Abstract
This study used a real-world population as a synthetic comparator for the single-arm TRANSCEND NHL 001 study (TRANSCEND; NCT02631044) to evaluate the efficacy of lisocabtagene maraleucel (liso-cel) compared with conventional (noncellular) therapies in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Inclusion and exclusion criteria for the real-world study closely matched the enrollment criteria in TRANSCEND. The analytic comparator cohort was created by matching and balancing observed baseline characteristics of real-world patients with those in TRANSCEND using propensity score methodology. Efficacy outcomes comparing liso-cel– (n = 257) and conventional therapy–treated (n = 257) patients, respectively, significantly favored liso-cel: overall response rate (74% vs 39%; p < 0.0001), complete response rate (50% vs 24%; p < 0.0001), median overall survival (23.5 vs 6.8 months; p < 0.0001), and median progression-free survival (3.5 vs 2.2 months; p < 0.0001). These results demonstrated a statistically significant and clinically meaningful benefit of liso-cel in patients with third- or later-line R/R LBCL relative to conventional therapies.
Clinical trial registration: ClinicalTrials.gov identifier: NCT02631044
Introduction
Patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL), a group of aggressive lymphomas comprised predominantly of diffuse large B-cell lymphoma (DLBCL), have few treatment options and poor prognosis [Citation1–4]. Historical data show little benefit from salvage therapies for patients with third- or later-line (3L+) LBCL [Citation3,Citation4]. Poor outcomes for patients treated with conventional (noncellular) therapies were demonstrated in SCHOLAR-1, a large, international, retrospective study that investigated real-world outcomes in patients with refractory DLBCL using a pooled multi-source clinical study and US real-world observational cohorts [Citation5]. The final analysis of SCHOLAR-1 consisted of patients who were refractory to induction or salvage chemotherapy or those with early relapse after hematopoietic stem cell transplantation (HSCT). In this group, objective and complete response (CR) rates were 26% and 7%, respectively, and median overall survival (OS) was 6.3 months [Citation5].
The introduction of chimeric antigen receptor (CAR) T-cell therapies is changing the treatment landscape for patients with R/R LBCL. Studies of CAR T-cell therapies in patients with 3L+ LBCL have reported objective response rates ranging from 52% to 82% and CR rates ranging from 40% to 54% [Citation6–8]. Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells. In the pivotal TRANSCEND NHL 001 (TRANSCEND) study, liso-cel treatment resulted in rapid and durable remission along with low rates of severe cytokine release syndrome and neurological events in patients with R/R LBCL [Citation6].
A recent study using a matching-adjusted indirect comparison (MAIC) approach evaluated the comparative efficacy of liso-cel versus salvage chemotherapy based on individual patient-level data from TRANSCEND and summary-level data from SCHOLAR-1. In this comparison, liso-cel had favorable efficacy compared with salvage chemotherapy in patients with R/R LBCL [Citation9]. However, the MAIC analysis was limited to summary-level data available from SCHOLAR-1 and was unable to adjust for many unreported patient-level baseline characteristics. The current analysis overcomes these 2 limitations by selecting an external control cohort from a large, pooled, real-world data set (NDS-NHL-001) matched at the individual level to patients from TRANSCEND, thus placing TRANSCEND study results into the context of conventional therapies for patients with R/R LBCL. This real-world comparator arm served to evaluate the comparative efficacy of liso-cel treatment in patients with LBCL in TRANSCEND versus conventional therapies in real-world patients.
Methods
Study design
This study was designed to compare the effectiveness of liso-cel (TRANSCEND) versus conventional therapies (NDS-NHL-001) through multiple endpoints. The primary endpoint was overall response rate (ORR). Secondary endpoints included complete response (CR), overall survival (OS), progression-free survival (PFS), and duration of response (DOR). For all endpoint analyses, 24-month follow-up was chosen to correspond with the maximum follow-up time in TRANSCEND.
Data sources
The primary data source for liso-cel was individual patient data from TRANSCEND, which is an open-label, multicenter, multicohort, seamless design clinical trial evaluating the efficacy and safety of liso-cel in adults with R/R DLBCL (de novo or transformed from any indolent lymphoma), high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (double-hit or triple-hit lymphoma), primary mediastinal B-cell lymphoma, or follicular lymphoma grade 3B [Citation6]. The data cutoff date for TRANSCEND was June 19, 2020, with a median (range) on study follow-up of 17.4 (0.2–24.0) months.
The primary data source for conventional therapy was patient-level data from the NDS-NHL-001 study. NDS-NHL-001 was a global, multicenter, retrospective, observational study that described treatment patterns and evaluated clinical outcomes of adults with R/R LBCL treated in the real-world setting. depicts the inclusion/exclusion criteria of NDS-NHL-001, which were selected to mirror those of TRANSCEND as closely as possible.
