Abstract
Polatuzumab vedotin (Pola) was approved for first-line and relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) in many countries. This means that retreatment with Pola for r/r DLBCL could be considered after first-line Pola treatment; however, there is currently no evidence on the effectiveness of Pola-retreatment. To address this, we established two Pola-resistant cells from DLBCL cells (SU-DHL-4 and STR-428) and evaluated the combination efficacy of Pola plus rituximab (Rit), the key component of DLBCL therapy. MDR1 overexpression and decreased Bim expression were suggested to be the resistant mechanisms to Pola in Pola-resistant SU-DHL-4 and Pola-resistant STR-428, respectively. In these cells, Pola significantly increased Rit-induced CDC sensitivity either with increased MAC formation or reduced Mcl-1 expression. Additionally, treatment with Pola + Rit significantly enhanced antitumor activity in Pola-resistant STR-428 xenograft mouse models. Based on these results, Pola + Rit retreatment could have preserved efficacy because of the effect of Pola on sensitizing cells to Rit.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of aggressive non-Hodgkin lymphoma [Citation1]. Although DLBCL is curable in the majority of cases, ∼40% of patients are either refractory or will relapse after first-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or a similar regimen [Citation2]. As a result of years of research, the treatment landscape for patients with relapsed/refractory (r/r) DLBCL has been evolving with the development of targeted therapies including CD19-directed chimeric antigen receptor (CAR) T-cell therapy, the anti-CD19 monoclonal antibody, and the anti-CD79b antibody drug conjugate (ADC) [Citation3–6]. However, it is not yet clear which drugs are appropriate for individual patients.
Polatuzumab vedotin (Pola) is a first in class ADC targeting CD79b, a transmembrane protein expressed on the surface of B cells [Citation7,Citation8]. On binding to CD79b, Pola is internalized and releases microtubule-disrupting agent, monomethyl auristatin E (MMAE) to cause a G2/M phase arrest and apoptosis [Citation9]. In the randomized open-label phase Ib/II study (GO29365), Pola in combination with bendamustine and rituximab (Pola + BR) showed a significant improvement in the complete response rate at end of treatment compared to BR in patients with r/r DLBCL. Based on these results, Pola + BR was approved for use in r/r DLBCL [Citation4,Citation10,Citation11]. In the phase III POLARIX study, Pola-R-CHP achieved a significantly improved progression-free survival compared to R-CHOP in previously untreated DLBCL [Citation12], and Pola-R-CHP recently also received regulatory approvals in first-line DLBCL. This means that after administration of Pola in first-line treatment, retreatment with Pola would be considered as a treatment option for r/r DLBCL. However, there is currently no evidence on the effectiveness of retreatment with Pola for patients who relapse after initial Pola treatment, since Pola-R-CHP was only recently approved and the data on the use of Pola at the time of relapse is lacking. This is an important issue to develop the next-line treatment to follow Pola-containing therapy.
To address this issue, in this study, we established two types of cells resistant to Pola with different resistance mechanisms using Pola-sensitive human DLBCL cell lines (SU-DHL-4 and STR-428) by long-term exposure to Pola in vitro. We then evaluated the combination efficacy of Pola plus rituximab (Rit), the key component in the treatment of r/r DLBCL, to determine the efficacy of retreatment with Pola in these Pola-resistant cells. Our current study provides insights into the molecular mechanisms underlying the efficacy of retreatment with Pola with respect to acquired Pola-resistant DLBCL with two different resistance mechanisms.
Materials and methods
Reagents
Monomethyl auristatin E and AZD5991 were purchased from Selleck Chemicals. Verapamil hydrochloride was purchased from Sigma-Aldrich. Anti-human CD79b antibody (SN8) was purchased from BD Biosciences.
Cell cultures
SU-DHL-4 cells were obtained from the American Type Culture Collection, and were maintained in RPMI-1640 (Sigma-Aldrich) with 10% fetal bovine serum (FBS; Nichirei Biosciences or Corning), 10 mM HEPES (Sigma-Aldrich), 0.45% D-glucose (Sigma-Aldrich), and 1 mM sodium pyruvate (Thermo Fisher Scientific). STR-428 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank and were cultured in RPMI-1640 ATCC modification (Thermo Fisher Scientific) with 10% FBS. All cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C.
Establishment of Pola-resistant cells
SU-DHL-4 cells were pretreated with 100 μg/mL of the mutagen N-ethyl-N-nitrosourea (Sigma-Aldrich) for 24 h to introduce random mutations in order to more rapidly establish Pola-resistant clones [Citation13]. SU-DHL-4 cells were then treated with polatuzumab vedotin (Chugai Pharmaceutical Co., Ltd.) at a dose sequentially increasing from 0.02 μg/mL to 1.62 μg/mL for 9 weeks. Cells that regrew in the Pola (1.62 μg/mL)-containing medium were single cell cloned, and established SU-DHL-4-Pola-R-2 and −8 cells. SU-DHL-4-Pola-R cells were cultured in the same medium as the parental SU-DHL-4 cells. STR-428 cells were treated with Pola at a dose sequentially increasing from 0.02 μg/mL to 0.5 μg/mL for 4 weeks. Cells that could grow under the Pola concentration of 0.5 μg/mL were single-cell cloned, and established STR-428-Pola-R-5 and −9 cells. STR-428-Pola-R cells were cultured in the same medium as the parental STR-428 cells. Pola concentration was increased as much as possible in our experimental settings to establish Pola-resistant cells.
