ABSTRACT
The exhaust emissions of 17 polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) were investigated in two spark-ignition light-duty vehicles, one gasohol-fueled and a flexible-fuel one fueled with hydrated ethanol. Gasohol is a mixture of gasoline and 22% ethanol. The influence of fuel type and quality, lubricant oil type, and use of fuel additives on the formation of these compounds was tested using standardized U.S. Federal Test Procedure (FTP)-75 cycle tests. The sampling of the PCDD/Fs followed the recommendations of a modified U.S. Environmental Protection Agency (EPA) Method 23 (http://www.epa.gov/ttn/emc/promgate/m-23.pdf) and the analysis basically followed the U.S. EPA Method 8290 (http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/8290a.pdf). Results showed that emission factors of PCDD/Fs for the gasohol vehicle varied from undetected to 0.068 pg international toxic equivalency (I-TEQ) km−1 (average of 0.0294 pg I-TEQ km−1), whereas in the ethanol vehicle they varied from 0.004 to 0.157 pg (I-TEQ) km−1 (average of 0.031 pg I-TEQ km−1). In the gasohol-powered vehicle, the use of fuel additive diminished the emission of Octachlorodibenzo-p-dioxin (OCDD) significantly, whereas in the ethanol vehicle no significant associations were observed between the investigated variables and the emissions.
The objective of this work was to analyze differences in emissions from a traditional fossil fuel (gasoline) and an alternative renewable fuel (ethanol from sugarcane), and the influence of fuel additives and lubricant oils on the formation of chlorinated dioxins and furans in spark-ignition light-duty gasohol and ethanol vehicles. Renewable fuels are very important in terms of climate change but the risk to the population's health must not increase. Thus the results of this work could help in the development of environmental impact studies as well as orienting policy-makers in formulating strategies for air pollution control.
INTRODUCTION
Vehicles are responsible for the emissions of many types of pollutants, some of high toxicity such as polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs), even at very low concentrations, can alter the morbidity and mortality rates of populations. PCDD/Fs are ubiquitous. They are resistant to environmental degradation through chemical, biological, and photolytic processes, and belong to the persistent organic pollutants (POPs) group. They are capable of long-range transport, depositing on land or water.3 Some PCDD/Fs are carcinogenic to mammalians. In humans they can cause chloracne, alterations in the ability of the liver to metabolize hemoglobin, lipids, sugar, and protein. Most of the effects are considered mild and are reversible. However, in some people these effects may last for many years. Slight increases in the risk of diabetes and abnormal glucose tolerance also have been reported.4,5
In Brazil there is also a concern about these substances. De Assunção et al.6,7 carried out evaluations of PCDD/Fs in the atmosphere of three distinct regions of São Paulo City. In a 2008 study, concentrations of PCDD/Fs ranging from 47 to 187 fg I-TEQ·Nm−3 were found in a region under the influence of vehicular emissions, and from 63 to 223 fg I-TEQ·Nm−3 in a region under the influence of vehicular and incinerator emissions, and from 47 to 751 fg I-TEQ·Nm−3 in a region under the influence of industrial and vehicular emissions. These results suggest a contribution of vehicles to atmospheric concentrations of PCDD/Fs.
According to the World Health Organization (WHO),8 PCDD/Fs are two series of almost planar tricyclic aromatic compounds with very similar chemical properties. The number of chlorine atoms can vary between 1 and 8. There are 75 PCDDs and 135 PCDFs, of which 17 are considered toxic. Toxicity equivalent factors (TEFs) were established for each 1 of these 17 PCDD/Fs by using a unitary factor for the 2,3,7,8-TCDD, which is considered the most toxic one. The TEF of the 17 congeners adopted by WHO9 are shown in .
Table 1. Toxicity equivalence factors and Method Detection Limit
According to Stanmore,10 PCDD/Fs are formed basically in combustion processes where chlorine, hydrogen, oxygen, and carbon atoms are present. The reactions are catalyzed by the presence of metals, such as copper and iron, among others. On the other hand, copper catalysis can be inhibited by amine-deactivating action on copper surfaces; chlorine action also may be inhibited by the presence of SO2 in the gaseous phase11; platinum promotes other reactions inhibiting PCDD/F formation.12 Gasoline containing ethanol in its formulation needs no addition of tetra-ethyl lead from gasoline. Tetra-ethyl lead additives are typically made with chlorinated hydrocarbons that could promote further PCDD/F formation during combustion13.
