Abstract
Extranodal marginal zone lymphoma of bronchus-associated lymphoid tissue (BALT) is a rare cancer for which optimal treatment strategies are undefined. Retrospective analyses suggest excellent outcomes with surgical resection for localized BALT lymphoma; however, the role of radiotherapy remains underexplored. We report the largest-to-date single-center analysis of 13 primary BALT lymphoma patients treated with radiotherapy. Of 15 treated lesions, we report a 100% response rate with complete response (CR) achieved in 67% of lesions. Among 10 lesions treated with very low-dose radiotherapy (VLDRT; 4 Gray [Gy]), 6 (60%) achieved a CR; among 5 lesions treated with full-dose radiotherapy (24–36 Gy), 4 (80%) achieved a CR. There were no local recurrences. Only one patient, treated with 30 Gy, developed an acute grade 3/4 toxic effect. There were no events of radiation-induced secondary malignancies. Our institutional experience indicates that radiotherapy, including VLDRT, is a safe and effective treatment for primary BALT lymphoma.
Introduction
Extranodal marginal zone lymphoma (MZL) of bronchus associated lymphoid tissue (BALT) is a rare, indolent malignancy for which the optimal treatment approach is not well defined [Citation1–4]. Oncologists may elect to manage BALT lymphoma expectantly because of its relatively favorable prognosis [Citation5,Citation6]; however, unlike other types of MZL, BALT lymphoma carries the risk of irreversible lung damage, local complications like atelectasis and recurrent pneumoniae, and, in rare cases, transformation into a more aggressive lymphoma and/or death with unchecked local progression.
Supporting the use of local therapies in localized BALT lymphoma are retrospective studies showing excellent long-term outcomes of surgical resection [Citation1,Citation2,Citation5,Citation7–12]. By contrast, there are scant data supporting the use of radiotherapy in the management of localized BALT lymphoma [Citation13–15].
A growing body of evidence indicates that extrapulmonary MZL can be effectively treated with very low-dose radiotherapy (VLDRT, 4 Gray [Gy] delivered in 2 fractions) [Citation16–20]. This approach represents a desirable treatment strategy in BALT lymphoma, as it would hopefully carry a reduced risk of morbidity compared to surgical resection or definitive radiation regimens of 24–36 Gy [Citation21–23]. However, to date, a single published case series that included 8 patients with primary, localized BALT lymphoma has explored management with VLDRT with favorable response rates [Citation13]. This encouraging initial report suggests that further efforts in this patient group are warranted.
Toward this end, we performed a single-institution retrospective study of patients with primary BALT lymphoma to systematically test the efficacy and safety of radiotherapy, including VLDRT, in its management.
Materials & methods
Patients
We screened 153 patients with BALT lymphoma treated at a single institution from 1995 to 2021. Inclusion criteria included age 18 years or older, histologically confirmed BALT lymphoma treated at our institution, and receipt of radiotherapy at initial presentation or disease progression/recurrence. We excluded patients with synchronous evidence of lymphoma involving non-thoracic extranodal sites and/or lymphadenopathy outside of the mediastinum and hilum, as these patients might represent MZL with secondary lung involvement as opposed to primary BALT lymphoma. All patients underwent baseline staging with FDG-positron emission tomography (PET)/computed tomography (CT). We extracted patient, tumor, imaging and treatment characteristics from the electronic medical record. We defined active surveillance as at least 3 months of follow up prior to initiating active therapy combined with documentation of this plan in the medical record. The Memorial Sloan Kettering Cancer Center (MSK)’s Institutional Review Board approved this study with a waiver of written informed consent under protocol #16-538 on May 24, 2016.
Treatment
Patients underwent CT simulation according to standard departmental procedures, typically immobilized with an Alpha Cradle (Smithers Medical Products, North Canton, OH) and allowed to breathe freely. All patients were treated with involved-site radiation therapy (ISRT) limited to the lesion only (). This is in contrast to guidelines for extranodal MZL involving non-pulmonary sites [Citation22], which include the entire organ at risk in the CTV. The smaller treatment volumes in the present series reflect a desire to maximally spare normal lung tissue. The gross tumor volume (GTV) comprised the CT abnormality, and an iGTV comprised the CT abnormality accounting for motion throughout the breathing cycle. The clinical target volume (CTV) included a 1–1.5 cm expansion on the iGTV respecting anatomical boundaries. The PTV comprised a 0.5–1 cm expansion on the CTV. Treatment plans were developed using the planning systems Eclipse (Varian Medical Systems, Palo Alto, CA) or Top Module (MSK, New York, NY). The median radiation dose was 4 Gy (range, 4–36 Gy). In general, patients treated with VLDRT (4 Gy) were treated more recently than those who received definitive-dose radiotherapy.
