Abstract
Ensuring improved leg health is an important prerequisite for broilers to achieve optimal production performance and welfare status. Broiler leg disease is characterized by leg muscle weakness, leg bone deformation, joint cysts, arthritis, femoral head necrosis, and other symptoms that result in lameness or paralysis. These conditions significantly affect movement, feeding and broiler growth performance. Nowadays, the high incidence of leg abnormalities in broiler chickens has become an important issue that hampers the development of broiler farming. Therefore, it is imperative to prevent leg diseases and improve the health of broiler legs. This review mainly discusses the current prevalence of broiler leg diseases and describes the risk factors, diagnosis, and prevention of leg diseases to provide a scientific basis for addressing broiler leg health problems.
Introduction
Broiler farming is popular worldwide due to the fast growth and production cycle of chicken.Citation1 In poultry farms around the world, broilers are mostly raised in modernized and intensive production systems,Citation2 where the time from hatching to slaughter is about 35-40 days, resulting in improved production efficiency of commercial broilers.Citation3,Citation4 However, the fast growth rate disrupts the original balance of tissue growth and development in broilers.Citation5 Due to rapid weight gain and delayed skeletal development, skeletal diseases, especially in the leg bones occurr in broilers.Citation6,Citation7 Currently, although large-scale intensive chicken production has improved the growth performance of broiler chickens, the issue of leg health still needs to be taken seriously as it has a detrimental impact on the economic benefits and welfare status of broiler farming.Citation8
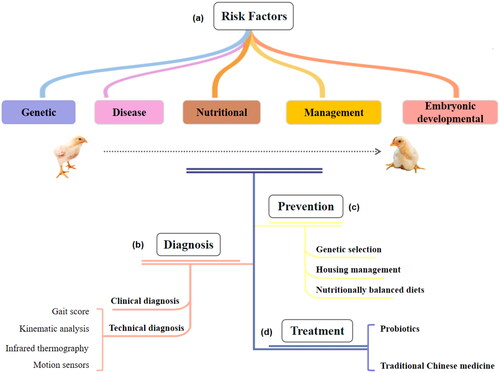
Generally, movement abnormalities in commercial broilers are referred to as leg diseases. Common leg diseases include viral arthritis (VA),Citation9 bacterial chondronecrosis with osteomyelitis (BCO),Citation10 tibial dyschondroplasia (TD),Citation11 and valgus-varus deformity (VVD).Citation12 Clinical symptoms are mostly characterized by leg weakness, reduced walking ability, unstable standing, frequent squatting, or lameness.Citation11,Citation13 The causes of leg diseases may be infectious or noninfectious, which contribute to leg abnormalities in broiler chickens and result in a series of health and welfare problems for the birds. Therefore, this review focuses on the current prevalence, risk factors, diagnosis, and treatment of broiler leg diseases to highlight issues related to poultry leg health ().
Figure 1. Risk factors, diagnosis, prevention and treatment of leg disease in broiler chickens. (a) Risk factors for broiler leg disease. (b) Diagnosis of leg disease in broiler chickens. (c) Prevention of leg disease in broiler chickens. (d) Treatment of leg disease in broiler chickens. Rightsholder: Shucheng Huang, College of Veterinary Medicine, Henan Agricultural University, Zhengzhou, 450002, China.
Prevalence of broiler leg disease
More than 66 billion broilers are reported to be slaughtered annually worldwide, with the United States, China and Brazil being the largest producers of broiler chickens.Citation14 Due to the increasing global population, the broiler industry faces pressure to improve efficiency and production. Consequently, breeding goals are excessively focused on broiler performance, leading to a rise in the incidence of leg disease in broilers, especially under large-scale farming conditions.Citation1
summarizes the research on the incidence of leg disease in broiler chickens on relevant farms in different countries. In a study, conducted on 28 broiler flocks in Denmark (nearly 8% of the national flocks), the average prevalence of TD, varus/valgus deformations, toe curvature and foot pad burn, and ankle-plantar asymmetric development were found to be 57.1%, 37.0%, 32.6%, and 42.0%, respectively.Citation15,Citation16 reported that statistics on the overall level of leg disease in broilers with locomotor disorders represented about 27.6% of the flock, with 3.3% of birds barely able to walk. In addition, a study byCitation17 reported that 30% of broilers tested in four representative commercial broiler flocks in southwestern Finland had a gait score ≥3 and the overall average incidence of TD in these broilers was 3.5%. Besides, a survey of 50 commercial broiler farms in Norway found that approximately 19% of broilers exhibited moderate to severe lameness (gait score ≥3) and that the occurrence of lameness was strongly associated with an increased prevalence of hock burns and footpad dermatitis.Citation18,Citation19 found a 10% prevalence of fractures due to chondromalacia in farm I, II, and III broiler flocks in fattening broiler flocks from seven farms in different regions of Bulgaria, of which about 7% occurred in the proximal tibia and about 3% in the proximal femur. On-farm V, the highest prevalence of TD was observed in crossbred strains A, B and C, and the highest prevalence was observed in strain C (27.7%), followed by strain B (26.5%) and strain A (24.22%). Furthermore, a recent study on VVD prevalence showed that the overall incidence of VVD was 1.75%, with 47.5% of cases in males and 52.5% in females.Citation12 Recently, studies conducted in various farms and countries have reported a prevalence of 5.5% to 48.8% in flocks with moderate to severe gait disorders (gait score of 3 or higher).Citation18,Citation20–Citation22
Table 1. Prevalence of broiler leg disease across various countries.
We have noticed that the incidence of leg disease in broiler chickens has not shown an increasing trend yet. In fact, more broilers are showing subclinical symptoms without showing obvious lameness, which breeders often overlook. At the same time, with the advancement of broiler scale and intensive breeding technology, the growth cycle of broilers will be further shortened to meet selling standards. However, rapid growth will further increase the load on broiler legs, thereby exacerbating issues related to broiler leg disease.Citation13
Risk factors for broiler leg disease
Genetic factors
It has been suggested that the incidence of leg disease in broiler chickens has a genetic basis. Four purebred turkey strains were reared in different environments, with strains A and C reared on farms in the United States and strains B and D housed on farms in the United Kingdom. The results showed that the heritability of TD incidence was significantly different between strains B and D (0.06-0.07) and strains A and C (0.11-0.12) in the four turkey strains A, B, C, and D.Citation23 Additionally, comparisons between strains with different growth rates showed that slower-growing broilers strains had better walking ability and a lower incidence of contact dermatitis compared to faster-growing broilers.Citation8,Citation24 Furthermore, a study on susceptibility to BCO in four broiler crosses indicated that crossbred broilers with rapid early growth had a higher incidence of BCO compared to slower-growing crossbred broilers.Citation25
Disease factors
Disease factors that cause leg diseases in broiler chickens include viruses,Citation9 bacteria, fungiCitation26 and parasites,Citation27 all of which can affect leg health in broilers. A summary of the effects of different disease factors on broiler leg health is provided in .
Table 2. Effects of disease factors on the health of broiler legs.
