Abstract
Chimeric antigen receptor T-cell (CAR-T) infusion settings may impact healthcare resource use (HRU) and reimbursement amounts. Adults with diffuse large B-cell lymphoma receiving CAR-T therapy were identified from the Centers for Medicare & Medicaid Services (CMS) 100% fee-for-service Medicare database and stratified into inpatient (IP; n = 380) and outpatient (OP; n = 50) cohorts based on CAR-T infusion setting. During the first month post-infusion, OP cohort had significantly fewer IP visits, IP days, intensive care unit (ICU) stays, ICU days, and significantly more OP, emergency room (ER) visits, than IP cohort. In subsequent months, HRU became comparable between cohorts. Medicare reimbursement amounts during the first month post-infusion were nominally higher in the OP vs. IP cohort and comparable in subsequent months. The reimbursement amounts did not reflect the reduced HRU with OP infusions, potentially due to differences in Medicare payment policies for OP vs. IP services.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) and accounts for more than 30% of newly diagnosed NHL cases in the United States (US) [Citation1,Citation2]. The standard first-line treatment for DLBCL is chemo-immunotherapy [Citation2,Citation3]. However, about one-third of patients either experience relapse or are refractory to first-line treatment [Citation4]. Younger patients with relapsed or refractory (r/r) DLBCL could receive salvage chemotherapy followed by autologous hematopoietic stem cell transplant. However, to benefit from such therapy, the disease must be chemo-sensitive [Citation3] and only about 35–45% of patients with relapsed disease would attain survival benefits with this approach [Citation5–7]. Prior to the introduction of chimeric antigen receptor T-cell (CAR-T) treatments, older patients and patients with refractory disease had limited treatment options [Citation3,Citation8].
CAR-T therapy is a novel treatment for lymphomas that employs genetically engineered autologous T cells [Citation2]. In recent years, three CAR-T therapies have been approved by the US Food and Drug Administration (FDA) – axicabtagene ciloleucel (axi-cel) in 2017, tisagenlecleucel (tisa-cel) in 2018, and lisocabtagene maraleucel (liso-cel) in 2021 – for treatment of r/r DLBCL patients with two or more prior lines of systemic therapy [Citation9–11]. While the majority of CAR-T infusions occur in an inpatient (IP) setting, CAR-T infusion can be performed as an outpatient (OP) procedure [Citation12,Citation13]. Of note, the pivotal trial of axi-cel (ZUMA-1) mandated administration in an IP setting [Citation13–15], while the trial of tisa-cel (JULIET) did not have similar restrictions, and both IP and OP administration were conducted [Citation13,Citation15]. A real-world analysis across eight US academic centers from 1 May 2017 to 31 July 2019 showed that 92% of patients received axi-cel infusion in an IP setting vs. 37% among patients who received tisa-cel [Citation16].
The infusion of CAR-T therapy in IP vs. OP settings could have important implications, including potential reduction in healthcare resource use (HRU) and improved accessibility to treatment with the OP care option [Citation12,Citation17]. To date, limited data are available on the impact of CAR-T administration setting on HRU and the associated payer reimbursement amounts. To address this gap in research, this study was conducted to assess and compare the real-world HRU and Medicare reimbursement amounts between IP and OP CAR-T infusions among patients with DLBCL.
Methods
Data source and study design
Data from the Centers for Medicare & Medicaid Services (CMS) 100% Medicare claims database were used. Medicare claims data used in this study included de-identified administrative claims from Medicare Parts A and B (2017Q1-2019Q4) and Part D (2017Q1-2018Q4) (Supplementary Methods). A retrospective, non-interventional cohort study was conducted. The index date was defined as the date of CAR-T therapy infusion. The index encounter for the IP cohort was defined as the IP admission episode in which CAR-T infusion occurred, whereas an index encounter for the OP cohort was defined as the OP visit in which CAR-T infusion occurred. The baseline period was defined as 3 months prior to the index date. The study period was defined from the index date to the end of health plan coverage based on insurance enrollment file or death, whichever occurred earlier. Patients were classified into the CAR-T IP and CAR-T OP cohorts based on the index CAR-T administration/infusion setting. Resource use and Medicare reimbursement amounts in the CAR-T IP and OP cohorts during the study period were assessed. An Institutional Review Board exemption letter for this research was acquired prior to data access.
