Abstract
In vitro embryos production from prepubertal heifers can help contribute to breeding programs; however, strategies are necessary to increase their embryo production. The aim of this study was to investigate the effects of two nutritional plans on oocyte recovery, embryo production and growth performance of prepubertal Nelore heifers. Thirty-four Nelore heifers with age of 6.5 months were divided into two feeding treatments (NP1 and NP2). The NP1 diets served as the control and NP2 diets were formulated to contain an average of 1.22-fold more energy than NP1. After 3 months of supplementation, the animals underwent follicular aspiration (ovum pick-up, OPU) every 21 d for 3 months and embryos were produced in vitro. Wither height, chest depth, body weight and subcutaneous fat of animals were measured. The number of retrieved and viable oocytes per OPU were 1.49-fold and 1.42-fold greater in NP2 heifers (p = 0.018 and p = 0.049, respectively) than those in NP1 heifers. Heifers administered NP2 produced 29.7% blastocysts, a percentage higher than NP1 animals that produced 24.40% embryos (p < 0.05). Consequently, females in the NP2 treatment showed improved body development. These results indicate a positive effect of a higher energy diet on assisted reproduction and body development in prepubertal heifers.
Introduction
Reducing the interval between generations represents a fundamental point in the genetic improvement of cattle herds. Owing to the exploration of reproduction in younger females, significant genetic gains are possible through bovine selection. The potential to produce embryos and calves from young prepubertal females has gained prominence owing to the development of genomic prediction evaluations for the traits of interest, both in dairy and beef cattle. The development of follicular aspiration equipment adapted for calves has led to an increased interest in in vitro embryo production as it does not involve harmful manipulation of the animals. Initially, studies with calves were performed using the laparoscopic aspiration technique through a surgical procedureCitation1,Citation2 and later using the ultrasound-guided transvaginal follicular aspiration technique (ovum pick-up, OPU) with a specific guide of adequate size for young females.Citation3 Prepubertal heifers may have a greater number of follicles available for aspirationCitation4; however, in vitro embryo production rates in these animals are lower than those of adult animals.Citation5
Among the factors that influence the reproductive performance of female beef cattle, special attention should be paid to nutrition, which can directly affect the ovarian follicles, oocytes and embryos.Citation6 Propionate, a volatile fatty acid produced in large quantities in diets with high energy concentrations, plays an important role in reproductive performance. Propionate serves as the main gluconeogenic substrate in ruminants and can modify reproductive hormone profiles through the association of glucose production with insulin-like growth factor 1 (IGF-1), which can improve embryonic developmentCitation7 and progesterone production.Citation8 Tropical pasture-based animals require protein and energy supplementation to meet their nutritional requirements, particularly during the dry season. During this period, Urochloa brizantha (syn. Brachiaria brizantha) pastures contain an average of 7% of crude protein (CP) and 1.8 Mcal of metabolizable energy (ME)/kg dry matter (DM).Citation9,Citation10
Body weight (BW) is a major factor affecting puberty in animals. Thus, restricted feeding, which slows down growth, increases the age at puberty.Citation11 A prepubertal heifer requires 8.75 Mcal of ME daily to achieve a moderate live weight gain of 400 g/d.Citation12 Therefore, during the winter, these animals must receive supplemental energy ranging from 0.5% to 0.7% of their BW.Citation12 In Brazil, pasture-raised cattle are usually supplemented with concentrates in the form of soybeans and corn because forage cannot fully meet their nutritional demand, especially during the dry season.
Heifers aged 4–9 months are more sensitive to the pubertal acceleration effects of improved nutrition,Citation13 which can improve the production and quality of oocytes and embryos. The most important nutritional factor affecting reproduction in cattle is the energy intake.Citation11 The growth of follicles and ovulation are dependent on the pulsatile secretion of LH,Citation14 insulin and IGF-I, among other factors.Citation11 However, with low-energy intake, the secretion of these hormones is inhibited, leading to slow growth and follicle development, which delays ovulation.Citation11,Citation15 Therefore, short-term supplementation with energy dense diets may improve reproduction in female cattle; increasing dietary energy density has a greater effect than increasing protein levels. The effects of high energy intake on follicular dynamics and the developmental competence of oocytes in cattle may be related to increased plasma insulin and IGF-I concentrations, compared to a low-energy diet.Citation16
According to Davis et al.,Citation17 owing to a rapid physiological response, the pre-breeding dietary energy supplementation strategy is of particular interest because it may present a method for enhancing the success of assisted reproductive technologies. This suggests that an increase in propionate production may improve reproductive outcomes. Ovarian tissues respond directly to metabolic inputs, with consequences for folliculogenesis, steroidogenesis and the development of the oocyte and embryo.Citation18 In addition to regulating follicular growth, short-term changes in dietary energy intake can also influence oocyte morphology and developmental potential.Citation19,Citation20
The nutritional status of an animal is usually assessed based on changes in its live weight and body condition. However, these are long-term changes and many reproductive events, such as ovulation, fertilization and placentation occur, over a short period of time.Citation11 In this context, the aim of this study was to determine the effects of feeding at two different energy levels on the quality and quantity of oocytes, in vitro embryo production and the body development of prepubertal Nelore heifers.