Cell cytotoxicity assay
Cells were cultured with Pola in varying concentrations for 3 days. After cultivation, Cell Counting Kit-8 solution (Dojindo Laboratories) was added to the cells in each well and absorbance at 450 nm was measured using a Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific) or CLARIOstar (BMG Labtech).
Immunoblotting
Cells were lysed with cell lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktail (Sigma-Aldrich). Total lysates were analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE) or Jess system (ProteinSimple). Immunoblotting was carried out using anti-caspase-3, anti-Bim, anti-Bcl-xL, anti-Mcl-1, anti-pERK1/2 (Thr202/Tyr204), anti-ERK1/2, anti-pAKT (Ser473), anti-AKT and anti-β-actin antibodies (Cell Signaling Technology). For SDS-PAGE, secondary antibodies were purchased from Cell Signaling Technology. Imaging for SDS-PAGE was performed with a ChemiDoc Touch imaging system (Bio-Rad Laboratories).
Complement-dependent cytotoxicity (CDC) assay
Target cells were prelabelled with calcein-AM (FUJIFILM Wako Pure Chemical Corporation) for 1 h at 37 °C. Specific cell lysis was assessed 4 h after incubation with 15% normal human serum (Complement Technology) and the indicated concentration of Rit at 37 °C. The total fluorescence intensity was determined using a Varioskan LUX Multimode Microplate Reader or EnVision 2105 Multimode Reader (PerkinElmer). %CDC was calculated as follows: (experimental release of calcein − background)/(maximum lysis − background) × 100. Here, ‘background’ means labeled Pola-pretreated cells with 15% normal human serum (without Rit) and ‘maximum lysis’ means labeled Pola-pretreated cells lysed with 1% Triton X-100 (Sigma-Aldrich).
Flow cytometry
Cells were stained with indicated antibodies. Anti-CD20 antibodies (clone: 2H7), IgG1κ isotype control (clone: MOPC-21), IgG2aκ isotype control (clone: G155-178), and IgG2bκ isotype control (clone: 27–35) were obtained from BD Biosciences. Anti-CD79b antibodies (clone: CB3-1) were purchased from Thermo Fisher Scientific. Anti-CD243 (anti-MDR1) antibodies (clone: UIC2) were purchased from BioLegend. For the analysis of MAC (C5b-9 complex), cells were incubated with Rit for 30 min and then with 15% normal human serum for 10 min at 37 °C. Cells were then washed and labeled with anti-C5b-9 antibody (clone: aE11) (Novus) or IgG2aκ isotype control (clone: MG2a-53) (BioLegend), and a secondary phycoerythrin-labeled antibody (clone: RMG2a-62) (BioLegend). Stained cells were analyzed with a BD LSRFortessa X-20 cell analyzer (BD Biosciences) and FlowJo v10 software (BD Biosciences). Mean fluorescence intensity (MFI) was calculated by subtracting the MFI value of the respective isotype control-stained cells from the observed MFI of labeled cells.
Lentivirus infection
Control or human Bim shRNA lentiviruses were purchased from VectorBuilder. The shRNA target sequence was control: CCTAAGGTTAAGTCGCCCTCG; Bim: TACTATGCAAGGAGGGTATTT. The cells were exposed to lentivirus for 1 day (MOI > 10), and then switched back into normal culture medium containing 500 μg/mL G418 (Nacalai Tesque).
Animals
Female 5-week-old C.B-17/Icr-scid/scidJcl (SCID) mice were obtained from CLEA Japan. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at Chugai Pharmaceutical Co., Ltd., which is an institute accredited by AAALAC international and conformed to the Guide for the Care and Use of Laboratory Animals published by the Institute for Laboratory Animal Research.
In vivo xenograft experiments
Indicated tumor cells (5 × 106 per mouse) were subcutaneously inoculated into SCID mice. After inoculation, mice were randomized by tumor volume and assigned to indicated study groups. To evaluate the antitumor activity of the test agents, tumor volume was evaluated as described previously [Citation14]. Control human IgG (HuIgG, MP Biomedicals), Pola, Rit, or Pola plus Rit was administered intravenously on Day 1.
Statistical analysis
Differences between two groups were analyzed with Student’s t-test. Dunnett’s test was used for multiple comparisons where the control group was compared with experimental groups. For tumor xenograft experiments, p-values were adjusted for the Wilcoxon rank sum test by the Holm–Bonferroni method. All statistical analyses were performed in JMP 15.0.0 software (SAS Institute).
