?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to investigate and characterize the spermatogonial stem cells (SSCs) in buffaloes at different stages of development, including prenatal, neonatal, prepubertal, and adult testes. We sought a comprehensive understanding of these cells through a combination of histological, immunohistochemical, and ultrastructural analyses. Specifically, we examined changes in the expression of two potential SSC markers, OCT4 and PGP9.5, using immunohistochemistry. Additionally, we conducted a real-time quantitative polymerase chain reaction (RT-qPCR) to assess the relative gene expression of OCT4 and PGP9.5. The relative expression of the OCT4 gene was down-regulated in the adult testes compared to its expression during prepubertal and neonatal life. The relative expression of the PGP9.5 gene was up-regulated in the neonatal testes and down-regulated in the prepubertal and adult testes. The spermatogonia were round, oval-to-ellipsoidal cells lying over the basement membrane (BM) with a round-to-oval nucleus. Based on the immunoexpression of the putative SSC markers, OCT4 and PGP9.5, we concluded that the proportion of stem cells was highest during the neonatal stage, followed by the prepubertal and prenatal stages. This finding sheds light on the dynamics of spermatogonial stem cells in buffalo testes at different developmental stages, providing valuable insights into these cells’ regulation and potential applications.
Introduction
The buffalo (Bubalus bubalis) is a significant domestic livestock of India, contributing major milk and meat production. Understanding the molecular mechanisms involved in developing and maturing reproductive organs is crucial for improving livestock breeding programs. As an essential reproductive organ, testis plays a vital role in male fertility. Investigating the genes expression in buffalo testes during prenatal and post-natal stages provides valuable insights into the regulatory processes underlying testicular development. Furthermore, understanding the temporal and spatial expression profiles of OCT4 and PGP9.5 in buffalo testes will help uncover their putative roles in testicular development. It may provide important insights into reproductive biology.
Among the genes involved in testicular development, OCT 4 and PGP 9.5 have gained significant attention due to their crucial role in cellular differentiation. OCT 4 is a pluripotency transcription factor. PGP 9.5 also known as UCHL1 (ubiquitin carboxyl-terminal esterase L1), is a gene that encodes a protein called ubiquitin carboxyl-terminal hydrolase isozyme L1, and is widely expressed in various tissues throughout the body, including the testes. PGP9.5 is expressed explicitly in Sertoli cells within the seminiferous tubules of the testes and plays an essential role in spermatogenesis.
After birth, the gonocytes relocate toward the basal membrane and resume proliferation, giving rise to the adult spermatogonial stem cells.Citation1 Recently, pluripotent, very small embryonic-like stem cells (VSELs) have also been reported in the testes of humans, Bhartiya et al. (2010)Citation2 and mice (Anand et al. 2014; Kaushik and Bhartiya 2020)Citation3,Citation4 in addition to the presence of spermatogonial stem cells (SSCs) as distinct stem cell population. Spermatogonial Stem Cells could be used as a tool for buffalo germplasm conservation.Citation5
The OCT4 and PGP9.5 gene expression in buffalo testes and temporal expression during prenatal and postnatal stages have not been studied till now in buffalo. The objective of this study is to employ molecular techniques such as reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and immunohistochemistry to analyze the expression levels of OCT4 and PGP 9.5 in buffalo testes at different developmental stages.
Material and Methods
Materials
Collection of embryos/fetuses and estimation of age
The present study was conducted on testes from prenatal and postnatal life of Indian buffaloes. The prenatal samples included the testes from first trimester to third trimester and the age of embryo/fetuses were calculated by known formula based on curved crown-rump length (CVRL). The postnatal life was divided into three subdivisions (neonatal, prepubertal and pubertal) based on age of the animal and confirmed by the histological structure.
Embryos/fetuses of different gestational ages were collected from the buffaloes presented with dystocia at the Teaching Veterinary Clinical Complex of Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, and abortion cases from the organized livestock farms in and around Ludhiana. The ages of fetuses were recorded by the history of insemination/natural service or by measuring the curved crown-rump length (CVRL). After measuring the curved crown-rump length (CVRL), the approximate ages were calculated using the formula.Citation6
Grouping of embryos/fetuses
Embryos and fetuses were classified into three groups based on the CVRL measurements representing early, mid, and late gestational stages: Group I: Embryos and fetuses of up to 19.5 cm CVRL; Group II: Fetuses more than 19.5 cm up to 40 cm CVRL; Group III: Fetuses more than 40 cm CVRL.
