Abstract
One of the most widely accepted conditioning regimens for hematopoietic stem cell transplantation (HSCT) is BEAM (carmustine, etoposide, cytarabine, melphalan). However, a recent increase in the cost of carmustine has limited its use bringing our institution to replace carmustine with bendamustine. This observational retrospective single-center study aims to report the efficacy and safety of the BeEAM regimen. 55 patients with diffuse large B-cell lymphoma (47%), Hodgkin lymphoma (25%), mantle cell lymphoma (25%), or follicular lymphoma (2%) were included. Progression-free survival (PFS) at 24 months was 75% and overall survival (OS) was 83%. Treatment-related mortality was 4%. The most common adverse effects were febrile neutropenia (98%), mucositis (72%) and colitis (60%). Our study demonstrated excellent efficacy of the BeEAM regimen. However, the toxicity profile of BeEAM significantly varies from one study to another, and guidelines suggesting optimal dose of bendamustine and supportive care are currently lacking.
Introduction
Autologous hematopoietic stem-cell transplantation (HSCT) is a standard therapeutic option for patients with Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL) presenting with initially refractory disease or those who relapsed after front-line therapy [Citation1]. It is also a first-line treatment for advance stages of mantle cells lymphoma (MCL), which tend to present with an aggressive clinical course [Citation2]. Although Chimeric antigen receptor T-cell therapy has become a new standard of care in primary refractory and early relapsing (< 12 months) diffuse large B-cell lymphoma (DLBCL), access to CAR T therapy in the second-line setting is still unaccessible in many countries [Citation3].
For suitable patients, the decision to proceed with autologous HSCT must be individualized according to the potential benefits versus adverse effects. Comorbidities and status of underlying disease should be considered. Among criteria of significance, the lymphoma must demonstrate a level of chemosensitivity, defined as a partial response or better to pre-intervention. In such cases, eligible patients will receive high-dose chemotherapy followed by stem-cell reinfusion.
Comparative studies between various conditioning regimens fail to identify an optimal treatment, as both long-term efficacy and short-term toxicities must be considered [Citation4]; One of the most frequently used regimens in North America and Europe is BEAM (carmustine (BCNU), etoposide, cytarabine, melphalan), with an initial reported 5-year overall survival (OS) of 55% [Citation5]. However, concerns over carmustine-induced toxicities, particularly pneumonitis and secondary malignancies, along with its rising cost and supply shortage, have limited its use.
Several hospitals worldwide, including ours, have switched to the bendamustine-based BeEAM regimen (bendamustine, cytarabine, etoposide, melphalan) as an alternative to BEAM. Bendamustine is an alkylating agent with a mustard group, which confers it its alkylating activity, and a purine analogue which is responsible for its antimetabolite effect. It also triggers DNA-damage stress response, apoptosis, and inhibition of mitotic checkpoints in tumor cells [Citation6].
To our knowledge, there are no prospective randomized studies comparing different types of conditioning regimen. Therefore, little data are available regarding the efficacy and safety of BeEAM. This study aims to evaluate the efficacy of BeEAM in terms of overall response rate (ORR), PFS, OS, and to report adverse events including mortality, in patients who received BeEAM at our institution.
Methods
Study design
We conducted an observational, retrospective, single-center study. Data were collected for 55 patients with HL or NHL who have received BeEAM followed by autologous HSCT between January 2015 and June 2020 at Hôpital du Sacré-Coeur de Montréal, Montreal, Canada. With exception of mantle cell lymphoma patients, all other patients were treated with autologous HSCT for primary chemotherapy-refractory disease or relapsed disease after first-line therapy. All data were collected from hospital chart reviews. Inclusion and exclusion criteria are listed in . This study was approved by the local ethics committee and research committee in accordance with the relevant guidelines and regulations.
Table 1. Inclusion and exclusion criteria.
Conditioning regimen
All patients received Benda-EAM regimen as followed: bendamustine 200 mg/m2 on days −8 and −7, etoposide 100 mg/m2 twice daily with cytarabine 200 mg/m2 twice daily on days −6 to −3, and melphalan 140/m2 on day −2. CD34-positive stem cells were reinfused on day 0. In some patients with comorbidities or advanced age at transplant, a reduced dose of bendamustine ranging from 100 mg/m2 to 160 mg/m2 was administered instead of 200 mg/m2.