Chlorine not removed from crude oil during the refining process can contribute to PCDD/F formation. Moreover, chlorine compounds can also be added to premium gasoline to improve engine performance.14
Lubricant oils are another probable chlorine source, coming by two potential routes: (i) oil film that remains on the internal surface of engine cylinders, and burned together with fuel; (ii) through the positive crankcase ventilation system, where the vapors of lubricant oil are mixed with air, before being admitted into the combustion chamber.15
Ethanol fuel may contain chlorine. In Brazil, ethanol comes primarily from sugarcane and there are 63 different kinds of chlorine substances that could be applied to sugarcane crops, apart from the use of micronutrients such as copper and zinc that are added to the soil together with fertilizers.16
Fuel additives are a potential source of organic chlorides,17 but their chemical compositions are not disclosed in Brazil. Product labeling is done in a generic way, although the Brazilian Federal Administration must be notified about substances that can cause damage to human health.18
Platinum is often used in automotive catalyst converters, which suggests that the presence of dioxins and furans are small in vehicle exhausts equipped with this kind of device.12 However, emissions of these pollutants could be significant in the initial operating time of the vehicle, a situation in which the catalytic converter is still below its normal working temperature and not so effective at PCDD/F conversion.
The objective of this work was to study the influence of fuels, fuel additives, and lubricant oils on the formation of chlorinated dioxins and chlorinated furans in spark-ignition light-duty gasohol- and ethanol-powered vehicles. Gasohol means gasoline that contains 20–25% of anhydrous ethanol. In the present study, gasohol with 22% of anhydrous ethanol was used.
EXPERIMENTAL METHODS
Vehicle Testing Conditions
Two in-use spark-ignition light-duty vehicles were tested, a gasohol-powered vehicle and an ethanol-powered one. The tested vehicles were half-life usage, with at least 50,000 km and equipped with catalytic converters and fuel injection systems. The catalytic converters were in good working condition. Their average age and mileage are very close to the average age and mileage of the vehicular fleet of the State of São Paulo, which corresponds to 36% of the Brazilian fleet.19
The gasohol-powered vehicle was manufactured in 1998 and it was equipped with a 1.6-L engine, with 15.1 kg·m of torque at 4500 rpm and 78 kW of power at 5500 rpm. Its weight was 1111 kg, and the odometer reading was 67,546 km at the starting time of the assays.
The other vehicle was a flexible fuel type, manufactured in 2004, equipped with a 1.6-L engine, having 14.4 kg·m of torque at 3000 rpm and 73 kW of power at 5750 rpm. Its weight was 1111 kg, and the odometer reading was 56,908 km at the beginning of the assays.
The assays were carried out in a vehicular emission laboratory in São Paulo City, Brazil. The standardized U.S. Federal Test Procedure (FTP)-75 driving cycle was adopted.20
The FTP-75 driving cycle begins with a cold start after an overnight cool down (from 12 to 36 hr) at ambient temperatures that ranged from 20 °C to 30 °C. The test includes a shutdown for 10 min with a repeat of the first 505 sec after a hot restart. The distance traveled corresponds to 17.77 km, performed in 2477 sec. The overall average speed is about 34.1 km hr−1, with a maximum speed of 91.2 km hr−1 and with the engine running at idle conditions for about 17.9% of the time.
Experimental Design and Parameters Tested
Experiments were performed using a statistical factorial design. This design economizes assays while extracting a maximum amount of information about the effects of factor changes on the results of the system being studied.21 The statistical treatment of results consisted of a preliminary evaluation for the determination of outlier values at the replicated points using Grubb's method. Coefficients and their standard errors were calculated for the models relating the PCDD/F concentrations to the factor levels investigated. Analysis of variance (ANOVA) was applied for the model validation. Statistics were applied by using the Statistica computer program.22
Gasohol-Powered Vehicle
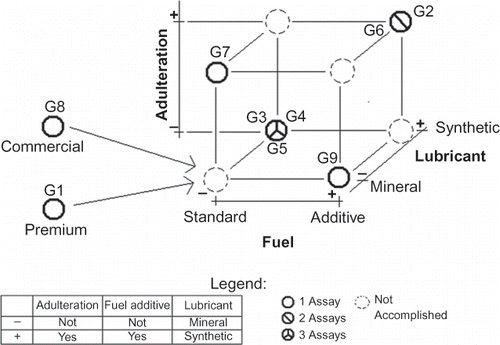
Nine assays were performed in this vehicle to test for the influence of fuel, fuel additive, and lubricant oils on the emission of PCDD/Fs, being 1 assay with regular gasohol and mineral oil; 1 assay with premium gasohol and mineral oil; 3 assays with standard gasohol for emission assays (SGEA; used for legal certification of new vehicle emissions) and synthetic oil; 1 assay with SGEA with fuel additive and mineral oil; 1 assay with adulterated SGEA and mineral oil; 2 assays with adulterated SGEA, fuel additive, and synthetic oil. All fuels, fuel additives, and lubricant oils were previously analyzed for total organic chloride by the potentiometric titration technique, in accordance with the Universal Oil Products (UOP) Method 588. The adulterated SGEA was obtained by mixing 80% of standard gasohol with 20% rubber solvent, taking into consideration the usual market adulteration formed according to field information from inspecting professionals. Two types of lubricant oils were tested: a synthetic one and a mineral one. Mineral and synthetic oils were aged to about 2500 and 5000 km, respectively, in a vehicle fueled only with gasohol. These are considered to be the half-life odometer readings for each type of lubricant oil.
