Abstract
Manganese acetate (MnAc) and manganese nitrate (MnN) were employed as precursors for the preparation of Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 by impregnation. These complexes were used as catalysts in the low-temperature selective catalytic reduction of NO with NH3. The influence of manganese precursors on catalyst characteristics, the reduction activity, and the stability of the catalysts to poisoning by H2O and SO2 were studied. Experiments showed that Mn(N) produced MnO2 with large grain sizes in Mn(N)/TiO2 catalyst. On the contrary, Mn(Ac) led to highly dispersed and amorphous Mn2O3 in Mn(Ac)/TiO2 catalyst, which had better catalytic activity and stability to SO2 at low temperatures. The doping of cerium reduced the differences in catalytic performance between the catalysts derived from different Mn precursors.
Developing catalyst for selective catalytic reduction (SCR) of NOx from stationary sources at low reaction temperature is an attractive and key area of alternative process for existing SCR technology due to economical and environmental reasons. Manganese-based catalysts were proven very active at low temperature. In this paper, comparative studies were conducted on how manganese precursors were affecting the properties of resultant Mn-based/TiO2 catalysts. Different precursors could lead to different grain size of active components, manganese oxide dispersion, and surface phase state of Mn/TiO2 catalyst, whereas these influences are reduced with the doping of cerium oxides into Mn/TiO2.
Introduction
Nitrogen oxides (NOx) remain a major source of air pollution. Over 90% NOx in the atmosphere comes from fossil fuel combustion and about 95% of NOx in flue gas is present as NO. Currently, selective catalytic reduction of NO with NH3 (NH3-SCR) is the most efficient technology in industry for the control of the emission of nitrogen oxides from stationary sources. Typically, the catalysts used in industrial applications are V2O5/TiO2 and V2O5-WO3/TiO2, which require operating temperatures of 300–400 °C (CitationBusca et al., 1998; CitationHeck, 1999). In order to meet the temperature requirements, SCR units are usually installed between a boiler's economizer and air pre-heater. Due to the position of the SCR unit, the catalyst's lifetime is unavoidably reduced due to attrition and the plugging of the micropores of the catalyst, caused by high concentrations of dust and other contaminants (CitationParvulescu et al., 1998; CitationTang et al., 2005; CitationValdés-Solýs et al., 2003). In order to move the SCR unit behind the pre-heater, it is important to develop low-temperature SCR catalysts with high activity and strong stability to SO2 and H2O poisoning.
In recent years, SCR catalysts based on MnOx/TiO2 have attracted a great deal of attention due to their high activity at low reaction temperatures (CitationEttireddy et al., 2007; CitationJiang et al., 2009; CitationSmirniotis et al., 2006; CitationWu et al., 2008; CitationWu et al., 2009). In our previous work, it was observed that catalyst preparation from manganese acetate and a calcination temperature of 500 °C led to a Mn-Ce/TiO2 catalyst with good activity, even at reaction temperatures below 200 °C (CitationLiu et al., 2006). Under the experimental conditions of 10,000 hr−1 GHSV and 2000 ppm NO, the catalyst was able achieve 90% NO conversion at 120 °C and 95% at 150 °C. The catalysts in the experiment also showed good stability to poisoning by SO2. However, the catalyst's performance became poor when both SO2 and H2O coexisted in the feed gas.
Manganese precursors for Mn-based SCR catalysts have great influence on the activity of the catalyst when prepared by impregnation. CitationKapteijn et al., (1994a, Citation1994b) found that a Mn/Al2O3 catalyst obtained from manganese acetate had better activity than a catalyst produced from manganese nitrate. This was attributed to highly dispersed manganese oxides on the catalyst surface. Similar work by (CitationPeña Donovan et al., 2004) showed that a Mn/TiO2 catalyst derived from manganese nitrate and calcinated at 400 °C had better activity at lower temperatures than a catalyst obtained from manganese acetate. However, CitationLi et al., ( 2007) study reached an opposite conclusion and found that a Mn/TiO2 catalyst with manganese acetate as a precursor had better activity. Currently, few studies have focused on the effect of the precursor on the catalyst's stability to poisoning by both SO2 and H2O. In this work, we prepared Mn/TiO2 and Mn-Ce/TiO2 catalysts with manganese acetate and manganese nitrate as precursors, respectively, and examined the effect of the precursors on the catalyst's performances. The effects of Ce doping in Mn/TiO2 were also investigated.