All patients received weight-based granulocyte-colony stimulating factor (G-CSF) at 5 mcg/kg starting at day +2 until absolute neutrophils count (ANC) exceeded 1 × 109/L for 2 consecutive days. Valacyclovir 500 mg PO twice daily was started on day +1 and continued for 30 days if serum herpes simplex virus (HSV) antibodies tested positive but serum varicella-zoster virus (VZV) antibodies tested negative, and no prior infection was documented. However, in case of a positive VZV serology or prior infection, the antiviral was continued for a period of 12 months. Fluconazole and trimethoprim/sulfamethoxazole (Pneumocystis jirovecci pneumonia prophylaxis) were started when ANC was below 0.5 × 109/L.
Statistical analysis and definitions
We used Kaplan-Meier method to estimate two-year progression-free survival (PFS) and overall survival (OS). OS was defined as the time from auto-HSCT to death or last follow-up. PFS was defined as the time from auto-HSCT to progression, death, or last follow-up. Overall response rates were defined according to the Lugano criteria on CT or PET scan. Toxicities were reported according to the Common Terminology Criteria for Adverse Events (CTCAE 5.0). We also performed a subgroup analysis to determine the impact of the BeEAM regimen on the different subtypes of lymphomas.
Results
Patients’ characteristics
Data were collected by reviewing hospital charts of 55 patients, including 26 (47.4%) with DLBCL, 14 (25.4%) with HL, 14 (25.4%) with MCL and 1 (1.8%) with FL. 71% of DLBCL were primary refractory as well as 57% of Hodgkin Lymphoma. The median age was 59 years (17-72), with a higher proportion of men (64%). Most patients had stage IV lymphoma (69%) according to the Lugano classification, with 39 (70.9%) having more than one extranodal involvement. The mean number of prior lines of therapy was 2.2. The median CD34 cell dose infused was 10.46 × 106/kg. The median times to neutrophil and platelet engraftment were 9.1 days (range, 11 to 15 days) and 12.4 days (range, 9 to 36 days), respectively. Clinical characteristics of the patients are summarized in .
Table 2. Patient characteristics.
Outcome
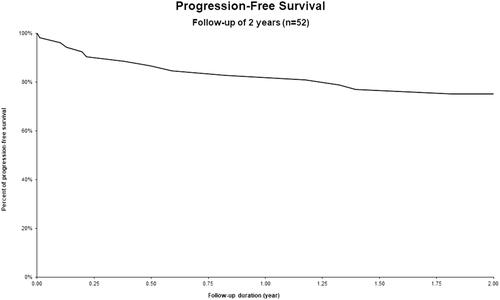
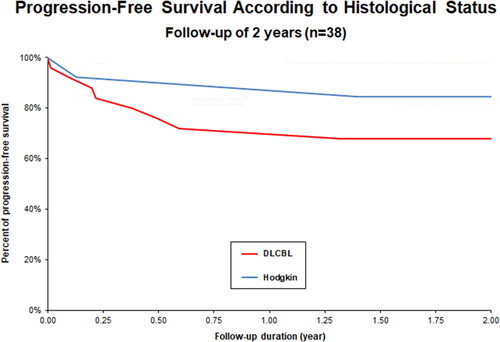
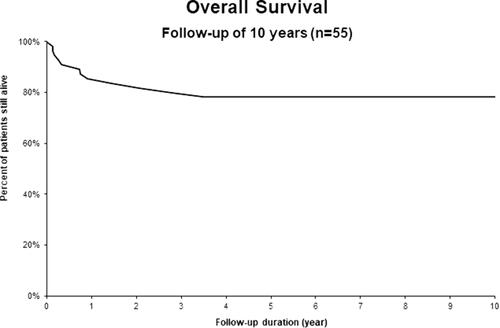
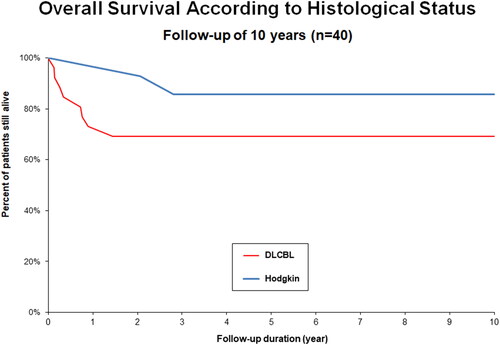
Global PFS rate at 24 months was 75% (). Subgroup histological status analysis showed a PFS rate of 77% for HL, 71% for MCL and 65% for DLBCL (). OS at 24 months was 83% (). Subgroup analysis showed an OS of 100% for HL, 93% for MCL and 69% for DLBCL (). Complete remission (CR) as best response to treatment was achieved in 73% of patients. Partial remission (PR) was obtained in 23% of patients, and 4% of them had progressive disease after treatment. Treatment-related mortality was 4% with 2 patients who died of infectious complications, including one case of Covid-19. Number of prior lines of therapy seemed to influence the prognosis. The mean number of lines of treatment for all patients was 2.2, while that for patients who progressed at 2 years was 2.7
Adverse events
