Abstract
This experiment was conducted to investigate the effects of JUNCAO Ganoderma lucidum polysaccharide peptide (JCGLPP) on slaughter performance and intestinal health of Minxinan black rabbits, which aimed to provide the basis for the application of JCGLPP in meat rabbits. One hundred male weaned Minxinan black rabbits of (33 ± 2) d [(initial body mass (655.65 ± 25.90) g] were randomly divided into four groups with five replicates per group and five rabbits per replicate. The diets were supplemented with 0 (control group), 50 (group I), 100 (group II) and 150 mg·kg−1 (group III) of JCGLPP, respectively. This experiment lasted for 56 days. The results are shown below: (1) The live weight before slaughter of groups I and III was significantly higher than that of control group (p < 0.05); The full net bore weight of group III was significantly higher than that of control group (p < 0.05). (2) pH value of group I was significantly higher than that of control group (p < 0.05); NH3–N content in experimental groups were significantly higher than that in control group(p < 0.05) while NH3-N content in group I was significantly higher than that in groups III and II (p < 0.05); The content of butyric acid in group II was significantly lower than that in control group (p < 0.05); There were no significant differences in acetic acid, isovaleric acid, isobutyric acid and propionic acid in experimental groups compared with control group (p > 0.05). (3) The Occludin content in duodenum, jejunum and ileum of groups I and II was significantly higher than that of control group (p < 0.05). (4) At the phylum level, Firmicutes and Bacteroidetes were the dominant phylum in each group. At the genus level, norank_f__norank_o__Clostridia_UCG-014 in group II were significantly higher than those in control group (p < 0.05). In conclusion, although dietary JCGLPP supplementation could not improve slaughter performance of Minxinan black rabbits, it could improve cecal fermentation parameters and intestinal flora structure and composition of Minxinan black rabbits to a certain extent. Our results revealed that 100 mg·kg−1 might be the optimal concentration obtained in dietary JCGLPP supplementation, which provided ideas and feasibility for drug combination.
Introduction
In recent years, the use of antibiotics in feed has brought about problems such as drug residues and serious environmental pollution, which seriously affected the ecological environment as well as the health of animals and humans.Citation1 At present, green and healthy breeding has become the focus of animal husbandry development. Therefore, it is urgent to find green, safe and efficient feed antibiotic substitutes, and plant extracts have become one of the effective ways to replace antibiotics. JUNCAO Ganoderma lucidum polysaccharide peptide (JCGLPP) is a glycoprotein derived from Ganoderma lucidum and consists of a variety of amino acids and monosaccharides. It can reduce oxidative stress and improve immunity, antioxidant capacity, anti-tumour and so on.Citation2 Ganoderma lucidum is environmentally friendly, safe, drug-free, toxic and side effects, which can be a substitute for antibiotics. In recent years, scholars around the world have studied the application of effective components of Ganoderma lucidum in animal husbandry.Citation3,Citation4
Intestinal microorganisms act as an important role in host immune regulation, nutritional metabolism and disease prevention during animal growth and development.Citation5 Studies have shown that Ganoderma lucidum polysaccharide peptides can ameliorate lipid metabolism disorders by regulating cecal microbe structure and genes involved in liver lipid and cholesterol metabolism.Citation6 Wang Yibing et al.Citation7 found that adding ganoderma spores powder to the diet could significantly reduce the plasma MDA content and increase the glutathione content of Wenchang chickens, thus improving the antioxidant capacity of Wenchang chickens. Yang Kai et al.Citation8 explored the regulatory function of oligosaccharides from Ganoderma lucidum spore powder on intestinal flora and found that Ganoderma lucidum spore powder could significantly promote the production of main short-chain fatty acids (acetic acid, propionic acid, butyric acid), improve the relative abundance of beneficial bacteria such as Bifidobacterium and Lactobacillus, and reduce the relative abundance of harmful bacteria such as Escherichia coli. The cecum of meat rabbits is rich in a large number of microorganisms, which maintain a relatively balanced and stable state with the host. Studies have shown that the digestion and absorption capacity of meat rabbits is closely related to the diversity of cecum microorganisms,Citation9 and the cecum microorganisms of meat rabbits can ferment cellulose and produce volatile fatty acids to provide energy for meat rabbits.Citation10
With the improvement of people’s living standard, the demand and requirement for meat quantity are increasing day by day. Minxinan black rabbits are famous for the characteristics of ‘Three High’ (high concentration of protein and phospholipid, with a high digestibility) and ‘Three Low’ (low concentration of fat, cholesterol and calories). The content of amino acid, protein and melanin in its meat is about twice that of ordinary rabbits, making it an ideal functional food for patients with obesity, hypertension and cardiovascular diseases.Citation11 However, with the implementation of the ban, digestive tract diseases in meat rabbits accounted for more than 50% of all disease types, resulting in 70% mortality and serious economic losses. What’s worse, gastrointestinal diseases often occurred in Minxinan black rabbits, which can lead to death in severe cases. Thus, improving the immune regulation ability, promoting intestinal health and enhancing disease resistance of meat rabbits are critical measures to reduce the occurrence and death of gastrointestinal diseases in meat rabbits.Citation12,Citation13
It was reported that plant extracts have the beneficial effects of antibacterial, anti-inflammatory, antioxidant and immunomodulatory.Citation14 Dietary with plant extracts can improve the performance, slaughter performance and immune function of rabbits. As a kind of plant extracts, researches on Ganoderma lucidum polysaccharide mainly focus on pharmacological effects of its components and polysaccharides, such as anticancer,Citation15 immune regulationCitation16 and antioxidant.Citation17 Studies have shown that ganoderma lucidum polysaccharide can regulate the balance of intestinal flora and maintain intestinal health to a certain extent.Citation18,Citation19 JCGLPP has strong immunomodulatory and antioxidant capacity. However, JCGLPP is rarely used in livestock and poultry production, especially in young rabbits. In this study, weaned Minxinan black rabbits were used as experimental animals to carry out JCGLPP feeding experiment to study the effects of JCGLPP on slaughter performance and intestinal health of Minxinan black rabbits. We aimed to explore the appropriate dosage of JCGLPP in the diet of rabbits and provide reference for the scientific use of JCGLPP in meat rabbit production.