Experimental
Catalyst preparation
Mn/TiO2 and Mn-Ce/TiO2 catalysts from different manganese precursors were prepared by an impregnation method. An anatase-type TiO2 (Nanjing Haitai Co., Nanjing City, China; SA = 80 m2/g, average size = 10 nm) was employed as a catalyst support, whereas analytical-reagent (AR) cerium nitrate (Ce(NO3)3·6H3O) was used as a source of cerium in the catalyst. Manganese precursors included manganese acetate (Mn(CH3COO)2·4H2O, AR) and manganese nitrate (Mn(NO3)2, AR). The amount of Mn and Ce that was used was based on the mass of TiO2 and was 15% and 50% by weight, respectively. Manganese precursor, cerium nitrate (if employed), and titanium dioxide were simultaneously mixed in distilled water, stirred for 1 hr, dried at 120 °C in an oven for 12 hr and calcined at 500 °C for 6 hr in an air atmosphere. After cooling to ambient temperature, the product was milled and filtrated to achieve catalyst particles between 40 to 80 meshes. Catalysts obtained from manganese acetate and manganese nitrate are denoted as Mn(Ac) and Mn(N), respectively, in the following text.
Catalyst activity and characterization
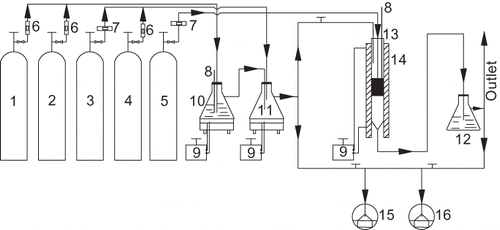
is the schematic diagram of the experimental installation. Catalyst evaluation was conducted in a tubular fixed bed reactor made of stainless steel 20 mm in internal diameter [i.d.]). H2O and SO2 were introduced into the reactor as needed. Ten milliliters of catalyst powder was placed on a sieve-like distributor in the middle of the reactor, which was settled in a tubular furnace equipped with a programmable temperature controller. Gas reactants were mixed online and fed into the reactor continually. NH3 was introduced into the reactor to initiate the reaction only when the NO concentrations at the inlet/outlet of the reactor were identical. This was done to remove the influence of catalyst adsorption on NO conversion. The NO and SO2 concentrations at the inlet/outlet of the reactor were continually monitored by a chemiluminescent 42C-HL NO-NO2-NOx analyzer and a 43C-HL SO2 analyzer (Thermo Environmental Instruments Inc., Franklin, MA). To avoid errors caused by the oxidation of ammonia by ozone in the converter of the NO-NO2-NOx analyzer, an ammonia trap filled with a phosphoric acid solution was installed at the sample inlet of the chemiluminescent analyzer.
Figure 1. Schematic diagram of the experimental installation. 1 = N2; 2 = O2; 3 = 10% NO/N2; 4 = 1.25% SO2/N2; 5 = 10% NH3/N2; 6 = rotor flowmeters; 7 = mass flowmeters; 8 = thermometers; 9 = temperature controllers; 10 = wash bottle (water); 11 = mix bottle; 12 = ammonia trap bottle (phosphoric acid); 13 = stainless steel tubular reactor; 14 = tube furnace; 15 = 42C-HL NO-NO2-NOx analyzer; 16 = 43C-HL SO2 analyzer.
N2, O2, SO2, and NO used in the experiments were commercial- grade gases of 99.99%, 99.99%, 99.9%, and 99.5% purity, respectively (Beijing Huayuan Gas Chemical Industry Co. Ltd., Beijing, China). The NH3 used in the experiment was liquid ammonia of 99.99% purity. Three mixed gases of 10% NO/N2, 10% NH3/N2, and 1.25% SO2/N2 were prepared and placed into a separate steel cylinder for the following experiments. Water vapor was generated by passing N2 through a gas-wash bottle containing deionized water. The water temperature in gas-wash bottle could be adjusted to meet the desired water content in the fed gas.
lists the standard reaction conditions for the evaluation of catalyst activity. The flow rates of N2 and O2 gases were regulated by rotameters. The control of the flow rates of NH3/N2 and NO/N2 was conducted by two mass flowmeters. N2, O2, and NO/N2 gases were mixed in a chamber before being supplied to the reactor. The gas flow of NH3/N2 was fed directly into the reactor to avoid a reaction between SO2 and NH3. The experimental data were collected when the SCR reaction had reached a steady state for at least 2 hr.
Table 1. Standard reaction conditions for catalyst activity measurement
Catalyst characterization
The specific surface areas of the fresh catalysts (Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2) were measured by the nitrogen adsorption method using a NOVA 4000 (Quantachrome Instruments, Boynton Beach, Florida) automated gas sorption system at −196 °C. The value of the BET surface area (specific surface area based on Brunauer-Emmett-Teller equation) was determined by the two-parameter BET equation. Prior to the surface area measurement, catalyst samples were degassed under vacuum at 400 °C for 24 hr.
Powder X-ray diffraction measurements (XRD) were carried out on a Rigaku D/max-3C system (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation under the following conditions: a lamp voltage of 40 kV, a current of 100 mA, a step of 0.02, a scan rate of 6°/min and a scan range 2θ = 10–70°.