Ethanol-Powered Vehicle
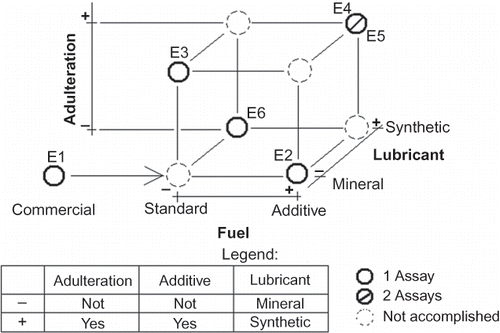
Six assays were performed with the ethanol-powered vehicle, with variations in fuels and lubricant oils, in the following way: 1 assay with regular hydrated ethyl alcohol fuel (HEAF) and mineral oil; 1 assay with standard HEAF for emission assays and synthetic oil; 1 assay with standard HEAF for emission assays, fuel additive, and mineral oil; 1 assay with adulterated standard HEAF for emission assays with mineral oil; 2 assays with adulterated standard HEAF for emission assays with fuel additive and synthetic oil. The mineral oil was aged to about 2500 km and the synthetic oil was aged to about 5000 km. Both aging processes were done in an ethanol-powered vehicle. The ethanol adulteration consisted of mixing 90% standard anhydrous ethyl alcohol fuel (AEAF) with 10% of chlorinated water prepared in laboratory, by adding 2 ppm of chlorine in distilled and deionized water, giving a concentration within the Brazilian standard for drinking water.23 Addition of chlorinated water from the tap into ethanol is considered to be one of the most common forms of ethanol adulteration in Brazil. Fuel additive was used according to manufacturer's recommendation: one flask of 375 mL to each 50 L of fuel.
Since the composition of commercial fuels can vary for many reasons, a mixture of samples from 10 gas stations of different brands, from different regions of São Paulo City, Brazil, were used with the purpose of getting more representative results of fuels in the market.
All these variations constitute a 2[3–1] fractional factorial design, which allows determination of the influence of the investigated variables.21 and show the experimental designs for both vehicles, and the respective identification for the experiments.
Collection and Analysis
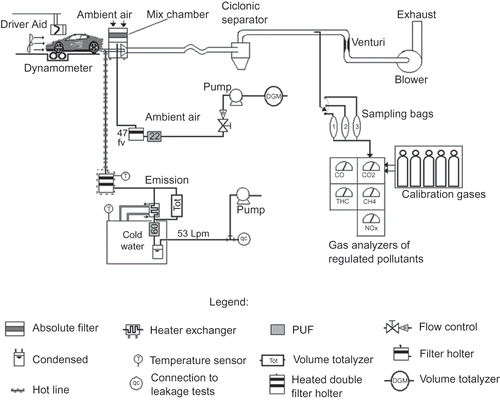
shows the schematic sampling diagram of the experiment. The collection of PCDD/Fs was carried out based on a modified U.S. Environmental Protection Agency (EPA) Method 231 and the work developed by Ryan and Gullett,24 adapted to the peculiarities of the vehicular emission laboratory. Raw sampling (with no dilution) of exhaust gases at a constant flow rate of 53 L·min−1 was chosen to be performed. Due to the possibility of particles being present in colloidal form an isokinetic sampling was chosen, based upon the average flow rate of the exhaust gases.
Using a hot probe and a hot double stainless steel filter holder, which had been kept at 120 °C, to avoid condensation into the sampling system, the solid phase was collected in 70-mm-diameter quartz fiber filters. For each assay, two filters were used in series. The gases passed through a glass heat exchanger after filtration, in order to reduce the temperature to 7 °C, followed by a 60-mm-diameter polyurethane foam (PUF) used to collect PCDD/Fs in the gaseous phase. The PUF's support was also kept cooled during the entire assay.
Prior to sampling, filters and PUF cartridges were cleaned and spiked with 4 ng of 13C6-1,2,3,7,8,9-HxCDD as field surrogate. Both filter and PUF were analyzed for the 17 2,3,7,8-substitued PCDD/Fs, together with a blank. The filters and PUFs used were placed in an original glass container at the end of the assays and wrapped with aluminum foil and kept cold until analysis.