Materials and methods
Moral statement
All experimental procedures and animals were approved by the College of Animal Sciences (College of Bee Science), Fujian Agriculture and Forestry University, which followed the recommendations of the European Commission (1997).
Preparation of JCGLPP
In February 2022, the mycorrhizal ganoderma lucidum seeds were sliced and dried in a constant temperature drying oven at 60 °C to a constant mass, then crushed with a pulverizer and passed through a 100 mesh sieve. JCGLPP was obtained by following steps. First, 500 g of JUNCAO Ganoderma lucidum powder was treated with ultrasonic assisted hot water extraction method. Then the compound was heated with 10 L of pure water in ultrasonic cleaning machine for 2.5h, filtered the extract for 3 times and poured it in a petri dish. Next, the compound was stored at −80 °C. Finally, JCGLPP was obtained freeze-drying after the compound was completely frozen. The viscosity of JCGLPP is related to its own molecular size. To reduce the viscosity, JCGLPP was treated by acid hydrolysis.
Experimental design and diet formulation
The feeding experiment was conducted in the rabbit farm of Tongxian Rabbit Industry Development Co., LTD., Longyan City, Fujian Province from April to May 2022. One hundred at the age of (33 ± 2) d [(initial body mass (655.65 ± 25.90) g] weaned male Minxinan black rabbits were randomly divided into four groups of five replicates each with five rabbits in each replicate using a one-way randomized design. The control group was fed by a basal diet (). We designed a basal diet with a low content of protein and high content of fibre, which was helpful to stimulate intestinal peristalsis and maintain intestinal health. The others in experimental groups I, II and III were fed diets supplemented with 50, 100 and 150 mg·kg−1 JCGLPP, respectively. The additive amount of JCGLPP was determined by the early pretest results, which relating to the body weight of Minxinan black rabbits. ln the experimental groups, the pre-mix was supplemented with JCGLPP and mixed well. All diets were made into pellets.
Table 1. Composition and nutrient levels of basal diets (air-dry basis).
Feeding management
Before the experiment, the rabbit house, the ground and the rabbit cages were cleaned and disinfected. Rabbits should be numbered uniformly and vaccinated. Rabbits were fed in a single cage for 7 days in the pre-feeding period and 56 days in the normal feeding period. Rabbits were disinfected and cleaned regularly every week. Rabbits were fed once a day at 7:00, 15:00 and 20:00 respectively.
Measurement indicators and methods
Slaughter performance
On the 56th morning after the start of the experiment, 5 rabbits in each group were randomly selected for fasting weighing. Then these rabbits were slaughtered, sampled and analyzed. Measurement indexes included live weight, half bore weight, full bore weight and slaughter rate.
Determination of cecal fermentation parameters
pH: The pH values of the cecum (upper, middle and lower segments) were determined by pH meter (PHSJ-3F, Shanghai).
Volatile fatty acids (VFA): The contents of acetic acid, isobutyric acid, isovaleric acid, propionic acid and butyric acid were determined by gas chromatograph (Agilent7890N, USA).
Ammoniacal nitrogen (NH3-N): The content of NH3-N was determined by phenol-sodium hypochlorite colorimetry.Citation20
Determination of intestinal compact junction protein content
The duodenum, jejunum and ileum of Minxinan black rabbits were cut out. The contents were washed with normal saline. The mucous membranes were scraped out with sterilizing slides and put into 1.5 mL sterilizing centrifuge tube and frozen with liquid nitrogen. These samples were stored at −80 °C for biochemical analysis.
Determination of microbe in cecum
After slaughter, cecal contents of Minxinan black rabbits were quickly removed. These samples were put into 5 mL sterilization centrifuge tube and frozen in liquid nitrogen. These samples were stored at −80 °C for microbiological analysis.