Figure 1. Study design. NDS-NHL-001 is a global, noninterventional, retrospective, multicenter study that collected data on a cohort of real-world patients with LBCL who had received prior treatment with an anthracycline and rituximab (or another CD20-targeting agent) and who had started a subsequent LOT. The figure shows the time frames during which baseline covariates and exclusion criteria were assessed relative to the index date. aSee Supplementary Table 8 for the list of comorbidities and associated time windows. bDay 0, index date (T0): Start date of each patient’s qualifying LOT after R/R disease to at least 2 LOTs and exposure to anthracycline and an anti-CD20–containing agent. CAR: chimeric antigen receptor; CNS: central nervous system; CrCl: creatinine clearance (Cockcroft and Gault); DHL: double-hit lymphoma; ECOG PS: Eastern Cooperative Oncology Group performance status; IPI: International Prognostic Index; KPS: Karnofsky Performance Scale; LBCL: large B-cell lymphoma; LOT: line of therapy; LVEF: left ventricular ejection fraction; MI: myocardial infarction; R/R: relapsed or refractory; SAP: statistical analysis plan; SCR: serum creatinine; T0: index date; THL: triple-hit lymphoma; ULN: upper limit of normal.
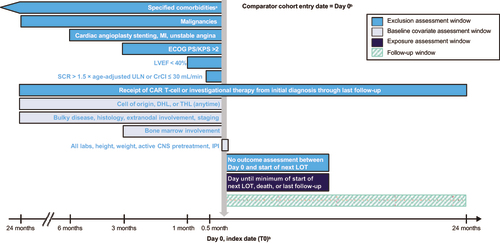
Real-world patient-level data for NDS-NHL-001 were acquired from the following sources: (1) 11 clinical sites (2 in North America and 9 in Europe) and (2) 3 external research database partners (COTA Real-World Evidence database, Flatiron Health database, and Guardian Research Network). Data from each source were integrated to conform to a standardized, common study data model, thus allowing variables of comparable constructs from disparate data sources to be harmonized into a pooled dataset. Consistent operational definitions were systematically applied during harmonization. Probabilistic methods were used to address potential duplication of patients across the disparate deidentified data sources. All real-world patient-level data collection was retrospective and did not influence clinical practice or patient visit schedules. The cutoff date for the real-world data was December 20, 2019, with a median (range) follow-up of 6.3 (0.4–24.0) months.
Each data source for NDS-NHL-001 was responsible for ensuring that data collection complied with applicable national and local ethical, legal, and privacy regulations. Data were acquired from multiple sources to achieve a patient population representative of different clinical care settings and geographic regions.
Patient cohorts
Cohort construction for all analyses is shown in and reflects the transparent methodology that was used to obtain a large enough sample to ensure generalizability and for which we could apply the inclusion/exclusion criteria of TRANSCEND to a real-world population. The liso-cel–treated analysis cohort (LTAC) included 257 patients from TRANSCEND who had received ≥1 dose of liso-cel and had evidence of positron emission tomography (PET)–positive disease before liso-cel infusion. For the real-world comparator cohorts from NDS-NHL-001, 3 consecutive comparator cohorts (initial comparator cohort [ICC], qualifying comparator cohort [QCC], and stratified analytic comparator cohorts [sACC]) were established. First, the ICC included 606 patients with documented LBCL (initial diagnosis between January 1, 2003, and September 30, 2018) of qualifying histology who had prior exposure to anthracycline and rituximab (or another CD20-targeting agent), completed ≥2 prior lines of therapy (LOT) and initiated a subsequent LOT, and had ≥1 outcome assessment between the index date and start of the subsequent LOT. Amongst the ICC, a QCC of 381 patients who met selected eligibility criteria () similar to those for TRANSCEND was established.
Figure 2. Cohort construction for all analyses. From a total of 1450 RW patients, 606 RW patients met the eligibility criteria for inclusion in the ICC. Additional eligibility criteria similar to those of TRANSCEND were then applied, resulting in the selection of 381 RW patients for the QCC. All 381 patients in the QCC were selected for the ACC by propensity score balancing. For comparison, the LTAC consisted of 257 liso-cel–treated patients from the LBCL cohort of TRANSCEND. Propensity scores were obtained for each imputed data set (LTAC and ACC), with 25 imputations used based on a moderate amount of missing information (≤15%) [Citation16,Citation17]. The primary analysis cohort and 2 sensitivity analysis cohorts contributed to the totality of evidence of the effectiveness of liso-cel compared with standard of care in RW settings. ALT: alanine aminotransferase; CAR: chimeric antigen receptor; DLBCL: diffuse large B-cell lymphoma; ECOG: Eastern Cooperative Oncology Group; FL3B: follicular lymphoma grade 3B; HGBCL: high-grade B-cell lymphoma; IPTW: inverse probability of treatment weights; LBCL: large B-cell lymphoma; LOT: line of therapy; LVEF: left ventricular ejection fraction; NOS: not otherwise specified; PMBCL: primary mediastinal B-cell lymphoma; RW: real-world; SaO2: oxygen saturation; SCR: serum creatinine; T0: index date; tFL: DLBCL transformed from follicular lymphoma; tiNHL: DLBCL transformed from indolent non-Hodgkin lymphoma other than follicular lymphoma; US: United States.