Collection of testes from postnatal life and grouping
The buffalo testes of all other age groups were collected from M K Overseas, Dera Basi, local abattoir at Bareilly, and from the buffaloes presented with dystocia at Teaching Veterinary Clinical Complex of Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab and were grouped into neonatal, prepubertal and adults animals. The testes were utilized for histomorphological, immunohistochemical, and ultrastructural studies. Testes were located either in the inguinal area or in the scrotum. The scrotum or inguinal canal was opened to collect the testes from neonatal calves. The scrotum was opened to collect the testes from prepubertal calves and pubertal adult buffalo bulls. Animals aged more than 20 months were tentatively classified in the adult stage, which was confirmed later by a histological picture of the testis with active spermatogenesis.
Methods
Histomorphological studies
The embryos/foetii and testis of buffaloes were fixed in 10% neutral buffered formalin and processed for paraffin sectioning by dehydrating in ascending grades of alcohols and acetone and were cleared in benzene and infiltrated and embedded in paraffin. Sections were cut at 4-5μm thickness for histological study and were subjected to hematoxylin and eosin stain for histomorphological details.Citation7
Immunohistochemical studies
The immunohistochemical staining protocol was used as described in our earlier publication.Citation8 Briefly, the paraffin sections were mounted on the positively charged slides and incubated at 60 °C for one hour for better adherence of the sections to the slides. The sections were deparaffinized using xylene and rehydrated to water using descending grades of alcohol. Heat-induced antigen retrieval was done in the microwave in citrate buffer solution at 95 °C-98 °C for 10 minutes. After washing in 0.1 M phosphate buffer saline (PBS), sections were incubated with 3% (v/v) H2O2 in methanol for 20 min to inhibit the endogenous peroxidase activity. To avoid the nonspecific binding of antibodies, 2.5% normal horse serum was employed for 30 minutes. The sections were treated overnight at 4 °C with ready-to-use primary antibodies (predetermined dilutions) from Biogenex, and universal secondary antibodies conjugated with enzyme horseradish peroxidase (Vector Laboratories) were used to identify any bound antibodies. The chromogen used was 3, 3′-diaminobenzidine tetrahydrochloride (DAB) and Gill’s III hematoxylin for nuclear counterstaining. The sections were washed in running tap water, dehydrated, cleared using xylene, and mounted with DPX mounting media.
Microphotography and quantification
Photomicrographs were taken at different magnifications for each section by a bright-field microscope with an attached camera and photography unit (Eclipse 80i, Nikon, Japan). 6-10 photomicrographs at 400 magnifications were taken to count the positive and negative cells. Images were processed, and positive cells were estimated using the Image J software using its cell counter plugin (version 1.49d).Citation9
Statistical analysis
Data on percentage positive cells for OCT4 and PGP9.5 was subjected to statistical analysis to determine the statistical difference between the different developmental stages. Test for analyzing mean of all data and correlation between OCT4 and PGP9.5, T-test, and Duncan’s multiple range tests were applied to the data using SAS software.
Transmission electron microscopic studies
The transmission electron microscopic study’s processing technique was carried out as per our previous publication instructions.Citation10 Briefly, the tissues from the testes of buffalo fetuses and adult buffaloes testes were trimmed to 1 mm3 size. And fixed for 2 hours in Karnovsky’s fixative, and then secondary fixation was done for 2 hours in 2% OsO4. The tissue samples were dehydrated in ascending grades of acetone (30% to absolute) and in dry acetone at room temperature. The clearing of the samples was accomplished by treatment with toluene. Subsequently, infiltration and embedding and polymerization were done. The prepared blocks’ semi-thin sections (.5-2.0 µm) were cut and stained with toluidine blue to scan the tissues for ultrathin sectioning. The ultrathin sections (70–90 nm) were sliced, and the grids with sections were stained for 15 minutes with uranyl acetate and then with lead citrate (10 min). The grids were then thoroughly investigated under a TEM, and the necessary pictures were taken.