Patient population
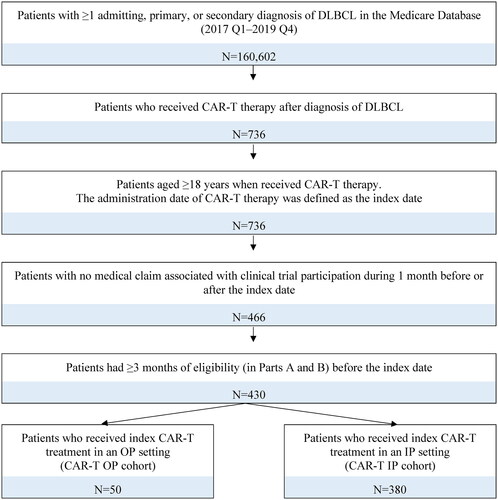
Adult patients were included in the study if they met the following criteria: had ≥1 diagnosis of DLBCL; received CAR-T therapy following DLBCL diagnosis; and had ≥3 months of continuous eligibility in Medicare Parts A and B prior to CAR-T administration. Patients with claims associated with a clinical trial during the 1 month before and after the index date were excluded. Diagnosis and procedure codes considered are listed in Supplementary Methods.
Study measures
The study outcomes included HRU and Medicare reimbursement amounts. In addition, baseline characteristics were measured at 3 months pre-index and included demographics, treatment characteristics, and comorbidities [Citation18]. HRU was assessed during the study period and included IP admissions, intensive care unit (ICU) stays, OP visits, and emergency room (ER) visits. The proportions of patients among the CAR-T IP and OP cohorts who were hospitalized each day during the first month after CAR-T infusion were described. Total reimbursement amounts (i.e. CAR-T infusion and post-infusion reimbursement amounts, including reimbursement for IP, ER, OP, other medical services and pharmacy drugs; pharmacy reimbursement might be an underestimation as 2019 Part D data were not available for the study) during the study period were assessed. Reimbursement amount refers to the amount paid by Medicare to the provider for a particular claim, which do not reflect the actual costs incurred by the hospital. All reimbursement amounts were inflated to 2020 United State Dollars. For the CAR-T IP cohort, reimbursement amounts were only assessed among patients who received CAR-T in non-Prospective Payment System (PPS)-exempt hospitals, because the reimbursement amounts for IP admissions in PPS-exempt hospitals were much lower compared to non-PPS-exempt hospitals due to specific policies in place for PPS-exempt hospitals and the fact that PPS-exempt hospitals are not common.
Statistical analysis
Baseline characteristics were reported as means and standard deviations for continuous variables and as frequency counts and percentages for categorical variables. Baseline characteristics were described and compared between CAR-T IP vs. CAR-T OP cohorts using Wilcoxon’s rank-sum tests for continuous variables and Chi-square tests or Fisher’s exact tests for categorical variables. HRU by month during the study period was expressed as monthly incidence, calculated as the total number of events divided by total follow-up months. HRU was compared between cohorts using generalized linear models (GLMs) with a Poisson distribution, with an offset to account for varying follow-up times between patients. Adjusted incidence rate ratios (IRRs) with their respective 95% confidence intervals (CIs) and p values were reported. Adjusted models controlled for patient age at the index date, gender, race (white vs. non-white race), and National Cancer Institute Comorbidity Index (NCICI). Total reimbursement amounts were described by month on a per-patient-per-month basis during the study period. Reimbursement amounts were compared using GLMs with a Tweedie distribution. Adjusted cost differences and p values comparing CAR-T OP vs. CAR-T IP non-PPS-exempt cohorts were reported, with the adjusted models controlled for patient age at the index date, gender, race (white vs. non-white race), and NCICI.