![Figure 2. Cohort construction for all analyses. From a total of 1450 RW patients, 606 RW patients met the eligibility criteria for inclusion in the ICC. Additional eligibility criteria similar to those of TRANSCEND were then applied, resulting in the selection of 381 RW patients for the QCC. All 381 patients in the QCC were selected for the ACC by propensity score balancing. For comparison, the LTAC consisted of 257 liso-cel–treated patients from the LBCL cohort of TRANSCEND. Propensity scores were obtained for each imputed data set (LTAC and ACC), with 25 imputations used based on a moderate amount of missing information (≤15%) [Citation16,Citation17]. The primary analysis cohort and 2 sensitivity analysis cohorts contributed to the totality of evidence of the effectiveness of liso-cel compared with standard of care in RW settings. ALT: alanine aminotransferase; CAR: chimeric antigen receptor; DLBCL: diffuse large B-cell lymphoma; ECOG: Eastern Cooperative Oncology Group; FL3B: follicular lymphoma grade 3B; HGBCL: high-grade B-cell lymphoma; IPTW: inverse probability of treatment weights; LBCL: large B-cell lymphoma; LOT: line of therapy; LVEF: left ventricular ejection fraction; NOS: not otherwise specified; PMBCL: primary mediastinal B-cell lymphoma; RW: real-world; SaO2: oxygen saturation; SCR: serum creatinine; T0: index date; tFL: DLBCL transformed from follicular lymphoma; tiNHL: DLBCL transformed from indolent non-Hodgkin lymphoma other than follicular lymphoma; US: United States.](/cms/asset/a2281018-fd8d-4ad2-b729-fc91a773b4e1/ilal_a_2160200_f0002_c.jpg)
The assignment of the index date differed between the LTAC and the QCC. The index date for the LTAC was assigned to the date of liso-cel infusion. The index date for the QCC was assigned to the start date of the first treatment after exposure to an anthracycline and rituximab (or other CD20-targeting agent) and the completion of ≥2 prior LOTs.
Differences in the number of prior LOTs between the LTAC and QCC necessitated creation of the sACC (). To generate the sACC (n = 257), a distribution of prior LOTs (2, 3, and ≥4) in the LTAC was generated. Patients in the QCC were then stratified by category of prior LOTs. Patients within each prior LOT stratum were randomly selected and combined to generate a QCC and subsequently the sACC. This resulted in the sACC and LTAC having a similar distribution of prior LOTs. Two sensitivity inverse probability of treatment weights (IPTW) analyses were conducted using the analytic comparator cohort (ACC), which included all 381 patients in the QCC and were created after multiple imputations for missing data and propensity score (PS) balancing on baseline characteristics.
Table 1. Patient demographics and baseline disease characteristics for the real-world and liso-cel–treated cohorts.
Statistical methods
Statistical methods for the comparison of TRANSCEND to the real-world cohorts were prespecified, including all details for evaluating and handling of missing data and creating the PS and the matches between cohorts, with the exception of the sACC that was conducted post hoc.
To ensure balance across baseline characteristics, the PS-stabilized IPTW was used to weight the real-world and respective cohorts from TRANSCEND. Potential prognostic factors for effectiveness and all-cause mortality were identified based on available demographic and baseline disease characteristics in TRANSCEND, with additional factors included based on literature review and medical review.
Prognostic factors with ≤15% overall missing data were included in the full multivariable logistic regression after multiple imputations for missing values. All possible models were evaluated with the best model fit being selected to obtain PS [Citation10]. PS were obtained for each of the imputed data sets. The final PS model included age, sex, months since diagnosis, number of prior LOTs per year since initial diagnosis to the index date, best response to any prior therapy, R/R to last therapy, prior HSCT, chemotherapy refractory or chemotherapy sensitive to last therapy, bulky disease, extranodal disease, and disease stage. The final PS obtained for each imputed data set and the PS-stabilized IPTW were then used to perform the balancing.