Quantitative Real-time PCR (qPCR)
Sample collection
Tissue samples were collected from prenatal, neonatal, prepubertal, and pubertal buffalo testes in RNAlater (Sigma-Aldrich, CA, USA) to preserve the RNA integrity of the samples.
RNA isolation and quality check
The total RNA isolation was performed as per our earlier study.Citation11 Briefly, tissues were thawed on ice for 5 mins. Thawed sample (∼100mg) was placed in a pre-labelled 2 ml nuclease-free tube containing 500 μl of RNAiso Plus, a total RNA extraction reagent. Each sample was homogenized using a Qiagen handheld homogenizer at its top speed and placed on ice. Then after adding the remaining 500 μl of RNAiso Plus and centrifugation at 12,000 × g for 5 min at 4 °C, the supernatant was transferred to a new tube containing 250 μl of chloroform and mixed by vigorous shaking. After incubating for 10 min, centrifugation was done at 12,000 × g for 15 mins at 4 °C. After centrifugation, the upper aqueous phase was transferred to the filter column and centrifuged for 1 min at 11,000 × g to shear large-sized DNA, and the filtrate was collected in a collection tube fitted with a filter. About 350 μl of 70% ethanol was added to the filtrate, and samples were loaded into the RNA binding column, followed by centrifugation for 35 sec. After washing, RNA was eluted with 40 μl of nuclease-free water after a couple of washing steps. The RNA purity and quantity were determined by NanoDrop ND-1000 spectrophotometer and ratios of OD (260/280 and 260/230) and concentrations were calculated.
cDNA synthesis and real-time qPCR
The isolated testicular RNA was used for the cDNA synthesis to check the expression of OCT4 and PGP9.5 genes. The cDNA was synthesized with the 1000 ng of total RNA using iScript RT Supermix (BioRad, Ca,USA) as per manufacturer instructions. RT-qPCR was performed in duplicate, with each reaction mixture (10 μl) containing 4 μl iScript RT supermix, and RNA template. The qPCR assays were performed using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, CA, USA) with the following cycling conditions: 95 °C for 3 min, 39 repeating cycles of 95 °C for 10 sec, 60 °C for 30 sec, 95 °C for 5 sec. The melt curve analysis was performed with a temperatureat 0.5 °C/sec from 65 °C to 95 °C. A 20 μl of reaction consisted of 10 μl of 2 × power SYBR green master mix buffer (Brilliant III Ultra-Fast SYBR QPCR, Agilent Technologies) and 0.5 μl of forward and 0.5 μl reverse primer (10 mM concentration of each primer), 2 μl of cDNA template and the remaining nuclease free water.
The primers used in this study were gifted from the Animal Stem Cells Lab of the College of Animal Biotechnology, GADVSU (). They were designed using Primer3 keeping one of the primers spanning between the exon and intron region to avoid DNA amplification.
Table 1. Target genes, primer sequence, annealing temperature ™ and amplicon size of RT-qPCR.
Results
The results of the present investigation were divided into two major developmental stages, viz. prenatal and postnatal.
Histomorphology and immunohistochemistry
Prenatal life
This study was conducted on fetuses from 38.5 days to 280 days of gestation to analyze the location of SSCs and the development of testicular cords. Upon opening the fetuses, testes were located in different areas in fetuses of various age groups.
Early prenatal life (CVRL 1.7 to 20 cm)
At 1.7 cm CVRL fetus, the genital ridge could not be visualized in the present study. At approximately 38.5 days of gestation (CVRL = 2.2 cm), the genital ridge was medio-ventral to the mesonephric kidney (). The mesonephric kidney was more significant at this stage than the metanephric kidney. The genital ridge comprised mesenchymal cells, immature RBCs, differentiating fibroblasts, and primordial germ cells (PGC). The PGCs were observed to be located between the mesenchymal cells. These cells were marked by their intense dark staining, prominent spherical to the oval nucleus, as seen in hematoxylin and eosin-stained sections. At this stage, the RBCs observed were nucleated.