Chemical analyses were carried out according to the U.S. EPA Method 8290 in a certified laboratory. Each pair of filters and the corresponding PUF were pooled and spiked with 17 13C6-PCDD/F internal standards, and then extracted in a Soxhlet apparatus with toluene/hexane for 16 hr. Each extract was then cleaned-up in a sulfuric acid–silica gel column using hexane as eluent and a Florisil column using dichloromethane as eluent. The extracts were concentrated to almost dryness and 13C6-1,2,3,4-TCDD was added in 15 µL of nonane, immediately before analysis. Extracts were analyzed in a Hewlett Packard model 6890 high-resolution gas chromatograph/VG Autospec Ultima mass spectrometer (HRGC/HRMS) equipped with a Hewlett Packard 7673 autosampler operating with electron impact ionization energy of 30 eV at a mass resolution of 5000. The GC was fitted with a J&W DB-5 capillary column (60 m × 0.25 mm internal diameter [id] fused silica column coated with 0.25 µm film thickness). The GC oven temperature program used was: 70 °C per 4 min, 15 °C min−1 to 220 °C, 1.5 °C min−1 to 240 °C, 2 min, 4 °C min−1 to 310 °C, 10 min. Helium was used as the carrier gas. Quantification was done by using 12 13C6-labeled internal standards. The labeled PCDD/F internal standards and their response factors were used for quantification of the unlabeled PCDD/Fs of homologous groups.
Ambient air concentration of regulated pollutants and PCDD/Fs were also quantified and were discounted from the results obtained in order to express the real results from engine combustion. Regulated pollutants were quantified after dilution (constant volume sampling method). Gas sampling from the exhaust pipe was continuously taken during the assays. The samples of regulated pollutants were collected in three plastic bags, one for each of the three phases of the test. Total mass of each phase was calculated and averaged.
RESULTS AND DISCUSSION
shows the results of the analyses for total organic chloride by the UOP Method 588.
Table 2. Results of total organic chloride analysis in the fuels, fuel additives, and oil lubricants
Although sugarcane crops could be a chlorine source, the chlorine concentrations in the ethanol samples were below the detection limits (DLs) of the method. These results are very similar to those obtained by Pereira et al.,25 in an evaluation of four samples of regular HEAF. They found chlorides in just one sample at a concentration very close to the DL, which was 0.16 mg kg−1. The fuel additive chlorine was also below of the detection limit of the method. The mixture of regular gasohols has presented the same chlorine concentration as the mixture of premium gasohols (0.4 ppm), whereas the SGEA has presented a higher chlorine concentration (0.6 ppm). The rubber solvent, used for the adulteration of the gasohol, presented much larger chlorine content when compared to the other fuels (3.5 ppm). With regards to the lubricant oils, those aged in ethanol-powered vehicles presented much lower chlorine content (4 and 3.8 ppm, respectively, for the mineral and synthetic oils) compared to those aged in gasohol-powered vehicles (14 and 40 ppm, respectively, for the mineral and synthetic oils).
Dioxin and Furan Emissions from Gasohol Vehicle
shows the experimental conditions for the gasohol vehicle, the results of the PCDD/F emissions as a function of distance, fuel consumption, their respective toxicity equivalents, regulated pollutants, and vehicle performance.
Table 3. Experimental conditions for the gasohol vehicle, its respective emissions of PCDD/Fs, TEFs, regulated pollutants, and performance
The conversion to equivalent toxicity was done by using the WHO toxicity factors listed in . The recoveries of these samples varied from 57.0% to 108%, with an average of 85.97% and standard deviation of 16.8%. The recoveries of the internal standards were used for correction of the native congeners. Of the 17 PCDD/Fs analyzed, only 1,2,3,4,6,7,8-HpCDD, OCDD, and OCDF were found. In all assays, the presence of chlorinated compounds in the fuels were observed, but no PCDD/Fs were detected in the G8 and G9 assays. The average emission of 1,2,3,4,6,7,8-HpCDD was 2.44 pg km−1, with a standard deviation of 2.94 pg km−1; in the case of OCDD, the average emission was 16.5 pg km−1, with a standard deviation of 13.0 pg km−1. OCDF was found only in the G3 assay. The average emission in terms of equivalent toxicity was 0.029 pg I-TEQ km−1, with a standard deviation of 0.032 pg I-TEQ km−1.
In terms of fuel consumption, the emission of total PCDD/F was 254 ng per metric ton (ng T−1) of fuel burned, with standard deviation of 209 ng T−1. This high variability also was observed among the replicated assays under identical conditions: G3, G4, and G5 showed PCDD/Fs total emissions of 43, 11, and 28 pg km−1, respectively, and in the G2 and G6 assays, emissions were 23 and 11 pg km−1, respectively. Although the results of the G3, G4, and G5 assays have quite different values, they were not considered discrepant on applying Grubb's test at the α = 5% significance level, so all results were included in the statistical analysis.