Assessment of balance for important baseline covariates between cohorts was conducted through a side-by-side comparison of baseline data for the TRANSCEND and real-world cohorts. For these comparisons, pooled standardized mean differences were computed using Rubin’s rules before and after balancing. For the standardized mean difference, a threshold of 0.2 was used to indicate potentially important imbalances [Citation11]. Stabilized IPTW and PS matching were used as the primary analysis methods. By using IPTW to adjust the LTAC and the ACCs, each patient in the LTAC and each real-world patient in individual ACCs was included in the comparison and weighted appropriately, thereby maximizing the sample size and robustness of the analyses. This methodology allowed for adjustment for the imbalances observed in the baseline characteristics between liso-cel–treated patients and real-world patients.
All real-world patients met the eligibility criteria by their third LOT (i.e., index date at start of third LOT; LOT-3L), resulting in different distributions of the number of prior LOTs between the QCC and LTAC. Three analyses (LOT-matched, LOT-unmatched, and LOT-3L–restricted) were conducted to address this methodologic index date bias and limitation introduced by the indexing rules for the real-world patients.
The LOT-matched analysis included real-world patients and liso-cel–treated patients with a similar categorical distribution of prior LOTs between the 2 cohorts, representing the full LTAC. This analysis was conducted to properly reflect the range of prior LOTs in real-world patients using the stratified random sampling method. After stratification, 49%, 26%, and 25% of patients in the sACC had received 2, 3, and ≥4 prior LOTs, respectively, which was similar to that of patients in the LTAC (46%, 26%, and 25%, respectively). As such, the LOT-matched analysis is the most appropriate and clinically relevant comparison. The LOT-unmatched and the LOT-3L–restricted analyses were conducted to determine how the distribution of prior LOTs impacted the results. In the LOT-unmatched analysis, all patients in the ACC were indexed at the third LOT, whereas some patients in the LTAC had an index date that corresponded to the start of later LOTs. As no adjustment for prior LOTs could be conducted for the LOT-unmatched analysis, the real-world cohort may have been biased toward longer survival outcomes by being indexed at an earlier LOT than the LTAC. The LOT-3L–restricted analysis limited patients in the LTAC to those who received only 2 prior LOTs and for whom liso-cel infusion was the third LOT (LTAC-3L).
Subgroup analyses based on baseline characteristics were conducted in the LOT-matched cohorts that compared ORR, OS, and PFS endpoints, wherever appropriate, between the LTAC and the sACC. Subgroups were chosen based on clinical relevance and adequate subgroup size. Forest plots were generated for subgroups considering the following variables: age (<65 vs ≥65 years), sex (male vs female), bridging therapy use (i.e., anticancer therapy for disease control) in the LTAC (yes vs no), disease stage (III–IV vs I–II), extranodal disease (yes vs no), lactate dehydrogenase (≥235 vs <235 U/L), and bulky disease (individual masses ≥10 cm in diameter; yes vs no).
All computations and generation of tables and data for figures were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline demographics and disease characteristics
For the real-world cohort, patients in the ICC had a median age of 63 years, 63% were male, and all were from the United States (77%) or Europe (23%; ). Most patients had a diagnosis of DLBCL not otherwise specified (NOS) (60%), an Eastern Cooperative Oncology Group performance status of ≤1 (39%) or missing (48%), stage III/IV disease (53%), extranodal disease (56%), and disease refractory to last prior therapy (90%), including 60% refractory to their last chemotherapy. Only 12% of patients had a prior HSCT and 22% had bulky disease, defined as individual masses ≥10 cm. Baseline patient demographics were similar for patients in the QCC ().
When comparing the LTAC with the real-world cohorts, patient demographics and disease characteristics were generally comparable in both the ICC and QCC (). However, a lower percentage of LTAC patients were ≥75 years of age (11% vs 18%, respectively) and all LTAC patients were from the United States, whereas the QCC included patients from Europe (25%).
DLBCL NOS was the most common diagnosis in both cohorts (LTAC, 51%; real-world QCC, 57%; ). For variables with adequate data, differences in disease characteristics between the LTAC and QCC, respectively, were observed for bulky disease (11% vs 21%), prior HSCT (34% vs 11%), disease refractory to last prior therapy (79% vs 90%), chemotherapy-refractory disease (49% vs 60%), chemotherapy-sensitive disease (33% vs 25%), disease relapse <12 months after autologous HSCT (18% vs 6%), and best response to prior therapy (56% vs 35%, with CR as best prior response).