Results
High-level expression of MDR1 is the mechanism of resistance in SU-DHL-4-Pola-R cells
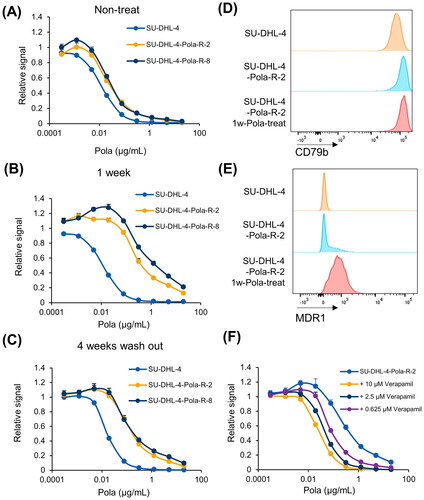
The Pola-resistant cells established from human DLBCL SU-DHL-4 cells (SU-DHL-4-Pola-R-2 and −8) had IC50 values 2.3- and 2.8-fold higher than those of the parental cells (). Interestingly, treating the Pola-resistant SU-DHL-4-Pola-R cells with 1 µg/mL of Pola for 1 week further increased their IC50 values (16.8- and 48.0-fold) (). However, this incremental Pola resistance in SU-DHL-4-Pola-R cells by additional 1 week treatment with Pola was partially released after cells were rested for 4 weeks in Pola-free culture media (). To determine the mechanisms underlying resistance to Pola in SU-DHL-4-Pola-R cells by comparing the parental SU-DHL-4 cells and SU-DHL-4-Pola-R cells with or without additional 1 week treatment with Pola, we analyzed the expression of known Pola-resistance factors; CD79blow, Bcl-xLhigh, and MDR1high. The expression of surface CD79b, the target of Pola, was retained in SU-DHL-4-Pola-R-2 cells (). The expression levels of Bcl-xL, a member of the Bcl-2 anti-apoptotic family proteins, has been reported to be correlated with reduced sensitivity to anti–CD79b ADC [Citation18], but the expression of Bcl-xL was not elevated in SU-DHL-4-Pola-R-2 cells (Supplementary Figure S1). On the other hand, there was an 1.8-fold increase in the expression of MDR1, a multidrug resistance pump that mediates MMAE efflux [Citation15], in SU-DHL-4-Pola-R cells without additional Pola treatment compared with parental cells, and treatment with Pola for 1 week further increased the expression of MDR1 (3.6-fold) in SU-DHL-4-Pola-R-2 cells (), as with the IC50 values. Also, the MDR1 specific inhibitor verapamil increased the sensitivity to Pola in SU-DHL-4-Pola-R-2 cells treated with Pola for additional 1 week, indicating that overexpression of MDR1 is one of the mechanisms of resistance to Pola in these cells ().
Figure 1. Pola-resistant SU-DHL-4 cells were resistant due to increased expression of MDR1. A, Sensitivity to Pola at the indicated concentrations was assessed in SU-DHL-4 and SU-DHL-4-Pola-R cells. Data points represent mean value + SD. n = 3 B, Sensitivity to Pola at the indicated concentrations was assessed in SU-DHL-4 cells and SU-DHL-4-Pola-R cells cultured in Pola-free media for 1 week following treatment with Pola (1 µg/mL) for 1 week. Data points represent mean value + SD. n = 3 C, Sensitivity to Pola was assessed at the indicated concentrations in SU-DHL-4 cells and SU-DHL-4-Pola-R cells cultured in Pola-free media for 4 weeks following the treatment with Pola (1 µg/mL) for 1 week. Data points represent mean value + SD. n = 3 D, E, Surface expression of CD79b (D) or MDR1 (E) on SU-DHL-4 cells, SU-DUL-4-Pola-R-2 cells, and SU-DUL-4-Pola-R-2 cells treated with Pola (1 µg/mL) for 1 week was analyzed by flow cytometry. F, SU-DUL-4-Pola-R-2 cells treated with Pola (1 µg/mL) for 1 week. Sensitivity to Pola was then assessed at the indicated concentrations in cells treated with verapamil. Data points represent mean value + SD. n = 3.