The most common infectious adverse events were febrile neutropenia (98%), colitis (60%) and mucositis (72%); for all these, grade 3 toxicities were most frequently encountered. All infectious side effects are summarized in . At least 1 pathogen was identified in 29 (54%) of the patients who had febrile neutropenia. The 3 most frequently encountered pathogens were Clostridium Difficile, Escherichia Coli and Candida. All pathogens are described in . Noninfectious toxicities included renal failure (19%), liver damage (13%), diarrhea (13%), atrial fibrillation (9%), upper/lower digestive hemorrhage (7%), adrenal insufficiency (7%) and deep venous thrombosis (5%). 21% of kidney injuries were grade 3 or more and 5% of them required temporary dialysis. Excluding patients who received a reduced dose of bendamustine, we observed a significant difference in the mean age of patients who had grade II or greater renal failure or who required a stay in intensive care. The average age of patients who suffered serious complications was 57 years in comparison with 51 years in the group without complication. However, pre-transplant renal function did not seem to influence the occurrence of renal failure. Blood products were administered to 93% of patients and total parenteral nutrition was required in 71% of them. The ICU admission rate was 15% and the readmission rate was 11% (). The mean length of stay post transplantation was 31 days. Refer to for the complete list of noninfectious adverse events.
Table 3. Infectious adverse events.
Table 4. Identified pathogens during febrile neutropenia.
Table 6. Noninfectious adverse events.
Table 5. Specific treatment, TRM and readmission rate.
Discussion
Several BCNU-based conditioning regimens are available in clinical practice, but little of them have been compared with each other in a prospective or randomized study. The CBV (cyclophosphamide (Cy), BCNU, etoposide), BEAC (BCNU, etoposide, aracytine, Cy) and BEAM (BCNU, etoposide, aracytine, melphalan) were most used, with a 2 to 5 years disease-free survival rates varying from 34–60% [Citation4]. Among these, the BEAM conditioning regimen was associated with less toxicity and lower TRM, explaining its more frequent use in auto-HSCT [Citation7]. Due to supply shortage and increase in the cost of BCNU, transplant centers needed to use alternative conditioning regimens [Citation1].
One of the alternative conditioning regimens of BEAM is BeEAM, with a 3-year PFS of 72% and a 100-day TRM of 0%, as published by Visani et al. [Citation8] The cumulative incidence of infectious complications was 60%, without grade 3–4 adverse events [Citation8,Citation9]. Therefore, BeEAM has become one of the most frequently used conditioning regimen worldwide. Since then, a few retrospective studies have been published to assess the efficacy and safety of this regimen.
Chantepie et al. has published a multicenter retrospective study evaluating the safety of bendamustine in 474 patients. The toxicity profile was acceptable with treatment-related mortality (TRM) of 3.3%. However, acute kidney injury was frequent (27.9%) and was associated with ICU admission, more toxicities and death. Multivariate analysis suggested that pretransplant chronic renal failure, bendamustine dose > 160 mg/m2, and age were independent prognostic factors for acute renal failure (ARF). In patients over 55 years old and with pre-transplant renal failure, a reduced bendamustine dose should be used because of a 23% risk of ARF with a full bendamustine dose [Citation9].