Results
Sample selection
A total of 430 adult patients with DLBCL who received CAR-T therapy and met the study criteria were identified. Based on the administration settings of the CAR-T therapy, 380 patients were assigned to the CAR-T IP cohort and 50 patients were assigned to the CAR-T OP cohort (). With data available in the current study, only tisa-cel and axi-cel were captured. Specific CAR-T regimens were not identifiable in the CAR-T IP cohort, as tisa-cel and axi-cel share the same procedure codes. For the CAR-T OP cohort, 41 (82%) patients had an identifiable CAR-T, the majority (>90%) of whom were treated with tisa-cel.
Baseline characteristics
Baseline patient characteristics at 3 months pre-index are summarized in . The results showed that age and gender were similar between the CAR-T IP and OP cohorts. The majority of patients were White in both CAR-T IP and OP cohorts (84.5% and 96.0%, respectively), while only 3.2% and 0% of patients were Black in the respective cohorts. Most patients received CAR-T therapy in 2019. NCICI score was significantly higher in the CAR-T IP cohort compared with the CAR-T OP cohort (p = 0.036). The most prevalent comorbidities in the IP and OP cohorts were hypertension, peripheral vascular disease, diabetes, and chronic obstructive pulmonary disease.
Table 1. Baseline characteristicsTable Footnotea.
Comparison of HRU in CAR-T IP vs. OP cohorts
The median length of follow-up for the CAR-T IP cohort and CAR-T OP cohort was 6.6 months (range: 0.0–24.2) and 6.0 months (range: 0.1–21.1), respectively. In the first month following the index date, 52.0% of patients in the CAR-T OP cohort had ≥1 IP visit. By definition, 100% of patients in the CAR-T IP cohort had ≥1 IP visit. In the second month following the index date, the proportion of patients with ≥1 IP visit was 21.7% in the CAR-T OP cohort and 34.6% in the CAR-T IP cohort. At the index encounter, i.e. hospitalization episode of CAR-T IP cohort, the mean length of stay (LOS) per episode was 16.8 days, including ICU stay; 27% of all patients in the CAR-T IP cohort had an ICU admission during the index encounter (mean LOS per ICU was 6.7 days). A total of 52.0% of CAR-T OP cohort ended up with a hospitalization during the first month following the index date; the mean LOS of first IP admission, including ICU stay, was 6.8 days per episode.
During the first month following the index date, the number of IP admissions was significantly lower among the CAR-T OP cohort compared to the CAR-T IP cohort (0.73 vs. 1.44; adjusted IRR [95% CI]: 0.51 [0.41, 0.63]; p < 0.001). During the second month following the index date, the number of IP admissions was nominally lower in the CAR-T OP cohort compared to the CAR-T IP cohort (0.36 vs. 0.47; adjusted IRR [95% CI]: 0.75 [0.39, 1.43]; p = 0.386) (; ). A similar trend was observed during the third and fourth month following the index date, while the number of IP admissions was comparable between the cohorts in subsequent months (). The number of IP days were significantly lower among the CAR-T OP cohort compared to the CAR-T IP cohort during first and second month following the index date (first month: 5.2 vs. 20.4 days; adjusted IRR [95% CI]: 0.26 [0.21, 0.32]; p < 0.001; second month: 1.6 vs. 4.6 days; adjusted IRR [95% CI]: 0.35 [0.13, 0.92]; p = 0.033) (; ); the number of IP days were nominally lower in the CAR-T OP cohort in subsequent months (). The number of ICU stays and ICU days were significantly lower among the CAR-T OP cohort compared to the CAR-T IP cohort in the first month following the index date. During the second month following the index date, the number of ICU stays was comparable between the CAR-T OP and CAR-T IP cohorts; the mean number of ICU days was nominally lower in the CAR-T OP cohort (; ). Meanwhile, the number of OP visits and ER visits during the first month following the index date were significantly higher among the CAR-T OP cohort (OP visits: 5.2 vs. 2.1; adjusted IRR [95% CI]: 2.51 [2.08, 3.04]; p < 0.001; ER visits: 0.32 vs. 0.03; adjusted IRR [95% CI]: 10.44 [4.23, 25.75]; p < 0.001). During the second month following the index date, the number of OP visits was nominally lower in the CAR-T OP cohort (3.20 vs. 3.81; adjusted IRR [95% CI]: 0.81 [0.65, 1.01]; p = 0.062), while the number of ER visits was nominally higher in the CAR-T OP cohort (0.14 vs. 0.10; adjusted IRR [95% CI]: 1.42 [0.52, 3.90]; p = 0.494); the number of OP visits and ER visits was generally comparable between the cohorts in subsequent months (; ).