X-ray photoelectron spectra (XPS) were recorded in a PHI-1600 ESCA system (PerkinElmer Inc., Waltham, MA) spectrometer with Mg Kα as a source of X-rays (E B = 1653.6 eV) and under a residual pressure of 5.0 × 10−6 Pa. The error of the binding energy (BE) was ±0.2 eV. Atomic sensitivity factors from CitationWagner (1977, Citation1972) were applied to the calibration of peak areas of binding energies. The peak areas were normalized to obtain the surface atom percentage. The charge effect was calibrated with contaminated carbon (C 1s peak at 284.6 eV).
Temperature-programmed reduction (TPR) measurements were conducted on a NOVA (Quantachrome Instruments, Boynton Beach, FL) physics chemistry adsorption system that required 30 mg of catalyst sample and H2/N2 mixed gas, in which H2 accounted for 5%. The mixed gas was used as a reductant and supplied to the system at a flow rate of 20 mL/min. During the experiment, the sample was heated from room temperature to 800 °C and the heating rate was set at 10 °C/min. A thermal conductivity detector (TCD) was used for the evaluation of the composition of the gas.
A Bio-Rad FTS 165 analyzer (Bio-Rad Laboratories, Hercules, CA) was employed for Fourier transform infrared spectrometer (FT-IR) analysis. Before characterization, catalyst samples were cleaned for 1 hr with N2 gas at the same temperature as the SCR reaction to wipe off physically adsorbed species. After being cooled to room temperature, the catalyst sample was mixed with KBr at a weight ratio of 1:150, ground, and pelleted. The FT-IR spectra were obtained from 4000 to 400 cm−1.
Results and Discussion
BET testing
The results of surface area measurements of fresh Mn/TiO2 and Mn-Ce/TiO2 catalysts are listed in The surface areas of the products from manganese acetate precursors are larger than those obtained from manganese nitrate. It was also noted from that Mn-Ce/TiO2 has larger surface area than Mn/TiO2 due to CeO2 in the catalysts.
Table 2. BET results of Mn/TiO2 and Mn-Ce/TiO2 with different precursors
XRD analysis
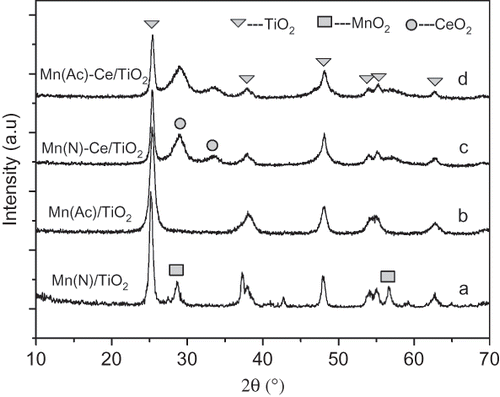
is the XRD patterns of fresh Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 catalysts. The Mn(N)/TiO2 catalyst has clear diffraction peaks from MnO2 and TiO2 of anatase type. However, Mn(Ac)/TiO2 only has diffraction peaks from TiO2, whereas the peaks attributed to MnO2 are not observed. This indicates that the manganese oxides in the Mn(Ac)/TiO2 catalyst are amorphous and well dispersed. After doping Ce into the Mn/TiO2 catalysts, the XRD spectra of Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 are almost identical, in which the only diffraction peaks observed are attributed to TiO2 and CeO2. Obviously, there is no manganese oxide in the crystalline phase on the catalysts.
XPS analysis
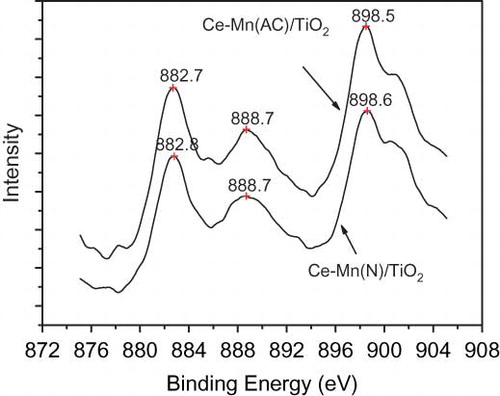
shows the Mn 2p binding energy (BE) spectra of fresh Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 catalysts. The Mn 2p3/2 binding energy of the Mn(Ac)/TiO2 and Mn(N)/TiO2 catalysts are 641.3 and 641.9 eV, respectively. Taking into account the XRD results and BE data, the majority of the manganese oxides present in the Mn(Ac)/TiO2 and Mn(N)/TiO2 catalysts are in the form of Mn2O3 and MnO2, respectively. The BE data of manganese oxides in literature were referenced and validated this conclusion (CitationAlvarez-Galván et al., 2004; Kapteijn et al., 1994). This result is also in accordance with Kapteijn et al., (1994) work on Mn/Al2O3 catalysts prepared from different manganese precursors.
Figure 3. Spectra of Mn 2p binding energy of Mn/TiO2 and Mn-Ce/TiO2 catalysts derived from different precursors.