In a single-center retrospective study, Saleh and al. compared dose-reduced BeEAM (bendamustine 100 mg/m2) to BEAM. A total of 102 patients were included in the study, with 49 in the BeEAM group. Of these, 56% had DLBCL. OS and PFS were not reached but seemed comparable between both groups [Citation10]. However, severe diarrhea and the number of days with fever were significantly higher in the BeEAM group [Citation10]. Aljohani et al. also compared dose-adjusted bendamustine (160 mg/m2) as a replacement for carmustine in autologous HSCT conditioning. Despite using a lower dose of bendamustine, OS and PFS were similar between both groups [Citation11]. There was no increase in cardiotoxicity or nephrotoxicity, although grade 3 mucositis as well as the need for parenteral nutrition was increased in the BeEAM group (82.4% vs 48%) [Citation11]. In a single-center retrospective study including a total of 237 patients, Frankiewicz et al. reported no difference in the probabilities of overall and progression-free survival when comparing BeEAM and BEAM regimens. Apart from arterial hypertension and severe hypokalemia, which occurred more frequently after BeEAM, the toxicity profile was comparable between the two groups [Citation6].
Most recently Lachance et al. published a retrospective study comparing BeEAM conditioning with BCNU-based conditioning. Development of grade > 3 mucositis (39.5% versus 7.8%) leading to a greater use of parenteral nutrition (48.9% versus 21.9%) as well as acute renal failure (15.3% versus 4.2%) and intensive care admission (6.9% versus 1.1%) were more frequent with BeEAM conditioning. There were no differences regarding cardiac, pulmonary of live toxicity between both groups. 2-years PFS (71% versus 61%) and OS (86% versus 71%) were significantly better in the BeEAM group [Citation12].
Other studies also showed an unfavorable toxicity profile of bendamustine with an increased risk of intensive care admission, renal failure, febrile neutropenia, and infections [Citation4,Citation9,Citation13–16]. Although, higher dose of bendamustine with 160–200mg/m2 were more frequently used and supportive care greatly differed across different studies.
BCNU drug shortage has also led to increased use of other myeloablative conditioning regimens such as TEAM (Thiotepa, Etoposide, Cytarabine, Melphalan), Treo/Flu (Treosulfan plus fludarabine) and FEAM (Fotemustine, Etoposide, Cytarabine, Melphalan). Duléry et al. published a multicenter prospective study reporting the efficacy and safety of the TEAM protocol prior to autologous HSCT. After a follow-up of 31 months, nonrelapse mortality (NRM) was 3.3%. The estimated three-year OS and PFS were 90.2% and 77.2%. Grade 1–4 mucositis (median 3) and diarrhea (median 1) were the most frequently encountered adverse effects in 100% and 98% of patients respectively. Other non-hematologic grade 3 adverse events occurred in 18% of patients and 27% had blood culture positive for Staphylococcus sp. [Citation17]. Frietsch et al. published a study comparing Treo/Flu versus TEAM as conditioning before autologous HSCT. Efficacy was similar between both regimens. However, Treo/Flu was associated with less infectious complications and platelets transfusion. It would thus be a valuable alternative in elderly patients with DLBCL [Citation18]. Olivieri et al. conducted a retrospective cohort study comparing the safety and efficacy of BEAM and FEAM regimens for ASCT. 1038 patients were included (BEAM = 607, FEAM = 431). FEAM conditioning was associated with higher rates of severe oral mucositis (44% vs 31%), sepsis and infection-related mortality. 2-years OS (83.9%) and PFS (70.3%) were not different between two groups [Citation19].
Since our data collection, new treatment strategies have been approved to improve lymphoma patient outcomes. In MCL, the addition of rituximab in maintenance after autologous HSCT has become a new standard of care, as it significantly lengthens PFS and OS [Citation20]. In Hodgkin’s lymphoma patients with high risk of relapse after autologous HSCT, maintenance with Brentuximab vedotin (BV) is now warranted, as supported by the ATHENA trial. It confers a sustained 5-year PFS benefit compared to placebo (59% vs 41%) and is safe and well tolerated [Citation21].