Materials and methods
Experimental design
The experimental design was randomized. In this study, 34 prepubertal heifers at 5.5 months of age received the same initial food supplementation in a creep-feeding system until weaning (6.5 months of age). After weaning, these animals were divided into two experimental groups according to age, weight and total economic genetic merit (MW120g: maternal effect of weight at 120 d of age (kg); DW210g: direct effect for weight at 210 d for age (kg); DW365g: direct effect for weight at 365 d of age (kg); DSC365g: direct effect for scrotal circumference at 365 d of age (cm); DCFg: carcass finishing (mm2); DSTAYg: stayability (%); D3Pg: probability of precocious calving (%)) and maintained on one of two nutritional plans (17 females per treatment) to stimulate oocyte production and provide adequate growth for mating. After 3 months of supplementation (i.e., at 9.5 months old), animals from both groups underwent follicular aspiration (OPU) every 21 d for three months without prior follicular wave synchronization. Embryos were produced in vitro (IEP). The total recovery of oocytes, quality of oocytes, viable oocytes and blastocyst rates on culture day 7 were measured. Adult cow oocytes obtained from local slaughterhouses were fertilized and used as controls.
The animals were weighed monthly and the average final weight gain was calculated. In addition, at the end of supplementation the rib eye area (REA), back fat thickness (BF) and rump fat thickness (RF) of each animal were measured via ultrasound. The carcass finish was measured after adding 35% BF and 65% RF.
Feed supplementation
Throughout the experimental period, all animals had access to feed bunks containing a mineral supplement and were provided clean water ad libitum (). All animals had ad libitum access to creep-feeding from weighing at 5.5 months of age until weaning at 6.5 months of age, with an average concentrate consumption of 0.568 g/d, equivalent to 0.38% BW. This creep feed contained 19.3% CP and 79.8% total digestible nutrients (TDN; 3.10 Mcal/kg of ME) and was formulated using soybean meal, ground corn and minerals. After weaning, the animals were divided into two experimental treatment groups: nutritional plan 1 (NP1, control) and nutritional plan 2 (NP2).
Table 1. Composition of diets NP1 and NP2 during dry and rainy period.
For NP1, animals were maintained in a deferred Urochloa brizantha (syn. Brachiaria brizantha) pasture during the dry season with an average consumption of concentrate (24.2% CP, 77.4% TDN, 3.0 Mcal/kg ME) of 1.4 kg/d, equivalent to 0.7% BW. This diet was formulated to achieve a gain of 400 g/d, according to the methods of Valadares Filho et al.Citation12 At the beginning of the wet season, animals were kept in the same pasture with an average concentrate (21.8% CP, 79.1% TDN, 3.07 Mcal/kg ME) consumption of 650 g/d, equivalent to 0.25% BW. This diet was used to achieve a weight gain of 500 g/d ().
For NP2, the animals received diets containing 26% and 19% more ME than NP1 during the dry and rainy seasons, respectively. During the dry season, these animals were kept confined after weaning and were fed an average of 11 kg of corn silage (33.0% DM) and 1 kg/d of concentrate (33.4% CP, 72.9% TDN, 2.8 Mcal/kg ME), equivalent to 0.33% BW. At the beginning of the rainy season, these animals were supplemented with 1.87 kg/d (0.35% BW) of the concentrate.
OPU and IEP
Before each OPU session, the presence of corpus luteum in the ovaries of heifers was evaluated using a B-mode ultrasound device (SSD 500; Aloka, Tokyo, Japan) equipped with a 7.5 MHz linear rectal probe to monitor the onset of puberty. Follicle aspiration was performed using a real-time B-mode ultrasound scanner (HS 1500; Honda®, Toyohashi, Japan) equipped with a 7.5 MHz convex array transducer (Honda®, Toyohashi, Japan).