According to E.Z.N.A. ® soil DNA kit (Omega Bio-tek, ms Norcross, GA, U.S.) instruction for microbial community total genomic DNA extraction, 1% agarose gel electrophoresis were used to detect the quality of the extraction of genomic DNA. Concentration and purity of DNA were determined by NanoDrop2000 (Thermo Scientific corporation, USA).
The purified PCR products were library-built using the NEXTFLEX Rapid DNA-Seq Kit and sequenced using Illumina’s Miseq PE300 platform.
OTUs (operational taxonomic units) clustering and species classification analysis based on valid data. Abundance of OTUs, Alpha diversity calculations, Venn diagrams to obtain information on species richness and evenness within samples, common and endemic OTUs between samples or groups were analyzed together. Principal Coordinate Analysis (PCoA) was performed to demonstrate sample community structure. LEfSe analysis to detect species differences between groups; and PICRUSt to standardize OTU abundance tables with greengene ids for each OTU. The community structure of the samples was demonstrated by PCoA. LEfSe analysis was used to detect species differences between groups. OTUs abundance tables were normalized by PICRUSt, and the OTUs were subsequently annotated with COG functions by the greengene id corresponding to each OTU.
Statistical analysis
The data were collated using WPS Office statistical software. One-way ANOVA and Duncan’s method of multiple comparisons were performed by GraphPad Prism (version 7.00) or SPSS 26.0. Error bars indicate standard error of the mean (SEM). p Values were calculated using Dunnett’s multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001). As for analysis of carcass weight, ANCOVA (Analysis of Covariance) was performed by SPSS 26.0. Live weight before slaughter was set as a covariable. The results were shown as mean ± standard deviation, and p < 0.05 was used as the criterion for determining significant differences.
Results
Growth and slaughter performance
No significant difference was found in growth performance (Table S1). The slaughter performance was shown in . The live weight before slaughter of Group I and Group III were significantly higher than control group (). There is a linear correlation between carcass weight (eviscerated weight with or without giblet) and pre-slaughter weight (). Thus, the pre-slaughter weight was taken as a covariable during the statistical analysis of carcass weight to decrease the risk of type I error.
Figure 1. Effects of JCGLPP on the slaughter performance of Minxinan black rabbits. (a) Adjusted p values were calculated using Dunnett’s multiple comparisons test (*p < 0.05, **p < 0.01, ***p < 0.001); (b) the p values of slope deviation from zero are both less than 0.01.
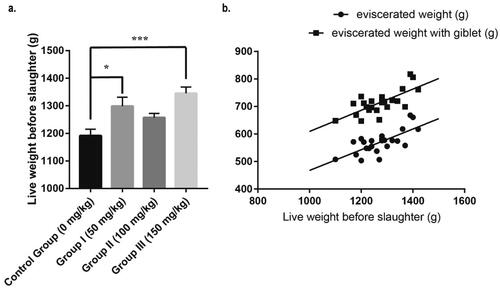
ANCOVA of carcass weight was taken and results was shown in . There were no significant differences in carcass weight in experimental groups compared with the control group (p > 0.05).
Table 2. Effects of JCGLPP on the carcass weight of Minxinan black rabbits.
Effect of JCGLPP on cecum fermentation parameters of Minxinan black rabbits
As shown in , the pH value of group I was significantly higher than that of the control group (p < 0.05), while there was no significant difference between experimental groups (p > 0.05). The NH3–N content of the experimental groups were significantly higher than that of the control group (p < 0.05), and the NH3–N content of group I was significantly higher than that of group III and group II (p < 0.05). The content of butyric acid in group II was significantly lower than that in the control group (p < 0.05), but there were no significant differences in acetic acid, isovaleric acid, isobutyric acid and propionic acid in the experimental groups compared with the control group (p > 0.05).
Table 3. Effect of JCGLPP on cecal fermentation of Minxinan black rabbits.
Effect of JCGLPP on intestinal tight junction protein of Minxinan black rabbits
As can be seen from , the Occludin content in duodenum, jejunum and ileum of group I and group II were both significantly greater than that of the control group (p < 0.05), furthermore, the content of duodenum and jejunum of group I was significantly greater than that of group II (p < 0.05).
Table 4. Effect of JCGLPP on occludin of Minxinan black rabbits(ng·mL−1).
Effect of JCGLPP on the cecal microbe of Minxinan black rabbits
Overview of cecal microbe microbiota sequencing
Sequencing was based on the Illumina Miseq PE300 platform. A total of 585, 610 optimized sequences containing 238,579,267 bases were obtained in this experiment, with an average sequence length of 407 bp.