Our results have shown excellent efficacy of the BeEAM conditioning regimen, especially for the Hodgkin’s lymphoma subgroup. Two-year OS and PFS are comparable to those obtain in other studies, such as Lachance et al. The median times to neutrophil and platelet engraftment was similar to data reported in the literature with BEAM induction protocol. As observed in the studies cited above, it is also associated with increased toxicity in terms of mucositis, febrile neutropenia, and infections. The rate of admission to ICU was significant (15%), despite a TRM (4%) not exceeding those previously reported. Available data seem to demonstrate a TRM rate up to 10% with the BEAM conditioning [Citation15]. Due to the increased toxicity observed with Bendamustine 200 mg/m2, the Transplant Center of Hôpital du Sacré-Coeur de Montréal issued new dosing recommendations based on age and patient comorbidities in 2019. Details of the dose adjustments can be found in . Reviewing the mortality cases following the use of the BeEAM regimen led us to believe that the most important risk factor associated with toxicity is the age of the patient at the transplant. The number of prior treatment lines could also have a potential impact on the morbidity and mortality associated with this conditioning regimen. Our study has certain limitations, the main one being its retrospective nature which limits the interpretation of the toxicities encountered, including their severity grade. Furthermore, we were not able to compare our data with the BEAM induction protocol since the BEAC protocol was the most frequently used in our center in recent years. It would be very interesting to compare toxicity and efficacy profile between different doses of bendamustine in a larger cohort. Lastly, long-term follow-up is necessary to evaluate the risk of secondary malignancies.
Table 7. Reduce dose BeEAM conditioning regimen.
With the arrival of CAR T-cell therapy, the place of autologous HSCT in first relapse or primary refractory DLBCL remains uncertain. Chimeric antigen receptors targeting the CD19 antigen on B cells are effective and safe in patients with relapse or refractory DLBCL, high-grade B cell lymphoma, DLBCL arising from transformed follicular lymphoma, and primary mediastinal B-cell lymphoma [Citation3]. The following three clinical trials, ZUMA-1, JULIET and TRANSCEND NHL001, supported the efficacy and safety of CAR T-cell therapies with an ORR ranging from 52% to 74% and 1-year OS rate of 48% to 59% [Citation3,Citation22–24]. In comparison, SCHOLAR-1 study demonstrated that patients with chemorefractory DLBCL treated with conventional therapies have a median OS of 6 months and 1-year OS rate of 28% [Citation25]. Most recently, three additional randomized phase-3 trials have evaluated second-line CAR T-cell therapy vs standard-of-care salvage chemotherapy followed by high dose chemotherapy and autologous HSCT [Citation26-29]. Results of the TRANSFORM trial (liso-cel vs standard of care) are particularly impressive with a median event-free survival of 10.1 months in the liso-cell group compared to 2.3 months with standard-of-care group [Citation30]. Treatment for relapsed or refractory B-cell lymphoma is rapidly changing as these results support the use of CAR T-cell therapy in primary refractory DLBCL as well as in early relapse (<12 months), although it’s not yet refunded as a second line of treatment in many countries.
Conclusion
Our study showed excellent PFS and OS outcomes of the BeEAM regimen, with results comparable to data available in the literature. However, this regimen is also associated with a high rate of mucositis, febrile neutropenia, and infections. Formal guidelines regarding optimal dosing of bendamustine and supportive care, including antibiotic prophylaxis, are currently lacking. Further studies are needed to determine the optimal doses in terms of safety and efficacy. With the arrival of CAR T-cell therapy, the role of autologous HSCT remains to be determined, particularly in the context of primary refractory or early relapsing DLBCL.
Disclosure statement
The corresponding author acts as a consultant at Janssen, BMS, Amgen, AbbVie and Sanofi. The other authors have no conflict of interest.
Additional information
Funding
References
- Lekakis LJ, Moskowitz CH. The role of autologous stem cell transplantation in the treatment of diffuse large B-cell lymphoma in the era of CAR-T cell therapy. Hemasphere. 2019;3(6):e295.
- Zoellner AK, Unterhalt M, Stilgenbauer S, et al. Long-term survival of patients with mantle cell lymphoma after autologous haematopoietic stem-cell transplantation in first remission: a post-hoc analysis of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2021;8(9):e648–e57.
- Westin JR, Kersten MJ, Salles G, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–1312.