The recovered cumulus–oocyte complexes (COCs) were classified by quality into grade 1, 2, 3 or 4 based on the number of layers, expansion of cumulus cells and cytoplasmic appearance in terms of colour, homogeneity and integrity. Only oocytes of grades 1–3, considered to have the greatest potential to develop into embryos,Citation21 were denominated as viable oocytes and matured and used for fertilization. Briefly, 30 COCs were cultured in a 2.0 mL cryotube with 600 µL of in vitro maturation media covered with 250 µL of mineral oil. Maturation media consisted of TCM199 with Earle’s salts (Thermo Fisher Scientific, USA) supplemented with 10% foetal bovine serum (FBS, v/v), 0.2 mM of pyruvate, 5 mg/mL of luteinizing hormone, 1 mg/mL of follicle-stimulating hormone, 75 μg/mL of amikacin and 1 mM of cystamine. The COCs were matured in drops covered with mineral oil for 22–24 h at 38.5 °C in 5% CO2 and high O2 tension. After this, COCs were transferred to 100 µL drops of fertilization media, consisting of Tyrode’s albumin lactate pyruvate supplemented with 6 mg/mL of bovine serum albumin (BSA), 0.2 mM of pyruvate, 30 μg/mL of heparin, 20 μM of penicillamine, 10 μM of hypotaurine, 1 μM of epinephrine and 75 mg/mL of amikacin. For fertilization (IVF), conventional commercial semen from a Nelore bull with proven in vitro fertility was used. Each COCs drop was fertilized with a final concentration of 1 × 106 spermatozoa/mL for 18 h at 38.5 °C, 5% CO2 and high O2 tension. Presumptive zygotes were transferred into drops (200 μL) of modified synthetic oviduct fluid media supplemented with 2.7 mM of myo-inositol, 0.2 mM of pyruvate, 2.5% of FBS (v/v), 5 mg/mL of BSA and 75 μg/mL of amikacin and cultured in the same conditions as the maturation and fertilization phases. Embryos were cultured for 7 d with evaluations on day 2 for cleavage and day 7 to determine blastocyst production rate. Adult cow oocytes from a local slaughterhouse were fertilized and used as the control group.
Assessment of lipid accumulation in the embryos
Twenty expanded blastocysts from each treatment group were evaluated for lipid accumulation. This evaluation was performed according to the methods of Faria et al.Citation22 with slight modifications for in vitro embryos. Firstly, embryos were fixed for 1 h in 4% paraformaldehyde, then washed thrice in PBS and stored for up to 1 week at 4 °C in 1% paraformaldehyde. After this fixation period, the embryos were washed twice in PBS supplemented with 0.3% polyvinylpyrrolidone (PVP) and then incubated for 30 min at room temperature in PBS supplemented with 0.2% Triton. Embryos were then washed thrice in PBS with 0.3% PVP and stained for 1 h at room temperature with Bodipy 493/503 (20 mg mL/L; Molecular Probes, Eugene, OR, USA) diluted in 50 mL of absolute ethanol and 950 mL PBS. Embryos were then washed thrice in PBS with 0.3% PVP, placed individually into 8 μL drops of an anti-fade solution (SlowFade™; Molecular Probes, Eugene, OR, USA) in a polystyrene petri dish (35.00 mm diameter × 10.00 mm depth) and viewed under a confocal microscope. All embryos were subjected to the same microscopic laser dissection conditions (LSM Leica Sp8 microscope; New Orleans, LA, EUA). All liquid droplets were analysed and photographed using a 20× objective and a 488-nm argon laser in a fluorescence spectrum between 495 and 505 nm. Embryos were processed one-by-one, creating 30 cross-sections at 2.96 mm intervals. Z-stacking was used to create images of overlapping sections. Final images were created for each embryo by adjusting the scale of grain (8-bit image) and correcting the background using ImageJ software (National Institutes of Health, Bethesda, MD, USA). No fluorescence compensation was applied and all samples were proven and photographed following a single set of parameters. Lipid quantification was calculated as the ratio of the area occupied by lipids to the total area of the embryo.
Morphometric traits
REA, BF and RF
Ultrasound scanning was used to obtain carcass traits, such as REA, BF and RF, in real time in vivo. REA and BF were measured between the 12th and 13th ribs, transversely over the longissimus dorsi muscle, while RF was measured at the rump, between the ileum and ischium bones, at the intersection of the gluteus medius and biceps femoris. These traits were obtained using Aloka 500 V ultrasound equipment (Tokyo, Japan) with a 17.5 cm linear probe and a frequency of 3.5 MHz with a silicone coupler and vegetable oil as an acoustic coupler.
Statistical analysis
All analyses were performed using R statistical software (version 3.5.1). The homogeneity of variance was assessed using Leven’s test and data normality was verified by the Shapiro–Wilk test. The variation in the number of COCs among the OPU sessions was determined due to the increase in animal age. When the ANOVA test detected statistical differences, the Student t-test was used to compare the treatment parameters. To compare rates the ANOVA test and Student t-test group by group were used. These results were confirmed by the Bonferroni test. All statistical tests were considered at the 5% probability level.