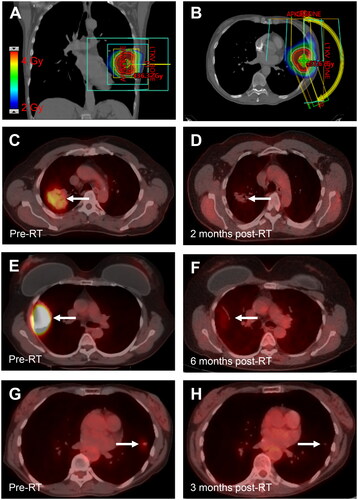
Figure 1. Response to very low-dose radiotherapy. (A,B) Coronal (A) and axial (B) images demonstrating the radiation plan for a 67-year-old woman with stage IE BALT lymphoma of the left upper lobe treated with 4 Gy in 1 fraction. Color wash shows radiation dose. (C,D) PET/CT images in a 64-year-old man with stage IE BALT lymphoma of the right upper lobe (RUL) before (A, SUV 3.8) and 2 months after treatment with 4 Gy in 2 fractions (B, SUV 1.2). (E,F) PET/CT images in a 60-year-old woman with recurrent BALT lymphoma of the RUL before (C, SUV 10.1) and 6 months after treatment with 4 Gy in 2 fractions (D, SUV 1.9). (G,H). PET/CT images for the patient shown in (A, B) before (A, SUV 1.6) and 3 months after radiotherapy (F, SUV 0.6).
Statistical analysis
We used the RECIL 2017 criteria to measure response to radiotherapy as documented on follow up CT or PET [Citation24]. We estimated the cumulative incidence of progression with death as a competing risk and defined time to progression as the time from the start of radiotherapy to disease progression confirmed by CT or FDG-PET scanning and review of the treating oncologist’s notes. Local recurrence was defined as reappearance of lymphoma within the PTV. Regional and distant recurrence were defined as radiographically and/or pathologically confirmed lymphoma recurrence within the thorax and in extrathoracic sites, respectively. Patients were censored at the last follow-up visit in the medical record.
Results
Patient characteristics
We reviewed the medical records of 153 consecutive patients with MZL involving the lung, of which 13 met inclusion criteria. Of excluded patients, 133 were managed without radiotherapy, 4 had extrathoracic involvement, and 3 did not receive treatment at MSKCC. The median age at diagnosis was 67 years (range, 33–80 years), and women predominated (n = 8, 62%) (). All patients except for one with chronic lymphocytic leukemia and one with localized bladder cancer had no evidence of disease from any second malignancy at the time of radiotherapy.
Table 1. Patient characteristics.
Most patients had a single lesion (n = 8, 62%) (). Of 15 treated lesions, the median diameter was 3.6 cm (range, 0.8–8.2 cm). Four patients (31%) had bilateral lung involvement, and one patient (8%) had thoracic nodal involvement. Baseline hematologic abnormalities were rare. Initial diagnosis was made by endobronchial resection (n = 3), percutaneous biopsy (n = 9), or wedge resection (n = 1). Radiotherapy was the initial treatment strategy in 5 patients (38%). The remaining patients were initially managed with systemic therapy (n = 3, 23%), surgical resection (n = 2, 14%), or active surveillance (n = 3, 23%) (). Four patients (31%) received radiotherapy after failing alternative first-line management strategies, and three patients (23%) received radiotherapy after failing both first- and second-line treatment strategies. One patient elected to pursue radiotherapy despite stable disease on active surveillance.
Table 2. Treatment outcomes.
Treatment response
Of 15 lesions treated with radiotherapy, 10 achieved a complete response (CR) (67%), and all others achieved a partial response (PR; n = 5, 33%) (, ). Among 10 lesions (67%) treated with VLDRT, 6 (60%) achieved a best response of CR; among 5 lesions treated with full-dose radiotherapy, 4 (80%) achieved a best response of CR.