Viral diseases
Viral diseases that cause leg disorders in broiler chickens include Marek’s disease (MD).Citation28,Citation29 avian encephalomyelitis (AE),Citation30 and VA.Citation9,Citation31 MD infection in broilers is capable of causing lymphoid tissue hyperplasia and tumors. Acute MD can cause nerve damageCitation28,Citation29 and it is likely to attack the sciatic nerve, resulting in paralysis of the legs and affecting drinking and feeding. In addition, AE mainly affects chicks and can cause encephalomyelitis, with affected chicks showing head and neck tremors, limb incoordination, and inability to stand and walk normally.Citation30 Furthermore, VA is caused by avian reovirus, and clinical signs of VA infection in broiler chickens include swelling of the wing joint with damage to the gastrocnemius and toe flexor tendons (hypertrophy, sclerosis, or rupture of the gastrocnemius tendon), leading to lameness.Citation9,Citation31
Bacterial diseases
Bacterial leg diseases are also prevalent in broiler farming. Infections withpathogenic bacteria such as Enterococcus cecorum, Mycoplasma synoviae, Salmonella enteritidis, and Staphylococcus can damage the leg health of broilers, resulting in significant economic losses for the farm. BCO, a leading infectious cause of lameness in broilers, causes bacterial infiltration, hematogenous colonization, and physitis of the femoral and tibial growth plates, necrosis and eventual lameness.Citation10,Citation25 Besides, broilers infected with Enterococcus cecum exhibit the clinical features of enterococcal spondylitis, including light paralysis and paresis, making it difficult for them to stand or walk when their legs are stretched forward.Citation33 The Mycoplasma synoviae and Salmonella enteritidis infections mainly cause infectious synovitis and purulent arthritis in broilers, which pose a serious health risks to their legs.Citation32 Furthermore, Staphylococcus infections in broilers commonly result in lameness and fever. Studies have shown that Staphylococcus agnetis can cause bacterial cartilage necrosis in broiler.Citation34,Citation35
Mycotoxicosis diseases
Mycotoxicosis has a serious impact on the health of poultry. It should be noted that wheat and other grains in broiler feed may be contaminated with more than one mycotoxin. The additive or synergistic effects of mycotoxins may enhance the adverse effects on the poultry skeletal system.Citation38 investigated fungal contamination of wheat in five regions of Poland and found that only 6% of the samples were contaminated with a single toxin, followed by 25% with two toxins. In the remaining 69% of the samples, at least three toxins were found in each sample. Importantly, a study has shown that eating mold-contaminated feed not only has a toxic effect on the broiler’s vital organs but can also increase the probability of lameness in chickens from 2.3% to 25%.Citation39 Mycotoxicosis had negative effects on the broiler’s phosphorus metabolism and bone mineralization at 42 days of age. Exposure to aflatoxins reduces the strength of tibia and increases the incidence of leg torsion.Citation26 (36) found that aflatoxin B1 (AFB1) significantly impaired the development of the tibial growth plate, as evidenced by a significant reduction in both the hyperplastic and hypertrophic areas of the growth plate.
Parasitic diseases
Poor housing conditions on farms can lead to parasitic infections that are detrimental to the welfare of broilers. Parasites compete with their hosts for nutrients in the feed, affecting the body’s digestion and absorption of nutrients, causing nutritional deficiencies and ultimately increasing the risk of leg disease.Citation40 Studies have shown that Eimeria spp. parasite infection causes disruption of the intestinal environment and imbalance in homeostasis, which can lead to the multiplication of pathogens and result in increased susceptibility to leg disease in broilers.Citation37 In addition, mite infestations are a constant threat to the health of broiler chickens. Mite infections can cause swollen hind metatarsal claws and hyperplasia of scaly skin in broilers. Besides, it can lead to the development of knee osteoarthritis in broiler chickens.Citation27
Nutritional factors
Dietary energy and protein levels
Dietary energy levels and protein levels are closely related to the body’s metabolism. Excess protein content in feed, especially animal protein, leads to impaired purine metabolism in the body. This results in the conversion of purines into large amounts of uric acid and urates, which are then deposited in internal organs and joints. This can cause swelling and deformation of the toes and leg joints, leading to lameness. Therefore, managing dietary energy and protein during broiler growth is critical to meet the demands of broiler growth and prevent leg diseases.Citation41 Dietary amino acid requirements for broilers have increased compared to the past in order to maintain increased protein turnover. Studies have shown that dietary amino acid levels of 3.17-3.48 g digestible lysine (Dlys)/Mcal during fattening feeding result in optimal protein gains in modern broiler lines.Citation42 Formulating broiler diets on ideal protein improves problems associated with energy expenditure in protein and amino acid metabolism, and increases protein utilization efficiency. In addition,Citation43 have shown that low-protein diets supplemented with free amino acids can improve growth performance, and reduce pad damage in broilers, as well as nitrogen emissions in the henhouse to some extent. When fed on an ideal protein and amino acid based diet, broilers can utilize more available energy for productive purposes as their metabolic system performs optimally, without having to expend energy to eliminate excess nitrogen. This also helps to maintain body temperature homeostasis.Citation44 Moreover, there are various inconsistencies in the results of studies on dietary energy requirements for optimal performance of broilers.Citation45 investigated the effect of six dietary nitrogen-corrected apparent metabolizable energy (AMEn) levels ranging from 3000 to 3150 kcal/kg, with a difference of 30 kcal between each diet, and showed that performance parameters such as body weight (BW), feed intake (FI) and feed conversion rate (FCR) were not affected. In another study, feeding 55 kcal/kg AMEn resulted in better performance and yield benefits than other treatment groups during broiler growth and development.Citation46
Dietary calcium-phosphorus content and ratio
The development of bones in broiler chickens is closely related to nutritional factors, as two essential minerals for the body, calcium (Ca) and phosphorus (P), play a vital role in healthy bone growth.Citation47 Ca and P deficiency can lead to chondroplasia and/or osteoporosis in broiler chickens. A study reported that the incidence of leg abnormalities is lowest when the ratio of Ca to P in broiler chicken starter and grower diets is about 2:1.Citation48 Significantly, broilers have a relatively high utilization of calcium from different sources in the diet, but P is usually present in the form of phytate P, resulting in a low utilization rate. Therefore, non- phytate P sources are usually considered for incorporation into diets.Citation49 Studies have shown that the addition of high non-phytate P to diets during the starter period increases plasma P concentrations and increases tibial ash and P content in the tibial.Citation50
Trace element content
The trace elements associated with broiler leg disease are manganese (Mn), zinc (Zn), and copper (Cu). Mn deficiency has been reported to affect the development of the tibial growth plate, and some research suggests that dietary interactions with high levels of Ca and P may be a complicating factor.Citation51 In addition, Zn deficiency can reduce the absorption of nutrients in the gut by reducing the diversity and abundance of microorganisms in the broiler’s gut, causing nutrient deficiency leg disease.Citation52,Citation53 noted that broilers supplemented with Cu, iron (Fe), Mn and Zn were able to achieve significantly higher feed conversions and increased total ash content in tibia. Furthermore, the role of Cu is mainly related to the formation of substances necessary for the formation of collagen fibers, and low levels of Cu in the broiler’s diet can reduce the structure of the collagen network and decrease the strength of mineralization.Citation54
Vitamin content
Vitamins are necessary for the maintenance of life, as well as the growth and development of poultry. Any deficiency or excess can cause serious leg disorders.Citation55 provides an overview of the physiological functions and effects of different vitamins on broiler bones. Studies have shown that a deficiency in vitamin B6 can affect the structural integrity of collagen in the bone matrix and alter fracture strength.Citation59 The bone matrix is responsible for the plastic deformation of tissues. In addition, vitamin D deficiency symptoms, such as chondroplasia and rickets, are caused by disordersin the body’s calcium and phosphorus metabolism. Insufficient levels of vitamin D in the diet can reduce the absorption of calcium and phosphorus.Citation64,Citation65 demonstrated that both 1, 25-dihydroxyvitamin D3 (1, 25OHD) and 25-hydroxyvitamin D3 (25OHD) upregulate the expression of osteogenesis-related genes in broiler embryos. Vitamin E possesses strong antioxidant properties and helps maintain normal muscle structure and peripheral blood vessel function. Thus, a deficiency in vitamin E can lead to muscle dystrophy, resulting in impaired mobility, and an increased incidence of leg deformities, particularly lateral or medial deviation of the distal tibia or proximal talus.Citation61
Table 3. Role of vitamins in the diet for the bone health of broiler chickens.