A statistical treatment to fit linear models to the results of factorial design was used to determine the effects of the factor levels on the individual PCDD/F and total PCDD/F emission rates. shows the model coefficients and standard errors for PCDD/F emitted by the gasohol vehicle only using standard fuel for emission assay (SFEA). The model in generic form is
Table 4. Model coefficients and standard errors for PCDD/F emissions from the vehicles using SFEA, in pg km− 1
where the bs are model coefficients, and the xs are codified variables, with x 1 indicating additive level (−1 absent, +1 present), x 2 the adulteration level (−1 absent, +1 present), and x 3 the type of lubricant oil (−1 mineral and +1 synthetic). OCDF is not shown in because it was only observed in the G3 assay. The total PCDD/F emission (pg km−1) as a function of the factor levels is given by
Statistically significant coefficients at the 90% confidence level or higher are given in bold face in . The average of the total PCDD/F emissions for the 2[3–1] factorial is 21.25 ± 5.98 pg km−1. This value is significant at the 95% confidence level. The effect of putting additive into the gasohol, −12.61 ± 5.98 pg km−1, is significant near the 90% confidence level. This effect represents a decrease in total PCDD/Fs emissions for gasohol with additive compared with gasohol without additive. The adulteration effect value, +7.32 ± 5.98 pg km−1, is just larger than its standard error and probably reflects experimental uncertainty rather than owing to gasohol adulteration. The average of all experiments with additives (G2, G6, and G9) is 11.5 pg km−1, about half of the average of the experiments without additives (G1, G3, G4, G5, G7, and G8). However the pooled standard deviation for these two groups of results is ±15.8 pg km−1, bigger than the difference in the average values. The high variability in the replicated experiments is a measure of the large experimental uncertainty in the measurements that could mask real physical effects.
The mathematical model for the 1,2,3,4,6,7,8-HpCDD emission does not contain any significant coefficients, not even the value representing the average emission of this compound. Only the G3, G5, G6, and G7 experiments showed 1,2,3,4,6,7,8-HpCDD emissions above the detection limit, from 4.14 to 6.22 pg km−1.
The OCDD emission values are highly correlated with the PCDD/F emissions, with a correlation coefficient of r = 0.99. As such the linear model for OCDD emission is
It is very similar to the one for PCDD/F emissions. Besides the average value, 18.0 pg km−1 for the 2[3–1] factorial experiments that is significant well above the 95% confidence level, the negative fuel additive effect, −10.8 pg km−1, is significant at the 90% confidence level. The coefficient is negative, indicating that either fuel additive diminished the emission of OCDD or this value is due to experimental uncertainty. However, additives can be rich in amines, which seem to inhibit the formation of PCDD/Fs.10
Regarding the emissions with the vehicle supplied with regular and premium commercial gasohol, significant coefficients were not determined in the statistical analyses. These results are similar to the behavior exhibited by emissions with the gasohol vehicle fueled with SFEA, indicating that the emissions obtained with the gasohol vehicle fueled with SFEA are close to real emissions obtained in the streets.
Dioxin and Furan Emissions from Ethanol Vehicle
shows the experimental conditions for the assays of the ethanol vehicle, and also the results of the PCDD/F emissions as a function of distance, fuel consumption and toxicity equivalents, regulated pollutants, and vehicle performance. From the 17 2,3,7,8-substituted PCDD/Fs analyzed, only 1,2,3,4,6,7,8-HpCDD and OCDD were detected. The recoveries of these samples varied from 55% to 95%, with an average of 68.2% and a standard deviation of 9.25%. The recoveries of the internal standards were used to correct the values of the native congeners. The presence of chlorine was observed just in assays with adulterated ethanol and even so PCDD/F emissions did occur in all assays. The average emission of OCDD was 21.3 pg km−1 with a standard deviation of 6.26 pg km−1, whereas 1,2,3,4,6,7,8-HpCDD was found just in the E2 assay. The average emission in terms of toxicity equivalent was 0.0310 pg I-TEQ km−1, with a standard deviation of 0.0619 pg I-TEQ km−1. Expressing the averages as a function of fuel consumption, the emission of the total PCDD/F was 186 ng per ton of fuel consumed, with a standard deviation of 99.8 ng T−1.