With a median follow-up of 36 months (95% CI, 22 months–not reached), only 3 patients experienced disease progression, none within the irradiated field. The 6-year cumulative incidence of progression was 18% (95% CI, 2.3%–46%) (Supplemental Figure 1). After radiotherapy, 2 patients developed radiographic progression of presumed low-grade lymphoma in the lungs, including one in a preexisting non-irradiated lesion (). There was one radiographic distant recurrence of presumptive low-grade lymphoma in retroperitoneal lymph nodes, subsequently treated with rituximab followed by rituximab-bendamustine therapy with a complete response ().
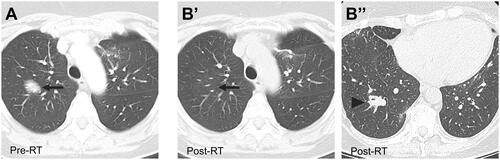
Figure 2. Regional Failure after radiotherapy for BALT lymphoma. (A) Axial CT image in a 48-year-old woman with recurrent BALT lymphoma of the RUL before treatment with 30 Gy in 15 fractions. (B) Axial CT images showing a CR in the treated lesion (arrow, B’) and regional recurrence in the right lower lobe (arrowhead, B’’) 17 months after radiotherapy.
Toxicity
Among all patients, the most common acute radiation-related toxicity was fatigue (n = 6, 46%) followed by dermatitis (n = 3, 23%). All acute toxicities were grade 1, except for a single patient, treated with 30 Gy, who developed grade 3 fatigue, which resolved on follow up. As expected, patients treated with VLDRT experienced minimal to no acute toxicities compared to patients treated with definitive doses (≥ 24 Gy). No radiation-related late toxicities occurred in any patients. There were no events of secondary lung cancers within or in proximity to the irradiated field.
Discussion
Primary BALT lymphoma is a rare type of extranodal MZL with an indolent clinical course. Consensus on appropriate management strategies is lacking and must balance the risk of irreversible lung damage from local progression with the potential morbidity of treatment. In the largest retrospective study to date of patients with primary BALT lymphoma treated with radiation, we show that radiotherapy, including VLDRT, is well tolerated and associated with excellent response rates, local control rates and progression outcomes. Caveats to this study include its small sample size and the variable dose and timing (i.e. as first-line or subsequent therapy) of radiation delivered.
We report an overall response rate of 100%, with CR achieved in 67% of treated lesions, and a 6-year cumulative incidence of progression of 18%, in line with prior studies. For example, the largest published series of BALT lymphoma patients reported a 6-year PFS of ∼80% among patients who underwent surgical resection (Sammassimo et al. 2016). The second largest series reported a 6-year event-free survival rate of 74% and for upfront resection [Citation5]. Compared to patients treated with an alternative local therapy (surgery), which is used as first-line treatment for BALT lymphoma in 30–40% of patients in large studies [Citation1, Citation5], our data suggest radiotherapy may achieve at least equivalent outcomes. This is particularly underlined by the overall more unfavorable patient population in the present study. Specifically, most patients in this study received radiotherapy after failing first- and/or second-line therapies. Furthermore, the median tumor diameter, which is inversely correlated with PFS [Citation5, Citation10], was larger (3.6 cm vs. 2.5 cm) in the present cohort compared to a recently published study on a similar patient population [Citation5].
VLDRT could be incorporated as part of an adaptive ISRT approach in primary BALT lymphoma, in which patients undergo VLDRT with short-interval PET/CT to evaluate whether additional ISRT should be offered, even in the setting of potentially curable disease [Citation18]. Such a response-adapted approach is desirable because it offers the advantages of hopefully fewer toxic effects, shorter treatment duration, and less financial toxicity [Citation25] for BALT lymphoma patients who have a CR or PR to VLDRT. In this treatment paradigm, patients who do not respond to VLDRT can still go on to receive definitive-dose radiotherapy, which our data suggest may achieve higher CR rates in BALT lymphoma.