Management factors
Many factors during rearing can cause leg disease. Poor quality broiler bedding, sharp foreign objects in the bedding, and rough handling during immunization and weighing can lead to mechanical fractures or sprains in the legs of broilers.Citation66 Furthermore, environmental problems such as a high temperature and high humidity environment in summer, cold and wet bedding in winter coops, poor ventilation, poisoning by harmful gases, and excessive feeding density can increase the incidence of leg disease.Citation67 Studies have shown that excessive feeding density leads to a decrease in the length and strength of the tibia and an increase in the curvature of the tibia.Citation68 Additionally, high-intensity light can stimulate the development of tibial growth and reduce the occurrence of leg disease.Citation69 showed that higher light intensity during feeding may have a beneficial effect on calcium homeostasis and bone formation in young Lohmann Brown-Lite chicken. However, too long light duration, too high light intensity, and high chicken activity can also predispose to leg disease.Citation70 proved that the use of alternative lighting schedules (2 L: 1D) can significantly increase the leg bone length of broilers after hatching. In addition, the insecticide thiram is known to be capable of causing TD in broiler chickens, which is one of the main causes of TD caused by feed sources.Citation71
Embryonic developmental factors
It is well known that embryonic development is completely dependent on the nutrients stored within the embryonated eggs laid by the hen, such as vitamin D, minerals and fatty acids. These specific nutrients play an irreplaceable role in early skeletal development.Citation72 Therefore, nutritional deficiencies can adversely affect embryonic development. Furthermore, poor incubation temperature and ventilation can also affect the uptake of nutrients from yolk during embryonic development.Citation73 The development of connective tissue and skeletal calcification begins during embryonic development, with the fastest skeletal growth usually occurring around the time of hatching. The first weeks of the incubation or brooding period therefore place high demands on hatching conditions. If the incubation temperature is too low without adequate preheating of the embryonated eggs before hatching or without providing good ventilation, skeletal development will be affected and the incidence of toe deformities and leg skeletons will increase.Citation74 Similarly, higher temperatures and hypoxia during the later stages of incubation will slow the development of the skeleton and increase bone asymmetry between the legs.Citation75
Diagnosis of leg disease in broiler chickens
Clinical diagnosis
Leg disease in broiler chickens is usually not a single disease but a combination of factors, making the diagnosis process very difficult. Clinical diagnosis relies on observing the flock’s clinical signs and typical necropsy lesions to confirm the diagnosis. Still, the diagnosis of specific leg diseases also needs consideration of characteristic clinical symptoms. Autopsy is the most commonly used method for diagnosing animal diseases due to its convenience, feasibility, directness, and objectivity compared to clinical and laboratory diagnostics.Citation76 Autopsy plays an important role in diagnosing diseases in herd animals. In particular, during the early stages of an infectious disease outbreak, a diagnosis can be made based on the pathological changes seen in the first sick animals that are culled. This enables early diagnosis and prevention, minimizing the damage caused by the disease.
During the early stage of VA infection, severe edema of the gastrocnemius tendon in the upper part of hip joint and metatarsal joint of broilers could be observed. This is followed by leg muscle rupture and bleeding.Citation77 Swelling is also present around the top and bottom of the tarsal joint, and an incision of the skin reveals edema of the upper peroneal tendon in the affected chicken’s joint. Congestion or punctate hemorrhage within the synovial membrane is usually found, and the joint cavity contains yellowish or blood-like exudates.Citation78 In chickens infected with infectious synovitis, the phalangeal joints become swollen, along with thickening of the skin. There is a large amount of viscous, grey-white or cheese-colored exudates in the synovial bursa and tendon sheaths, and a pale yellow cheese-like exudate can also be present for a prolonged period.Citation79 Pathological histology reveals the presence of heterophilic leukocytes and fibrinous infiltrates.Citation76,Citation80 In addition, broilers with gout may experience swelling in the legs, wings and toe joints. Incision of the joint capsule shows the flow of viscous white liquid and urate deposition can be observed in the in articular cartilage and surrounding tissues. The presence of urate worsens and often leads to ulceration of the joint surfaces or necrosis of the joint capsule.Citation81
Technical diagnosis
Gait score
In broiler chickens, the gait scoring criteria ofCitation82 are mostly used as a reference for lameness studies. According to this criteria, GS0 (indicating normal walking) to GS5 (indicating inability to walk), a score equal to or greater than 3 is considered painful, indicating potential compromise of the welfare of broiler chickens.Citation83 The gait scoring method is a practical method to assess broiler lameness in the field. It has the advantage of being fast, does not requiring specialized equipment and being widely used on farms. However, the method is strongly subjective, serving as a research tool and the actual relationship between this impaired walking ability and specific leg problems remains unclear.
Kinematic analysis
Traditional kinematic measurements have provided quantitative means of assessing gait in poultry, using techniques such as plantar recorders,Citation84 force plates,Citation85 and piezoelectric pressure sensing padsCitation86 to collect kinetic data. However, these studies have identified technical shortcomings that have led to instability in broiler locomotion data. The development of kinematic analysis has shown promise in studying poultry gait, as it can detect subtle changes in gait patterns that are not easily quantifiable through visual observation alone. This method also allows for rapid assessment of data suitability and the ability to selectively collect useful data from broilers with different locomotor speeds. A study has showed that computer-aided 3D kinematic gait analysis can effectively record and quantify gait patterns. Specifically, the jungle chicken exhibits a short standing stage, less double support, less leg raising, and less back displacement, while the broiler displays more double support, more leg movement, and more vertical back movement.Citation87
Infrared thermography
Infrared thermography (IRT), also known as thermal imaging, is used as a noninvasive, noncontact diagnostic technique to determine the infrared radiation or surface heat of an object.Citation88 IRT has been widely used in animal studies, especially for detecting lameness in cows.Citation89 However, few studies have been conducted to assess and diagnose lameness caused by leg disease in broilers. Many of the health problems affecting broiler legs are associated with inflammation, and inflammation leads to an increase in tissue temperature.Citation90 Although IRT cannot identify specific pathologies, it can detect localized areas of increased heat due to inflammation or decreased heat caused by decreased blood flow.Citation91 Studies have shown that the IRT technique can detect a decrease in surface temperature in broilers affected by BCO, which results from a decrease in oxygen saturation of the blood in the legs.Citation35,Citation92,Citation93 Therefore, IRT could be a new technique used for early detection of leg abnormalities or to predict subclinical foot lesions in broiler chickens.