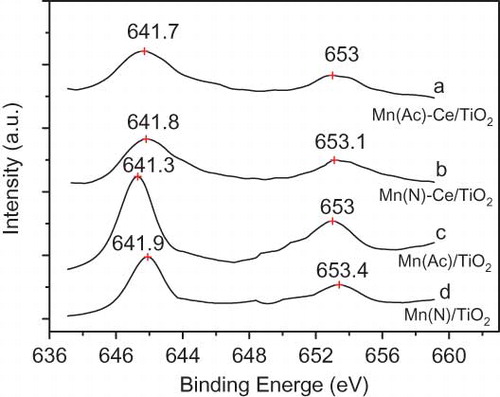
The Mn 2p3/2 BEs of Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 are similar to each other. Each Mn 2p region consists of a spin-orbit doublet, with Mn 2p1/2 having a BE of nearly 653.1 eV and Mn 2p3/2 with a BE of nearly 641.7 eV. These values are characteristic of a mixed-valence manganese system (Mn4+ and Mn3+) (CitationWu et al., 2008; CitationZhang et al., 2007). This phenomenon suggests that the doping of Ce reduced the valence state of manganese in Mn(N)/TiO2 catalyst and some of the MnO2 was converted to Mn2O3. The interaction between manganese and cerium forces an electron to move from cerium to manganese, thus reducing the valence state of manganese and enhancing the concentration of Ce4+ (CitationChen et al., 2001). shows the Ce3d BE spectra of Mn(Ac)-Ce/TiO2 and Ce-Mn(N)/TiO2 catalysts. The BEs of cerium in the catalysts obtained from the two manganese precursors are almost identical, indicating that the cerium oxides in the two catalysts are CeO2.
gives the surface atom ratios of manganese to titanium in the catalysts according to XPS analysis. Compared with the catalysts derived from Mn(N), catalysts obtained from Mn(Ac) have a higher concentration of surface manganese.
Table 3. XPS results of the catalysts
H2-TPR analysis
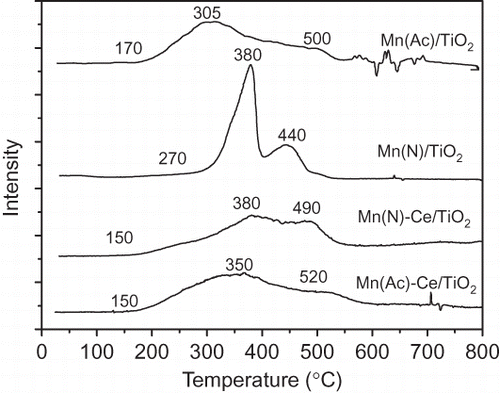
According to the literature (CitationLiang et al., 2008; CitationLiu et al., 2007; CitationYao et al., 2006), in the reduction of MnOx and CeO2, pure MnO2 has reduction peaks at 370 and 604 °C. For pure Mn2O3, reduction peaks appear at 468 and 534 °C, which are attributed to the reduction of MnO2/Mn2O3 to Mn3O4 and further reduction of Mn3O4 to MnO. Reduction peaks of pure CeO2 are observed at 501 and 746 °C. Thus, the peak near 500 °C is assigned to reduction of CeO2 at the surface. The peak near 750 °C represents the reduction of lattice CeO2 in bulk phase. The reduction peak of TiO2 is very weak, and it is observed at 600 °C (CitationLiang et al., 2008; CitationLiu et al., 2007; CitationYao et al., 2006).
shows the TPR patterns of fresh Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 catalysts. According to above analysis from the literature, the two reduction peaks below 500 °C in the TPR patterns of Mn(N)/TiO2 and Mn(Ac)/TiO2 of are assigned to reduction of manganese oxides. In Mn(N)/TiO2 of , a reduction peak in the low temperature range appears at 380 °C, which is the characteristic of the reduction of MnO2 (CitationLiang et al., 2008; CitationLiu et al., 2007; CitationYao et al., 2006). This peak is sharp and has a strong intensity, indicating that most manganese species within the Mn(N)/TiO2 catalyst are MnO2 in an aggregated state, with large grain sizes. The reduction peak of Mn(N)/TiO2 at 440 °C is weak, and may result from the reduction of a small amount of Mn2O3.
Mn(Ac)/TiO2 yields weak reduction peaks within wide temperature range of 170∼510 °C. Taking into account the results of XPS analysis, these reduction peaks are attributed to the reduction of Mn2O3. The starting temperature of 170 °C and the peak point of low-temperature reduction at 305 °C are much lower than pure Mn2O3. This suggests a highly dispersed manganese species on TiO2 support, and the interaction between TiO2 support and manganese makes Mn–O bond easier to reduce.