OTUs (operational taxonomic units)
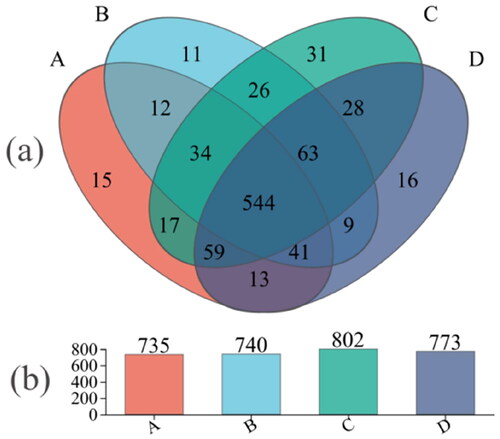
As shown in , there were 735, 740, 802 and 773 core OTUs in the control group, group I, group II and group III, respectively. A total of 3050 core OTUs were obtained. There were 15, 11, 31 and 16 unique core OTUs in control group, group I, group II and group III, respectively. There were 544 core OTUs in all groups, accounting for 17.82% of the total OTUs.
Rarefaction curve analysis
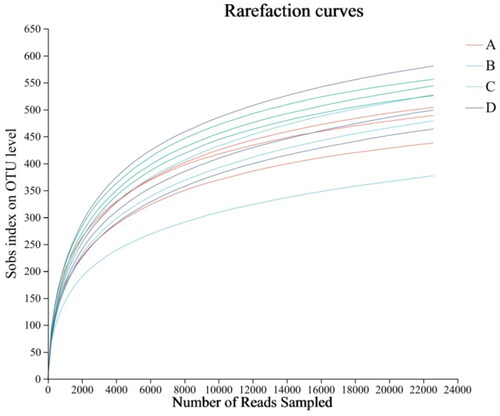
The Rarefaction curve can be used to judge whether the depth of sequencing is sufficient. The dilution curve of each group was relatively stable (shown in ). These results indicated that the sequencing data amount of cecal microflora of Minxinan black rabbits in this experiment was enough. The sequencing columns were sufficient to cover the composition of microbial community species, which could truly reflect the proportional relationship among various species in the community.
Alpha diversity analysis
The coverage rate of all groups reached 99% (shown in ), indicating that the library constructed in this study could truly reflect the species and structural diversity of cecal bacterial community of Minxinan black rabbits. There were no significant differences in Ace, Chao, Sobs and Simpson indices between the experimental groups and the control group (p > 0.05). Shannon index of group I was significantly lower than that of control group (p < 0.05), indicating that the community diversity of group I was significantly lower than that of control group.
Table 5. Effect of JCGLPP on cecal microbial Alpha diversity of Minxinan black rabbits.
Beta diversity analysis
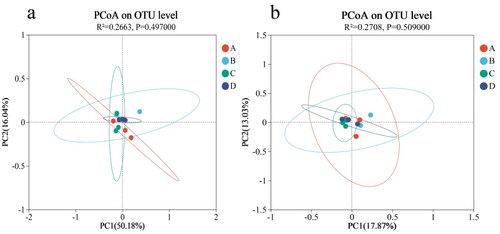
As shown in , the contribution of the first principal component was 50.18 and 17.87%, and the contribution of the second principal component was 16.04 and 13.03%. The distribution distance between the groups was close, indicating that there was little difference in the structure of cecal microflora between the experimental groups and the control group.
Figure 4. Beta diversity analysis of microflora in the cecum of Minxinan black rabbits. (a) PCoA of rumen microbial composition based on weighted Unifrac; (b) PCoA of rumen microbial composition based on Unweighted Unifrac. Note: A = Control (0 mg·kg−1), B = Group I (50 mg·kg−1), C = Group II (100 mg·kg−1), D = Group III (150 mg·kg−1).
Cecal microbial species composition and abundance
As shown in and , the phylum Firmicutes and Bacteroidetes were the dominant phylum at the phylum level. The relative abundance of Firmicutes was the highest, with 86.02, 82.30, 83.42 and 84.51% in the control I, II and III groups, respectively. Followed by Bacteroidetes, it accounted for 5.43, 6.33, 6.48 and 6.89%, respectively. They accounted for 91.45, 88.63, 89.90, 91.4% of the total flora, but there was no significant difference (p > 0.05).
Figure 5. (a) Relative abundance of OTUs about the cecal microflora of Minxinan black rabbits on phylum level; (b) relative abundance of OTUs about the cecal microflora of Minxinan black rabbits on genus level. Note: A = Control (0 mg·kg−1), B = Group I (50 mg·kg−1), C = Group II (100 mg·kg−1), D = Group III (150 mg·kg−1).
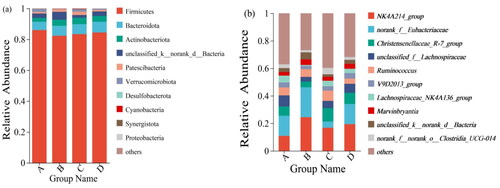
Table 6. Relative abundance of microorganisms at phylum level (%).