Motion sensors
Motion sensors have been used to study different aspects of locomotion. In the case of broilers with imbalanced gait, piezoelectric crystal sensors were used to detect the asymmetry in peak force on each foot by measuring the maximum vertical force during walking. This method provides a real-time assessment of broiler gait.Citation94,Citation95 used a 3-axis ADL335 accelerometer positioned within a laying hen to calculate landing force, jump height, and the timing of onset and landing during jumps from perches of varying heights onto lower landing surface. To validate the measurements, a pressure mapping system and camera were also installed as validation methods to measure landing force and record the jumps. This technology not only allows for the assessment of leg health through landing force in laying hens but also contributes to the improvement of housing and perch design, addressing longstanding issues in poultry housing systems and promoting laying hen welfare.
Biomarker
The biomarker is generally a characteristic biochemical indicator of a pathological or therapeutic process that can be objectively measured and evaluated, and its determination can inform the biological processes that the organism is currently undergoing.Citation96 The detection of biomarkers can not only accurately detect and reflect the health status of broiler legs but also contribute to the study of the pathogenesis of broiler leg disease.Citation97 Currently, few studies have reported on biomarkers of broiler leg health. However, the gut microbiota is closely related to the growth and development of bones,Citation98 therefore, the detection of gut microorganisms may have some significance for the diagnosis of leg health problems in broiler chickens. Research on broiler gut health has focused on multiple biomarkers, including DNA metabarcoding, metagenomic, metatranscriptomic, metaproteomic, and metabolomic approaches. Especially the combination of multi-omics technologies will be the future direction.Citation99 Studies have shown that noninvasive sampling of biomarkers, screening of fecal microbial markers, and metabolic biomarkers of intestinal microorganisms provide new avenues in the early diagnosis of TD in broiler chickens. The authors identified 4-hydroxybenzaldehyde as a key biomarker to differentiate TD broilers from normal broilers through interaction analysis of the fecal microbiota and metabolites by multi-omics techniques. They also elucidated that the molecular mechanism of broiler TD pathogenesis may be due to dysregulation of gut microbes and regulation of host fecal metabolism.Citation100 As biomarkers should have the advantages of reliability, specificity, and sensitivity, many researchers have embarked on the study of poultry candidate biomarkers in recent years.Citation101,Citation102 However, the validation of biomarkers will be a major challenge due to the complexity of intestinal microbes.
Prevention and treatment of leg disease in broiler chickens
Genetic selection
Although it has been argued that good management strategies can alleviate leg problems better than genetic improvement.Citation103 However, the important contribution that genetic selection for broiler traits can make to improving leg health in modern broilers cannot be ignored. Long-term selection for improved leg health has been demonstrated in broilers.Citation104 Results from studies assessing genetic parameters of leg health and mobility in purebred turkeys and their genetic correlation with body weight have shown that leg health can be effectively improved in turkeys using balanced breeding objectives based on a balance of production traits and leg health traits.Citation23 In addition, a study comparing the degree of heritability of two broiler lines differing in growth capacity and adult body weight, foot pad dermatitis (FPD) and hock burn (HB), found relatively high heritability for FPD in female fast-growing broilers.Citation105 Therefore, genetic selection of broilers is necessary for the prevention and improvement of leg health.
Housing management
Housing management provides a suitable growth environment for chickens and an effective way to prevent leg diseases in broiler chickens. The relationship between temperature, humidity and ventilation should be addressed in both summer and winter to ensure that chicken houses are well ventilated, thus preventing the spread of diseases. In addition, maintaining a suitable feeding density is very important. When rearing chickens to expand the flock, it is recommended to keep broilers older than 3 to 4 weeks old at no more than 12 per square meter.Citation106 Moreover, it is advised to provide less feed and more frequent meals to increase the feeding and exercise time of broilers. Furthermore, the height of the water-feeding line should be adjusted in a timely manner according to the different ages of the chickens, and high-quality bedding should be selected to prevent bacterial growth, especially mold contamination. It is worth noting that ehen catching and immunizing chickens, it is essential to handle them gently to reduce stress and minimize the risk of leg deformities in broilers.Citation22 Finally, a reasonable light program and light intensity should be maintained in the chicken house to ensure that chickens rest and move in a specific pattern, thus reducing mortality caused by leg diseases.
Nutritionally balanced diets
With the development of the broiler nutrition and feed industry, the formulation and processing of feed tend to be mature. The biggest trigger for leg abnormalities due to unbalanced diets may be problems with the quality of raw material components, digestion and absorption. Therefore, strict control of feed ingredient quality, meeting the minerals and vitamins required by broilers, is the key to reducing leg disease in broilers. The manganese (Mn) requirement for poultry production under normal ration conditions is generally considered to be 40 mg/kg. Mn deficiency leads to the synthesis of mucopolysaccharides, reduces the amount of mucopolysaccharides in bones, hinders the formation of a calcified matrix, and affects bone strength.Citation107 In addition, high concentrations of Ca and P can lead to the formation of calcium phosphate precipitates in the intestine, which adsorb Mn and excrete it with feces, exacerbating manganese deficiency. Therefore, to safely adapt to changes in Ca and P intake, 50 mg/kg Mn supplementation is required in the diet. The recommendations for the requirements of several different forms of essential trace elements that are important in bone formation are provided in . (57) reported that vitamin D3 supplementation in broiler diets was effective in preventing the development of TD. For broilers under 14 days of age, vitamin D3 requirements might be in the range of 35–50 μg/kg of feed to ensure proper cortical bone quality, provided that the calcium and effective phosphorus content of the diet is adequate. Furthermore, 25-hydroxycholecalciferol (25-HCC) is the active metabolite of vitamin D, which can enhance collagen maturation in the bones and facilitate bone calcification. Studies have shown that the addition of 250 μg/kg of 25-HCC in the diet can significantly reduce the incidence of TD in broilers.Citation62
Table 4. Broiler requirements for various forms of essential trace elements crucial for bone formation (mg/kg).
Microbioecologics
Recently, microbioecologics (probiotics, prebiotics and synbiotic) have shown great potential in the preventing leg diseases in broiler chickens.Citation115 It is well known that probiotics are closely related to the gut microbiota, and a few studies have reported that broilers fed probiotics exhibit better growth performance, immune response, intestinal morphology, and expression of immune-related genes.Citation116 The possible mechanism of microbioecologics action in leg disorders is by regulating the composition of the poultry intestinal flora, indirectly improving bone metabolism, and promoting healthy bone growth by regulating calcium and phosphorus, hormones, and nutrients.Citation117 Research has indicated that dietary supplements containing probiotics, prebiotics, and synbiotics significantly improved bone mineral metabolism.Citation118 The addition of synbiotics (containing a prebiotic-fructooligosaccharides and a probiotic mixture of 4 microbial strains - Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, and Lactobacillus reuteri) to the diet can significantly increase the bone mineral density, bone mineral content, and bone area of heat-stressed broilers, and reduce the gait score.Citation119 In addition, it was found that synbiotics (Lactobacillus B94, Lactobacillus casei, Lactobacillus acidophilus) were able to significantly improve broiler performance intestinal histomorphology, cecum volatile fatty acids, meat quality, and leg bone strength.Citation120,Citation121 demonstrated that Bacillus subtilis increased bone mass and density in broilers. Also, Lactobacillus rhamnosus JYLR-005 was able to prevent TD by improving bone-related growth performance in broiler chickens.Citation122 Therefore, the addition of microbioecologics restorative nutritional supplements for bone damage may be an effective, safe, and inexpensive method for the prevention and treatment of leg diseases in broiler chickens.Citation123
Traditional Chinese medicine
Common skeletal metabolic disorders in broiler chickens are characterized by dysregulation of bone resorption and bone formation, low bone mass, and abnormal bone tissue structure. A variety of traditional Chinese medicines, such as polysaccharides, flavonoids, and glycosides, have been shown to be therapeutically effective in the prevention and treatment of broiler leg disease.Citation124–Citation127 In addition, previous studies have confirmed that flavonoids have estrogen-like effects,Citation128 which can act synergistically with estrogen to promote intestinal calcium absorption, affect calcium and phosphorus metabolism, and protect tibial growth and development. Quercetin can improve broiler TD by regulating calcium and phosphorus metabolism.Citation129 Assemble flavone of Rhizome Drynaria can increase body weight, tibial weight, and tibial length, and reduce growth plate width in broilers with TD, indicating its skeletal strengthening effect.Citation11 Additionally, Icariin ameliorates broiler TD by regulating the expression of the wingless-type MMTV integration site family, member 4 (WNT4), and vascular endothelial growth factor (VEGF) genes.Citation130 Chlorogenic acid-like components of various herbs can ameliorate broiler leg disease by regulating the molecular mechanisms of Caspases, Beclin 1 expression and extracellular matrix (ECM) degradation.Citation131 Therefore, the use of herbs for the prevention of leg diseases in broiler chickens is an effective and safe strategy.