Pola enhances anti-tumor effects of Rit in SU-DHL-4-Pola-R cells
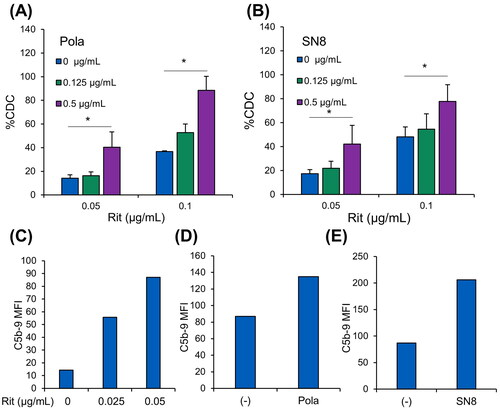
We then investigated the efficacy of combination therapy of Pola plus Rit against SU-DHL-4-Pola-R cells by cell-based CDC assays, because CDC is the predominant mechanism of action of Rit [Citation16]. Pretreatment with Pola or the anti-CD79b antibody (SN8) before the assays significantly increased Rit-induced CDC sensitivity in SU-DHL-4-Pola-R-2 cells ( and Supplementary Figure S2), but pretreatment with MMAE did not (Supplementary Figure S3). Our previous study suggested that treatment with Pola upregulates CD20 expression in Pola-refractory cells [Citation17]; however, neither Pola nor anti-CD79b antibody (SN8) increased CD20 expression in SU-DHL-4-Pola-R-2 cells (Supplementary Figure S4). When antibodies bind to surface antigens on target cells, the classical complement pathway is activated by protein C1q binding to these antibodies, leading to formation of C5b-9 complex, also known as the membrane attack complex (MAC) and lysis of the target cell [Citation18,Citation19]. To determine the point of action of Pola in Rit-induced CDC, we then assessed the formation of MAC in the CDC reaction by flow cytometry. It was confirmed that surface MAC expression was increased after CDC was induced by Rit treatment (). Under these conditions, pretreatment with Pola or anti-CD79b antibody (SN8) before the assays enhanced the surface amounts of Rit-induced MAC during complement activation in SU-DHL-4-Pola-R-2 cells ().
Figure 2. In vitro combination effect of Pola with Rit in SU-DHL-4-Pola-R cells. A, B, SU-DHL-4-Pola-R-2 cells treated with Pola (1 µg/mL) for 1 week were pretreated with Pola (A) or anti-CD79b antibody (SN8) (B) for 3 days at the indicated concentrations. Cells were then collected, and CDC assay was performed with Rit. The results are presented as mean values + SD. n = 3, * p < 0.05 by Dunnett’s t-test compared to control (Pola or SN8: 0 μg/mL). C, SU-DHL-4-Pola-R-2 cells were treated with Pola (1 µg/mL) for 1 week. Cells were then treated with Rit (30 min) followed by normal human serum (10 min). MAC (C5b-9 complex) binding was measured by flow cytometry. D, E, SU-DHL-4-Pola-R-2 cells treated with Pola (1 µg/mL) for 1 week were pretreated with Pola (D) or anti-CD79b antibody (SN8) (E) at 0.5 μg/mL for 3 days. Cells were then treated with Rit (0.05 μg/mL) for 30 min followed by normal human serum (10 min). MAC (C5b-9 complex) binding was measured by flow cytometry.
Downregulation of Bim confers resistance to Pola in STR-428-Pola-R cells
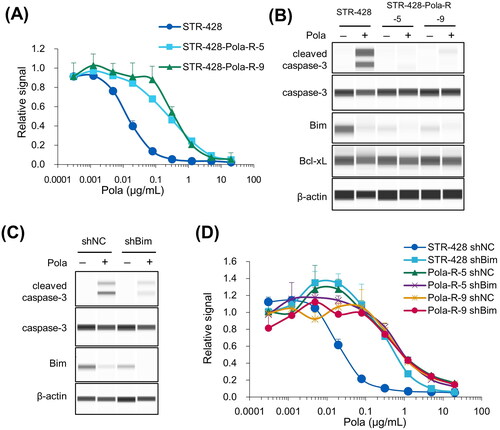
Pola-resistant cells derived from human DLBCL STR-428 cells (STR-428-Pola-R-5 and −9) displayed IC50 values 17.6- and 27.7-fold higher than the parental cells (). To elucidate the mechanisms of resistance to Pola in these cells, we assessed several factors that could regulate sensitivity to Pola. Flow cytometry analysis showed that loss of CD79b was not observed in either STR-428-Pola-R-5 or −9 cells (Supplementary Figure S5(A)), and that expression of MDR1 was not elevated (Supplementary Figure S5(B)). The expression levels of Bcl-xL were not upregulated in STR-428-Pola-R cells (). We then explored the expression of other apoptosis regulatory genes belonging to the Bcl-2 protein family, because it is possible that they might also affect the sensitivity to Pola or MMAE-induced apoptosis. Immunoblotting analysis revealed that the expression of Bim, an apoptosis-inducing factor, was downregulated both in STR-428-Pola-R-5 and −9 cells (). Expression of Bim was downregulated by 24 h treatment with Pola (). To examine the effect of Bim on the cell sensitivity to Pola, we established STR-428-Pola-R cells stably expressing control or Bim-shRNA. Stable knockdown of Bim reduced the Pola-induced caspase-3 cleavage () and the sensitivity to Pola () in parental STR-428 cells, indicating the hitherto unreported possibility that decreased Bim expression could be a mechanism of resistance to Pola.