As can be seen from and , at the genus level, the dominant species were g_NK4A214_group, g_norank_f_Eubacteriaceae, hristensenellaceae_R-7_group, unclassified-f-Lachnospiraceae, and Ruminococcus. The relative abundance of NK4A214_group was the highest, with 10.8, 24.52, 16.75 and 19.36% in the control I, II and III groups, respectively. In the unclassified Eubacteriaceae, it occupied 14.69, 21.70, 4.55 and 14.71%, respectively. It made up 6.71, 4.10, 9.78, 8.13% in the Christensenellaceae_R-7_group, respectively, and 8.04, 3.44, 5.24, 6.60% in the unclassified-f-Lachnospiraceae, respectively. The proportion of Ruminococcus was 5.72, 5.53, 7.54, 3.74%, respectively. They accounted for 46.01, 59.29, 43.86 and 52.54% of the whole flora, respectively. The abundance of g__norank_f__norank_o__Clostridia_UCG-014 was significantly higher in group II than in the control group (p < 0.05). The abundance of g__Lachnospiraceae_NK4A136_group and the g__ unclassified_f__Lachnospiraceae in group I were significantly less than the control group (p < 0.05). In all, there was a difference in the abundance of cecum microflora between the experimental groups and control group.
Table 7. Relative abundance of microorganisms at genus level (%).
Analysis of different species among groups
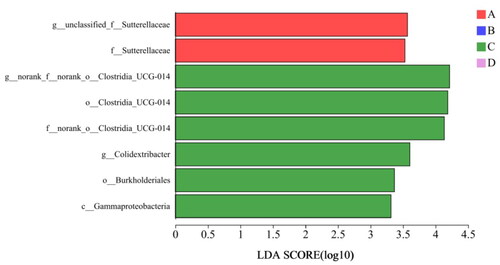
As shown in , LEfSe analysis (LDA threshold value: 3) showed that a total of 8 different bacteria genera were noted from phylum to genus level. Compared with the control group, Clostridia-UCG-014 was significantly enriched in group II.
Prediction and analysis of flora function
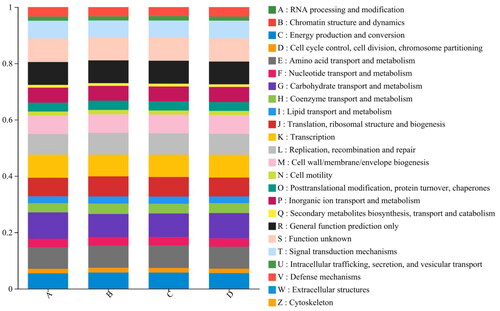
The functional composition of COG was similar in all samples compared to the species composition (). There showed no significant difference although the functional abundance of microflora involved in transport, replication, repair, cellular process and signal transduction was similar in all the cecum of Minxinan black rabbits. The results indicated that JCGLPP supplementation did not significantly change the metabolic pathway factors of cecal microflora in Minxinan black rabbits.
Discussion
Effects of JCGLPP on slaughter performance of Minxinan black rabbits
Slaughter performance is the main index that can directly reflect the production performance and economic value of animals, including live weight before slaughter, half-eviscerated weight, full-eviscerated weight, etc. Half-eviscerated percentage and full-eviscerated percentage are the key evaluation indexes of meat production performance of meat rabbits.Citation21 Zhang Haijun et al.Citation22 found that selenium methionine combined with ganoderma lucidum spores powder could improve the slaughter rate of broilers. However, in this experiment, there was no significant difference between the semi-eviscerated rate and eviscerated rate among all groups, indicating that the effective components of Ganoderma lucidum had different effects on the slaughter performance of different kinds of animals.
Effects of JCGLPP on cecum fermentation parameters of Minxinan black rabbits
The rabbit cecum was developed well with a volume of about 40% of the total volume of the digestive tract. The environment in the cecum can reflect the health of the intestine, which would affect the digestion, absorption and utilization of nutrients in meat rabbits. There are three main parameters which could evaluate the cecum environment and be used to reflect the degree of cecum fermentation: pH, VFA and NH3–N. These three parameters are closely related to each other and maintain the dynamic balance of microorganisms in the gut.Citation23 The pH value is within the right range to ensure that the digestive enzymes in the intestine enzymes are active, thus facilitating the digestion and absorption of nutrients. The pH value of cecum ranged from 6.0 to 6.6. If the pH value is too high, it will affect the activity of digestive enzymes and lead to the proliferation of pathogenic bacteria. A high value of pH in cecum will also increase the diarrhea rate of meat rabbits.Citation24 VFA is mainly consists of acetic acid, propionic acid and butyric acid, which are absorbed by the organism and provide energy to the organism. A higher fibre level in diet will lead to a higher VFA concentration in cecum.Citation25 The increase of VFA content can improve intestinal barrier function and nutrient absorption rate, stimulate the growth and reproduction of mucosal epithelial cells and improve intestinal immune function.Citation26 Acetic acid can provide energy for the body and also form cholesterol and fat through liver metabolism. Propionic acid is closely related to carbohydrate metabolism and can inhibit cholesterol synthesis. Butyric acid can promote epithelial cell proliferation and development, and is associated with genes related to intestinal epithelial cell apoptosis.Citation27 NH3–N is one of the products formed after protein and urea decomposition, which can serve as a nitrogen source for the synthesis of microbial proteins by the cecal microbiota. NH3–N can also be utilized by meat rabbits through soft manure.