To explore the comparative effectiveness of liso-cel versus available conventional therapies, cohorts were assembled that accounted for discrepancies in the distribution of prior LOTs. For these cohorts, differences in baseline characteristics between groups were generally consistent with those noted for the LTAC and QCC, except for distribution of prior LOTs. In the LOT-matched analysis, 49% of patients in the LTAC and sACC had 1–2 prior LOTs, and in the LOT-3L–restricted analysis, all patients in the LTAC-3L and ACC had 2 prior LOTs (Supplementary Table 1). The most common index treatments in the ICC and QCC are presented in Supplementary Table 2. In the QCC, 15 patients received allogeneic HSCT as the index treatment, and an additional 8 patients received allogeneic HSCT as subsequent therapy. HSCT received before and after treatment in the TRANSCEND efficacy-evaluable population is presented in Supplementary Table 3.
The following covariates had potentially important imbalances: months since diagnosis to index date, best response to any prior therapy, R/R status to last therapy, prior HSCT, and bulky disease. For the LOT-matched analysis, after balancing using stabilized IPTW, all selected baseline covariates were well balanced with an overall standardized mean difference of <0.1 (Supplementary Table 4). Covariate balances for the LOT-unmatched and LOT-3L–restricted analyses are shown in Supplementary Tables 5 and 6. For all analyses, PS balancing was performed using the same covariates, except for number of prior LOTs, which was only included in the LOT-matched analysis based on relevance for corresponding matching criteria.
Efficacy outcomes
Primary endpoint analyses
For the LOT-matched analysis, the ORR adjusted for stabilized IPTW was significantly higher in the LTAC compared with the sACC (74% vs 39%, respectively; p < 0.0001; ). The ORR adjusted for stabilized IPTW was consistent for the LOT-unmatched and LOT-3L–restricted analyses (), when patients who had received investigational agents as third-line therapy were included in the ACC population (n = 563; ORR, 44%; relative risk [95% confidence interval (CI)] vs LTAC, 1.7 [1.5–2.0]; p < 0.0001), and in the total liso-cel leukapheresed cohort (Supplementary Table 7).
Table 2. Summary of effectiveness results, adjusted for stabilized IPTW of real-world and liso-cel–treated analysis cohorts.
Secondary endpoint analyses
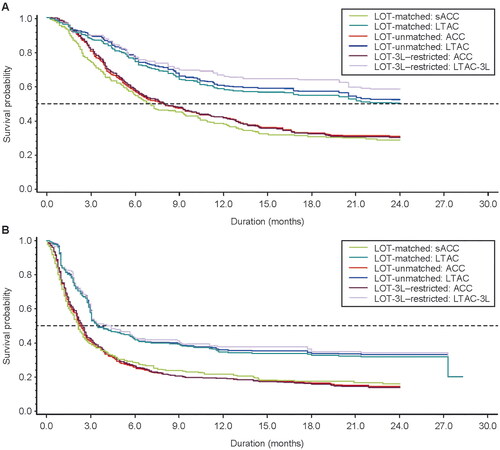
For the LOT-matched analysis, the CR rate adjusted for stabilized IPTW was significantly higher in the LTAC versus the sACC (50% vs 24%, respectively; p < 0.0001; ). After a median follow-up of 24.0 and 17.9 months in the LTAC and sACC, 52% and 33% of patients, respectively, were still alive. The median OS was significantly longer in the LTAC compared with the sACC (23.5 vs 6.8 months, respectively; p < 0.0001; and ). After a median follow-up of 17.4 and 5.3 months for all patients in the LTAC and sACC, 32% and 19% of patients were progression free, respectively. The median PFS was significantly longer in the LTAC compared with the sACC (3.5 vs 2.2 months, respectively; p < 0.0001; and ). Of note, comparisons of CR rate, OS, and PFS between the ACC and liso-cel leukapheresis cohort were consistent with the LOT-matched analyses reported above (Supplementary Table 7).
Figure 3. OS and PFS adjusted for stabilized IPTW of real-world and liso-cel–treated analysis cohorts. Results shown for the LOT-matched analysis, LOT-unmatched analysis, and LOT-3L–restricted analysis. (A) OS was defined as the time from the index date to all-cause death or the end of the follow-up period. Multiple imputations were performed to create 25 data sets. Estimates for the analyses were then obtained using Rubin’s rule to combine the individual estimates from each data set. (B) PFS, according to European Medicines Agency censoring rules, was defined as the time from the index date to the first documented disease progression, relapse, death from any cause, or end of the follow-up period, whichever occurred first. Multiple imputations were performed to create 25 data sets. Estimates for the analyses were then obtained using Rubin’s rule to combine the individual estimates from each data set. ACC: analytic comparator cohort; IPTW: inverse probability of treatment weights; LOT: line of therapy; LOT-3L: third line of therapy; LTAC: liso-cel–treated analysis cohort; LTAC-3L: liso-cel–treated analysis cohort who received only 2 prior lines of therapy; OS: overall survival; PFS: progression-free survival; sACC: stratified analytic comparator cohort.