Figure 2. Comparison of HRU between CAR-T OP vs. CAR-T IP during the study period. (A) IP admission, (B) IP days, (C) ICU stays, (D) ICU days, (E) OP visits, and (F) ER visits. CAR-T: chimeric antigen receptor T cells; CI: confidence interval; ER: emergency room; HRU: healthcare resource use; ICU: intensive care unit; IP: inpatient; IRR: incidence rate ratio; OP: outpatient. *Statistical significance (p < 0.05).
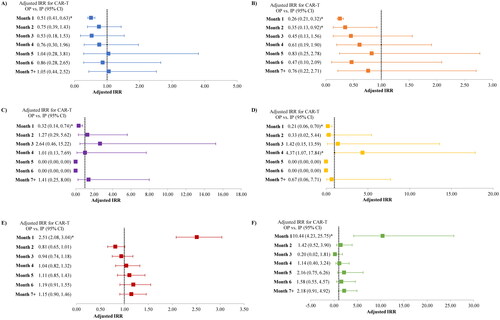
Table 2. HRU in CAR-T IP and OP cohorts during the follow-up period (monthly incidence)Table Footnotea,Table Footnoteb.
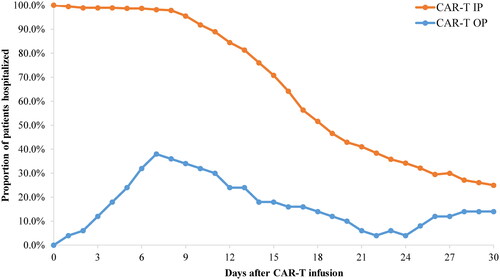
Proportion of patients hospitalized during first month post-infusion
Over the first month following CAR-T infusion, the proportion of patients hospitalized at any given day was higher in the CAR-T IP cohort compared to the CAR-T OP cohort (). In the CAR-T IP cohort, the proportion of hospitalized patients decreased over time during the first month post-infusion, and 25.0% of patients were hospitalized at day 30 post-infusion. In the CAR-T OP cohort, the proportion of hospitalized patients peaked at day 7 (38%) and decreased over time thereafter. Twelve percent of the CAR-T OP cohort was hospitalized at day 3 after the infusion date. At day 30 post-infusion, 14.0% of patients in the CAR-T OP cohort were hospitalized. The proportion of patients who were ever hospitalized during 30 days post-infusion was 52.0% for the CAR-T OP cohort.