Results
Prepubertal Nelore cows that received the NP2 diet had 49% more recovered oocytes than NP1 animals (p = 0.018; ). There was no statistical difference in the number of grades 1, 2 or 3 oocytes between treatments; however, the NP2 group had more grade 4 (poor quality) oocytes than the NP1 group (p = 0.008; ). The total number of viable oocytes per OPU was approximately 42% higher in NP2 animals than that in NP1 animals (p = 0.049; ). A low variation in the number of oocytes between the OPU treatments was also observed (12.30% for NP1 and 13.35% for NP2), indicating that the increased age of the animals at each OPU had no influence.
Table 2. Number of recovery oocytes, viable oocytes and quality of oocytes (average ± standard deviation), obtained per OPU from Nelore prepubertal heifers feeding with different nutritional plan.
There was no difference in the cleavage rate between treatments (p = 0.57; ); however, NP2 animals had D7 blastocyst rates greater than those of NP1 animals and lower than those of the control group, which used oocytes from the ovaries of adult cows (p = 0.047; ).
Table 3. Cleavage and blastocyst rate at D7 from prepubertal heifers feeding of different nutritional plan.
Lipid accumulation was equally dispersed throughout the cytoplasm and was not concentrated in a specific area of the ooplasm in any sample. No difference in lipid accumulation was observed between the NP1 and NP2 embryos (p = 0.066; and ).
Figure 1. Representative confocal microscopy (LSM Leica sp8; 488-nm argon laser, 20× objective) of embryos stained with Bodipy 493/503. (A) Lipid analysis of embryo from NP2 treatment and (B) lipid analysis of embryo from NP1 treatment.
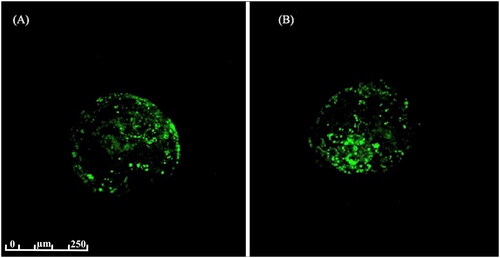
Table 4. Percentage of the cytoplasm area occupied by lipids in relation to total area of oocytes matured from prepubertal heifers feeding different nutritional plan.
Considering the difference between the initial average weight and the final average weight, NP2 animals showed a higher average weight gain than NP1 animals (p = 0.0133; ). The accumulated weight of the animals was slightly different between groups, with NP2 animals weighing approximately 15 kg more than NP1 animals (284.88 ± 20.19 vs 298.19 ± 25.15 for NP1 and NP2, respectively).
Table 5. Parameters (mean ± standard deviation) of body development performance between prepubertal Nelore heifers submitted to two nutritional plans.
Although NP2 animals reached the suggested weight to begin reproduction, no heifers from either treatment presented with a corpus luteum in their ovaries; thus, they remained prepubertal throughout the experiment.
The results of the morphometric trait analyses are presented in . REA and WH values did not differ between the two treatment groups. Animals fed NP2 had a mean BF of 3.70 ± 0.59 mm, which was significantly higher than that of NP1 animals (3.11 ± 0.48 mm; p = 0.013). Likewise, the mean RF of NP2 animals was significantly higher than that of NP1 animals, with a value 1.11 mm greater than that of NP1 animals (p = 0.001; ).
The mean CD of NP2 animals was higher than that of NP1 animals (p = 0.009; ). In addition, NP2 animals had improved carcass finishing compared to NP1 animals (p < 0.0001; ).
Discussion
The results of this study support the hypothesis that short-term supplementation with higher energy density diets may promote an increase in the number of total oocytes retrieved, viable oocytes and blastocyst rates on D7, in association with improved body development in prepubertal Nelore heifers.
In the present study, we observed that the number of retrieved COCs per OPU was 1.49-fold greater with 1.42-fold more viable oocytes in Nelore prepubertal heifers fed the NP2 diet containing greater ME levels. Although not measured, these findings indirectly indicate that a larger follicular population was present in the ovaries of these animals, especially because a low variation in COCs number among OPUs was observed. These results indicate that positive effects of higher dietary energy could be related to major body development, fat deposition and, consequently, reproductive hormone production. According to Sartori et al.,Citation23 management factors, such as diet type and feed intake, are known to have a great impact on oocyte quality and energy intake; these factors can affect circulating levels of insulin and IGF-1 and are directly involved in the function of the hypothalamic–pituitary–gonadal axis.
Female heifers administered the NP2 diet had 1.8-fold more grade 4 oocytes than NP1 animals; these oocytes are considered inviable for IVF. It is possible that this could be attributed to the greater amount of oocytes recovered from available follicles of different sizes in the ovary of NP2 animals. Despite this increased number of low-quality oocytes, NP2 diets increased the number of viable oocytes recovered compared to NP1 diets (113.25 ± 21.40 vs 79.75 ± 14.82 in NP2 and NP1, respectively). This result was superior to those reported by Silva et al.Citation24 who obtained 20.60 ± 5.12 viable oocytes per OPU from prepubertal Nelore heifers of the same age as the animals in this study. We hypothesize that this increase in the oocytes quantity and quality in heifers fed NP2 diets is linked to improved body development and moderate fat deposition promoted by this food supplementation; consequently generating greater availability of metabolites and improving hormone balance in prepubertal females. According to Sartori et al.Citation23 insulin and IGF-1 are directly involved in the functioning of the hypothalamic–pituitary–gonadal axis and are controlled by nutritional status and level of DM intake.
