Treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and other low-grade B-cell malignancies has changed dramatically over the past decade, mainly due to the identification of B-cell receptor signaling and related targeted therapies. Zanubrutinib is a highly selective, potent, irreversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target inhibition of TEC, interleukin-2-inducible kinase, and epidermal growth factor receptor family kinases [Citation1]. Zanubrutinib is approved in multiple regions for treatment of patients with various B-cell malignancies. A pivotal, single-arm, multicenter, phase II study of zanubrutinib monotherapy in patients with relapsed/refractory (R/R) CLL/SLL was conducted at 12 study centers in China, and the results have been published previously [Citation2]. Here, we report the final results after extended follow-up and provide an update on the resistance study and exploratory correlative analysis of lymphocytosis on prognostic factors of CLL/SLL. This study was designed and monitored according to sponsor procedures and in compliance with the ethical principles of Good Clinical Practice, International Conference on Harmonization guidelines, the Declaration of Helsinki, and applicable local regulatory requirements. All patients provided written informed consent. The protocol, amendments, and informed consent forms were approved by the institutional review boards/independent ethics committees at each study site. The study is registered at ClinicalTrials.gov (NCT03206918).
Ninety-one patients diagnosed with R/R CLL/SLL were enrolled and received oral zanubrutinib 160 mg twice daily continuously until disease progression or unacceptable toxicity (see supplementary materials Table S1, for baseline patient demographics and disease characteristics). Median age was 61 years (range 35–87). All patients received at least one line of prior therapy (median 1, range 1–9). Most patients had at least one unfavorable prognostic factor, including unmutated immunoglobulin heavy chain variable (IGHV) region gene (56.0%), del(17p) or TP53 mutation (24.2%), and del(11q) (22.0%). As of the final data cutoff, the median follow-up period was 34 months (range 0.8–41.4), and the median duration of treatment was 33 months (range 0.2–41.4). Most patients (n = 60 [66%]) were still on zanubrutinib treatment at study closure and were transferred to a long-term extension trial. Of the 31 discontinuations, 15 were due to progressive disease, 14 were due to adverse events (AEs), one patient discontinued at the investigator’s discretion, and one patient withdrew consent for further treatment.
Efficacy responses were assessed according to the International Workshop on Chronic Lymphocytic Leukemia guidelines [Citation3] or the Lugano Classification [Citation4] for CLL and SLL, respectively. With a median follow-up of 34 months, the primary endpoint was overall response rate (ORR; 87.9%, 95% confidence interval [CI] 79.4–93.8%) assessed by an independent review committee (IRC) and defined as the proportion of patients achieving a complete response (CR; 6.6%), a CR with incomplete bone marrow recovery (0%), a partial response (PR; 69.2%), or a PR with lymphocytosis (PR-L; 12.1%). The median duration of response assessed by IRC was not reached. The ORR as assessed by the investigator was 92.3%, with 13.2% of patients achieving a CR. The response to treatment increased and deepened over time (). The ORR was generally consistent across all subgroups analyzed, including those with unfavorable prognostic factors. Patients with del(17p) or TP53 mutation and those with IGHV unmutated status achieved high response rates: 90.9% (95% CI 70.8–98.9%) and 88.2% (95% CI 76.1–95.6%), respectively. All patients harboring del(11q) achieved a response (ORR 100%, 95% CI 83.2–100%) ().
Figure 1. Clinical efficacy outcomes of patients treated with zanubrutinib. (A) Overall response rate assessed by IRC and INV over time. (B) Forest plot of ORR by a predefined subgroup analysis. (C) Kaplan–Meier curves of progression-free survival for the safety population and patients with selected chromosomal abnormalities. For IGHV mutation status, 17 patients were excluded due to the following reasons: three patients with IGHV gene rearrangement undetected, 13 patients with multiclonal IGHV gene rearrangement detected, and one patient with test failed. aTwo-sided Clopper–Pearson 95% CIs. CI: confidence interval; CLL: chronic lymphocytic leukemia; CR: complete response; IGHV: immunoglobulin heavy chain variable region gene; INV: investigator; IRC: independent review committee; NA: not applicable; nPR: nodular partial response; ORR: overall response rate; PFS: progression-free survival; PR: partial response; PR-L: partial response with lymphocytosis; SLL: small lymphocytic lymphoma.