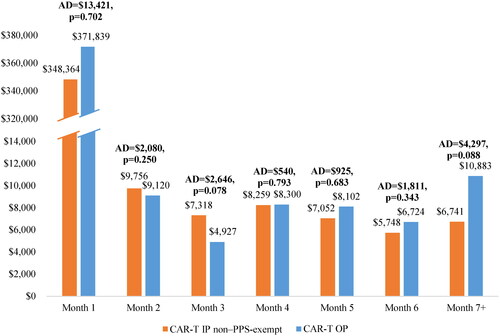
Total Medicare reimbursement amounts in CAR-T OP vs. IP non-PPS-exempt cohorts
The total Medicare reimbursement amounts among the CAR-T OP and IP non-PPS-exempt cohorts during the study period are shown in . During the first month following the index date (including the index encounter), total reimbursement amount was nominally higher in the CAR-T OP cohort compared to the CAR-T IP non-PPS-exempt cohort, with an adjusted difference of $13,421 between the cohorts. The difference in reimbursement amounts was primarily due to CAR-T infusion-related costs (e.g. CAR-T product costs and associated hospitalization) and payments for subsequent hospital stays and OP visits. Specifically, the adjusted CAR-T infusion-related reimbursement amount was nominally higher in the CAR-T OP cohort than in the CAR-T IP non-PPS-exempt cohort by $4913, while the reimbursement amount of IP admissions was significantly higher in the CAR-T IP non-PPS-exempt cohort compared to the CAR-T OP cohort by $334,308 (p < 0.001), and that of OP visits was significantly higher in the CAR-T OP cohort compared to the CAR-T IP non-PPS-exempt cohort by $363,607 (p < 0.001). During the second month following the index date, total reimbursement amount was nominally lower in the CAR-T OP cohort, with an adjusted difference of –$2080 when compared to the CAR-T IP non-PPS-exempt cohort. Similarly, the total reimbursement amount from month 3 onward was not significantly different between the CAR-T OP and CAR-T IP non-PPS-exempt cohorts.
Discussion
This retrospective cohort study found that among Medicare beneficiaries with DLBCL, those who received CAR-T therapy in an IP setting had significantly higher resource use in respect to the number of IP and ICU stays and duration of IP and ICU during the first month post-infusion compared with those who received CAR-T therapy in an OP setting. Specifically, the average IP days in the first month following CAR-T infusion, including index encounter, were 20.4 days for the CAR-T IP cohort and 5.2 days for the CAR-T OP cohort, in which >90% of patients received tisa-cel. These findings are in line with those reported by Riedell et al. in an abstract published by Transplantation and Cellular Therapy Conference. The authors conducted a real-word analysis of axi-cel and tisa-cel use and outcomes across eight academic centers in the US [Citation19]. Their study showed that median IP days within the 28-day period post-infusion was 16 days among axi-cel-treated patients (92% of whom received IP infusion) and 2 days among tisa-cel-treated patients (37% of whom received IP infusion).
The current study revealed an increased use of OP administration of CAR-T from 2018 to 2019 (9% of all infusions were OP infusion in 2018, which increased to 13% in 2019). In fact, OP administration of CAR-T therapy is not uncommon in the real world [Citation20,Citation21], with reported rates from 5% to 8% for axi-cel to 37% for tisa-cel [Citation16,Citation22,Citation23]. The feasibility of OP administration relies on several factors, including availabilities of infrastructures and resources, patient circumstances and preferences, as well as the severity and time to onset of adverse events of different therapies [Citation12,Citation13]. With increased experience in OP administration of CAR-T therapy supplemented by real-world data, healthcare providers begin to have a better understanding on the safety profiles of CAR-T therapies, which will increase their ability to perform routine OP infusion. Of note, the safety profiles of CAR-T therapies generally include cytokine release syndrome (CRS) and neurotoxicity [Citation14,Citation24,Citation25]. Axi-cel is associated with 11% and 32% of grade ≥3 CRS and neurotoxicity, respectively, in the ZUMA-1 trial [Citation15], with similar rates observed in real-world studies [Citation16,Citation23,Citation26]; these events occurred in 22% and 12% of patients treated with tisa-cel in the JULIET trial [Citation27], and the rates appeared lower in real-world settings [Citation16,Citation28]. Real-world data from the Center for International Blood and Marrow Transplant Research (CIBMTR) cellular therapy registry revealed that 9% and 58% of patients treated with axi-cel had grade ≥3 CRS and