Some research laboratories have successfully produced viable embryos from prepubertal heifersCitation25; however, oocytes from young females have shown a lower capacity for embryonic development than oocytes from adult females,Citation26,Citation27 which may be attributed to cytoplasmicCitation28 and/or nuclearCitation29 differences. Furthermore, the oocytes of prepubertal heifers are known to have a lower number of transcripts than those of pubertal bovine females.Citation30 In the current study, NP2 animals had a higher D7 blastocyst rate than NP1 animals and a lower rate than the control group (p < 0.05), which used oocytes from the ovaries of adult cows. Animals receiving more ME likely produced more propionate and, consequently, more IGF-1 and progesterone, which could have altered heifer blastocyst development. One positive effect that supports this idea is that NP2 animals produced 43% more embryos than NP1 animals. Our results reinforce the theory of Santos et al.,Citation31 who stated that nutritional manipulation can influence the competence of oocytes within follicles and the ability of fertilized ova to result in embryos with high developmental potential.
The D7 blastocyst rate of NP2 heifers (29.7%) was higher than that of NP1 heifers (24.4%). This result indicates a beneficial effect of feeding energy dense diets short-term, which may be related to the increased production of hormones, such as insulin. However, high and excessive energy intake over a long period of time has been shown to have a negative effect on the blastocyst rate.Citation32 The blastocyst formation rate of NP2 heifers was higher than that reported by Silva,Citation33 who obtained a 19.2% blastocyst rate in 12-month-old Nelore heifers. The blastocyst production rate found in the present study was also higher than that reported by Baruselli et al.,Citation34 who showed that prepubertal females between 8 and 12 months of age had a 20.2% blastocyst rate, and Silva et al.,Citation24 who reported a 27.1% blastocyst rate in 13-month-old Nelore heifers.
In our study, greater animal growth and fat deposition in NP2 animals may have positively influenced the increased number of viable oocytes, allowing them to present the same potential for embryonic development as oocytes from adult cows. Heifers fed diets that do not meet their nutritional requirements produce lower quality oocytes and reduced embryo cleavage and blastocyst rates compared to animals of a moderate body condition score fed diets that meet their requirements. Similarly, obesity in heifers is correlated with low-quality embryos and reduced pregnancy rates.Citation35
According to Ashworth et al.,Citation36 optimal nutrition of replacement heifers provides the opportunity for increased reproductive capacity by improving gamete quality and embryonic competence, whereas overnutrition can lead to obesity, which may result in the reduced performance of these animals.Citation37 The effects of obesity on oocyte quality and fertility are complex. However, there are reports that obesity is related to mitochondrial damage, endoplasmic reticulum stress response and dysregulated hormonal synthesis in granulosa cells (GCs) obtained from individuals with obesity, whereas none of these changes occur in individuals with normal weight.Citation38 GCs play important roles in the development of oocytes and follicles, including the secretion of essential nutrients and hormones for follicular growth, thereby providing a suitable microenvironment for meiosis and oocyte maturation.Citation39 Another factor that may lead to poor oocyte quality in individuals with obesity is the exposure of ovarian cells to high levels of fatty acids. This exposure can result in an inflammatory response within ovarian follicles, excessive production of ovarian androgens through ineffective oxidation of free fatty acids in the mitochondria,Citation40 insulin resistance,Citation41 oxidative damage to DNA, decreased oestradiol levels, low ovulation rates and compromised oocyte quality.Citation38,Citation42 In our study, the NP2 diet was sufficient to promote adequate body development and moderate fat deposition, however, without generating overnutrition and harming the reproduction of the animals.
As heifers fed the NP2 diet received a greater supply of energy, it was hypothesized that a greater accumulation of lipids could occur in the embryos, which could compromise their cryopreservation quality. However, no differences in lipid deposition were observed between the embryos of heifers subjected to the two treatments.
Considering weight gain, NP2 animals had a greater accumulated gain at the end of the trial, with an average gain 15 kg greater than that of NP1 animals and their final weight was within the recommended weight for artificial insemination. According to Mousquer et al.,Citation43 the reproductive cyclicity of bovine females is a consequence of a series of hormonal events and is more closely related to BW than age, which was confirmed by Gregianini et al.,Citation44 who evaluated Nelore heifers with a mean age of 12 months. In those studies, the mean weight of heifers that became pregnant was 287.27 ± 30.28 kg, while that of females who were not pregnant was 274.84 ± 29.14 kg, indicating an influence of weight on reproductive efficiency. However, in the present study, females from both treatments did not have a corpus luteum in the ovary and remained prepubertal. For adequate body development, a hormonal protocol may be used to induce puberty, followed by artificial insemination.
