time and across the continuum of transfusion-independent, transfusion-requiring, and transfusion-dependent patients can provide meaningful clinical insights into a greater spectrum of therapeutic benefits. The presented findings support momelotinib as a potential treatment option for the significant number of MF patients with anemia or at risk of developing anemia due to progressive disease and/or exacerbation by currently approved JAK inhibitors, including ruxolitinib and fedratinib.
Momelotinib therapy elicits mechanistically driven and differentiated anemia benefits through its inhibition of ACVR1/ALK2, in addition to JAK1 and JAK2, thereby improving the iron-restricted anemia of inflammation. Momelotinib’s anemia benefits are currently being further investigated in MOMENTUM (NCT04173494), a global phase 3 clinical trial in symptomatic and anemic patients previously treated with an approved JAK inhibitor, that is intended to support potential registration of momelotinib for the treatment of MF. In addition to assessments of constitutional symptoms, transfusion-independence, and splenomegaly, MOMENTUM will provide an opportunity to evaluate associations between anemia benefit, transfusion burden, and patient-reported measures of clinical benefit.
Acknowledgments
Momelotinib is sponsored by Sierra Oncology. Editorial support was provided by Impact Communication Partners, Inc.
Disclosure statement
R. M. has received clinical research funding from Celgene/Bristol Myers Squibb, Incyte, Samus, Genentech, Promedior, and CTI BioPharma, and has served as a consultant for Novartis, Sierra Oncology, and La Jolla Pharmaceutical Company. S. T. O has served as a consultant for Incyte, Gilead Sciences, Novartis, Celgene/Bristol Myers Squibb, Blueprint Medicines, Kartos Therapeutics, Disc Medicine, and CTI BioPharma. A. T. G has served as a consultant for Celgene/Bristol Myers Squibb, Pfizer, Kartos Therapeutics, Promedior, and CTI BioPharma. V. G has received clinical research funding from Novartis and Incyte, and has served as a consultant for Novartis, Sierra Oncology, AbbVie, Celgene/Bristol Myers Squibb, CTI Biopharma, Roche, and Pfizer. F. C has served as a consultant for Celgene/Bristol Myers Squibb on its advisory board and has served on the speakers bureau for Celgene/Bristol Myers Squibb and Novartis. T. D has served as a consultant for Novartis, Celgene/Bristol Myers Squibb and Incyte on their advisory boards. J. –J. K. has served as a consultant for Novartis, Celgene/Bristol Myers Squibb, and AOP Orphan Pharmaceuticals on their advisory boards. E. L. -M. has served as a consultant for Roche, Amgen, Gilead Sciences, AbbVie, Janssen-Cilag, Takeda, and Novartis on their advisory boards. D. M. has served on the speakers bureau for Novartis, Celgene/Bristol Myers Squibb, and Jazz Pharmaceuticals. J. P. has received research funding from PharmaEssentia, Sierra Oncology, CTI BioPharma, Protagonist Therapeutics, and Incyte, and has served as an advisor for Sierra Oncology, CTI BioPharma, and PharmaEssentia. U. P. has served as a consultant for Celgene/Bristol Myers Squibb, Novartis, Amgen, AbbVie, and Takeda. J. T. has served as a consultant for Novartis, Amgen, Takeda, Novo Nordisk, Baxalta, Sobi, and Roche. K. S. has served on the speakers bureau for Celgene/Bristol Myers Squibb, Takeda, and Novartis. KD’H has served as a consultant for Sierra Oncology. S. V. has received clinical research funding from Incyte, Roche, NS Pharma, Celgene/Bristol Myers Squibb, Gilead, Promedior, CTI BioPharma, Genentech, Blueprint Medicines, Novartis, Sierra Oncology, PharmaEssentia, AstraZeneca, Italfarmaco, and Protagonist Therapeutics, and has served as a consultant for Sierra Oncology, Incyte, Novartis, and Celgene/Bristol Myers Squibb. R. D. and M. K. are employees of Sierra Oncology. J. C. and M. H. report no conflicts of interest.
Data availability statement
Sierra Oncology, Inc., supports the principles of clinical trial data transparency, including registration and disclosure of clinical trial results in external registries and publication of results in peer-reviewed journals. Clinical trial results for SIMPLIFY-1 (NCT01969838) have been previously published (Mesa RA, et al. J Clin Oncol. 2017;35:3844-3850) and will be posted to ClinicalTrials.gov and other public registries in accordance with the requirements of each jurisdiction in which the study was conducted. Inquiries regarding availability of data and clinical trial documentation should be directed to: [email protected].
Additional information
Funding
References
- Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo clinic experience. Mayo Clin Proc. 2012;87(1):25–33.
- Elena C, Passamonti F, Rumi E, et al. Red blood cell transfusion-dependency implies a poor survival in primary myelofibrosis irrespective of IPSS and DIPSS. Haematologica. 2011;96(1):167–170.
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (international working group for myeloproliferative neoplasms research and treatment). Blood. 2010;115(9):1703–1708.
- Nicolosi M, Mudireddy M, Lasho TL, et al. Sex and degree of severity influence the prognostic impact of anemia in primary myelofibrosis: analysis based on 1109 consecutive patients. Leukemia. 2018;32(5):1254–1258.
- Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009;97(3):185–197.
- Bartoszko J, Panzarella T, Lau A, et al. Effect of red blood cell transfusion dependence on the natural history of myeloproliferative neoplasm-associated myelofibrosis. Clin Lymphoma Myeloma Leuk. 2015;15(11):e151–e156.
- Naymagon L, Mascarenhas J. Myelofibrosis-related anemia: current and emerging therapeutic strategies. Hemasphere. 2017;1(1):e1.
- Cervantes F, Correa JG, Hernandez-Boluda JC. Alleviating anemia and thrombocytopenia in myelofibrosis patients. Expert Rev Hematol. 2016;9(5):489–496.
- Hernandez-Boluda JC, Correa JG, Garcia-Delgado R, et al. Predictive factors for anemia response to erythropoiesis-stimulating agents in myelofibrosis. Eur J Haematol. 2017;98(4):407–414.
- Luo X, Xu Z, Li B, et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer J. 2018;8(1):9.
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844–3850.
- Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73–e81.
- Asshoff M, Petzer V, Warr MR, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129(13):1823–1830.
- Oh ST, Talpaz M, Gerds AT, et al. Hepcidin suppression by momelotinib is associated with increased iron availability and erythropoiesis in transfusion-dependent myelofibrosis patients. Blood. 2018;132(Supplement 1):4282–4282.