neurotoxicity, respectively, compared with 4.3% for each of the event among patients treated with tisa-cel [Citation28,Citation29]. The potential relationship between the higher proportion of OP infusions with tisa-cel [Citation16,Citation22,Citation23] and the reduced frequency of these severe adverse events in the real world warrants further investigations. Extensive experience has been accumulated from multiple prospective and retrospective studies to aid the better management of complications associated with CAR-T infusion. For instance, both clinical trial and real-world evidence have demonstrated the benefits of earlier use of tocilizumab and corticosteroids in reducing the risk of developing high-grade CRS and neurotoxicity [Citation13,Citation30–33]. Collectively, the continuous advancements in knowledge of adverse event management may further increase physicians’ and patients’ confidence in CAR-T infusions provided in OP settings. Nevertheless, the administration of CAR-T therapy requires close monitoring of patients and rapid responses from clinical teams to manage potential adverse events that may arise. In this regard, specific clinical guidelines as well as trainings for providers, patients, and caregivers are necessary for the successful delivery of CAR-T infusions in the OP setting [Citation13].
Our analysis shows the majority of payer reimbursement for CAR-T-treated patients are for CAR-T infusion-related costs (including CAR-T product costs and associated hospitalization) and payment for subsequent hospital stays. Based on the study findings, while resource use is generally lower in the CAR-T OP cohort, total reimbursement amount is nominally higher than that in the CAR-T IP cohort. Under the Medicare program, hospital IP reimbursement is calculated based on a prospectively determined base payment rate using an assigned payment classification known as Medicare Severity Diagnosis-Related Group (MS-DRG), with additional reimbursements covered through the new technology add-on payment (NTAP) and outlier payments [Citation34]. Prior to 2021, there was no specific DRG code for IP stays associated with CAR-T therapy, and such stays were assigned to DRG 016 (autologous bone marrow transplant with CC/MCC or T-cell immunotherapy). Even with adjustments of NTAP and outlier payments, Medicare reimbursement for CAR-T IP cases often failed to cover total hospital costs [Citation35]. CMS has recognized the disconnection and a new MS-DRG code specific for CAR-T treatment stay has been generated in 2021, which increased the base payment rate to $239,929 compared to $43,094 in the prior year [Citation20,Citation34]. Nonetheless, the amount covered under the new code remains insufficient to measure up the total costs incurred by CAR-T IP administration, and hospitals still bear significant financial burden for providing the treatment [Citation34]. Meanwhile, OP reimbursement is covered under Medicare Part B, which covers the full cost of the product plus handling and storage costs, as well as part of the physician’s service cost determined based on the Outpatient Prospective Payment System (OPPS) [Citation34]. The differences in reimbursement approaches for IP and OP services and the period covered in the current study (i.e. prior to the introduction of the new MS-DRG code in 2021) may have contributed to the observed differences in reimbursement between the current CAR-T IP and OP cohorts. In line with this concept, a recent real-world study [Citation17] assessing the costs associated with CAR-T therapy for r/r DLBCL from healthcare practitioner perspective (i.e. the costs to all practitioners involved in a patient’s treatment) found that the total cost incurred by OP infusion in nonacademic specialty oncology networks was about 40% less compared with IP infusion in academic hospitals. Nonetheless, our findings based on Medicare reimbursement amounts suggest that CAR-T infusion in IP settings may be subject to the risk of inadequate reimbursement, which leads to financial strain for some medical centers. Taken together, additional efforts are needed to continue improve the reimbursement structures and care policies for CAR-T therapy for all infusion settings to increase providers’ incentive to deliver this life-saving treatment, which will ultimately benefit patients.