The effects of Ce in Ce-doped Mn(Ac)/TiO2 and Mn(N)/TiO2 catalysts are different. On one hand, the spectra of Mn(Ac)-Ce/TiO2 and Mn(Ac)/TiO2 are similar, with the exception that the initial temperature of reduction reaction decreases to 150 °C and the peak point of low-temperature reduction shifts up to 350 °C after Ce doping into the Mn(Ac)/TiO2 catalyst. H2 consumption peak at 450∼600 °C is the result of overlap of further reduction of manganese oxides and reduction of surface CeO2. The peak near 750 °C related to the reduction of lattice oxygen of CeO2 in bulk phase was not observed. From these results and the XRD data, it is deduced that cerium in the doped catalyst (Mn(Ac)-Ce/TiO2) interacts with manganese oxides and decreases reducibility of manganese. However, cerium doping increases the amount of CeO2 at the surface and enhances the reducibility of lattice oxygen of CeO2 in bulk phase.
On the other hand, the TPR pattern of the Mn(N)-Ce/TiO2 catalyst is quite different from that of Mn(N)/TiO2. Reduction peaks of the Mn(N)-Ce/TiO2 catalyst become much wider and weaker. The initial temperature of the reduction reaction is lower than that of the catalyst without incorporation of Ce. This suggests that doping of cerium greatly improves dispersity of manganese on the catalyst. The peak point of low-temperature reduction appears at 380 °C, which is at the same position as observed in Mn(N)/TiO2, suggesting that there is a small amount of MnO2 in Mn(N)-Ce/TiO2. In comparison with Mn(Ac)-Ce/TiO2 catalyst, reduction peaks of Mn(N)-Ce/TiO2 have shifted to higher temperatures, and a new but weak reduction peak appears near 720 °C. This peak could be attributed to the reduction of lattice oxygen of CeO2 in bulk phase, suggesting that dispersity of manganese and reducibility of cerium in Mn(N)-Ce/TiO2 are lower than that in Mn(Ac)-Ce/TiO2.
Catalyst activity
The activity tests showed that Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 catalysts were stable when there are no H2O and SO2 in the feed gas. presents NO conversions for the four catalysts at reaction temperatures ranging from 100 to 210 °C. shows that Mn(Ac)/TiO2 reaches 78% of NO conversion at 100 °C and 93% at 210 °C, which is much higher than that of Mn(N)/TiO2. CitationLietti et al., (1996) (CitationLietti and Forzatti, 1994) stated that NH3 is prone to adsorb on catalyst at low temperatures, but activation of adsorbed NH3 is restricted by the low temperature. However, catalyst surface with strong oxidizability could help to promote the activation of adsorbed NH3 to from NH2(ads) by dehydrogenation. Therefore, the catalyst's redox capability is crucial for its activity at low temperatures. According to the results of catalyst characterization, Mn(Ac)/TiO2 possesses good dispersity of manganese species, and high reducible surface. Both do contribute to the higher activity of Mn(Ac)/TiO2 for NH3-SCR at low temperatures than that of Mn(N)/TiO2.
Figure 6. NO conversion over Mn/TiO2 and Mn-Ce/TiO2 catalysts from different precursors. NO = 1000 ppm, NH3 = 1100 ppm, O2 = 6%, GHSV = 5000 hr−1, balance N2.
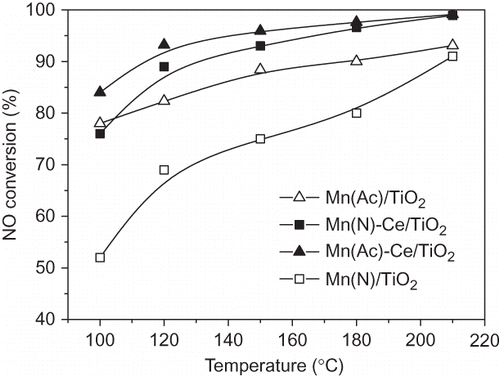
The curves of Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 are similar to each other, suggesting that activity difference between the two catalysts has become smaller after doping with cerium. This is considered to be due to an increase in the dispersity of manganese species in the catalysts and enhanced catalytic activity, which is a result of the interaction between cerium and manganese. According to the TPR results, the activity of the surface species of CeO2 in Mn(Ac)-Ce/TiO2 catalyst is higher than that in Mn(N)-Ce/TiO2. Mn(Ac)-Ce/TiO2 catalyst still shows higher activity than Mn(N)-Ce/TiO2.
The effects of H2O and SO2 on catalyst activity
The effects of 200 ppm SO2 and 10% H2O on activity of Mn(Ac)/TiO2, Mn(N)/TiO2, Mn(Ac)-Ce/TiO2, and Mn(N)-Ce/TiO2 catalysts were investigated at 150 °C, as shown in The NO conversion data were collected 2 hr after introduction of SO2 or SO2 and H2O.
Figure 7. The impact of the introduction of SO2/H2O for 2 hr on Mn/TiO2 and Mn-Ce/TiO2 catalysts from different precursors. NO = 1000 ppm, NH3 = 1100 ppm, O2 = 6 %, GHSV = 5000 hr−1, SO2 = 200 ppm, H2O = 10% (if used), balance N2, T = 150 °C.