Table 5. Experimental conditions for the ethanol vehicle, its respective emissions of PCDD/Fs, TEFs, regulated pollutants, and performance
contains the mathematical model coefficients and their standard errors for the formation of OCDD and total PCDD/F emitted by the ethanol vehicle. The b 0 values are significant around the 95% level but none of the effect values for fuel additive; adulteration and lubrication are significant, not even at the 90% confidence level. Considering all the experimental results for ethanol vehicle in , including the E1 value not included in the 2[3-1] factorial design, the average for the experiments without adulteration is 17.0 pg km−1, whereas the average of the experiments with adulteration is 30.3 pg km −1. Even though this seems to be a large difference, the pooled standard deviation of these two groups of experiments is 10.2 pg km−1. As such this difference is not significant at the 90% confidence level.
Regarding the emissions with the vehicle supplied with regular ethanol, significant coefficients were not determined in the statistical analyses. Their emission results are similar to the behavior exhibited by emissions with the ethanol vehicle fueled with HEAF, indicating that the emissions obtained with the ethanol vehicle fueled with HEAF are close to real emissions obtained in the streets. Comparing the emission of PCDD/Fs with regulated pollutants in both vehicles, no correlation coefficient above 0.75 was found.
Dyke et al.26 also observed irregular behavior in PCDD/F formation in their study and concluded that the emissions of PCDD/Fs could not be related to the level of chlorine in the lubricating oil or fuel, indicating that other factors control the emissions. The difficulties to establish correlations between chlorine routes and PCDD/F formation in both vehicles suggest that chlorinated compounds in the atmosphere could be significant. This hypothesis had been considered negligible before the test, but after analysis of the results, we consider that ambient air could be a significant source of chlorine in PCDD/F formation and we suggest more detailed studies in this direction. The concentration of chlorides may vary significantly from one locality to another, being higher in localities under the influence of ocean breezes. However, stronger winds enable salt particles to cover long distances before settling.27
Graedel and Keene28 calculated a typical background concentration of about 1.5 ppbv. At this atmospheric concentration, in a driving cycle identical to the one carried out in this work, the vehicles would consume about 1.5 µg of chlorine from the atmosphere, i.e., mass more than necessary to reach these emissions levels.
Chang et al.29 evaluated a tunnel predominantly used by gasoline vehicles in Taiwan and estimated average emission of 22.93 pg I-TEQ km−1 and standard deviation of 4.93 pg I-TEQ km−1. Chuang et al.,30 using the same driving cycle than this study, obtained average emission of 101 pg I-TEQ km−1 and standard deviation of 64.3 pg I-TEQ km−1. Marklund et al., cited by Geueke et al.,31 estimated that the emission rate of total PCDD/F in catalyzed vehicles fueled with nonleaded gasoline was of the order of 0.36 I-TEQ pg km−1. Hagenmaier, cited by Geueke et al.,31 found an emission rate of PCDD/Fs of the order of 0.7 pg I-TEQ km−1 for vehicles under these same conditions. In summary, the average emission rate obtained in this work was at least 10 times smaller, with the average emission rate of 0.03 ± 0.03 pg I-TEQ km−1 for the gasohol vehicle and 0.03 ± 0.06 I-TEQ pg km−1 for the ethanol vehicle, showing that the ethanol vehicle emit practically the same amount as the gasohol vehicle in this study and also suggests that the improvement in fuel and emission control technology have really contributed to lower rates, compensating somewhat for the growth of the global fleet of vehicles.
Comparing the magnitude of the PCDD/Fs obtained is this work with other pollutants, one could say these values are negligible; however, it is important to consider that these pollutants belong to the POP group, and can remain in the environment for several years.
Besides global fleet of vehicles number close to 1 billion units, a significant part of which have no emission control of pollutants. In recent years, the fleet has consumed more than 5 Gt of oil equivalent tons per year, in oil, gas, and renewable fuels in the world.19,32
The results of the two vehicles and the input variables selected in this work do not fully explain PCDD/F formation; however; PCDD/F levels in São Paulo atmosphere are significant, so it is important to deepen studies of PCDD/F formation and identify the main chloride routes in vehicle combustion.6,7
Finally, the emission results for the PCDD/Fs were about 150 times lower than those considered by United Nations Environmental Programme (UNEP)13 for vehicles without catalysts, showing the importance of pollutant control devices and fuel quality improvement for the emission of less dioxins and furans into the atmosphere.
ACKNOWLEDGMENTS
The authors express their gratitude to the Sao Paulo Environmental Company, CETESB, for the support given in vehicle testing and to the Sao Paulo Foundation for Research Support, FAPESP, for providing the necessary financial support for this project (grant 2004/02623-6). R.E.B. thanks the Brazilian National Research Foundation (CNPq) for a research fellowship.