Figure 1. Paraffin sections of prenatal buffalo fetus (CVRL = 2.2 cm) showing genital ridge (outline), which is located on the ventromedial aspect of mesonephros (MsK) [a]. at 2.7 cm CVRL, the gonadal ridge (shape) shows an increase in size and development of tunica albuginea (TA) and testicular parenchyma (TP) [B, C]. at 3.7 and 4 cm CVRL, an indifferent gonad located between the mesonephric kidney (MsK) and metanephric kidney (MtK) [D, E] and comprises mesenchymal cells, immature RBCs, differentiating fibroblasts (F) and primordial germ cells (arrow) [F]. a strongly positive reaction was seen by PGCs (arrow) for immunostaining with OCT4 [G] and PGP9.5 [H].
![Figure 1. Paraffin sections of prenatal buffalo fetus (CVRL = 2.2 cm) showing genital ridge (outline), which is located on the ventromedial aspect of mesonephros (MsK) [a]. at 2.7 cm CVRL, the gonadal ridge (shape) shows an increase in size and development of tunica albuginea (TA) and testicular parenchyma (TP) [B, C]. at 3.7 and 4 cm CVRL, an indifferent gonad located between the mesonephric kidney (MsK) and metanephric kidney (MtK) [D, E] and comprises mesenchymal cells, immature RBCs, differentiating fibroblasts (F) and primordial germ cells (arrow) [F]. a strongly positive reaction was seen by PGCs (arrow) for immunostaining with OCT4 [G] and PGP9.5 [H].](/cms/asset/525616cd-1a56-41db-a74b-f31e08213984/labt_a_2285509_f0001_c.jpg)
At 41 days of gestation (CVRL = 2.7 cm), the genital ridge was noted as a roughly quadrilateral mass () and was still located near the mesonephric kidney. By this stage, the mesenchymal cells appeared to be making a round, whorl-like pattern of testicular cord formation along with the PGCs. Immature nucleated RBCs were scattered in the parenchyma of the indifferent gonad. Immunostaining at this stage for anti-OCT4 antibodies showed an intensely positive reaction () anddid not stain with PGP9.5 antibody. The immunopositive stained cells were large spherical cells with a high nucleus-to-cytoplasm ratio and strong cytoplasmic and nuclear staining.
At 45 days of gestation (CVRL = 3.7 cm), the indifferent gonad was seen a little away from the mesonephric kidney and between the metanephric and mesonephric kidneys. The parenchyma of the testes had developed, giving it a more oval shape, as seen in its cut cross-section. The mesonephros, located laterally to the testes, degenerated compared to earlier stages. On the other hand, the metanephric kidney was developing medial to the testes ( and ). At 46.6 days of gestation (CVRL = 4 cm), the indifferent gonad had increased in size and was found between the metanephric kidney laterally and the mesonephric kidney medially in the lumbar region. The size of the mesonephros was observed to have decreased drastically compared to the extent seen at 45 days of gestation (). At this stage, the PGCs stained intensely positive for OCT4 (Figure G) and PGP9.5 (). The metanephric kidney had developed substantially at this stage with a distinct cortex, medulla, and glomeruli. The size of the indifferent gonad also increased due to the development of the testicular parenchyma. The indifferent gonad was now seen as an elongated oval structure. In stained slides, mesenchymal cells were organized around PGCs, similar to cord formation. Immunostaining for Oct4 protein showed the presence of darkly stained PGCs, while that for anti-PGP9.5 also gave strong positive results ( and ).
By 52 days of gestation (CVRL = 5.2 cm), the formation of prospective seminiferous cords was noticed (). Immunostaining for OCT4 showed a strong positive reaction by PGCs, indicating their undifferentiated state (). Immunostaining for anti-PGP9.5 also showed a strong positive response by PGCs. (). The testis had reached the pelvic cavity by 62.8 days of gestation (CVRL = 7.6 cm). The blood vessels and testicular cords enclosing germ cells and supporting cells () were observed. Immunostaining on OCT4 revealed strong positive results (), and with PGP9.5 () resulted in intensely stained germ cells. At 71.4 days of gestation (CVRL = 9.5 cm), complete organization of testicular cords was seen (). Gonocytes were seen at the center, and a few were seen toward the periphery of the testicular cords. Immunostaining with OCT4 showed strong positive results (), and immunostaining with PGP9.5 showed intense staining of gonocytes, indicating their development into male germ cells ().