- Damaj G, Cornillon J, Bouabdallah K, et al. Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: a review. Bone Marrow Transplant. 2017;52(7):941–949.
- Chopra R, McMillan AK, Linch DC, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin’s disease. A single-center eight-year study of 155 patients. Blood. 1993;81(5):1137–1145.
- Frankiewicz A, Saduś-Wojciechowska M, Najda J, et al. Comparable safety profile of BeEAM (bendamustine, etoposide, cytarabine, melphalan) and BEAM (carmustine, etoposide, cytarabine, melphalan) as conditioning before autologous haematopoietic cell transplantation. Contemp Oncol (Pozn). 2018;22(2):113–117.
- Puig N, de la Rubia J, Remigia MJ, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006;47(8):1488–1494.
- Visani G, Stefani PM, Capria S, et al. Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3-year PFS in resistant lymphoma. Blood. 2014;124(19):3029–3031.
- Chantepie SP, Garciaz S, Tchernonog E, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from lymphoma study association (LYSA) centers. Am J Hematol. 2018;93(6):729–735.
- Saleh K, Danu A, Koscielny S, et al. A retrospective, matched paired analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: results from a single center experience. Leuk Lymphoma. 2018;59(11):2580–2587.
- AlJohani NI, Nasani M, Ahmed HE, et al. Dose-adjusted bendamustine as a replacement for carmustine in autologous stem cell transplant conditioning for patients with relapsed lymphoma: a retrospective single-center study. Hematol Oncol Stem Cell Ther. 2021;14(4):327–335.
- Lachance S, Bourguignon A, Boisjoly JA, et al. Impact of implementing a Bendamustine-Based conditioning regimen on outcomes of autologous stem cell transplantation in lymphoma while novel cellular therapies emerge. Transplant Cell Ther. 2023;29(1):34. e1- e7.
- Garciaz S, Coso D, Schiano de Collela JM, et al. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant. 2016;51(2):319–321.
- Hueso T, Gastinne T, Garciaz S, et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from lymphoma study association (LYSA) centers. Bone Marrow Transplant. 2020;55(6):1076–1084.
- Isidori A, Christofides A, Visani G. Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and hodgkin lymphoma: alternatives to BEAM conditioning. Leuk Lymphoma. 2016;57(11):2499–2509.
- Lucijanic M, Prka Z, Jaksic O, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation is associated with high rate of febrile neutropenia and higher mortality. Am J Hematol. 2019;94(2):E42–E43. E3.
- Mohty M, Belhocine R, Aoudjhane M, et al. TEAM conditioning (thiotepa, etoposide, cytarabine, melphalan) prior to autologous hematopoietic stem cell transplantation for Hodgkin and non-Hodgkin lymphoma: final results from a prospective multicenter study. Blood. 2019;134(Supplement_1):786–786.
- Frietsch JJ, Miethke J, Linke P, et al. Treosulfan plus fludarabine versus TEAM as conditioning treatment before autologous stem cell transplantation for B-cell Non-Hodgkin lymphoma. Bone Marrow Transplant. 2022;57(7):1164–1170.
- Olivieri J, Mosna F, Pelosini M, et al. A comparison of the conditioning regimens BEAM and FEAM for autologous hematopoietic stem cell transplantation in lymphoma: an observational study on 1038 patients from fondazione italiana linfomi. Biol Blood Marrow Transplant. 2018;24(9):1814–1822.
- Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous Stem-Cell transplantation in Mantle-Cell lymphoma. N Engl J Med. 2017;377(13):1250–1260.
- Moskowitz CH, Walewski J, Nademanee A, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–2642.
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–2544.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2019;380(1):45–56.
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808.
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-Cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38(27):3119–3128.
- Riedell PA, Hwang WT, Nastoupil LJ, et al. Patterns of use, outcomes, and resource utilization among recipients of commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. 2022;28(10):669–676.
- Bishop MR, Dickinson M, Purtill D, et al. Second-Line tisagenlecleucel or standard care in aggressive B-Cell lymphoma. N Engl J Med. 2022;386(7):629–639.
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as Second-Line therapy for large B-Cell lymphoma. N Engl J Med. 2022;386(7):640–654.
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308.