Conclusion
Briefly, this review summarizes the risk factors that cause leg disorders, as well as the diagnosis, prevention, and treatment of leg diseases in broiler chickens. It is commonly considered that the main cause of leg disease in broiler chickens is their rapid growth, as their leg bones, joints, tendons, ligaments, and other tissues are weak and unable to support their weight, leading to the disorder. However, there are multiple factors associated with genetics, diseases, nutrition, and embryonic development. These risk factors may even interact with each other to influence the development of leg diseases. The key issue to reduce the incidence of leg diseases in broilers is to pay attention to poultry welfare, strengthen feeding management, create a comfortable and relatively stable growth environment for broilers, implement comprehensive control measures, and try to eliminate factors unfavorable for broiler growth. In addition, proper diagnosis and effective treatment are crucial for broilers with locomotor disorders, as the problem can be effectively addressed by identifying the factors that disrupt the function of the skeletal system.
Authors’ contributions
S.C.H. contributed to the conceptualization, resources, funding acquisition, and revision and editing of the manuscript. K.L.L. performed the literature review, and drafted the manuscript. Y.F.H and B.W.X contributed to the revision and editing of the manuscript. L.X.L., P.C., M.K.I. and K.M. collected the literature and reviewed the text. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors would like to thank Dr. Mujeeb Ur Rehman, from Hainan University, China for his generous time to edit the manuscript for language and spelling errors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mottet A, Tempio G. Global poultry production: current state and future outlook and challenges. World Poult Sci J. 2017;73(2):245–256.
- Bessei W. Impact of animal welfare on worldwide poultry production. World Poult Sci J. 2018;74(2):211–224.
- Cartoni Mancinelli A, Mattioli S, Bosco AD, Aliberti A, Amato MG, Castellini C. Performance, behavior, and welfare status of six different organically reared poultry genotypes. Animals (Basel). 2020;10(4):550.
- Olschewsky A, Riehn K, Knierim U. Suitability of slower growing commercial Turkey strains for organic husbandry in terms of animal welfare and performance. Front Vet Sci. 2020;7:600846.
- Weng K, Huo W, Li Y, et al. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult Sci. 2022;101(1):101537.
- Huang SC, Zhang LH, Zhang JL, et al. Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens. Sci Rep. 2018;8(1):3680.
- Santos MN, Widowski TM, Kiarie EG, Guerin MT, Edwards AM, Torrey S. In pursuit of a better broiler: tibial morphology, breaking strength, and ash content in conventional and slower-growing strains of broiler chickens. Poult Sci. 2022a;101(4):101755.
- Dixon LM. Slow and steady wins the race: the behaviour and welfare of commercial faster growing broiler breeds compared to a commercial slower growing breed. PLoS One. 2020;15(4):e0231006.
- Sellers HS. Current limitations in control of viral arthritis and tenosynovitis caused by avian reoviruses in commercial poultry. Vet Microbiol. 2017;206:152–156.
- Wideman RF. Jr. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci. 2016;95(2):325–344.
- Xu T, Zheng J, Jin W, et al. Total flavonoids of rhizoma drynariae ameliorate bone growth in experimentally induced tibial dyschondroplasia in chickens via regulation of OPG/RANKL axis. Front Pharmacol. 2022a;13:881057.
- Guo Y, Tang H, Wang X, et al. Clinical assessment of growth performance, bone morphometry, bone quality, and serum indicators in broilers affected by valgus-varus deformity. Poult Sci. 2019;98(10):4433–4440.
- Huang S, Kong A, Cao Q, Tong Z, Wang X. The role of blood vessels in broiler chickens with tibial dyschondroplasia. Poult Sci. 2019a;98(12):6527–6532.
- Phibbs DV, Groves PJ, Muir WI. Leg health of meat chickens: impact on welfare, consumer behaviour, and the role of environmental enrichment. Anim Prod Sci. 2021;61(12):1203–1212.
- Sanotra GS, Lund JD, Ersøll AK, Petersen JS, Vestergaard KS. Monitoring leg problems in broilers: a survey of commercial broiler production in Denmark. World Poult Sci J. 2001;57(1):55–69.
- Knowles TG, Kestin SC, Haslam SM, et al. Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS One. 2008;3(2):e1545.
- Kaukonen E, Norring M, Valros A. Perches and elevated platforms in commercial broiler farms: use and effect on walking ability, incidence of tibial dyschondroplasia and bone mineral content. Animal. 2017;11(5):864–871.
- Granquist EG, Vasdal G, de Jong IC, Moe RO. Lameness and its relationship with health and production measures in broiler chickens. Animal. 2019;13(10):2365–2372.
- Dinev I, Kanakov D, Kalkanov I, Nikolov S, Denev S. Comparative pathomorphologic studies on the incidence of fractures associated with leg skeletal pathology in commercial broiler chickens. Avian Dis. 2019;63(4):641–650.
- Kittelsen KE, David B, Moe RO, Poulsen HD, Young JF, Granquist EG. Associations among gait score, production data, abattoir registrations, and postmortem tibia measurements in broiler chickens. Poult Sci. 2017;96(5):1033–1040.
- Santos MN, Widowski TM, Kiarie EG, Guerin MT, Edwards AM, Torrey S. In pursuit of a better broiler: walking ability and incidence of contact dermatitis in conventional and slower growing strains of broiler chickens. Poult Sci. 2022b;101(4):101768.
- Vasdal G, Moe RO, de Jong IC, Granquist EG. The relationship between measures of fear of humans and lameness in broiler chicken flocks. Animal. 2018;12(2):334–339.
- Kapell DNRG, Hocking PM, Glover PK, Kremer VD, Avendaño S. Genetic basis of leg health and its relationship with body weight in purebred Turkey lines. Poult Sci. 2017;96(6):1553–1562.
- Castellini C, Mugnai C, Moscati L, et al. Adaptation to organic rearing system of eight different chicken genotypes: behaviour, welfare and performance. Ita J Anim Sci. 2016;15(1):37–46.