Figure 3. Pola-resistant STR-428 cells were resistant due to reduced expression of Bim. A, Sensitivity to Pola at the indicated concentrations was assessed in STR-428 and STR-428-Pola-R cells. Data points represent mean value + SD. n = 3 B, STR-428 and STR-428-Pola-R cells were treated with Pola (0.5 μg/mL) for 24 h, and cell lysates were then collected and evaluated by immunoblotting. C, STR-428 cells expressing shRNA targeting Bim (shBim) or control (shNC) were treated with Pola (0.5 μg/mL) for 24 h. Cell lysates were then evaluated by immunoblotting. D, Sensitivity to Pola at the indicated concentrations was assessed in cells expressing shRNA targeting Bim (shBim) or control (shNC). Data points represent mean value + SD. n = 3.
Treatment with Pola plus Rit showed significant combination effect in STR-428-Pola-R cells in vitro and in vivo
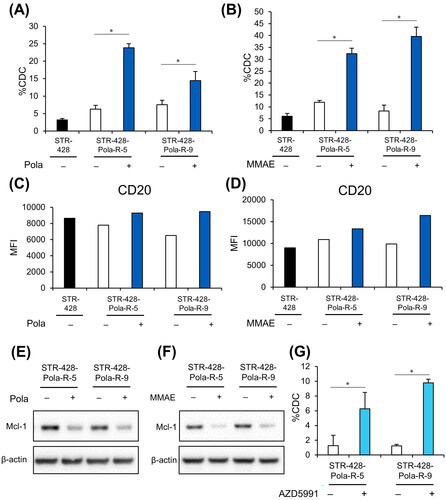
We next used CDC assays to investigate the efficacy of Pola in combination with Rit in STR-428-Pola-R cells. STR-428-Pola-R cells were pretreated with Pola, MMAE, or anti-CD79b antibody (SN8) for 3 days before the assays. Pretreatment of STR-428-Pola-R cells with concentrations of Pola or MMAE that would kill parental cells significantly increased sensitivity to Rit-mediated CDC (); however, pretreatment with anti-CD79b antibody (SN8) did not (Supplementary Figure S6). We previously reported that in several Pola-refractory DLBCL cells, treatment with Pola upregulates CD20 expression and sensitivity to Rit-induced CDC by activating both AKT and ERK signaling [Citation17]. Treatment with Pola or MMAE slightly upregulated the surface expression of CD20 on STR-428-Pola-R cells (). We also found that AKT and ERK phosphorylation was increased after Pola treatment in these cells (Supplementary Figure S7). We then explored the possibility that additional factors were involved in the regulation of CDC sensitivity by Pola. Mcl-1, a member of the Bcl-2 family proteins that prevents induction of apoptosis, is known to attenuate CDC sensitivity [Citation20]. Immunoblotting analysis revealed that treatment with Pola or MMAE for 3 days downregulated the expression of Mcl-1 in STR-428-Pola-R cells (). Inhibition of Mcl-1 using AZD5991 significantly increased the sensitivity to Rit-induced CDC in these cells (), indicating that Pola-mediated downregulation of Mcl-1 could upregulate the sensitivity to CDC in these cells.
Figure 4. In vitro combination effect of Pola plus Rit in STR-428-Pola-R cells. A, B, Cells pretreated with Pola (0.5 μg/mL) (A) or MMAE (2 nM) (B) for 3 days were collected, and CDC assay was performed with Rit (0.04 μg/mL). The results are presented as mean values + SD. n = 3, * p < 0.05 by Student’s t-test. C, D, Surface expression of CD20 on cells pretreated with Pola (0.5 μg/mL) (C) or MMAE (2 nM) (D) for 3 days was analyzed by flow cytometry. E, F, STR-428-Pola-R cells were treated with Pola (0.5 μg/mL) (E) or MMAE (2 nM) (F) for 3 days. Cell lysates were then evaluated by immunoblotting. G, STR-428-Pola-R cells were pretreated with AZD5991 (20 μM) for 30 min before the assay. CDC assay was then performed with Rit (0.01 μg/mL). The results are presented as mean values + SD. n = 3, * p < 0.05 by Student’s t-test.
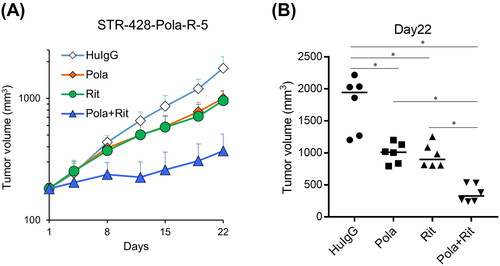
Furthermore, the combination effect of Pola plus Rit was examined in a SCID mouse xenograft model derived from STR-428-Pola-R-5 cells. The study period was adjusted according to the clinical treatment cycle (21 days). Treatment with Pola (1 mg/kg) in combination with Rit (10 mg/kg) showed more significant antitumor activity compared with each single agent on Day 22 (21 days after starting treatment) ().