The findings of this study should be interpreted in light of several limitations. The samples were identified from the Medicare database, and thus the results might not be generalizable to patients outside the Medicare population (i.e. elderly and disabled Medicare beneficiaries). Additionally, the sample size is limited, especially for the CAR-T OP cohort, which reduces the power of the study. The study is also subject to common limitations that are inherent to retrospective observational studies using claims data, which include the lack of clinical information, such as disease severity or prognostic factors, and potential coding errors or data omissions.
Collectively, evidence suggests that OP CART-T infusion is feasible and is increasingly used in the US. Among patients who received CAR-T in the OP setting in the current study, over nine in 10 patients received tisa-cel. During the first month following CAR-T infusion and including the index encounter, IP and ICU resource use was significantly higher in the CAR-T IP cohort. Despite an overall lower HRU, reimbursement during the first month was nominally higher for CAR-T OP cohort than IP cohort, which reflects different Medicare reimbursement policies for IP and OP CAR-T infusions.
Author contributions
All authors were involved in the following aspects of the research: the conception and design of the study, or analysis and interpretation of the data; drafting of the paper and revising it critically for intellectual content; and the decision to submit the manuscript for publication. All authors agree to be accountable for all aspects of the work.
ilal_a_2147395_sm5135.docx
Download MS Word (46.8 KB)Acknowledgements
Medical writing assistance was provided by Flora Chik, PhD, an employee of Analysis Group, Inc.
Disclosure statement
Vamsi Bollu, Stephen Lim, Mimi Tesfaye, and Anand A. Dalal are employees of Novartis Pharmaceutical Company and may own stock or stock options. Hongbo Yang, Angela Lax, and Sakshi Sethi are employees of Analysis Group Inc. and Jing Zhao is a former employee of Analysis Group Inc. and received payments from Novartis to conduct the research.
Additional information
Funding
References
- Cheson BD, Nowakowski G, Salles G. Diffuse large B-cell lymphoma: new targets and novel therapies. Blood Cancer J. 2021;11(4):68.
- Susanibar-Adaniya S, Barta SK. 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. 2021;96(5):617–629.
- American Cancer Society (ACS). Treating non-Hodgkin lymphoma. Atlanta (GA): American Cancer Society (ACS); 2018. p. 1–49.
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011(1):498–505.
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190.
- Hamlin PA, Zelenetz AD, Kewalramani T, et al. Age-adjusted international prognostic index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102(6):1989–1996.
- Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19(2):406–413.
- Maziarz RT, Hao Y, Guerin A, et al. Economic burden following allogeneic hematopoietic stem cell transplant in patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2018;59(5):1133–1142.
- U.S. Food and Drug Administration (FDA). FDA approves axicabtagene ciloleucel for large B-cell lymphoma. Silver Spring (MD); 2021 [updated 2017 Oct 18; cited 2021 Jul 26]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma
- U.S. Food and Drug Administration (FDA). FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. Silver Spring (MD); 2021 [updated 2018 May 1; cited 2021 Jul 26]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma
- U.S. Food and Drug Administration (FDA). FDA approves lisocabtagene maraleucel for relapsed or refractory large B-cell lymphoma. Silver Spring (MD); 2021 [updated 2021 Feb 5; cited 2021 Jul 26]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-large-b-cell-lymphoma
- Smith S, Essell J. Evolving the delivery of CAR T-cell therapies to the outpatient setting. J Clin Pathways. 2018;4(8):42–47.
- Myers GD, Verneris MR, Goy A, et al. Perspectives on outpatient administration of CAR-T cell therapy in aggressive B-cell lymphoma and acute lymphoblastic leukemia. J Immunother Cancer. 2021; 9(4):e002056.
- YESCARTA® (axicabtagene ciloleucel) prescribing information. Santa Monica (CA): Kite Pharma, Inc.; 2021.
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42.