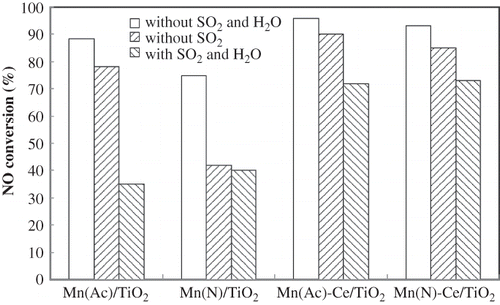
With SO2 present in the feed gas, the decrease in catalytic activity was observed in all the tests. Compared with Mn(Ac)/TiO2, Mn(N)/TiO2 had much greater loss in activity, from 75% to 42%. However, Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 catalysts demonstrated similar behaviors. They were less affected, and maintained NO conversions over 85%.
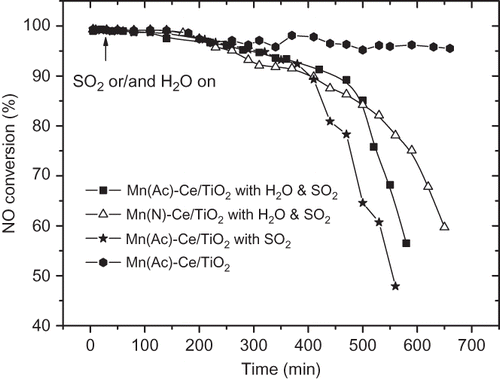
When both SO2 and H2O were conducted into the SCR system, the behaviors of Mn(Ac)/TiO2 and Mn(N)/TiO2 were similar. Both suffered severe deactivation or inhibition. It was observed that NO conversion dropped to 35% with Mn(Ac)/TiO2 catalyst. The effects of H2O and SO2 on Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 catalysts were analogous. Activities of the two catalysts fell to around 70% in experiments. Catalyst activity tests were also conducted at extended reaction times to verify the effects of SO2 and H2O on Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2, as shown in The two catalysts behaved almost identically in the presence of both SO2 and H2O, except that Mn(Ac)-Ce/TiO2 showed a faster decrease in NO conversion than Mn(N)-Ce/TiO2 after 500 min. As a comparison, another two sets of NO conversion data over Mn(Ac)-Ce/TiO2 catalyst, with SO2 only, and without SO2 and H2O in the feed gas, are also given in The data show that Mn(Ac)-Ce/TiO2 could maintain its activity for a long time without the influence from SO2 and H2O. However, when there was SO2 in the feed gas, NO conversion fell sharply in 9 hr, which is similar to that observed with SO2 and H2O in the feed gas.
Figure 8. The effect of SO2 and H2O for extended times on Mn-Ce/TiO2 catalysts. NO = 1000 ppm, NH3 = 1100 ppm, O2 = 6 %, GHSV = 5000 hr−1, SO2 = 200 ppm, H2O = 10 %, balance N2, T = 170 °C.
In order to investigate the cause for the difference between Mn(Ac)/TiO2 and Mn(N)/TiO2 catalysts in their stabilities to SO2 poisoning, the oxidation of SO2 over Mn(Ac)/TiO2 and Mn(N)/TiO2 was simulated under conditions with/without H2O in the feed gas. These experiments were conducted at reaction temperature of 150 °C. Reaction gases (N2, NO, O2, and SO2) with the same concentrations in previous experiments were fed into the reactor. Three hours later, the catalysts were removed from the reactor for FT-IR analysis, and the results are shown in
Figure 9. FT-IR spectra of spent Mn/TiO2 from different precursors under the influence of SO2 and H2O.
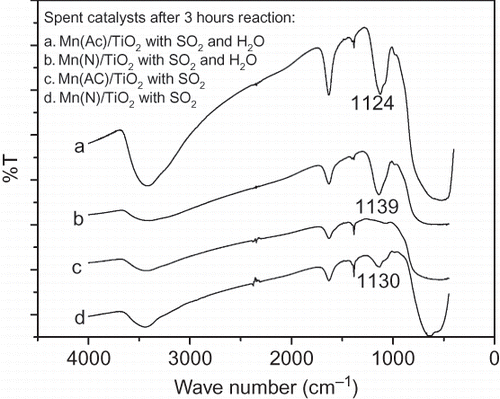
Hug (CitationHug, 1997) and others (CitationNakamoto, 1986) reported that FTIR spectrum of SO4 2− has characteristic bands near 1200, 1130, 1050, and 970 cm−1, whereas HSO4 2− gives characteristic bands near 1200, 1040, and 900 cm−1. Obviously, in a FTIR spectrum of (bi)sulfate on catalyst, the band near 1130 cm−1 could be assigned to SO4 2−, and the band near 900 cm−1 is HSO4 2−. In , there are absorption peaks near 1130 cm−1 (1124 cm−1, 1139 cm−1, and 1130 cm−1), but no peak near 900 cm−1 could be found. This suggests that the S-containing salt on the four tested catalysts is sulfate salt, not bisulfate salt.