Figure 5. Combination efficacy of Pola plus Rit in the STR-428-Pola-R-5 xenograft model. A, B, SCID Mice bearing STR-428-Pola-R-5 cells were randomly divided into each group (n = 6/group). mice were treated with Pola (1 mg/kg), Rit (10 mg/kg), or Pola (1 mg/kg) plus Rit (10 mg/kg) on Day 1. As a control, HuIgG was administered. (A) Data points are mean + SD. (B) Tumor volumes measured on Day 22 are shown. Dots represent individual values and bars represent median. Statistically significant differences are shown as * p < 0.05 by Wilcoxon rank sum test with Holm-Bonferroni method applied.
Discussion
In this work, we revealed that retreatment with Pola is effective against established Pola-resistant DLBCL cells (SU-DHL-4-Pola-R and STR-428-Pola-R) with two different resistance mechanisms, high expression of MDR1 or low expression of Bim, through upregulation of sensitivity to Rit-induced CDC. Our study also identified the novel mechanisms of the combination efficacy of Pola plus Rit in both Pola-resistant cells other than the upregulation of the CD20 expression as we reported previously [Citation17].
In SU-DHL-4-Pola-R-2 cells, overexpression of MDR1 was shown to cause resistance to Pola. A previous study suggested that expression of MDR1 is elevated in non‐Hodgkin lymphoma cells resistant to anti-CD79b ADC [Citation21]. In a phase I study in Hodgkin lymphoma, the combination therapy of brentuximab vedotin, an ADC with structure similar to that of Pola, plus the MDR1 inhibitor cyclosporine A showed positive results in patients with relapsed/refractory to brentuximab vedotin [Citation22]. Therefore, high MDR1 expression would be an important clinical resistance factor toward Pola. In SU-DHL-4-Pola-R-2 cells, pretreatment with Pola or anti-CD79b antibody (SN8) enhanced sensitivity to Rit-induced CDC. Pola-mediated upregulation of CD20 was not observed in these cells. The reason for this remains to be elucidated but is possibly due to more active MMAE efflux evoked by the higher expression of MDR1 in SU-DHL-4-Pola-R cells. In our previous study [Citation17], we reported that the Pola-mediated upregulation of the sensitivity to Rit-induced CDC was not observed in RC-K8 cells, which have multiple mechanisms of refractoriness to Pola, including low CD79b expression, high Bcl-xL expression and high MDR1 expression [Citation17]. However, based on the results of this study, the combination of Pola plus Rit could be also effective when resistance to Pola is caused by high MDR1 expression without the involvement of other factors such as CD79b and Bcl-xL. One novel mechanism of action that we identified here was that Pola in combination with Rit increases the amount of MAC in the process of complement activation in the absence of the Pola-mediated upregulation of the CD20 expression. The amount of MAC on the cell surface is intricately regulated by intracellular signaling, membrane-bound complement inhibitors (CD46, CD55, and CD59), caveolae and dynamin, and the mitochondrial stress protein mortalin/GRP75 [Citation18,Citation23,Citation24]. Further analysis is needed to determine how Pola or anti-CD79b antibodies regulate the amounts of MAC on the surface of SU-DHL-4-Pola-R cells.
STR-428-Pola-R cells were shown to acquire resistance to Pola through downregulation of Bim expression. This is the report to suggest that decreased expression of Bim could be one of the mechanisms causing resistance to Pola. Bim is a pro-apoptotic member of the Bcl-2 protein family and interacts with anti-apoptotic Bcl-2 family proteins, such as Bcl-2, Mcl-1, and Bcl-xL. Bim is also known as a tumor suppressor, and is frequently downregulated in cancers, giving tumor cells a growth advantage [Citation25]. In some cases of non‐Hodgkin lymphoma, Bim has also been reported to be silenced through homozygous deletion or promoter methylation [Citation26]. The genomic region encoding the miR-17-92 microRNA, which downregulates Bim, is known to be commonly overexpressed in DLBCL [Citation27–29]. Furthermore, it has been reported that the expression of Bim is reduced by a negative feedback loop in the Bim-caspase axis [Citation30], and we confirmed that a reduction of Bim expression by Pola was observed in STR-428-Pola-R cells. These results suggest that the reduced expression of Bim could be an important factor for resistance or refractoriness to Pola in clinical practice. In STR-428-Pola-R cells, pretreatment with Pola or MMAE increased the sensitivity to Rit-induced CDC by upregulating the expression of CD20 or reducing the expression of Mcl-1, an anti-apoptotic member of the Bcl-2 protein family that promotes cell survival. Mcl-1 is known to be degraded by MMAE via the ubiquitin/proteasome system in non‐Hodgkin lymphoma cells [Citation31]. Although the detailed mechanism remains unclear, downregulation of Mcl-1 has been reported to increase the sensitivity to Rit-mediated CDC [Citation20]. Our results also showed that the Mcl-1 inhibitor AZD5991 enhanced CDC sensitivity in STR-428-Pola-R cells, indicating that downregulation of Mcl-1 is one factor that explains the combination effect of Pola plus Rit in these cells. In contrast, our data showed that the sensitivity to Rit-induced CDC was not reduced in STR-428-Pola-R cells with low expression of Bim, another member of Bcl-2 family, compared to the parental STR-428 cells, hence the expression levels of Bim did not affect the sensitivity to CDC in these cells. Therefore, the contribution of apoptosis-related factors to complement activation and CDC may differ among proteins. The role of Mcl-1 in apoptosis is well defined; however, it also broadly participates in non-apoptotic signaling pathways including calcium flux, DNA damage response, autophagy, and mitochondrial quality control [Citation32–36]. Thus, functions of Mcl-1 other than apoptosis regulation may also be involved in the process of Pola or MMAE mediated upregulation of CDC sensitivity in STR-428-Pola-R cells.