- Riedell PA, Walling C, Nastoupil LJ, et al. A multicenter retrospective analysis of clinical outcomes, toxicities, and patterns of use in institutions utilizing commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B-cell lymphomas: Oncology Learning Network; 2021 [cited 2021 Jul 26]. Available from: https://www.hmpgloballearningnetwork.com/site/onc/videos/car-t-therapy-efficacy-b-cell-lymphomas-similar-commercial-and-trial-settings
- Lyman GH, Nguyen A, Snyder S, et al. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020;3(4):e202072.
- Stedman MR, Doria-Rose P, Warren JL, et al. The impact of different SEER-Medicare claims-based comorbidity indexes on predicting non-cancer mortality for cancer patients; Bethesda, MD:National Cancer Institute (NCI) 2017 https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity.html, and the technical report itself can be found at this link: https://healthcaredelivery.cancer.gov/seermedicare/considerations/comorbidity-report.pdf
- Riedell PA, Hwang WT, Nastoupil LJ, et al. Patterns of use, outcomes, and resource utilization among recipients of commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. 2022; 28(10):669–676.
- Geethakumari PR, Dhakal B, Ramasamy DP, et al. CAR T-cell therapy: current practice and future solutions to optimize patient access. J Clin Pathways. 2021;7(2):54–62.
- Nasta SD, Namoglu EC, Hughes ME, et al. Hospitalization patterns with commercial CAR T-cell therapy: a single institution experience. Blood. 2019;134(Suppl. 1):3240.
- Kilgore K, editor. Medicare patients receiving chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma: a first real-world look at patient characteristics, healthcare utilization and costs. 61st Annual Meeting and Exposition; 2019 Dec 7–10; 2019. ASH.
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–3128.
- BREYANZI® (lisocabtagene maraleucel) prescribing information. Bothell (WA): Bristol-Myers Squibb Company; 2021.
- KYMRIAH® (tisagenlecleucel) prescribing information. East Hanover (NJ): Novartis Pharmaceuticals Corporation; 2021.
- Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–3106.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- Jaglowski S, Hu Z-H, Zhang Y, et al. Tisagenlecleucel chimeric antigen receptor (CAR) T-cell therapy for adults with diffuse large B-cell lymphoma (DLBCL): real world experience from the Center for International Blood & Marrow Transplant Research (CIBMTR) cellular therapy (CT) registry. Blood. 2019;134(Suppl. 1):766.
- Pasquini MC, Locke FL, Herrera AF, et al. Post-marketing use outcomes of an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, axicabtagene ciloleucel (Axi-Cel), for the treatment of large B cell lymphoma (LBCL) in the United States (US). Washington (DC): American Society of Hematology; 2019.
- Topp M, Meerten TV, Houot R, et al. Earlier steroid use with axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory large B cell lymphoma. Blood. 2019;134(Suppl. 1):243.
- Dholaria BR, Bachmeier CA, Locke F. Mechanisms and management of chimeric antigen receptor T-cell therapy-related toxicities. BioDrugs. 2019; 33(1):45–60.
- Vitale C, Strati P. CAR T-cell therapy for B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia: clinical trials and real-world experiences. Front Oncol. 2020;10:849.
- Myers RM, Kadauke S, Li Y, et al. Risk-adapted preemptive tocilizumab decreases severe cytokine release syndrome (CRS) after CTL019 CD19-targeted chimeric antigen receptor (CAR) T-cell therapy for pediatric B-cell acute lymphoblastic leukemia (B-ALL). Biol Blood Marrow Transplant. 2020;26(3):S39.
- American Society of Clinical Oncology. CAR-T therapy policy brief. Alexandria (VA): American Society of Clinical Oncology; 2020. p. 1–4.
- American Society of Hematology. CAR T-cell therapy: an update on coverage and reimbursement. Washington (DC); 2019 [cited 2021 Jul 8]. Available from: https://www.hematology.org/advocacy/policy-news/2019/car-t-cell-therapy-an-update-on-coverage-and-reimbursement