Among the 74% of patients who achieved a response in the LTAC, the median DOR was 10.4 months (median follow-up, 17.4 months), while the median DOR was 9.8 months (median follow-up, 5.3 months) among the 39% of responders in the sACC. Results for median DOR numerically favored the LTAC over the sACC; however, the results were not statistically significant (p = 0.3938; ).
Overall, results for the LOT-unmatched analysis and the LOT-3L–restricted analysis were similar to the LOT-matched analysis, with CR remaining significantly higher in the LTAC versus the ACC (). In these analyses, OS and PFS also remained significantly longer in the LTAC versus the ACC ( and ). As in the LOT-matched analysis, although median DOR was numerically longer in the LTAC compared with the real-world ACC, the results were not statistically significant in either the LOT-unmatched analysis or the LOT-3L–restricted analysis ().
Subgroup analyses of baseline characteristics in the LOT-matched analysis
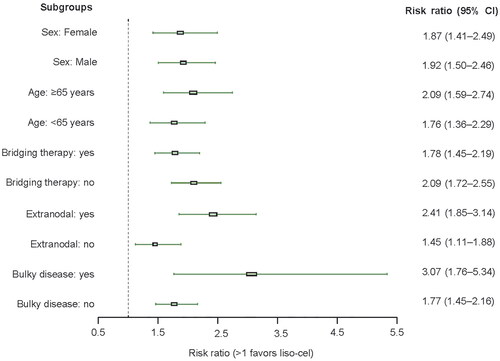
For all subgroups evaluated, the ORR results demonstrated a benefit of liso-cel compared with conventional therapies and were consistent with the overall results for the ORR (). Similarly, the OS and PFS outcomes by subgroup were generally consistent with the overall results for OS and PFS, respectively, and the benefit of liso-cel compared with conventional therapies was maintained for most variables (Supplementary Figures 1 and 2). However, OS outcomes for females (34% of the LTAC and 37% of real-world patients) and patients who received bridging therapy (58% of the LTAC) were not statistically significant in the subgroup analysis.
Figure 4. Risk ratio by subgroup for ORR adjusted for stabilized inverse probability of treatment weights. The index date was defined as the day the patient received the new therapy. ORR was defined as the percentage of patients who achieved a best overall response of partial response or complete response as assessed by the investigator (treating physician for real-world patients). Multiple imputation procedures created 25 data sets. Estimates were then obtained using Rubin’s rules to combine the individual estimates from each data set. Subgroups for bridging therapy (i.e., anticancer therapy for disease control) were applied to the LTAC only to compare with the real-world patients. Stabilized weights used for all analyses were those of the overall ACC and LTAC. Bulky disease was defined as the presence of individual masses ≥10 cm in diameter. ACC: analytic comparator cohort; CI: confidence interval; liso-cel: lisocabtagene maraleucel; LTAC: liso-cel–treated analysis cohort; ORR: overall response rate.
Discussion
In order to evaluate the comparative effectiveness of liso-cel versus available conventional therapies, an external control cohort of real-world patients was constructed using inclusion and exclusion criteria designed to closely match the patient population in TRANSCEND [Citation6]. After matching and adjusting for imbalances in baseline characteristics observed between real-world patients and liso-cel–treated patients, efficacy outcomes of ORR, CR, OS, and PFS significantly favored liso-cel over conventional therapies.
Three analyses were conducted to examine the impact of differences in index date definitions between studies on efficacy outcomes. The LOT-matched analysis, which included real-world and liso-cel–treated patients with a similar categorical distribution of prior LOTs, represents the most clinically relevant comparison and demonstrated significantly higher efficacy outcomes of ORR, CR, OS, and PFS in the LTAC compared with the sACC. The LOT-unmatched analysis did not adjust for prior LOTs since all real-world patients met eligibility criteria at their third LOT. As a result, the unmatched analysis may be biased toward improved survival outcomes among real-world patients indexed at an earlier LOT than in the LTAC due to the retrospective versus prospective nature of index data assignment. The LOT-3L–restricted analysis limited patients in the LTAC to those with 2 prior LOTs, resulting in both real-world and LTAC patients indexed at the same LOT. This resulted in a similar, albeit smaller, subset of the overall TRANSCEND patient population. Regardless of the method used, the results from all 3 analytic approaches clearly demonstrated a treatment benefit for liso-cel compared with real-world conventional therapies, irrespective of the number of prior LOTs.