Animals with higher mature BWs tend reach sexual maturity laterCitation45 as they begin fat deposition and the physiological processes that trigger puberty at an older age. However, the greater BW of the heifers in the NP2 group reflects an increased speed of growth and body development, which could lead to reaching the body condition that triggers puberty. Studies have shown that the genetic correlation between BW and the probability of precocious calving and/or age at first calving in Nelore cattle is low to moderate but favourable, especially when evaluated at younger ages.Citation46 This is likely because younger animals with a faster growth rate have greater body development, including fat deposition, a hormone precursor tissue that leads to the development of the reproductive system and sexual maturity.Citation44,Citation47–49 These reports support the results obtained in the present study and suggest that nutritional strategy improvements that lead to greater BW and anticipate animal development enable the production of heifers with precocious development and a younger age at first calving.
Animals that received a higher supply of energy (NP2) showed a higher accumulation of general subcutaneous fat (BF) and subcutaneous fat in the rump (RF) than those that received less energy (NP1). Body fat is important for the initiation and maintenance of puberty, nutrition and the rate of weight gain that acts on intermediary metabolism and can modulate the generation of gonadotropin hormone-releasing hormone (GnRH) pulses in the central nervous system, initiating the activity of the hypothalamic axis, and triggering puberty.Citation50 Animals fed the NP2 diet not only had higher body fat than NP1 animals, but likely also had a higher proportion of visceral fat; this total body fat content could influence precocious puberty in females. According to Basarab et al.,Citation51 body fat may be responsible for differences in intermediary metabolism and reproductive maturation rates in young animals. However, no methodology is currently available to estimate the composition of visceral tissues in vivo.
Conclusions
Prepubertal Nelore heifers that received short-term higher energy density diets (NP2) presented an increase in the number of recovered oocytes, viable oocytes and embryos produced in vitro. Moreover, these heifers presented greater weight and fat deposition in the carcass, allowing females to reach the suggested mating weight at 12–14 months of age. The use of short-term nutrition with higher-density diets is an option for maximum exploitation of the reproduction in immature Nelore females for the production of embryos in vitro and preparation for an artificial insemination program.
CRediT authorship contribution statement
R.B. de Toledo: Methodology, investigation, data curation, writing—original draft; O.A.C. de Faria: Methodology, investigation; L.O. Leme: Methodology, investigation, data curation. C.U. Magnabosco: Methodology, investigation; R. Guimarães Jr: Investigation, writing—review and editing; E.C. Eifert: Methodology, investigation, writing—original draft; I.R. dos Santos: Investigation; R.V. Oliveira: Investigation; M.A.N. Dode: Methodology, investigation; J.V. Malaquias: Data curation, formal analysis; I. Pivato: Methodology and C.F. Martins: Conceptualization, methodology, investigation, supervision and writing—original draft, writing—review and editing, funding acquisition.
Ethical approval
All experimental procedures were performed in accordance with current Brazilian laws and were approved beforehand by the Ethics Committee on Animal Use at Embrapa Cerrados (protocol no. 800-4372-1/2020).
Disclosure statement
The authors declare that they have no conflict of interests.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Baldassarre H, Currin L, Michalovic L, et al. Interval of gonadotropin administration for in vitro embryo production from oocytes collected from Holstein calves between 2 and 6 months of age by repeated laparoscopy. Theriogenology. 2018;116:64–70.
- Batista EOS, Guerreiro BM, Freitas BG, et al. Plasma anti-Müllerian hormone as a predictive endocrine marker to select Bos taurus (Holstein) and Bos indicus (Nelore) calves for in vitro embryo production. Domest Anim Endocrinol. 2016;54:1–9.
- Elliff FM, Guimarães EC, Féres LF, Bayeux BM, Colli MA, Baruselli PS. Effect of treatment with follicle-stimulating hormone on in vitro embryo production of Gyr (Bos indicus) calves, pubertal heifers and adult cows. Reprod Fertil Dev. 2019;31(1):191.
- Landry DA, Bellefleur AM, Labrecque R, et al. Effect of cow age on the in vitro developmental competence of oocytes obtained after FSH stimulation and coasting treatments. Theriogenology. 2016;86(5):1240–1246.
- Guerreiro BM. Produção in Vitro de Embriões de Doadoras Pré-Púberes da Raça Holandesa [dissertação (mestrado)]. São Paulo: Universidade de São Paulo; 2015.
- D’Occhio MJ, Baruselli PS, Campanile G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology. 2019;125:277–284.