Our data further suggest that VLDRT could potentially serve as a bridging strategy for indolent pulmonary lymphomas, such as relapsed follicular lymphoma [Citation26, Citation27], planned for CD19-targeted chimeric antigen receptor T-cell (CAR-T) therapies. Many patients who receive CAR-T therapy require treatment for active disease between leukapheresis and CAR-T infusion (termed ‘bridging’ therapy). Among various bridging strategies (e.g. chemotherapy, immunotherapy, steroids), radiotherapy represents an attractive option both for its ability to achieve rapid cytoreduction in hematologic malignancies [Citation28] and for its immunomodulatory properties [Citation29], suggesting the possibility for immune synergies with CAR-T therapies. VLDRT has the added benefit of minimizing toxicity. Retrospective studies indicate that bridging radiotherapy for diffuse large B-cell lymphoma patients planned for CAR-T therapy is safe [Citation30–35] and may improve outcomes among select patients [Citation30, Citation31], and its safety and efficacy is being tested in ongoing prospective studies (NCT00574114, NCT04726787). Considered in this context, our work suggests future opportunities for incorporating VLDRT in bridging regimens for indolent pulmonary lymphomas.
Our findings show that radiotherapy, including VLDRT, is an effective and safe management strategy for BALT lymphoma and could be considered for patients at increased risk for progression and/or with tumors causing or presenting a risk for morbidity including bronchial obstruction or infection.
glal-2023-0547-File006.pptx
Download MS Power Point (133.8 KB)Acknowledgments
The authors gratefully acknowledge MSK’s DataLine team and Jisun Lee for help querying the medical record.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Sammassimo S, Pruneri G, Andreola G, et al. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of international extranodal lymphoma study group (IELSG). Hematol Oncol. 2016;34(4):177–183. doi:10.1002/hon.2243
- Oh SY, Kim WS, Kim JS, et al. Pulmonary marginal zone B-cell lymphoma of MALT type–what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy?: consortium for improving survival of lymphoma (CISL) study. Ann Hematol. 2010;89(6):563–568. doi:10.1007/s00277-009-0875-7
- Zucca E, Arcaini L, Buske C, et al. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17–29. doi:10.1016/j.annonc.2019.10.010
- Stefanovic A, Morgensztern D, Fong T, et al. Pulmonary marginal zone lymphoma: a single Centre experience and review of the SEER database. Leuk Lymphoma. 2008;49(7):1311–1320. doi:10.1080/10428190802064933
- Joffe E, Leyfman Y, Drill E, et al. Active surveillance of primary extranodal marginal zone lymphoma of bronchus-associated lymphoid tissue. Blood Adv. 2021;5(2):345–351. doi:10.1182/bloodadvances.2020003213
- Troch M, Streubel B, Petkov V, et al. Does MALT lymphoma of the lung require immediate treatment? An analysis of 11 untreated cases with long-term follow-up. Anticancer Res. 2007;27:3633–3637.
- Borie R, Wislez M, Thabut G, et al. Clinical characteristics and prognostic factors of pulmonary MALT lymphoma. Eur Respir J. 2009;34(6):1408–1416. doi:10.1183/09031936.00039309
- Wöhrer S, Kiesewetter B, Fischbach J, et al. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single-center analysis. Ann Hematol. 2014;93(8):1287–1295. doi:10.1007/s00277-014-2042-z
- Lee H, Yang B, Nam B, et al. Treatment outcomes in patients with extranodal marginal zone B-cell lymphoma of the lung. J Thorac Cardiovasc Surg. 2017;154(1):342–349. doi:10.1016/j.jtcvs.2017.03.043
- Xu Y, Zheng M, Guo Q, et al. Clinical features and survival outcome of Early-Stage primary pulmonary MALT lymphoma after surgical treatment. Front Surg. 2021;8:713748. doi:10.3389/fsurg.2021.713748
- Wang L, Ye G, Liu Z, et al. Clinical characteristics, diagnosis, treatment, and prognostic factors of pulmonary mucosa-associated lymphoid tissue-derived lymphoma. Cancer Med. 2019;8(18):7660–7668. doi:10.1002/cam4.2683
- Zhao S, Zhang L, Gu Z, et al. Clinical manifestations of pulmonary mucosa-associated lymphoid tissue lymphoma: single-center experience with 18 patients. Onco Targets Ther. 2018;11:555–561. doi:10.2147/OTT.S147275