Efficacy results for the real-world population in our analysis differed somewhat from those of SCHOLAR-1 [5]. SCHOLAR-1 reported an ORR of 26% (range, 20%–31%), a CR of 7% (range, 2%–15%), and a median OS of 6.3 months (95% CI, 5.9–7.0 months) [Citation5]. The directionally similar, yet higher magnitude estimates in our study, may be attributable to a number of differences in study design, such as inclusion of mostly refractory patients who were matched on a patient-level by the number of prior LOTs to the patient population in TRANSCEND. An important distinction between our analysis and SCHOLAR-1 is that we applied rigorous patient-level inclusion and exclusion criteria, as well as PS matching to the real-world population to mimic the TRANSCEND clinical trial population, which may account for some of the differences in efficacy outcomes between studies. For example, our analysis focused on patients with 3L+ LBCL and included patients with late relapses as well as refractory disease, whereas SCHOLAR-1 included patients with refractory disease after induction or salvage chemotherapy and limited patients with relapsed disease to those who relapsed within 12 months after autologous HSCT [Citation5]. Studies evaluating patients with relapsed LBCL, including those who relapsed within 1 year after autologous HSCT, reported an ORR of 46%–48% and CR rate of 29%–33% [Citation12,Citation13]. Compared with refractory patients from SCHOLAR-1, these data indicate that patients with relapsed disease may have potentially better outcomes than those with refractory disease. In addition to real-world data, SCHOLAR-1 also included clinical trial data where response endpoints were centrally reviewed, potentially impacting outcomes.
In studies comparing CAR T-cell therapies with SCHOLAR-1 using matching and adjusted indirect treatment comparisons, the CAR T-cell therapies liso-cel and axicabtagene ciloleucel were found to significantly increase the odds of CR and reduce the risk of death compared with conventional regimens for R/R disease [Citation9,Citation14]. Similarly, in a retrospective observational study of 215 patients with R/R LBCL from Memorial Sloan Kettering (MSK) Cancer Center, response rates and survival outcomes for patients treated with commercial CAR T-cell therapies (both axicabtagene ciloleucel and tisagenlecleucel) were superior to those in the historical population treated with alternate therapies. In this study, the number of prior LOTs also differed between the CAR T-cell and alternate therapy cohorts, but the benefit of CAR T-cell therapies persisted regardless of the number of prior LOTs. However, in contrast to our analysis, after adjusting for unfavorable baseline factors, only response rate remained superior in the CAR T-cell therapy group of the MSK study, with no statistically significant differences found for PFS or OS [Citation15].
This analysis was strengthened by the NDS-NHL-001 design, which applied stringent inclusion and exclusion criteria to best allow for patients with comparable baseline features. The patient population from NDS-NHL-001 was matched, adjusted, balanced, and weighted to maximize the sample size and robustness of the analyses. The study approach of casting a wide net to identify the matched patient population was an added strength, as it afforded the added ability to evaluate the generalizability of the findings to a broader cohort of real-world patients with 3L+ LBCL.
Given the retrospective and nonrandomized nature of this study, there were some inherent limitations. Despite extensive efforts to select real-world patients similar to patients in TRANSCEND, any ‘unmeasured’ confounders could not be controlled and balanced between the 2 groups. As part of the stringent inclusion criteria, real-world patients treated with investigational agents in any line of therapy were excluded from all but the post hoc analysis of the primary endpoint. This was important as these investigational treatments are not considered standard of care; however, it diverges from a true real-world data set where many patients are treated on clinical trials due to the poor outcomes with conventional therapies. Of note, ORR analyses including patients who received investigational therapy were consistent with results excluding these patients. Real-world patients treated with commercially available CAR T-cell therapies were also excluded to avoid potential confounding of the liso-cel and conventional therapies comparison. Additional ORR analyses were conducted excluding patients treated with unknown therapies or therapies other than established standard of care as their index line of therapy, which yielded strikingly similar results to the primary overall and stratified results. The feasibility of intention-to-treat analyses comparing the real-world and TRANSCEND leukapheresed cohorts was evaluated; however, a matched comparison to replicate all analyses using the intention-to-treat cohorts was not possible due to an insufficient sample size in the real-world cohort when matching on the line of therapy distribution of the total leukapheresed cohort in TRANSCEND (n = 345). The treatment landscape in R/R DLBCL has continued to evolve since the data cutoff date for these analyses (December 2019), potentially limiting the generalizability of these results.
Potential bias could also occur because of the variable timing and availability of real-world clinical assessments versus scheduled investigator clinical trial assessments in TRANSCEND. As real-world data collection included all clinical and imaging assessments available in the electronic health records, not only those associated with progression events, the analysis was afforded considerable data density to reflect a patient’s longitudinal disease journey. Further, the endpoint analyses were blinded such that the analysts did not have visibility to the response data when conducting the PS matching. This likely obviated non-differential misclassification of study outcomes. In addition, statistical comparisons for select subgroup analyses and DOR were limited by the small number of patients in these subgroup analyses, coupled with relatively small proportions of responders in the sACC. Finally, while the evidence of longer OS with liso-cel was clear, we note the shorter follow-up time for real-world patients as a limitation.