Figure 2. Paraffin sections of prenatal testicular tissue at 5.2 cm CVRL showing the formation of prospective seminiferous cords [A] with PGCs, which stain immunopositive for OCT4 [B] and PGP9.5 [C]. at 7.6 cm CVRL, fully formed cords were seen with gonocytes (arrow) located in the center and toward the periphery of the cords [D]. These gonocytes stained strongly positive for OCT4 [E] and PGP9.5 [F]. at 9.5 cm CVRL organized testicular cords were seen with gonocytes (arrow) localized in the center of cords while few were seen toward the periphery [G]. These cells gave a strong positive result for OCT4 [H] and PGP9.5 [I, J]. at 22 cm CVRL, well-organized testicular cords were seen with gonocytes in the center and toward the periphery of the cords [K], noted by the extension of their cytoplasmic processes seen when immunostained with OCT4 [L]. by 255 days of gestation, extensive and tortuous seminiferous cords were seen with more gonocytes [M] which stains strongly positive for PGP9.5 [N, O].
![Figure 2. Paraffin sections of prenatal testicular tissue at 5.2 cm CVRL showing the formation of prospective seminiferous cords [A] with PGCs, which stain immunopositive for OCT4 [B] and PGP9.5 [C]. at 7.6 cm CVRL, fully formed cords were seen with gonocytes (arrow) located in the center and toward the periphery of the cords [D]. These gonocytes stained strongly positive for OCT4 [E] and PGP9.5 [F]. at 9.5 cm CVRL organized testicular cords were seen with gonocytes (arrow) localized in the center of cords while few were seen toward the periphery [G]. These cells gave a strong positive result for OCT4 [H] and PGP9.5 [I, J]. at 22 cm CVRL, well-organized testicular cords were seen with gonocytes in the center and toward the periphery of the cords [K], noted by the extension of their cytoplasmic processes seen when immunostained with OCT4 [L]. by 255 days of gestation, extensive and tortuous seminiferous cords were seen with more gonocytes [M] which stains strongly positive for PGP9.5 [N, O].](/cms/asset/cce83813-6bb8-4f1b-ab2b-7033c5f8f2e0/labt_a_2285509_f0002_c.jpg)
At 114.1 days of gestation (CVRL = 19 cm), well-organized testicular cords were seen. Gonocytes were seen in the center and toward the periphery. The gonocytes gave strong positive results for immunohistochemistry on OCT4 and stained intensely positive when immunostained with PGP9.5.
Mid prenatal life (CVRL 20 to 40 cm)
By approximately 123.176 days of gestation (CVRL = 22 cm), testicular cords were seen to have better organized. Gonocytes were seen in the center and toward the cords’ periphery, which was noted by the extension of their cytoplasmic processes (). These gonocytes also stained positively for OCT4 () and PGP9.5 ().
Late prenatal life (CVRL >40 cm)
At eight months of gestation, seminiferous cords were seen to have become extensive and tortuous compared to early and mid-prenatal life, i.e., the development of cords had increased vastly in late prenatal life (). More gonocytes in this stage are seen toward the periphery, although not in contact with the basement membrane. The proportion of gonocytes in this prenatal stage had increased drastically compared to early and mid-prenatal life. Numerous intensely positive gonocytes are seen in the seminiferous cords in the testicular parenchyma when immunostained with PGP9.5 ( and ). On the other hand, the mediastinum testis reacted negatively to any immunostaining, indicating the absence of gonocytes in the developing rete testis. The gonocytes at this stage were also positive for OCT4.
Postnatal life
As mentioned earlier, the postnatal life of buffalo testes was divided into neonatal, prepubertal, and adult stages.