In this experiment, the pH value of group I was significantly higher than that of the control group. Besides, there was no significant difference between the experimental groups. However, the cecum intestinal pH of all groups was within the normal pH range, indicating that the addition of JCGLPP did not have a significant effect on the pH in the intestine of Minxinan black rabbits. The NH3–N content of the experimental groups was significantly higher than that of the control group, and group I showed the highest NH3–N content. We held the opinion that the addition of JCGLPP to the diet would improve the utilization of protein and increase the decomposition products in the cecum of Minxinan black rabbits, which led to an increasing content of NH3–N.Citation28 There were no significant differences in acetic acid, isovaleric acid, isobutyric acid and propionic acid among the experimental groups and control group, as all groups of Minxinan black rabbits were fed the same basic diet (same diet composition and nutrient level) and the same fermentation substrate.Citation29
Effects of JCGLPP on intestinal compact junction protein content in Minxinan black rabbits
The epithelium forms a mechanical barrier to the diffusion of toxins, pathogens and allergens from the lumen to the mucosal tissue. In the event of damage to the barrier, inflammatory response will appear. The intestinal barrier system relies on a mucus gel layer, antimicrobial peptides, sIgA and tight intercellular junctions for its maintenance. It is the key factor for the tight cellular junctions to maintain the intestinal mechanical barrier. The tight junction structure is mainly composed of Claudin, Oclaudin, JAM, and ZO-1. Tight junctions are a multi-protein complex composed of different transmembrane proteins. Among them, Oclaudin contains four transmembrane structural domains, and its expression is positively correlated with intestinal barrier function.Citation30,Citation31 In this study, the contents of Oclaudin in duodenum, jejunum and ileum were significantly higher in groups I and II than those in the control group. These results were consistent with the findings of Mengxi Ying et al.Citation32 and Jie Han et al.,Citation33 indicating that low doses of JCGLPP is beneficial for maintaining the mucosal barrier function in Minxinan black rabbits.
Effects of JCGLPP on cecal microbe of Minxinan black rabbits
The intestine is the largest immune system in the animal organism. Around 70% of lymphocytes and nearly 80% of antibodies originating from the intestine. The health of the gut reflects the health status of the animal. Besides, intestinal flora has very important physiological functions, such as nutrient absorption, metabolic regulation, accelerating the development and maturation of the immune system, etc.Citation34 The digestion of cellulose in rabbits depends on the cecum and the balance of the flora structure is closely related to the physiological function of the cecum. Therefore, a dynamic balance of flora in the cecum is essential to maintain healthy growth in rabbits. The structure of the flora in the gut is influenced by the host’s genetics, diet, age, environment and other factors, which interact to maintain relative host stability.Citation35 Research shows that dysbiosis of intestinal flora can trigger weaning diarrhea.Citation36 The coverage rate of all groups reached 99% in this study, indicating that the library constructed in this study can accurately reflect the diversity of the cecum flora of the Minxinan black rabbits. There showed no significant difference in the Ace, Chao, Sobs and Simpson indices of the experimental groups compared with the control group. The Shannon index of group I was significantly smaller than that of the control group, indicating that group I had less community diversity than the control group. According to the Beta diversity analysis, there was no significant difference in bacterial community structure among the groups. This result revealed that adding JCGLPP to the diet of Minxinan black rabbits had little effect on the diversity of cecal microbial communities.
The intestinal microbiota is complex and diverse in composition, consisting mainly of bacteria, archaea, fungi and viruses. There are four main groups of intestinal microflora: Firmicutes, Actinobacteria, Bacteroidetes and Proteobacteria, of which the main dominant groups are Firmicutes and Bacteroidetes. The conversion rate of the animal organism to the diet is closely related to the thick-walled phylum. The phylum Bacillus can promote the breakdown of plant cell. The phylum Bacillus can promote the decomposition of plant cell walls, enhance the abundance of the phylum Bacillus and improve the barrier function of the intestinal mucosa, thus improving the immunity of the organism.Citation37 In this experiment, the dominant groups of cecal microbiota in each group were Firmicutes and Bacteroidetes, which is basically consistent with the research results of Cao Qi.Citation38 The relative abundance of Firmicutes ranges from 82.30 to 86.02%, while that of Bacteroidetes ranges from 5.43 to 6.89%. These microflora accounted for 87.73–92.91% of the overall microbial community, which suggested that obesity alters the composition and metabolic function of the intestinal flora.Citation39 In the intestinal flora of obese bodies, the ratio of thick-walled to bacteriophage phylum is significantly increased. As a result, more nutrient was absorbed by the intestinal flora and more energy was provided to the body, which promoted lipid synthesis and storage and resulted in excess energy in the body. Excess energy in the body will lead to obesity and weight gain, which in turn increases the pre-slaughter live weight of the rabbits in the experimental groups.Citation40,Citation41 Based on LEfSe analysis, this study conducted a microbial community difference analysis between the control group and the experimental groups. We found that the Clostridia UCG-014 in Group II was significantly enriched. Clostridia-UCG-014 was significantly and negatively associated with diarrhea rates and has been identified as having both beneficial and harmful effects. In this study, it’s more likely to be beneficial.Citation42
Conclusion
Although the addition of JCGLPP to the diet cannot improve the slaughter performance of Minxinan black rabbits, it can lower the intestinal pH, increase the content of NH3-N and tight junction protein Oclaudin, improve the Cecal flora diversity to a certain extent and promote intestinal health. There showed no significant difference among these experimental groups and control group in growth performance and slaughter performance. After comprehensive consideration of intestinal flora, blood antioxidant indexes and immune indexes, we recommended 100 mg·kg−1 as the relatively optimal substitution level during these three levels of JCGLPP addition. Based on these results, a future study in JCGLPP which could determine a precise additive amount with suitable substitution level is recommended. Besides, our results might provide some ideas and feasibility for drug combination together with JCGLPP.