- Girinsky T, Paumier A, Ferme C, et al. Low-dose radiation treatment in pulmonary mucosa-associated lymphoid tissue lymphoma: a plausible approach? A single-institution experience in 10 patients. Int J Radiat Oncol Biol Phys. 2012;83(3):e385-389–e389. doi:10.1016/j.ijrobp.2012.01.005
- Hashemi SMS, Heitbrink MA, Jiwa M, et al. A patient with endobronchial BALT lymphoma successfully treated with radiotherapy. Respir Med. 2007;101(10):2227–2229. doi:10.1016/j.rmed.2006.11.028
- Demir M, Güven DC, Kiliçkap S. Same disease, different approaches: a report on six lymphoma cases with extranodal marginal zones in rare sites. J Oncol Sci. 2021;7(1):34–38. doi:10.37047/jos.2020-79452
- Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15(4):457–463. doi:10.1016/S1470-2045(14)70036-1
- Cerrato M, Orlandi E, Vella A, et al. Efficacy of low-dose radiotherapy (2 Gy × 2) in the treatment of marginal zone and mucosa-associated lymphoid tissue lymphomas. Br J Radiol. 2021;94(1123):20210012. doi:10.1259/bjr.20210012
- Imber BS, Chau KW, Lee J, et al. Excellent response to very-low-dose radiation (4 Gy) for indolent B-cell lymphomas: is 4 Gy suitable for curable patients? Blood Adv. 2021;5(20):4185–4197. doi:10.1182/bloodadvances.2021004939
- Chelius M, Chau K, Yang J, et al. Low grade, indolent lymphomas of the head and neck: comparative toxicity of standard versus very low dose radiation therapy. Hematol Oncol. 2021;39(3):304–312. doi:10.1002/hon.2865
- Hoskin P, Popova B, Schofield O, et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021;22(3):332–340. doi:10.1016/S1470-2045(20)30686-0
- Teckie S, Qi S, Chelius M, et al. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol. 2017;28(5):1064–1069. doi:10.1093/annonc/mdx025
- Yahalom J, Illidge T, Specht L, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):11–31. doi:10.1016/j.ijrobp.2015.01.009
- Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100(1):86–92. doi:10.1016/j.radonc.2011.05.013
- Younes A, Hilden P, Coiffier B, et al. International working group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28(7):1436–1447. doi:10.1093/annonc/mdx097
- Zafar SY, Abernethy AP. Financial toxicity, part I: a new name for a growing problem. Oncology (Williston Park). 2013;27(80–81):149.
- Ng AK, Yahalom J, Goda JS, et al. Role of radiation therapy in patients with relapsed/refractory diffuse large B-Cell lymphoma: guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2018;100(3):652–669. doi:10.1016/j.ijrobp.2017.12.005
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi:10.1038/nature14292
- Hubbeling H, Silverman EA, Michaud L, et al. Bridging radiation rapidly and effectively cytoreduces High-Risk relapsed/refractory aggressive B cell lymphomas prior to chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2023;29(4):259.e1–259.e10. doi:10.1016/j.jtct.2022.12.021
- Qu C, Ping N, Kang L, et al. Radiation priming chimeric antigen receptor T-Cell therapy in relapsed/refractory diffuse large B-Cell lymphoma with high tumor burden. J Immunother. 2020;43(1):32–37. doi:10.1097/CJI.0000000000000284
- Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4(13):2871–2883. doi:10.1182/bloodadvances.2020001837
- Wright CM, LaRiviere MJ, Baron JA, et al. Bridging radiation therapy before commercial chimeric antigen receptor T-Cell therapy for relapsed or refractory aggressive B-Cell lymphoma. Int J Radiat Oncol Biol Phys. 2020;108(1):178–188. doi:10.1016/j.ijrobp.2020.05.014
- Sim AJ, Jain MD, Figura NB, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-Cell lymphoma. Int J Radiat Oncol Biol Phys. 2019;105(5):1012–1021. doi:10.1016/j.ijrobp.2019.05.065
- Imber B, Palomba ML, DeSelm C, et al. MSKCC early experience using radiotherapy as a bridging strategy for relapsed diffuse large B cell lymphoma before CD19 CAR T therapy. Blood. 2019;134(Supplement_1):3238–3238. doi:10.1182/blood-2019-131449
- Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. doi:10.1016/S1470-2045(21)00591-X
- Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325–332. doi:10.1038/s41591-021-01622-0