- Thatcher W, Guzeloglu A, Meikle A, et al. Regulation of embryo survival in cattle. Reprod Suppl. 2003;61:253–266.
- Spicer LJ, Chamberlain CS. Influence of cortisol on insulin-and insulin-like growth factor 1 (IGF-1)-induced steroid production and on IGF-1 receptors in cultured bovine granulosa cells and thecal cells. Endocrine. 1998;9(2):153–161.
- Euclides VPB, de Medeiros SR. Valor nutritivo das principais gramíneas cultivadas no Brasil. Campo Grande, MS: Embrapa Gado de Corte; 2003: 43 p., Documentos 139. https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPGC/10919/1/Doc139.pdf.
- Valadares Filho SC, Lopes SA, Chizzotti ML. Brazilian Feed Composition Tables for Ruminants; 2018. www.cqbal.com.br.
- Amin RU. Nutrition: its role in reproductive functioning of cattle-a review. Vet Clinic Sci. 2014;2:01–09.
- Valadares Filho SC, Lopes SA, Chizzotti ML. BR Corte: Nutrient Requirements of Zebu and Crossbred. 3rd ed. Viçosa, MG: UFV, DZO; 2016; 314 p. https://brcorte.com.br/livro2016en.
- Cardoso CR, Alves BRC, Williams GL. 2018. Neuroendocrine signaling pathways and the nutritional control of puberty in heifers. In: Proceedings of the 10th International Ruminant Reproduction Symposium (IRRS 2018); September 16–20, Foz do Iguaçu, PR, Brazil.
- Canfiel RW, Butler WR. Energy balance and pulsatile LH secretion in early postpartum dairy cattle. Domest Anim Endocrinol. 1990;7(3):323–330.
- Imakawa K, Day ML, Zalesky DD, Clutter A, Kittok RJ, Kinder JE. Effects of 17 betaestradiol and diets varying in energy on secretion of luteinizing hormone in beef heifers. J Anim Sci. 1987;64(3):805–815.
- Armstrong DG, McEvoy TG, Baxter G, et al. Effect of dietary energy and protein on bovine follicular dynamics and embryo production in vitro: associations with the ovarian insulin-like growth factor system. Biol Reprod. 2001;64(6):1624–1632.
- Davis TC, Amirault KE, Stewart JW, et al. Effect of dietary energy source on pregnancy rates and reproductive physiology of pastured beef heifers. Front Anim Sci. 2023;4(1):1–10.
- Chagas LM, Bass JJ, Blache D, et al. New perspectives on the roles of nutrition and metabolic priorities in the subfertility of highproducing dairy cows. J Dairy Sci. 2007;90(9):4022–4032.
- McEvoy TG, Robinson JJ, Aitken RP, Findlay PA, Palmer RM, Robertson IS. Dietary-induced suppression of preovulatory progesterone concentrations in superovulated ewes impairs the subsequent in vivo and in vitro development of their ova. Anim Reprod Sci. 1995;39(2):89–107.
- O’Callaghan D, Yaakub H, Hyttel P, Spicer L, Boland M. Effect of nutrition and superovulation on oocyte morphology, folicular fluid composition and systemic hormone concentrations in ewes. J. Reprod Fertil. 2000;118(2):303–313.
- Stojkovic M, Machado SA, Stojkovic P, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64(3):904–909.
- Faria OAC, Kawamoto TS, Dias LRO, et al. Maturation system affects lipid accumulation in bovine oocytes. Reprod Fertil Dev. 2021;33(5):372–380.
- Sartori R, Spies C, Wiltbank MC. Effects of dry matter and energy intake on quality of oocytes and embryos in ruminants. Reprod Fertil Dev. 2016;29(1):58–65.
- Silva MO, Borges MS, Fernandes LG, et al. Effect of Nellore (Bos indicus) donor age on in-vitro embryo production and pregnancy rate. Reprod Domest Anim. 2022;57(9):980–988.
- Taneja M, Bols PE, de Velde AV, et al. Developmental competence of juvenile calf oocytes in vitro and in vivo: influence of donor animal variation and repeated gonadotropin stimulation. Biol Reprod. 2000;62(1):206–213.
- Majerus V, De Roover R, Etienne D, et al. Embryo production by ovum pick up in unstimulated calves before and after puberty. Theriogenology. 1999;52(7):1169–1179.
- Presicce GA, Jiang S, Simkin M, et al. Age and hormonal dependence of acquisition of oocyte competence for embryogenesis in prepubertal calves. Biol Reprod. 1997;56(2):386–392.
- Salamone DF, Damiani P, Fissore RA, Robl JM, Duby RT. Biochemical and developmental evidence that ooplasmic maturation of prepubertal bovine oocytes is compromised. Biol Reprod. 2001;64(6):1761–1768.