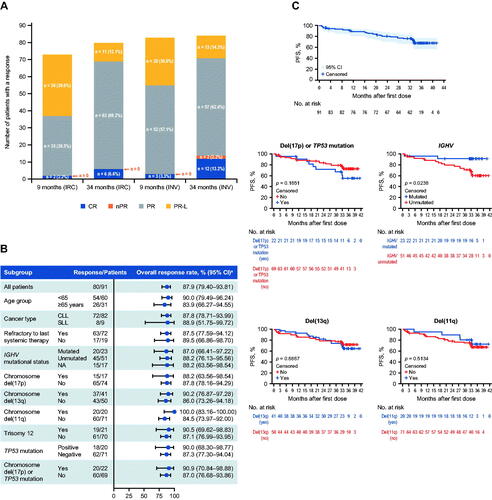
A total of 26 (28.6%) patients had either progressed (20.9%) or died (7.7%) as of the study closure. The median progression-free survival (PFS) assessed by IRC was not reached. The estimated PFS event-free rates by IRC at 24, 30 and 36 months were 80.5% (95% CI 70.5 − 87.4%), 75.7% (95% CI 65.2 − 83.4%), and 68.1% (95% CI 56.6 − 77.2%), respectively. The PFS curves were comparable among patients carrying unfavorable chromosomal abnormalities versus wild types, including del(17p) or TP53 mutation, del(13q), and del(11q). IGHV unmutated status remained a prognostic factor for patients treated with zanubrutinib (hazard ratio for unmutated versus mutated, 4.63 [95% CI 1.33–29.16]; p = 0.0238 from log-rank test; ).
Median overall survival was not reached. Estimated survival rates at 24, 30 and 36 months were 89.8% (95% CI 81.3 − 94.6%), 88.6% (95% CI 79.8 − 93.7%) and 86.5% (95% CI 76.6 − 92.4%), respectively.
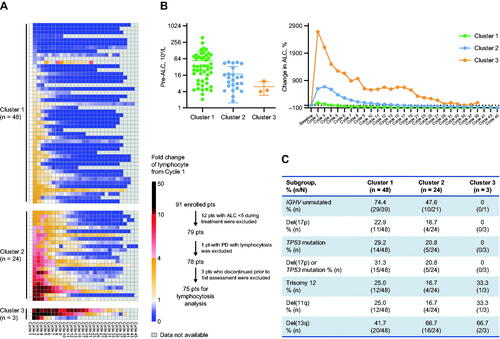
In the current study, 75 of 91 (82.4%) patients with absolute lymphocyte count (ALC) ≥5 × 109/L during treatment were selected to explore zanubrutinib-induced lymphocytosis patterns using unsupervised cluster analysis and to explore their association with baseline risk factors. Three lymphocytosis patterns were identified (). Cluster 1 had higher baseline ALC counts, smaller ALC increases and faster ALC resolution after treatment compared to Clusters 2 and 3 (). Clusters 2 and 3 were characterized by persistent lymphocytosis, tended to have less unfavorable prognostic factors such as IGHV unmutated status, TP53 mutation, and del(17p) (); and did not show inferior PFS compared with Cluster 1 (Supplementary Materials, Figure S1). A total of 11 (12.1%) patients maintained PR-L after a median follow-up of 34 months; nine (81.8%) of these patients showed Cluster 2 or 3 lymphocytosis patterns. Overall, zanubrutinib-induced prolonged lymphocytosis was associated with baseline IGHV mutation and normal TP53 function and did not predict suboptimal response to zanubrutinib.
Figure 2. Zanubrutinib-induced lymphocytosis and association with prognostic factors. (A) Identification of three different lymphocytosis patterns by unsupervised cluster analysis using ALC fold change from baseline. (B) Baseline ALC comparison among three lymphocytosis patterns plotted in mean ± SD (left); and percentage change in ALC over different treatment time periods shown in mean value (right). (C) Comparison of baseline prognostic factors among three lymphocytosis patterns. ALC: absolute lymphocyte count; CLL: chronic lymphocytic leukemia; IGHV: immunoglobulin heavy chain variable region gene; PD: progressive disease; pts: patients; SD: standard deviation; SLL: small lymphocytic lymphoma.
AEs were graded for severity based on National Cancer Institute Common Toxicity Criteria version 4.03. No new safety signals were identified. In total, 83.5% of patients had at least one Grade ≥3 AE, and 51.6% reported at least one serious AE. Grade ≥3 AEs reported in ≥5% of patients included neutrophil count decreased (49.5%); pneumonia (24.2%); upper respiratory tract infection (12.1%); anemia (11.0%); platelet count decreased (8.8%); neutrophil percentage decreased, thrombocytopenia, white blood cell count decreased and hypokalemia (7.7% each); and hyponatremia (5.5%).
Second primary malignancies were reported in five patients (two gastric adenocarcinomas; and one each of colon cancer, breast cancer, and rectal cancer). One patient experienced atrial fibrillation (Grade 2). Hypertension was reported in 11 patients (12.1%), including 3.3% Grade 3 events. While minor mucocutaneous bleeding events were relatively common (72.5%), other bleeding AEs observed were all Grade 1 or 2 events.
Fourteen (15.4%) patients experienced AEs that led to discontinuation of study drug, most commonly due to pneumonia (n = 4) and hepatitis B (n = 2). Six (6.6%) patients experienced an AE leading to death (detailed information provided in Table S2). Among these fatal events, two events of pneumonia were assessed as possibly related to study drug by the investigator. The other fatal events were assessed as unlikely related or not related to study drug, and most occurred early after treatment initiation (one each of cardiopulmonary failure, brain herniation, and multiple organ dysfunction syndrome; and one due to cardiac failure, pneumonia, and respiratory failure).