Neonatal stage
Testes that were elongated in shape had descended to the abdominal cavity, located in the scrotum in the neonatal phase of postnatal life. The distinction of cells as germline stem cells (GSCs)/gonocytes and supporting sustentacular cells was evident in the highly coiled seminiferous cords placed in the testicular parenchyma. Gonocytes were observed toward the center of the seminiferous cords loosely supported by Sertoli cells. The SSCs were round cells with distinct spherical to the oval nucleus. The cytoplasm was stained homogeneously, while the nucleus showed the loose distribution of heterochromatin and euchromatin (). Immunostaining for anti-OCT4 () and anti-PGP9.5 () both found strong positive and confirming the presence of male germ cells and their pluripotency. The reactivity was nuclear as well as cytoplasmic. As advanced age, the number of GSCs increased, and migration of GSCs was noticed toward the periphery of seminiferous cords, which kept growing. The GSCs close to the basement membrane seemed to be held in an adluminal compartment made by cytoplasmic extensions of the Sertoli cells, suggestively for the SSCs to remain pluripotent.
Figure 3. Paraffin sections of neonatal buffalo testes showing gonocytes in the center and toward the periphery of developed seminiferous tubules loosely supported by Sertoli cells [a], Immunostaining for OCT 4, inset with a magnified view of OCT4 immunoreaction [B], and PGP 9.5 [C] both yielded strong positive reactions confirming the presence of male germ cells and their pluripotency. Paraffin sections of prepubertal buffalo testes show that amination had occurred [D]. The proportion of cells positive for OCT4 [E] had reduced in prepubertal life, while a good number of PGP 9.5 [F] positive cells were seen. Paraffin sections of adult buffalo testes show large seminiferous tubules with SSCs located at the periphery of the basement membrane [G], fewer cells were positive for OCT4 [H], but good numbers of SSCs were positive for PGP 9.5 [I].
![Figure 3. Paraffin sections of neonatal buffalo testes showing gonocytes in the center and toward the periphery of developed seminiferous tubules loosely supported by Sertoli cells [a], Immunostaining for OCT 4, inset with a magnified view of OCT4 immunoreaction [B], and PGP 9.5 [C] both yielded strong positive reactions confirming the presence of male germ cells and their pluripotency. Paraffin sections of prepubertal buffalo testes show that amination had occurred [D]. The proportion of cells positive for OCT4 [E] had reduced in prepubertal life, while a good number of PGP 9.5 [F] positive cells were seen. Paraffin sections of adult buffalo testes show large seminiferous tubules with SSCs located at the periphery of the basement membrane [G], fewer cells were positive for OCT4 [H], but good numbers of SSCs were positive for PGP 9.5 [I].](/cms/asset/cc41dd74-3788-468c-a621-642c2cad2bc9/labt_a_2285509_f0003_c.jpg)
Prepubertal stage
Testes of prepubertal buffalo were relatively large and elongated in shape. A hematoxylin and eosin-stained section of the testis revealed that lumen formation had occurred by one year of age due to liquefaction of the ground substance of seminiferous cords (). The seminiferous tubules in this stage were also relatively more extensive than in the late neonatal stage, probably due to the lumen formation. At this stage of development, few SSCs were seen toward the center of the forming tubules and migrating. At the same time, most SSCs were located toward the periphery of seminiferous tubules where the SSCs had established contact with the basement membrane while settling in between the care of Sertoli cells. A good number of SSCs were immunopositive for PGP9.5, and comparatively fewer SSCs were positive for OCT4. Immuno-positive cells expressing OCT4 () were seen in moderate amounts compared to the neonatal stage, with even staining of the nucleus and cytoplasm. In contrast, PGP9.5 expression was reduced in the prepubertal stage ().
Adult stage
Histomorphology of adult testis revealed large-sized seminiferous tubules, and spermatogenesis was noticed to be fully established by the presence of a good number of spermatids in varied phases of spermiogenesis (). Basally located SSCs were seen partly flattened from the point of contact with the basement membrane dispersed between Sertoli cells. The expression of OCT4 () was drastically reduced in adult buffaloes. In contrast, the expression of PGP9.5 () was increased compared to prepubertal buffalo’s testes expression. However, it was downregulated compared with the neonatal immunopositive reaction of cells.
The variation in the percentage of positive cells for OCT4 and PGP9.5 in prenatal and postnatal life was observed (). The protein expression of OCT4 was higher in early prenatal life, while that of PGP9.5 was high in both prenatal and postnatal life. The correlation of OCT4 and PGP9.5 from prenatal to adult life showed a significant difference. While OCT4 positive cells decreased from prenatal to postnatal stage, the proportion of PGP9.5 positive cells remains unchanged (; ).