REFERENCES
- U.S. Environmental Protection Agency. Method 23: Determination of Polychlorinated Dibenzo-p-dioxins and Polychlorinated Dibenzofurans from Municipal Waste Combustors; U.S. EPA: Research Park Triangle, NC, 1997; p 34 http://www.epa.gov/ttn/emc/promgate/m-23.pdf (http://www.epa.gov/ttn/emc/promgate/m-23.pdf) (Accessed: 24 March 2011 ).
- U.S. Environmental Protection Agency. Method 8290: Analytical Procedures and Quality Assurance for Multimedia Analysis of Polychlorinated Dibenzo-p-Dioxins and Dibenzo-p-Furans by High-Resolution Gas Chromatography/High-Resolution Mass Spectrometry; U.S. EPA: Research Park Triangle, NC, September 1994 http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/8290a.pdf (http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/8290a.pdf) (Accessed: 24 March 2011 ).
- Agency for Toxic Substances and Disease Registry. Polycyclic Aromatic Chlorinated Dibenzo-p-dioxins (CDDs) http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=363&tid=63 (http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=363&tid=63) (Accessed: 24 March 2011 ).
- World Health Organization . 1989 . Polychlorinated Dibenzo-para-dioxins and Dibenzofurans; Environmental Health Criteria, 88; World Health Organization , Geneva : International Program on Chemical Safety .
- Kogevinas , M. 2001 . Human Health Effects of Dioxins: Cancer, Reproductive and Endocrine System Effects . Hum. Reprod. Update , 7 : 331 – 339 .
- De Assunção , J.V. , Pesquero , C.R. , Bruns , R.E. and Carvalho , L.R.F . 2005 . Dioxins and furans in the atmosphere of São Paulo City, Brazil . Chemosphere , 58 : 1391 – 1398 .
- De Assunção , J.V. 2008 . Pesquero, C.R.; Carvalho, L.R.F.; Nóbrega, R.P.; Abrantes, R. de; Sant'ana, R.A. Dioxins and Furans in Sao Paulo City, Brazil: 2006 Levels, Comparison with 2000–2001 Levels, and Discussion Of Potential Emission Sources . Organohalogen Compd. , 70 : 1518 – 1521 .
- World Health Organization. Polychlorinated Dibenzodioxins and Dibenzofurans http://www.euro.who.int/__data/assets/pdf_file/0017/123065/AQG2ndEd_5_11PCDDPCDF.pdf (http://www.euro.who.int/__data/assets/pdf_file/0017/123065/AQG2ndEd_5_11PCDDPCDF.pdf) (Accessed: 24 March 2011 ).
- Van Den Berg, M.; Birnbaum, L.; Denison, M.; de Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L. The 2005 World Health Organization Re-evaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds; Toxicol. Sci. J. 2006, 93(2): 223-241 http://toxsci.oxfordjournals.org/cgi/reprint/kfl055v1?ijkey=pio0gXG6d ghrndD&keytype=ref (http://toxsci.oxfordjournals.org/cgi/reprint/kfl055v1?ijkey=pio0gXG6d ghrndD&keytype=ref)
- Stanmore , B.R. 2004 . The Formation of Dioxins in Combustion Systems . Combust Flame. , 136 : 398 – 427 .
- Raghunathan , K. and Gullet , B.K. 1996 . Role of Sulfur in Reducing PCDD and PCDF Formation . Environ. Sci. Technol. , 30 : 1827 – 1834 .
- Hiraoka , M. 1991 . Takeda, N.; Kasakura, T.; Imoto, Y.; Tsuboi, H.; Iwasaki T. Catalytic destruction of PCDDs/PCFFs in municipal solid waste flue gas . Chemosphere , 23 : 1445 – 1452 .
- United Nations Environment Programme. Standardized Toolkit for Identification and Quantification of Dioxin and Furan Releases; United Nations Environment Programme: Geneva, 2005 http://www.pops.int/documents/guidance/toolkit/en/Toolkit_2005_En.pdf (http://www.pops.int/documents/guidance/toolkit/en/Toolkit_2005_En.pdf)
- World Health Organization . 1982 . Chlorine and Hydrogen Chloride; Environmental Health Criteria, 21 , Geneva : World Health Organization, International Programme on Chemical Safety .
- Sher , E. 1998 . Handbook of Air Pollution from Internal Combustion Engines , London : Academic Press Limited .
- Armas E.D. Biogeodinâmica de herbicidas utilizados em cana-de-açúcar (Saccharum spp.) na sub-bacia do rio Corumbataí [Biogeodinamics of Herbicides Used in Sugarcane (Saccharum spp.) in the Sub-basin of the Corumbataí River]; Ph.D. thesis in Ecology and Agribusiness, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo: Piracicaba, 2006 http://www.teses.usp.br/teses/disponiveis/91/91131/tde-03102006-170029/ (http://www.teses.usp.br/teses/disponiveis/91/91131/tde-03102006-170029/) (Accessed: 24 March 2011 ).