Ethical approval
The study was conducted according to the Institutional Animal Care and Use Committee Guidelines.
Author contributions
Conceptualization, J.Q.Q. and Q.H.L; data curation, L.W.Q.; methodology, F, Z.; software, X.Y.F.; formal analysis, H.Y.J.; validation, Y.K.G., W.W.L. and Z.J.D.; writing original draft preparation, J.Q.Q.; review and editing, Q.H.L, J.Q.Q and C.C.Y. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (12.5 KB)Acknowledgments
Authors acknowledge National Engineering Research Center of JUNCAO Technology for supplying Ganoderma lucidum, and Longyan Tongxian Rabbit Industry Development Co., China, for providing Minxinan black rabbits.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Q.H.L.], upon reasonable request.
Additional information
Funding
References
- Scott GI, Porter D, Sean E, et al. Antibiotics as CECs: An overview of the hazards posed by antibiotics and antibiotic resistance. Front. Mar. Sci. 2016;3:e24.
- Handayani O, Siwi K, Widya A, et al. PS 16-11 Ganoderma lucidum polysaccharide peptides: A potent protective endothelial vascular and anti-lipid in atherosclerosis. J Hypertens. 2016;34(Suppl. 1):e468.
- Miao J, He XB, Fang SM, Lin SX, Liang XW. The effect of Ganoderma lucidum polysaccharides on male lamb liver immunity based on RNA-seq technology. Heilongjiang Anim Sci Vet Med. 2021;6:118–123.
- Chen HW, Yu YH. Effect of Ganoderma lucidum extract on growth performance, fecal microbiota, and bursal transcriptome of broilers. Anim Feed Sci Technol. 2020;267:114551.
- Rolhion N, Chassaing B. When pathogenic bacteria meet the intestinal microbiota. Phil Trans R Soc B. 2016;371(1707):e504.
- Xu CL, Wei LG, Lu L, Xiao DY, Bin L. Polysaccharide peptides from Ganoderma lucidum ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet-fed rats. J Funct Foods. 2019;57:48–58.
- Wang YB, Gu LH, Ye JL, et al. Effects of spore powder of Ganoderma lucidum and soybean isoflavone on growth performance, meat quality, and antioxidant capacity of Wenchang chicken. J China Agric Univ. 2021;26:148–156.
- Yang k, Zhang YJ, Zhang S, et al. Preparation of Ganoderma lucidum spore oligosaccharide and its regulation on Gutmicrobiota. Food Ferment Indus. 2020;46:37–42.
- Wang J, Zhao K, Kang Z, et al. The multi-omics analysis revealed a metabolic regulatory system of cecum in rabbit with diarrhea. Animals. 2022;12(9):1194.
- Dorota M, Króliczewska BK, Pecka-Kiełb E, et al. Comparative in vitro study of caecal microbial activity in brown hares and domestic rabbits which were offered the same diet. Mammal Res. 2018;63:285–296.
- Liu WL. Germplasm innovation and efficient breeding techniques of Minxinan black rabbits. Chin Livestock Poult Breed. 2022;18:70–72.
- Liu CH. Suggestions on prevention and control of digestive tract diseases in rabbits after feed resistance prohibition. Chin J Rabbit Farm. 2021;5:35–36.
- Tang L, Lei M, Ji Y, et al. Review of several feed additives for preventing rabbit diarrhea. Chin J Rabbit Farm. 2021;4:18–22.
- Wu ZQ. Research progress on the application of plant extracts in rabbit production. Chin J Rabbit Farm. 2023;(3):18–20.
- Didem S, Shile H. Ganoderma lucidum Polysaccharides as an anti-cancer agent. Anti-Cancer Agent Med Chem. 2018;18:667–674.
- Jia L, Feifei G, Chao C, et al. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int J Biol Macromol. 2020;143(C):806–813.
- Si J, Meng G, Wu Y, Ma H-F, Cui B-K, Dai Y-C. Medium composition optimization, structural characterization, and antioxidant activity of exopolysaccharides from the medicinal mushroom Ganoderma lingzhi. Int J Biol Macromol. 2019;124:1186–1196.