In conclusion, a comparison between the effectiveness of liso-cel used in a clinical trial setting and conventional therapies used in real-world settings showed a significant improvement in ORR, CR rate, OS, and PFS with liso-cel treatment, and a numerically longer DOR in favor of liso-cel. None of the baseline characteristics examined, such as number of prior LOTs, had an impact on the favorable efficacy of liso-cel. Taken together, these results support the conclusion that liso-cel provided a significant and meaningful efficacy benefit for patients with 3L+ R/R LBCL relative to conventional therapies.
Author contributions
HVL and KVNB contributed to study conceptualization, formal analysis, investigation, and methodology. GSN, DS, JR, WT, HG, TM, EP, and CPF contributed to investigation. LY and MDB contributed to formal analysis and methodology. FFL contributed to study investigation. CS and JH contributed to study conceptualization and formal analysis. All authors contributed to writing, reviewing, and editing of the manuscript.
Supplement_Van_Le_et_al_RWE_3L_LBCL_ms_7FEB2023_FINAL__1_.pdf
Download PDF (634.9 KB)Disclosure statement
HVL was an employee of Bristol Myers Squibb when the work was completed and holds stock in Bristol Myers Squibb. KVNB, FFL, LY, and MDB are employees of Bristol Myers Squibb and hold stock in Bristol Myers Squibb. GSN has received compensation for consulting from Bantam Pharmaceutical, Blueprint Medicines, Celgene/Bristol Myers Squibb, Curis, Daiichi Sankyo, Genentech, Incyte, Karyopharm Therapeutics, Kymera Therapeutics, Kite Pharma, MorphoSys, Roche, Ryvu Therapeutics, Selvita, TG Therapeutics, and Zai Laboratory and research support (to the institution) from Celgene/Bristol Myers Squibb, Genentech, NanoString Technologies, and Roche. DS received consulting fees from Syros Pharmaceuticals. JR has received compensation for Advisory Board membership from ADC Therapeutics, AstraZeneca, and Takeda, and holds stock in ADC Therapeutics and AstraZeneca. WT is supported by funding from the National Institute for Health Research University College Hospitals Biomedical Research Centre. HG has received compensation for consulting from Celgene, a Bristol-Myers Squibb Company, Gilead Sciences, and Roche; honoraria from Celgene, a Bristol-Myers Squibb Company, Gilead Sciences, and Janssen; and compensation for travel, accommodations, or expenses from Celgene, a Bristol-Myers Squibb Company, Gilead Sciences, Roche, and Takeda. TM has received honoraria from Amgen, Atara Biotherapeutics, Celgene, a Bristol-Myers Squibb Company, Daiichi Sankyo, Kite/Gilead, Janssen, Novartis, Pfizer, Roche, Servier, and Takeda, and research funding from AstraZeneca, Janssen, and Novartis. EP has nothing to disclose. CPF has received consulting fees from AbbVie, AstraZeneca, Atara Biotherapeutics, Celgene, a Bristol-Myers Squibb company, Gilead/Kite, Incyte, Roche, and Takeda; honoraria from Atara Biotherapeutics, Genmab, Gilead/Kite, Incyte, Roche, and Takeda; support for attending meetings and/or travel from Roche; participation in Data Safety Monitoring Board or Advisory Board from Genmab and MorphoSys; and grants for research from BeiGene. CS and JH were employees of Celgene, a Bristol-Myers Squibb Company, when the work was completed and hold stock in Bristol Myers Squibb.
Data availability statement
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Martelli M, Ferreri AJ, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146–171.
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–v125.
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52(2):216–221.
- van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35(5):544–551.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- Salles G, Spin P, Liu FF, et al. Indirect treatment comparison of liso-cel vs. salvage chemotherapy in diffuse large B-cell lymphoma: TRANSCEND vs. SCHOLAR-1. Adv Ther. 2021;38(6):3266–3280.
- Rosenbaum P, Rubin DB. The Central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107.
- González-Barca E, Boumendil A, Blaise D, et al. Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2020;55(2):393–399.
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the international CORAL study. Bone Marrow Transplant. 2016;51(1):51–57.
- Neelapu SS, Locke FL, Bartlett NL, et al. A comparison of two-year outcomes in ZUMA-1 (axicabtagene ciloleucel) and SCHOLAR-1 in patients with refractory large B cell lymphoma. Blood. 2019;134(suppl 1):4095.
- Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4(19):4669–4678.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213.
- van Buuren S. How many imputations?. In: van Buuren S, editor. Flexible imputation of missing data. 2nd ed. New York: Chapman & Hall/CRC; 2018. p. 58–60.