- Wideman RF, Al-Rubaye A, Jr, Gilley A, et al. Susceptibility of 4 commercial broiler crosses to lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult Sci. 2013;92(9):2311–2325.
- Bai S, Wang L, Luo Y, et al. Effects of corn naturally contaminated with aflatoxins on performance, calcium and phosphorus metabolism, and bone mineralization of broiler chicks. J Poult Sci. 2014;51(2):157–164.
- Sparagano O, Song B, Aziz U, et al. Poultry mites: ubiquitous, spreading, and still a growing threat. Avian Dis. 2022;66(3):1–7.
- Biggs PM, Nair V. The long view: 40 years of Marek’s disease research and Avian Pathology. Avian Pathol. 2012;41(1):3–9.
- Zhang Z, Zhang S, Wang G, et al. Role of microRNA and long non-coding RNA in Marek’s disease tumorigenesis in chicken. Res Vet Sci. 2021;135:134–142.
- Welchman DdB, Cox WJ, Gough RE, et al. Avian encephalomyelitis virus in reared pheasants: a case study. Avian Pathology: journal of the W.V.P.A. 2009;38(3):251–256.
- Sharafeldin TA, Mor SK, Bekele AZ, Verma H, Goyal SM, Porter RE. The role of avian reoviruses in turkey tenosynovitis/arthritis. Avian Pathol. 2014;43(4):371–378.
- Oh JY, Kang MS, An BK, Song EA, Kwon JH, Kwon YK. Occurrence of purulent arthritis broilers vertically infected with Salmonella enterica serovar Enteritidis in Korea. Poult Sci. 2010;89(10):2116–2122.
- Robbins KM, Suyemoto MM, Lyman RL, Martin MP, Barnes HJ, Borst LB. An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum. Avian Dis. 2012;56(4):768–773.
- Szafraniec GM, Szeleszczuk P, Dolka B. A review of current knowledge on Staphylococcus agnetis in poultry. Animals (Basel). 2020;10(8):1421.
- Weimer SL, Wideman RF, Scanes CG, Mauromoustakos A, Christensen KD, Vizzier-Thaxton Y. Impact of experimentally induced bacterial chondronecrosis with osteomyelitis (BCO) lameness on health, stress, and leg health parameters in broilers. Poult Sci. 2021;100(11):101457.
- Oznurlu Y, Celik I, Sur E, Ozaydın T, Oğuz H, Altunbaş K. Determination of the effects of aflatoxin B1 given in ovo on the proximal tibial growth plate of broiler chickens: histological, histometric and immunohistochemical findings. Avian Pathol. 2012;41(5):469–477.
- Madlala T, Okpeku M, Adeleke MA. Understanding the interactions between Eimeria infection and gut microbiota, towards the control of chicken coccidiosis: a review. Comprendre l’interaction entre l’infection à Eimeria et le microbiote intestinal pour lutter contre la coccidiose du poulet: une synthèse. Parasite. 2021;28:48.
- Bryła M, Waśkiewicz A, Podolska G, et al. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins (Basel). 2016;8(6):160.
- Okiki PA, Ojiezeh TI, Ogbimi AO. Effects of feeding diet rich in mycotoxins on the health and growth performances of broiler chicken. International J of Poultry Science. 2010;9(12):1136–1139.
- Murillo AC, Abdoli A, Blatchford RA, Keogh EJ, Gerry AC. Parasitic mites alter chicken behaviour and negatively impact animal welfare. Sci Rep. 2020;10(1):8236.
- Heijmans J, Duijster M, Gerrits WJJ, Kemp B, Kwakkel RP, van den Brand H. Impact of growth curve and dietary energy-to-protein ratio on productive performance of broiler breeders. Poult Sci. 2021;100(7):101131.
- Maharjan P, Mullenix G, Hilton K, et al. Effect of digestible amino acids to energy ratios on performance and yield of two broiler lines housed in different grow-out environmental temperatures. Poult Sci. 2020;99(12):6884–6898.
- van Harn J, Dijkslag MA, van Krimpen MM. Effect of low protein diets supplemented with free amino acids on growth performance, slaughter yield, litter quality, and footpad lesions of male broilers. Poult Sci. 2019;98(10):4868–4877.
- Maharjan P, Martinez DA, Weil J, et al. Review: physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production. Animal. 2021;15 Suppl 1:100284.
- Dozier WA, III, Gehring CK. Growth performance of Hubbard × Cobb 500 and Ross × Ross 708 male broilers fed diets varying in apparent metabolizable energy from 14 to 28 days of age. J Appl Poult Res. 2014;23(3):494–500.
- Zhai W, Peebles ED, Mejia L, Zumwalt CD, Corzo A. Effects of dietary amino acid density and metabolizable energy level on the growth and meat yield of summer-reared broilers. J Appl Poult Res. 2014;23(3):501–515.
- Li T, Xing G, Shao Y, et al. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilization parameters of broilers. Poult Sci. 2020;99(6):3207–3214.
- Whitehead CC. Nutrition and poultry welfare. World Poult Sci J. 2002;58(3):349–356.
- Scheideler SE, Ferket PR. Phytase in broiler rations-effects on carcass yields and incidence of Tribal Dyschondroplasia. J Appl Poult Res. 2000;9(4):468–475.
- Baradaran N, Shahir MH, Asadi Kermani Z. Subsequent bone and metabolic responses of broilers to high-non-phytate phosphorus diets in the starter period. Br Poult Sci. 2017;58(4):435–441.
- Noetzold TL, Vieira SL, Favero A, Horn RM, Silva CM, Martins GB. Manganese requirements of broiler breeder hens. Poult Sci. 2020;99(11):5814–5826.
- Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7(12):9768–9784.
- Singh AK, Ghosh TK, Haldar S. Effects of methionine chelate- or yeast proteinate-based supplement of copper, iron, manganese and zinc on broiler growth performance, their distribution in the tibia and excretion into the environment. Biol Trace Elem Res. 2015;164(2):253–260.
- Opsahl W, Zeronian H, Ellison M, Lewis D, Rucker RB, Riggins RS. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J Nutr. 1982;112(4):708–716.
- Horvath-Papp I. Practical Guide to Broiler Health Management. Budapest: BetűVet Limited; 2008.
- Waldenstedt L. Nutritional factors of importance for optimal leg health in broilers: a review. Anim Feed Sci Tech. 2006;126(3-4):291–307.
- Whitehead CC, McCormack HA, McTeir L, Fleming RH. High vitamin D3 requirements in broilers for bone quality and prevention of tibial dyschondroplasia and interactions with dietary calcium, available phosphorus and vitamin A. Br Poultry Sc. 2004;45(3):425–436.
- Thompson JN, Howell JM, Pitt GA, McLaughlin CI. The biological activity of retinoic acid in the domestic fowl and the effects of vitamin A deficiency on the chick embryo. Br J Nutr. 1969;23(3):471–490.
- Massé PG, Rimnac CM, Yamauchi M, et al. Pyridoxine deficiency affects biomechanical properties of chick tibial bone. Bone. 1996;18(6):567–574.
- Pesti GM, Benevenga NJ, Harper AE, Sunde ML. Factors influencing the assessment of the availability of choline in feedstuffs. Poult Sci. 1981;60(1):188–196.