Resistance mutations were assessed in disease progression and paired screening blood samples from eight progressive patients with on-treatment durations ranging from 11.4 months to 33.4 months. BTK C481S, A428D mutations which inhibit the covalent binding affinity of BTK inhibitors [Citation5] and PLCG2 D993H/G, M1141K mutations which were found in ibrutinib progressive CLL patients [Citation6] were detected in progression but not screening samples in four and two patients, respectively (Supplementary Material, Table S3 and Figure S2).
Zanubrutinib is a novel inhibitor of BTK with a mechanism of action similar to that of ibrutinib. It achieves high plasma concentrations and sustains complete BTK occupancy in peripheral blood mononuclear cells and lymph nodes in vivo, and, compared with ibrutinib, it has demonstrated greater selectivity for BTK versus other TEC and EGFR family kinases in vitro. The current study demonstrated deepened and durable clinical benefit with zanubrutinib in patients with R/R CLL/SLL and it is noteworthy that the response to zanubrutinib increased over time (ORR: from 80.2% at initial analysis with a median follow up duration of 9.10 months to 87.9% at final analysis; CR: from 2.2 to 6.6%). This trend toward response improvement has also been observed with other BTK inhibitors, including ibrutinib and acalabrutinib [Citation7–11], suggesting that continued use of BTK inhibitors could provide clinical benefit for patients with R/R CLL/SLL. Transient/prolonged, treatment-induced lymphocytosis is a class effect of BTK inhibitors. Previous evidence has demonstrated that persistent lymphocytosis during ibrutinib treatment is not a barrier to patients achieving CR/PR [Citation12,Citation13]. The present study showed for the first time that survival in patients receiving zanubrutinib was comparable between patients harboring chromosomal abnormalities and those who were wild type, except for IGHV mutation status where unmutated status remained a poor prognostic factor.
With extended follow-up, the toxicity profile of zanubrutinib monotherapy was mostly mild or moderate in severity and was generally consistent with the known toxicity profile of BTK inhibitors. The most common AEs were decreased blood cell counts and infectious complications, which are well-known conditions associated with indolent hematologic malignancies managed with long-term treatment. Notably, only one patient reported atrial fibrillation in the current study, and a similarly low incidence was observed in zanubrutinib-treated patients in another head-to-head clinical trial of zanubrutinib versus ibrutinib [Citation14].
In summary, results with longer follow-up continued to show a high response rate for zanubrutinib. Deep and durable responses were achieved in all patient subgroups, including patients with high-risk prognostic factors and those with prolonged lymphocytosis. Data support the tolerability of long-term zanubrutinib treatment in R/R CLL/SLL patients, with no new safety signals identified. Zanubrutinib may represent an important treatment option for these patients.
GLAL-2022-0640-File004.docx
Download MS Word (120.8 KB)Acknowledgments
We sincerely thank the patients who participated in the study, their supporters, the investigators, and clinical research staff from the study centers. We also thank Dr. Jianfeng Zhou for his dedication to the study and for the care he provided to his patients.
Disclosure statement
Meng Ji, Haiyi Guo, Xia Zhao, Binghao Wu, Yiling Yu, Yu Wang, Jane Huang, and William Novotny are employees of and own stock in BeiGene. No potential conflict of interest was reported by the author(s).
Data availability statement
On request, and subject to certain criteria, conditions, and exceptions, BeiGene will provide access to individual de-identified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to [email protected]
Additional information
Funding
References
- Noy A, de Vos S, Thieblemont C, et al. Targeting bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–2232.
- Xu W, Yang S, Zhou K, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48.
- Hallek M, Cheson BD, Catovsky D, International Workshop on Chronic Lymphocytic Leukemia, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the national cancer Institute-Working group 1996 guidelines. Blood. 2008;111(12):5446–5456.
- Cheson BD, Fisher RI, Barrington SF, United Kingdom National Cancer Research Institute, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3068.
- Wang E, Mi X, Thompson MC, et al. Mechanisms of resistance to noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735–743.
- Sedlarikova L, Petrackova A, Papajik T, et al. Resistance-associated mutations in chronic lymphocytic leukemia patients treated with novel agents. Front Oncol. 2020;10:894.
- Munir T, Brown JR, O'Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363.
- Byrd JC, Brown JR, O'Brien S, RESONATE Investigators, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223.
- Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332.
- Byrd JC, Hillmen P, O'Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs. ofatumumab. Blood. 2019;133(19):2031–2042.
- Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135(15):1204–1213.
- Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817.
- Barrientos JC, Burger JA, Byrd JC, et al. Characterizing the kinetics of lymphocytosis in patients with chronic lymphocytic leukemia treated with single-agent ibrutinib. Leuk Lymphoma. 2019;60(4):1000–1005.
- Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs. ibrutinib in symptomatic waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–2050.