- Ding L. The Study on Immune-Enhancing of Ganoderma lucidum Poilysaccharide in Chicken [Master’s thesis]. Nanjing, China: Nanjing Agricultural University; April 1.
- Sang TT, Guo CJ, Guo DD, et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr Polym. 2021;256:117594–117594.
- Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63(1):64–75.
- Ding Y, Jiang X, Yao X, et al. Effects of feeding fermented mulberry leaf powder on growth performance, slaughter performance, and meat quality in chicken broilers. Animals. 2021;11(11):3294–3294.
- Zhang HJ, Wu SG, Qi GH. Effects of immune modulators on performance and immune function of broilers. China Feed. 2007;11:12–14.
- Abu HSH, Mahmoud AEM, Fayed AMA, Abdel, AAA, S. The effect of exogenous lysozyme supplementation on growth performance, caecal fermentation and microbiota, and blood constituents in growing rabbits. Animals. 2022;12(7):899–899.
- Lai NJ, Wang H, Su SL, et al. Effects of yeast peptide and Bacillus licheni formis on growth performance, nutrient apparent digestibility, slaughter performance, meat quality and intestinal morphology of meat rabbits. Chin J Anim Nutr. 2023;35:546–555.
- Gidenne T, Jehl N, Segura M, Michalet-Doreau B. Microbial activity in the caecum of the rabbit around weaning: impact of a dietary fibre deficiency and of intake level. Anim Feed Sci Technol. 2002;99:107–118.
- Wei YC, Wang FX, Zhang C, et al. Effects of bacillus subtilis on intestinal structure, cecal volatile fatty acid contents and microbial diversity of meat rabbits. Chin J Anim Nutr. 2021;33:7021–7032.
- Lv JZ. Effect of Arginine and Inulin on Intestinal Morphology, Caecal Microflora and Growth Performance of Meat Rabbits. [Ph.D. thesis]. Chongqing, China: Southwest University, May 25.
- Tian G, Lu YY, Yu B, et al. Effects of dietary replacement of alfalfa meal by Coix Lacryma-Jobi CV. Daheishan Mealon intestinal morphology, digestive enzyme activities and cecal fermentation parameters of growing meat rabbits. Chin J Anim Nutr. 2018;30:4210–4218.
- Wang GZ, Yu ZK, Wu FY, et al. Effects of dietary supplemented with combined yeast-Bacillus coagulans fermentation culture on intestinal morphology, intestinal permeability, cecal fermentation and microflora of rex rabbits. Chin J Anim Nutr. 2023;35:1–14.
- Sun K, Lei Y, Wang R, Wu ZL, Wu GY. Cinnamicaldehyde regulates the expression of tight junction proteins and amino acid transporters in intestinal porcine epithelial cells. J Anim Sci Biotechnol. 2017;8(1):66.
- Udo H, Anja S. Structural features of tight-junction proteins. Int J Mol Sci. 2019;20:6020.
- Ying MX, Zheng B, Yu Q, et al. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem Toxicol. 2020;138:111244.
- Han J, Xu Y, Yang D, Yu N, Bai ZS, Bian L. Effect of polysaccharides from acanthopanax senticosus on intestinal mucosal barrier of Escherichia coli lipopolysaccharide challenged mice. Asian-Australas J Anim Sci. 2016;29(1):134–141.
- Fang S, Chen X, Pan J, et al. Dynamic distribution of gut microbiota in meat rabbits at different growth stages and relationship with average daily gain (ADG). BMC Microbiol. 2020;20(1):116.
- Liu H, Zhang B, Li F, et al. Effects of heat stress on growth performance, carcass traits, serum metabolism, and intestinal microflora of meat rabbits. Front Microbiol. 2022;13:998095–998095.
- Raphaële G, Frédérique C, Mickaël AF, Tom VW, Evelyne F, Stéphanie B. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873.
- Sun H. Effect of the Synbiotic of Yuping Feng Polysaccharides on the Immunity Function and Intestinal Microflora in Rex Rabbit [Master’s thesis]. Chengdu, China: Sichuan Agricultural University, June 1.
- Cao Q, Zhang KG, Fan ZB, Wang YY, Jiang YB. Effect of plant essential oils on growth performance, slaughter performance, intestina morphology and microflora of meat rabbits. Feed Research. 2022;45:57–61.
- Ludovico A, Emidio S, Carmela C, et al. Gut microbiota and obesity: A role for probiotics. Nutrients. 2019;11:2690.
- Cheng Z, Zhang L, Yang L, Chu H. The critical role of gut microbiota in obesity. Front Endocrinol. 2022;13:1025706.
- Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251–1262.
- Miao XY, Wnag YJ, Zhao X, Zhang ZQ, Xi SM, Zhao S. Effect of Wumeisan on gut lactase activity and microflora diversity of mice with dysbacteriosis diarrhea. Chin J Experim Trad Med Formula. 2023;29(4):33–42.