- Summers JD, Shen H, Leeson S, Julian RJ. Influence of vitamin deficiency and level of dietary protein on the incidence of leg problems in broiler chicks. Poult Sci. 1984;63(6):1115–1121.
- Rennie JS, Whitehead CC. Effectiveness of dietary 25- and 1-hydroxycholecalciferol in combating tibial dyschondroplasia in broiler chickens. Br Poult Sci. 1996;37(2):413–421.
- Elliot MA, Edwards HM. Jr. Effect of 1,25-dihydroxycholecalciferol, cholecalciferol, and fluorescent lights on the development of tibial dyschondroplasia and rickets in broiler chickens. Poult Sci. 1997;76(4):570–580.
- Ledwaba MF, Roberson KD. Effectiveness of twenty-five-hydroxycholecalciferol in the prevention of tibial dyschondroplasia in Ross cockerels depends on dietary calcium level. Poult Sci. 2003;82(11):1769–1777.
- Chen C, White DL, Marshall B, Kim WK. Role of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in chicken embryo osteogenesis, adipogenesis, myogenesis, and vitamin D3 metabolism. Front Physiol. 2021;12:637629.
- Tahamtani FM, Pedersen IJ, Riber AB. Effects of environmental complexity on welfare indicators of fast-growing broiler chickens. Poult Sci. 2020;99(1):21–29.
- Pedersen IJ, Tahamtani FM, Forkman B, Young JF, Poulsen HD, Riber AB. Effects of environmental enrichment on health and bone characteristics of fast growing broiler chickens. Poult Sci. 2020;99(4):1946–1955.
- Buijs S, Van Poucke E, Van Dongen S, Lens J, Baert L, Tuyttens FA. The influence of stocking density on broiler chicken bone quality and fluctuating asymmetry. Poult Sci. 2012;91(8):1759–1767.
- Sadvakassova G, Ghaly M, Chew JA, et al. Research Note: effect of light intensity of calcium homeostasis in pullets. Poult Sci. 2022;101(9):101982.
- van der Pol CW, Molenaar R, Buitink CJ, et al. Lighting schedule and dimming period in early life: consequences for broiler chicken leg bone development. Poult Sci. 2015;94(12):2980–2988.
- Huang SC, Li L, Rehman MU, et al. Tibial growth plate vascularization is inhibited by the dithiocarbamate pesticide thiram in chickens: potential relationship to peripheral platelet counts alteration. Environ Sci Pollut Res Int. 2019b;26(36):36322–36332.
- Yair R, Uni Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult Sci. 2011;90(7):1523–1531.
- Hulet RM. Managing incubation: where are we and why? Poult Sci. 2007;86(5):1017–1019.
- Güz BC, Molenaar R, de Jong IC, Kemp B, van Krimpen M, van den Brand H. Effects of eggshell temperature pattern during incubation on tibia characteristics of broiler chickens at slaughter age. Poult Sci. 2020;99(6):3020–3029.
- Meijerhof R. Design and operation of commercial incubators. In Practical Aspects of Commercial Incubation. Lincolnshire UK: Ratite Conference Books; 2002.
- Riber AB, Herskin MS, Foldager L, Sandercock DA, Murrell J, Tahamtani FM. Post-mortem examination of fast-growing broilers with different degrees of identifiable gait defects. Vet Rec. 2021;189(7):e454.
- Stalker MJ, Brash ML, Weisz A, Ouckama RM, Slavic D. Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J Vet Diagn Invest. 2010;22(4):643–645.
- Mirzazadeh A, Abbasnia M, Zahabi H, Hess M. Genotypic characterization of two novel avian orthoreoviruses isolated in Iran from broilers with viral arthritis and malabsorption syndrome. Ir J Vet Res. 2022;23(1):74–79.
- Yadav JP, Tomar P, Singh Y, Khurana SK. Insights on Mycoplasma gallisepticum and Mycoplasma synoviae infection in poultry: a systematic review. Anim Biotechnol. 2022;33(7):1711–1720.
- Louton H. Exploring the association between slight gait defects and sex, body morphology and leg pathology in broilers. Vet Rec. 2021;189(7):284–285.
- Hong F, Zheng A, Xu P, et al. High-protein diet induces hyperuricemia in a new animal model for studying human gout. IJMS. 2020;21(6):2147.
- Kestin SC, Knowles TG, Tinch AE, Gregory NG. Prevalence of leg weakness in broiler chickens and its relationship with genotype. Vet Rec. 1992;131(9):190–194.
- Hartcher KM, Lum HK. Genetic selection of broilers and welfare consequences: a review. World Poult Sci J. 2020;76(1):154–167.
- Corr SA, McCorquodale CC, Gentle MJ. Gait analysis of poultry. Res Vet Sci. 1998;65(3):233–238.
- Sandilands V, Brocklehurst S, Sparks N, et al. Assessing leg health in chickens using a force plate and gait scoring: how many birds is enough? Vet Rec. 2011;168(3):77–77.
- Nääs IdA, Paz ICdLA, Baracho MdS, et al. Assessing locomotion deficiency in broiler chicken. Sci. agric. (Piracicaba, Braz.). 2010;67(2):129–135.
- Caplen G, Hothersall B, Murrell JC, et al. Kinematic analysis quantifies gait abnormalities associated with lameness in broiler chickens and identifies evolutionary gait differences. PLoS One. 2012;7(7):e40800.
- Moe RO, Bohlin J, Flø A, Vasdal G, Stubsjøen SM. Hot chicks, cold feet. Physiol Behav. 2017;179:42–48.
- LokeshBabu, D. S., S. Jeyakumar, P. J. Vasant, M. Sathiyabarathi, A. Manimaran, A. Kumaresan, H. A. Pushpadass, M. Sivaram, K. P. Ramesha, M. A. Kataktalware, and Siddaramanna. 2018. Monitoring foot surface temperature using infrared thermal imaging for assessment of hoof health status in cattle: a review.J Therm Biol. 78: 10–21.
- Shepherd EM, Fairchild BD. Footpad dermatitis in poultry. Poult Sci. 2010;89(10):2043–2051.
- Michel V, Prampart E, Mirabito L, et al. Histologically-validated footpad dermatitis scoring system for use in chicken processing plants. Br Poult Sci. 2012;53(3):275–281.
- Weimer SL, Wideman RF, Scanes CG, Mauromoustakos A, Christensen KD, Vizzier-Thaxton Y. The utility of infrared thermography for evaluating lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult Sci. 2019;98(4):1575–1588.
- Weimer SL, Wideman RF, Scanes CG, Mauromoustakos A, Christensen KD, Vizzier-Thaxton Y. Broiler stress responses to light intensity, flooring type, and leg weakness as assessed by heterophil-to-lymphocyte ratios, serum corticosterone, infrared thermography, and latency to lie. Poult Sci. 2020;99(7):3301–3311.
- De Jong I, Berg C, Butterworth A, Estevéz I. Scientific report updating the EFSA opinions on the welfare of broilers and broiler breeders. EFS3. 2012;9(6):295E.
- Banerjee D, Daigle CL, Dong B, et al. Detection of jumping and landing force in laying hens using wireless wearable sensors. Poult Sci. 2014;93(11):2724–2733.
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466.
- Chen J, Tellez G, Richards JD, Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front Vet Sci. 2015;2:14.
- Lu L, Chen X, Liu Y, Yu X. Gut microbiota and bone metabolism. Faseb J. 2021;35(7):e21740.