Previous studies have shown that there are various resistant mechanisms for ADCs against hematological malignancies, such as downregulation of the target-antigens, high expression of the drug efflux transporters, and abnormalities in the apoptosis regulators [Citation37–40]. Consistent with these previous observations, we and others found that low CD79b expression, high MDR expression, and change in the expression of apoptosis regulators could serve as resistant mechanisms for Pola [Citation7,Citation17]. Notably, the present study is the first to assess Bim as an apoptosis regulator that contributes to Pola-resistance.
Our previous and present study showed that Pola has the ability to increase the sensitivity to Rit-induced CDC in Pola-refractory and resistant cells [Citation17]. In some cell lines, Pola was proposed to increase CDC sensitivity through the upregulation of the expression of CD20, the target antigen of Rit. On the other hand, in the present study we also proposed that Pola increased Rit-mediated CDC sensitivity by mechanisms other than increasing CD20 expression: reducing the expression of Mcl-1 and increasing the amount of MAC. Although these results could contribute to understanding the molecular basis of the Pola-Rit combination, there are still few studies that have examined this issue, thus further studies are warranted to fully understand the molecular rationale for the Pola-Rit combination.
Moreover, comprehensive analyses using data from both cell lines and clinical samples are needed to uncover the relationship between resistance/combination mechanisms and cell status, such as mutation profile, expression profile, and genomic rearrangements, which could help us to understand what type of resistance would appear after Pola administration and whether the combination effects confirmed in this study could occur in clinical practice. Despite these limitations, the data presented here suggests that retreatment with Pola may be considered or could yield responses even in the presence of Pola resistance.
Supplemental Material
Download MS Power Point (265.7 KB)Acknowledgments
The authors thank Osamu Kondoh, Koh Furugaki, Sei Shu, Takaaki Fujimura, and Xiaoxiao Liu (Chugai Pharmaceutical Co., Ltd.) for their helpful support and advices. The authors also thank Kumiko Kondoh (Chugai Pharmaceutical Co., Ltd.) for her technical assistance with the experiments.
Disclosure statement
All authors are employees of Chugai Pharmaceutical Co., Ltd.
Additional information
Funding
Notes on contributors
Natsumi Kawasaki
N. Kawasaki: Conceptualization, data curation, investigation, methodology, writing–original draft, writing–review and editing. M. Tomita: Data curation, investigation, methodology, writing–original draft, writing–review and editing. Y. Yamashita-Kashima: Data curation, investigation, methodology, writing–review and editing. Y. Yoshimura: Supervision, writing–review and editing. S. Yoshiura: Conceptualization, data curation, investigation, methodology, supervision, writing–original draft, writing–review and editing.
References
- Sehn LH, Salles G. Diffuse large B-Cell lymphoma. N Engl J Med. 2021;384(9):842–858.
- Sawalha Y. Relapsed/refractory diffuse large B-Cell lymphoma: a look at the approved and emerging therapies. J Pers Med. 2021;11(12):1345.
- Cheson BD, Nowakowski G, Salles G. Diffuse large B-cell lymphoma: new targets and novel therapies. Blood Cancer J. 2021;11(4):68.
- Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-Cell lymphoma. Am Soc Clin Oncol Educ Book. 2022;42(2):1–14.
- Salles G, Długosz-Danecka M, Ghesquières H, et al. Tafasitamab for the treatment of relapsed or refractory diffuse large B-cell lymphoma. Expert Opin Biol Ther. 2021;21(4):455–463.
- Abramson JS, Ghosh N, Smith SM. ADCs, BiTEs, CARs, and small molecules: a new era of targeted therapy in Non-Hodgkin lymphoma. Am Soc Clin Oncol Educ Book. 2020;40:302–313.
- Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood. 2009;114(13):2721–2729.
- Palanca-Wessels MCA, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody–drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16(6):704–715.
- Bourbon E, Salles G. Polatuzumab vedotin: an investigational anti-CD79b antibody drug conjugate for the treatment of diffuse large B-cell lymphoma. Expert Opin Investig Drugs. 2020;29(10):1079–1088.
- Sehn LH, Hertzberg M, Opat S, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6(2):533–543.
- Terui Y, Rai S, Izutsu K, et al. A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2021;112(7):2845–2854.
- Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-Cell lymphoma. N Engl J Med. 2022;386(4):351–363.
- Fujimura T, Yamashita-Kashima Y, Kawasaki N, et al. Obinutuzumab in combination with chemotherapy enhances direct cell death in CD20-Positive obinutuzumab-resistant Non-Hodgkin lymphoma cells. Mol Cancer Ther. 2021;20(6):1133–1141.
- Ishikura N, Sugimoto M, Yorozu K, et al. AntiVEGF antibody triggers the effect of antiPDL1 antibody in PDL1(low) and immune desertlike mouse tumors. Oncol Rep. 2022;47(2):36.
- Liu-Kreyche P, Shen H, Marino AM, et al. Lysosomal P-gp-MDR1 confers drug resistance of brentuximab vedotin and its cytotoxic payload monomethyl auristatin E in tumor cells. Front Pharmacol. 2019;10:749.
- Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182(1):29–45.
- Kawasaki N, Nishito Y, Yoshimura Y, et al. The molecular rationale for the combination of polatuzumab vedotin plus rituximab in diffuse large B-cell lymphoma. Br J Haematol. 2022;199(2):245–255.
- Fishelson Z, Kirschfink M. Complement C5b-9 and cancer: mechanisms of cell damage, cancer counteractions, and approaches for intervention. Front Immunol. 2019;10:752.
- Reis ES, Mastellos DC, Ricklin D, et al. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018;18(1):5–18.
- Hussain SR, Cheney CM, Johnson AJ, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13(7):2144–2150.
- Yu SF, Zheng B, Go M, et al. A novel anti-CD22 Anthracycline-Based Antibody-Drug conjugate (ADC) that overcomes resistance to Auristatin-Based ADCs. Clin Cancer Res. 2015;21(14):3298–3306.
- Chen R, Herrera AF, Hou J, et al. Inhibition of MDR1 overcomes resistance to brentuximab vedotin in hodgkin lymphoma. Clin Cancer Res. 2020;26(5):1034–1044.
- Moskovich O, Herzog LO, Ehrlich M, et al. Caveolin-1 and dynamin-2 are essential for removal of the complement C5b-9 complex via endocytosis. J Biol Chem. 2012;287(24):19904–19915.
- Saar Ray M, Moskovich O, Iosefson O, et al. Mortalin/GRP75 binds to complement C9 and plays a role in resistance to complement-dependent cytotoxicity. J Biol Chem. 2014;289(21):15014–15022.
- Akiyama T, Dass CR, Choong PF. Bim-targeted cancer therapy: a link between drug action and underlying molecular changes. Mol Cancer Ther. 2009;8(12):3173–3180.
- Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2007;109(1):271–280.
- Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–414.
- Inomata M, Tagawa H, Guo YM, et al. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113(2):396–402.
- Dal Bo M, Bomben R, Hernandez L, et al. The MYC/miR-17-92 axis in lymphoproliferative disorders: a common pathway with therapeutic potential. Oncotarget. 2015;6(23):19381–19392.
- Wakeyama H, Akiyama T, Takahashi K, et al. Negative feedback loop in the bim-caspase-3 axis regulating apoptosis and activity of osteoclasts. J Bone Miner Res. 2007;22(10):1631–1639.
- Lasater EA, Amin DN, Bannerji R, et al. Targeting MCL-1 and BCL-2 with polatuzumab vedotin and venetoclax overcomes treatment resistance in R/R NHL: results from preclinical models and a phase Ib study. Am J Hematol. 2023;98(3):449–463.
- Widden H, Placzek WJ. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun Biol. 2021;4(1):1029.
- Vervliet T, Clerix E, Seitaj B, et al. Modulation of Ca(2+) signaling by anti-apoptotic B-Cell lymphoma 2 proteins at the endoplasmic Reticulum-Mitochondrial interface. Front Oncol. 2017;7:75.
- Jamil S, Stoica C, Hackett TL, et al. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell Cycle. 2010;9(14):2843–2855.
- Germain M, Slack RS. MCL-1 regulates the balance between autophagy and apoptosis. Autophagy. 2011;7(5):549–551.
- Hollville E, Carroll RG, Cullen SP, et al. Bcl-2 family proteins participate in mitochondrial quality control by regulating parkin/PINK1-dependent mitophagy. Mol Cell. 2014;55(3):451–466.
- Walter RB, Gooley TA, van der Velden VH, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168–4170.
- Takeshita A, Shinjo K, Yamakage N, et al. CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug resistant cells: analyses in cell lines and cells from patients with B-cell chronic lymphocytic leukaemia and lymphoma. Br J Haematol. 2009;146(1):34–43.
- Chen R, Hou J, Newman E, et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol Cancer Ther. 2015;14(6):1376–1384.
- Haag P, Viktorsson K, Lindberg ML, et al. Deficient activation of bak and bax confers resistance to gemtuzumab ozogamicin-induced apoptotic cell death in AML. Exp Hematol. 2009;37(6):755–766.