- Scott, F.J. Compositions and Methods of Employing Same to Prevent Corrosion of Metal Surfaces in Contact with Hot Gaseous Combustion Products. 1978 http://www.google.com.br/patents?hl=pt-BR&lr=&vid=USPAT4131433&id=BskzAAAAEBAJ&oi=fnd&dq=chlorine+fuel+additive&printsec=abstract#v=snippet&q=chlorine&f=false (http://www.google.com.br/patents?hl=pt-BR&lr=&vid=USPAT4131433&id=BskzAAAAEBAJ&oi=fnd&dq=chlorine+fuel+additive&printsec=abstract#v=snippet&q=chlorine&f=false) (Accessed: 13 April 2011 ).
- Agência Nacional do Petróleo . March . Portaria n.º 41, de 12 de março de 1999. [Establish the selling rules of automotive fuel additives and automotive additivities fuels] , Vol. 15 , March , 1999 Brasília , DF : Diário Oficial da União .
- Associação Nacional dos Fabricantes de Veículos Automotores. Statistical Annual of the Brazilian Automobile Industry [Report Online] http://www.anfavea.com.br/Index.html (http://www.anfavea.com.br/Index.html) (Accessed: 24 March 2011 ).
- Associação Brasileira de Normas Técnicas . 2005 . NBR 6601: Veículos Rodoviários Automotores Leves [Light Self-Driven Vehicles: Determination of Hydrocarbons, Carbon Monoxide, Nitrogen Oxides, Carbon Dioxide and Particulate Matter in the Exhaust Pipe] , Rio de Janeiro : Associação Brasileira de Normas Técnicas .
- Bruns , R.E. , Scarminio , I.S. and Barros Neto , B. 2006; Chapter 4 . Statistical Design—Chemometrics , Elsevier : Amsterdam . pp 147-198
- Statsoft . 1999 . Statistica for Windows [computer program. 1 CD-ROM] , Tulsa , OK : Statsoft, Inc .
- Ministério da Saúde . March 2004 . Portaria n.º 518, de 28 de março de 2004; [It establishes the procedures and responsibilities for the control and monitoring of water quality for human consumption and its potability standard, and gives other steps] , Vol. 26 , March , Brasília , DF : Diário Oficial da União .
- Ryan , J.V. and Gullett , B.K. 2000 . On-Road Emission Sampling of Heavy-Duty Diesel Vehicle for Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans . Environ. Sci. Technol. , 34 : 4483 – 4489 .
- Pereira , E.A. 2006 . Tavares, M.F.M.; Stevanato, A.; Cardoso, A.A. Avaliação de Contaminantes Inorgânicos e Orgânicos em Álcool Combustível Utilizando Eletroforese Capilar [Evaluation of Inorganic and Organic Contaminants in Alcohol Fuel Using Capillary Electrophoresis] . Quím. Nova. , 29 : 66 – 71 .
- Dyke , P.H. , Sutton , M. , Wood , D. and Marshall , J. 2007 . Investigations on the Effect Of Chlorine in Lubricating Oil and the Presence of a Diesel Oxidation Catalyst on PCDD/F Releases FROM An Internal Combustion Engine . Chemosphere , 67 : 1275 – 1286 .
- Meira , G.R. 2007 . Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba Jr, J.C. Salinity of Marine Aerosols in a Brazilian Coastal Area: Influence of Wind Regime . Atmos. Environ. , 41 : 8431 – 8441 .
- Graedel , T.E. and Keene , W.C. 1995 . Tropospheric Budget of Reactive Chlorine . Global Biogeochem. Cycles , 9 : 47 – 78 .
- Chang , M.B. , Chang , S.H. , Chen , Y.W. and Hsu , H.C. 1994 . Dioxin Emission Factors for Automobiles from Tunnel Air Sampling in Northern Taiwan . Sci. Total Environ , 325 : 129 – 138 .
- Chuang , S-C. , Chen , S-J. , Huang , K-L. , Wu , E.M-Y. , Chang-Chien , G-P. and Wang , L-C. 2010 . Gas/Particle Partitioning of Dioxins in Exhaust Gases from Automobiles . Aerosol Air Qual. Res , 10 : 489 – 496 .
- Geueke , K.J. 1999 . Gessner, A.; Quass, U.; Broker, G.; Hiester, E. PCDD/F Emissions from Heavy Duty Vehicle Diesel Engines . Chemosphere , 38 : 2791 – 2806 .
- International Energy Agency . 2008 . Key World Energy Statistics; International Energy Agency Paris