- Ducatelle R, Goossens E, De Meyer F, et al. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res. 2018;49(1):43.
- Huang S, Zhang C, Xu T, et al. Integrated fecal microbiome and metabolomics reveals a novel potential biomarker for predicting tibial dyschondroplasia in chickens. Front Physiol. 2022;13:887207.
- Cui Y, Han C, Li S, et al. High-throughput sequencing-based analysis of the intestinal microbiota of broiler chickens fed with compound small peptides of Chinese medicine. Poult Sci. 2021;100(3):100897.
- Tong X, Rehman MU, Huang S, Jiang X, Zhang H, Li J. Comparative analysis of gut microbial community in healthy and tibial dyschondroplasia affected chickens by high throughput sequencing. Microb Pathog. 2018;118:133–139.
- González-Cerón F, Rekaya R, Aggrey SE. Genetic relationship between leg problems and bone quality traits in a random mating broiler population. Poult Sci. 2015;94(8):1787–1790.
- Kapell DN, Hill WG, Neeteson AM, McAdam J, Koerhuis AN, Avendaño S. Twenty-five years of selection for improved leg health in purebred broiler lines and underlying genetic parameters. Poult Sci. 2012;91(12):3032–3043.
- Kjaer JB, Su G, Nielsen BL, Sørensen P. Foot pad dermatitis and hock burn in broiler chickens and degree of inheritance. Poult Sci. 2006;85(8):1342–1348.
- Tullo E, Fontana I, Peña Fernandez A, et al. Association between environmental predisposing risk factors and leg disorders in broiler chickens. J Anim Sci. 2017;95(4):1512–1520.
- Wang CY, Xia WH, Wang L, Wang ZY. Manganese deficiency induces avian tibial dyschondroplasia by inhibiting chondrocyte proliferation and differentiation. Res Vet Sci. 2021;140:164–170.
- Carvalho BRd, Ferreira Junior HdC, Viana GdS, et al. In-feed organic and inorganic manganese supplementation on broiler performance and physiological responses. Anim Biosci. 2021;34(11):1811–1821.
- Shinde PL, Ingale SL, Choi JY, Kim JS, Pak SI, Chae BJ. Efficiency of inorganic and organic iron sources under iron depleted conditions in broilers. Br Poult Sci. 2011;52(5):578–583.
- da Cruz Ferreira Júnior H, da Silva DL, de Carvalho BR, et al. Broiler responses to copper levels and sources: growth, tissue mineral content, antioxidant status and mRNA expression of genes involved in lipid and protein metabolism. BMC Vet Res. 2022;18(1):223.
- Kumar A, Hosseindoust A, Kim M, et al. Nano-sized zinc in broiler chickens: effects on growth performance, zinc concentration in organs, and intestinal morphology. J Poult Sci. 2021;58(1):21–29.
- Wei C, Lin X, Zhang Y, et al. Effects of inorganic and organic selenium sources on the growth performance of broilers in China: a meta-analysis. Open Life Sci. 2021;16(1):31–38.
- Feng C, Lin H, Li J, Xie B. Effects of dietary inorganic chromium supplementation on broiler growth performance: a meta-analysis. PeerJ. 2021a;9:e11097.
- Feng C, Wuren Q, Zhang X, Sun X, Na Q. Effects of dietary chromium picolinate supplementation on broiler growth performance: a meta-analysis. PLoS One. 2021b;16(4):e0249527.
- Neveling DP, Dicks L. Probiotics: an antibiotic replacement strategy for healthy broilers and productive rearing. Probiotics Antimicrob Proteins. 2021;13(1):1–11.
- Fazelnia K, Fakhraei J, Yarahmadi HM, Amini K. Dietary supplementation of potential probiotics Bacillus subtilis, Bacillus licheniformis, and Saccharomyces cerevisiae and synbiotic improves growth performance and immune responses by modulation in intestinal system in broiler chicks challenged with Salmonella Typhimurium. Probiotics Antimicrob Proteins. 2021;13(4):1081–1092.
- Xu T, Yue K, Zhang C, et al. Probiotics Treatment of Leg Diseases in Broiler Chickens: a Review. Probiotics Antimicrob Proteins. 2022b;14(3):415–425.
- Houshmand M, Azhar K, Zulkifli I, Bejo MH, Meimandipour A, Kamyab A. Effects of non-antibiotic feed additives on performance, tibial dyschondroplasia incidence and tibia characteristics of broilers fed low-calcium diets. J Anim Physiol Anim Nutr (Berl). 2011;95(3):351–358.
- Yan FF, Mohammed AA, Murugesan GR, Cheng HW. Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poult Sci. 2019;98(3):1083–1089.
- Yalçın S, Ramay MS, Güntürkün OB, et al. Efficacy of mono- and multistrain synbiotics supplementation in modifying performance, caecal fermentation, intestinal health, meat and bone quality, and some blood biochemical indices in broilers. J Anim Physiol Anim Nutr (Berl). 2023;107(1):262–274.
- Abdelqader A, Al-Fataftah AR, Daş G. Effects of dietary Bacillus subtilis and inulin supplementation on performance, eggshell quality, intestinal morphology and microflora composition of laying hens in the late phase of production. Anim Feed Sci Tech. 2013;179(1–4):103–111.
- Liu F, Kong A, Fu P, et al. Lactobacillus rhamnosus JYLR-005 prevents thiram-induced tibial dyschondroplasia by enhancing bone-related growth performance in chickens. Probiotics Antimicrob Proteins. 2021a;13(1):19–31.
- Chen P, Xu T, Zhang C, et al. Effects of probiotics and gut microbiota on bone metabolism in chickens: a review. Metabolites. 2022;12(10):1000.
- Liu B, Li Y, Mehmood K, et al. Role of oxidative stress and antioxidants in thiram-induced tibial dyschondroplasia. Pak Vet J. 2021b;41(1):1–6.
- Qamar H, Li A, Zeng Z, et al. Effect of grape seed extract on tibial dyschondroplasia incidence, liver weight, and tibial angiogenesis in chickens. PVJ. 2020;40(02):187–194.
- Zhang C, Xu T, Lin L, et al. Morinda officinalis polysaccharides ameliorates bone growth by attenuating oxidative stress and regulating the gut microbiota in thiram-induced tibial dyschondroplasia chickens. Metabolites. 2022;12(10):958.
- Zhang H, Wang Y, Mehmood K, Chang YF, Tang Z, Li Y. Treatment of tibial dyschondroplasia with traditional Chinese medicines: “lesson and future directions”. Poult Sci. 2020;99(12):6422–6433.
- Lv ZP, Yan SJ, Li G, Liu D, Guo YM. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poult Sci. 2019;98(12):7022–7029.
- Wang B, Wang S, Ding M, Lu H, Wu H, Li Y. Quercetin regulates calcium and phosphorus metabolism through the Wnt signaling pathway in broilers. Front Vet Sci. 2022;8:786519.
- Zhang H, Mehmood K, Li K, et al. Icariin ameliorate thiram-induced tibial dyschondroplasia via regulation of WNT4 and VEGF expression in broiler chickens. Front Pharmacol. 2018;9:123.
- Zhang J, Huang S, Tong X, et al. Chlorogenic acid alleviates thiram-induced tibial dyschondroplasia by modulating caspases, BECN1 expression and ECM degradation. Int J Mol Sci. 2019;20(13):3160.