- Aston KI, Li G-P, Hicks BA, et al. The developmental competence of bovine nuclear transfer embryos derived from cow versus heifer cytoplasts. Anim Reprod Sci. 2006;95(3-4):234–243.
- Dorji, Ohkubo Y, Miyoshi K, Yoshida M. Gene expression differences in oocytes derived from adult and prepubertal Japanese black cattle during in vitro maturation. Reprod Domest Anim. 2012;47(3):392–402.
- Santos JEP, Cerri RLA, Sartori R. Nutritional management of the donor cow. Theriogenology. 2008;69(1):88–97.
- Baruselli PS, Sá Filho MF, Ferreira RM, et al. Manipulation of follicle development to ensure optimal oocyte quality and conception rates in cattle. Reprod Domest Anim. 2012;47(s4):134–141.
- Silva LGD. Produção in vitro de embriões de novilhas Nelore (Bos indicus) de 12 e 24 meses de idade tratadas ou não com FSH [dissertação (mestrado)]. São Paulo: Universidade de São Paulo; 2020.
- Baruselli PS, Batista EOS, Vieira LM, et al. Factors that interfere with oocyte quality for in vitro production of cattle embryos: effects of different developmental & reproductive stages. Anim Reprod. 2016;13(3):264–272.
- Horn EJ, Read CC, Edwards JL, et al. Preovulatory follicular fluid and serum metabolome profiles in lactating beef cows with thin, moderate, and obese body condition. J Anim Sci. 2022;100:skac152.
- Ashworth CJ, Toma LM, Hunter MG. Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philos Trans R Soc Lond B Biol Sci. 2009;364(1534):3351–3361.
- Bagley CP. Nutritional management of replacement beef heifers: a review. J Anim Sci. 1993;71(11):3155–3163. PMID: 8270540.
- Si C, Wang N, Wang M, Liu Y, Niu Z, Ding Z. TMT-based proteomic and bioinformatic analyses of human granulosa cells from obese and normal-weight female subjects. Rep Biol Endocrinol. 2021;19:1–11.
- Komatsu K, Masubuchi S. Mouse oocytes connect with granulosa cells by fusing with cell membranes and form a large complex during follicle development. Biol Reprod. 2018;99(3):527–535.
- Gervais A, Battista MC, Carranza-Mamane B, Lavoie HB, Baillargeon JP. Follicular fluid concentrations of lipids and their metabolites are associated with intraovarian gonadotropin-stimulated androgen production in women undergoing in vitro fertilization. J Clin Endocrinol Metab. 2015;100(5):1845–1854.
- Xu L, Wang W, Zhang X, et al. Palmitic acid causes insulin resistance in granulosa cells via activation of JNK. J Mol Endocrinol. 2019;62(4):197–206.
- Chaube SK, Shrivastav TG, Tiwari M, Prasad S, Tripathi A, Pandey AK. Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus. 2014;3(1):464.
- Mousquer CJ, Fernandes FFD, Fernandes GA, et al. Desempenho reprodutivo de matrizes Nelore. PUBVET. 2014;8(3):1–15.
- Gregianini HAG, Junior JMC, Neto AP, et al. Precocidade sexual de novilhas Nelore em rebanho sob seleção no Estado do Acre. Res Soc Dev. 2021;10(4):e16310413945.
- Lacerda VV, Campos GS, Roso VM, Souza FRP, Brauner CC, Boligon AA. Effect of mature size and body condition of Nelore females on the reproductive performance. Theriogenology. 2018;118:27–33.
- Kluska S, Olivieri BF, Bonamy M, et al. Estimates of genetic parameters for growth, reproductive, and carcass traits in Nelore cattle using the single step genomic BLUP procedure. Livest Sci. 2018;216:203–209.
- Andrade EF, Ferreira DF, Santos PEF, Eustáquio Filho A. Main factors that affect the precocity of Nellore heifers and the classification of the early production system: a review. Recital. 2020;2:58–72.
- Brunes LC, Baldi F, Costa MFOE, et al. Early growth, backfat thickness and body condition has major effect on early heifer pregnancy in Nellore cattle. An Acad Bras Cienc. 2022;94(1):e20191559.
- Pires BC, Tholon P, Buzanskas ME, et al. Genetic analyses on bodyweight, reproductive and carcass traits in composite beef cattle. Anim Prod Sci. 2017;57(3):415–421.
- Randel RD, Welsh Jr TH. Joint Alpharma-Beef Species Symposium: interactions of feed efficiency with beef heifer reproductive development. J Anim Sci. 2013;91(3):1323–1328.
- Basarab JA, Colazo MG, Ambrose DJ, Novak S, McCartney D, Baron VS. Residual feed intake adjusted for backfat thickness and feeding frequency is independent of fertility in beef heifers. Can J Anim Sci. 2011;91(4):573–584.