In , curves a, b, and d have clear absorption peaks near 1130 cm−1, which is attributed to SO4 2−. However, this peak does not appear in curve c. Obviously, the oxidation of SO2 over Mn(Ac)/TiO2 is very weak when H2O is not present in the feed gases. There should be smaller amount of SO4 2− formed on the catalyst than others. On the other hand, Mn(N)/TiO2 has a catalytic effect on the oxidization of SO2, as shown in curve d. This is related to MnO2 in aggregation state in Mn(N)/TiO2 catalyst. Compared with curve d, the stronger peaks near 1130 cm−1 in curves a and b indicate that H2O promotes the oxidation of SO2 over the two Mn/TiO2 catalysts. In particular, this is observed especially over Mn(Ac)/TiO2 catalyst. Vadjić and Gentilizza (CitationVadjić and Gentilizza, 1985) reported similar phenomenon and found that the high relative humidity of ambient air increased the oxidization of SO2 over MnO2.
Conclusions
| 1. | Manganese precursors have great effects on the state and dispersity of manganese species in resultant Mn/TiO2 catalysts. Manganese species in Mn(N)/TiO2 is present as MnO2 with aggregated state and large grain size, whereas that in Mn(Ac)/TiO2 catalyst is amorphous Mn2O3 with high dispersity. Thus, Mn(Ac)/TiO2 catalyst has a higher surface concentration of active constituents, and is easier to be reduced, leading to better performance at low temperature in the SCR process. | ||||
| 2. | Doping of cerium into Mn/TiO2 catalysts can eliminate performance differences between the catalysts derived from manganese acetate and manganese nitrate precursors. Due to the higher activity of surface oxygen species from CeO2, Mn(Ac)-Ce/TiO2 shows better activity than Mn(N)-Ce/TiO2. | ||||
| 3. | Mn(Ac)/TiO2 catalyst shows better stability to SO2 than Mn(N)/TiO2. The impact of SO2 on Mn(Ac)-Ce/TiO2 and Mn(N)-Ce/TiO2 catalyst are similar, which is weaker than on Mn/TiO2 catalyst. When SO2 and H2O coexist in the feed gas, all four catalysts suffered severe deactivation. This is due to the promotion effect of H2O on the oxidization of SO2, no matter what kind of manganese precursor was taken. | ||||
Acknowledgments
This work was supported by the Science Foundation for the Excellent Youth Scholars of Ministry of Education of China (20070010017) and the Foundation for Key Discipline Construction of Beijing.
References
- Alvarez-Galván , M.C. , Pawelec , B. , De La PeÌa O'Shea , V.A. , Fierro , G. and Arias , P.L. 2004 . Formaldehyde/Methanol Combustion on Alumina-Supported Manganese-Palladium Oxide Catalyst . Applied Catalysis B: Environmental , 51 : 83 – 91 .
- Busca , G. , Lietti , L. , Ramis , G. and Berti , F. 1998 . Chemical and Mechanistic Aspects of the Selective Catalytic Reduction of NO x by Ammonia over Oxide Catalysts: A Review . Applied Catalysis B , 18 : 1 – 36 .
- Chen , H.Y. , Sayari , A. , Adnot , A. and Larach , F.V. 2001 . Composition–Activity Effects of Mn-Ce-O Composites on Phenol Catalytic Wet Oxidation . Applied Catalysis B , 32 : 195 – 204 .
- Ettireddy , P.R. , Ettireddy , N. , Mamedov , S. , Boolchand , P. and Smirniotis , P.G. 2007 . Surface Characterization Studies of TiO2 Supported Manganese Oxide Catalysts for Low Temperature SCR of NO with NH3 . Applied Catalysis B , 76 : 123 – 134 .
- Heck , R.M. 1999 . Catalytic Abatement of Nitrogen Oxide-Stationary Applications . Catalysis Today , 53 : 519 – 523 .
- Hug , S.J. 1997 . In Situ Fourier Transform Infrared Measurements of Sulfate Adsorption on Hematite in Aqueous Solutions . Colloid Interface Science , 188 : 415 – 422 .
- Jiang , B.Q. , Liu , Y. and Wu , Z.B. 2009 . Low-Temperature Selective Catalytic Reduction of NO on MnOx/TiO2 Prepared by Different Methods . Journal of Hazardous Materials , 162 : 1249 – 1254 .
- Kapteijn , F. , Singoredjo , L. and Vandriel , M. 1994a . Alumina-Supported Manganese Oxide Catalysts: II. Surface Characterization and Adsorption of Ammonia and Nitric Oxide . Journal of Catalysis , 150 : 105 – 116 .
- Kapteijn , F. , Vanlangeveld , A.D. and Moulijn , J.A. 1994b . Alumina-Supported Manganese Oxide Catalysts: I. Characterization: Effect of Precursor and Loading . Journal of Catalysis , 150 : 94 – 104 .
- Li , J.H. , Chen , J.J. , Ke , R. , Luo , C.K. and Hao , J.M. 2007 . Effects of Precursors on the Surface Mn Species and the Activities for NO Reduction Over MnOx/TiO2 Catalysts . Catalysis Communications , 8 : 1896 – 1900 .
- Liang , F.X. , Zhu , H.Q. , Qing , Z.F. and Wang , H. 2008 . Preparation and Characterization of CeO2-TiO2 Composite Oxide and its Catalytic Performance for CO Oxidation . Chinese Journal of Catalysis , 29 : 264 – 268 .
- Lietti , L. , Alemany , J.L. , Forzatti , P. , Busca , G. , Ramis , G. and Giamello , E. 1996 . Reactivity of V2O5-WO3/TiO2 Catalysts in the Selective Catalytic Reduction of Nitric Oxide by Ammonia . Catalysis Today , 29 : 143 – 148 .
- Lietti , L. and Forzatti , P. 1994 . Temperature Programmed Desorption/Reaction of Ammonia over V2O5/TiO2 de-NOxing Catalysts . Journal of Catalysis , 147 : 241 – 249 .
- Liu , Y. , Meng , M. , Yao , J.S. and Zha , Y.Q. 2007 . Preparation and Characterization of the Multicomponent Mesoporous Mixed Oxides Catalysts La-Mn-Ce-O . Acta Physico-Chimica Sinica , 23 : 641 – 646 .
- Liu , W. , Tong , Z.Q. and Lu , J. 2006 . Low-Temperature Selective Catalytic Reduction of NO with NH3 over Ce-Mn/TiO2 Catalyst . Acta Science Circumcision , 26 : 1240 – 1245 .
- Nakamoto , K. 1986 . Infrared and Raman Spectra of Inorganic and Coordination Compounds , 4th , John Wiley & Sons : New York .
- Parvulescu , V.I. , Grange , P. and Delmon , B. 1998 . Catalytic Removal of NO . Catalysis Today , 46 : 233 – 316 .
- Peña , D.A. , Uphade , B.S. and Smirniotis , P.G. 2004 . TiO2-Supported Metal Oxide Catalysts for Low-Temperature Selective Catalytic Reduction of NO with NH3: I. Evaluation and Characterization of First Row Transition Metals . Journal of Catalysis , 221 : 421 – 431 .
- Smirniotis , P.G. , Sreekanth , P.M. , Pen , D.A. and Jenkins , R.G. 2006 . Manganese Oxide Catalysts Supported on TiO2, Al2O3, and SiO2: A Comparison for Low-Temperature SCR of NO with NH3 . Industrial and Engineering Chemistry Research , 45 : 6436 – 6443 .
- Tang , X.L. , Hao , J.M. , Xu , W.G. and Li , J.H. 2005 . Low-Temperature SCR of NOx for Stationary Source . Acta Science Circumcision , 25 : 1297 – 1305 .
- Vadjić , V. and Gentilizza , M. 1985 . The effect of MnO2 and Some Manganese Salts on the Behaviour of Sulphur Dioxide in the Air Investigated on Model Systems . Science of the Total Environment , 44 : 245 – 251 .
- Valdés-Solýs , T. , Marbán , G. and Fuertes , A.B. 2003 . Low-Temperature SCR of NOx with NH3 over Carbon-Ceramic Supported Catalysts . Applied Catalysis B: Environmental , 46 : 261 – 271 .
- Wagner , C.D. 1972 . Sensitivity of Detection of the Elements by Photoelectron Spectrometry . Analytical Chemistry , 44 : 1050 – 1053 .
- Wagner , C.D. 1977 . Factors Affecting Quantitative Determinations by X-ray Photoelectron Spectroscopy . Analytical Chemistry , 49 : 1282 – 1290 .
- Wu , Z.B. , Jin , R.B. , Liu , Y.H. and Wang , Q. 2008 . Ceria Modified MnOx/TiO2 as a Superior Catalyst for NO Reduction with NH3 at Low-Temperature . Catalysis Communications , 9 : 2217 – 2220 .
- Wu , Z.B. , Jin , R.B. , Wang , H.Q. and Liu , Y. 2009 . Effect of Ceria Doping on SO2 Resistance of Mn/TiO2 for Selective Catalytic Reduction of NO with NH3 at Low Temperature . Catalysis Communications , 10 : 935 – 939 .
- Yao , J.S. , Meng , M. , Luo , J.Y. and Zha , Y.Q. 2006 . Structures, Properties and Support Effects of Highly Dispersed Manganese Oxide Catalysts . Journal of Molecular Catalysis , 20 : 300 – 305 .
- Zhang , X. , Ji , L.Y. , Zhang , S.C. and Yang , W.S. 2007 . Synthesis of a Novel Polyaniline-Intercalated Layered Manganese Oxide Nanocomposite as Electrode Material for Electrochemical Capacitor . Journal of Power Sources , 173 : 1017 – 1023 .