Figure 4. Proportion of positive cells for OCT4 (A) and PGP9.5 (B) in all prenatal and postnatal life stages.
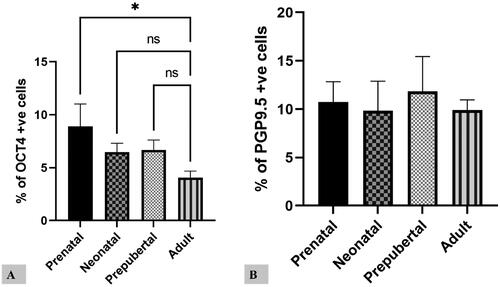
Table 2. Average proportion of positive cells in prenatal and postnatal stages.
Comparatively higher proportions of cells were favorable for OCT4 in prenatal life. In contrast, the balance of cells positive for PGP9.5 showed no significant difference in prenatal or postnatal life (). The percentage of OCT4-positive germ cells was significantly decreased from prenatal to postnatal stages. Among subgroups of prenatal life, group I was considerably higher for OCT4. In contrast, the postnatal life adult group was significantly lower for OCT4 (). The maximum proportion of PGP9.5 positive cells was observed in the prepubertal stage. Among subgroups of prenatal life, group I was significantly higher for PGP9.5. In contrast, there was no significant difference in postnatal life ().
Relative mRNA expression of OCT4 and PGP9.5
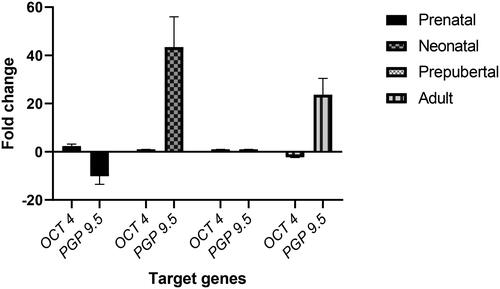
The present study was conducted to evaluate the relative gene expression of the spermatogonial stem cell markers (OCT4 and PGP9.5) to support the findings of immunohistochemical studies on spermatogonial stem cell markers in testes during different developmental stages. Both the genes were successfully amplified with a single peak in the melt curve analysis, and a single amplicon was visualized in the agarose gel electrophoresis (figures not shown). The mean relative expression (delta Ct) of genes varied among the different stages of development. The expression of the OCT4 gene was down-regulated in the adult testes compared to the prepubertal and neonatal stages. The relative expression of the PGP9.5 () was up-regulated (p < 0.05) in the neonatal testes, compared to all the post-natal stages.
Transmission electron microscopic studies
At the early stage of development (5.2 cm CVRL), the primordial germ cells were seen in between the mesenchymal cells, fibroblasts developing into peritubular myoid cells ( and ). At places, they looked to form testicular cords. The PGCs were large, round cells with large nuclei and a thin rim of cytoplasm. Heterochromatin was dispersed in between the euchromatin in the nucleus. The developing myoid cells had a sizeable elongated nucleus that occupied most parts of the cell.
Figure 6. Transmission electron micrographs of prenatal testicular tissue illustrating the morphology of spermatogonial stem cells (SSC) ultrastructure. A: Semi-thin section of the early stage of development (5.2 cm CVRL) showing PGCs in between the mesenchymal cells and fibroblasts, which were developing into peritubular myoid cells. At places, they seemed to be forming testicular cords (dotted outline). Toluidine blue stain × 1000; B: The PGCs (arrow) were large, round cells with large nuclei and a thin rim of cytoplasm. Heterochromatin was dispersed in between the euchromatin in the nucleus. The developing myoid cells (M) had a sizeable elongated nucleus that occupied most parts of the cell. Toluidine blue stain × 1000; C: Electron micrograph of prenatal development at 7.6 cm CVRL showing testicular cords with gonocytes (G) and Sertoli cells surrounded by a thin basement membrane. The gonocytes were comparatively larger cells with large nuclei. The basement membrane separated the testicular cords from the blood vessels (BV). Several Leydig cells were observed in between the testicular cords. × 570; C: Electron micrograph of prenatal development at 7.6 cm CVRL showing testicular cords with gonocytes (G) and Sertoli cells surrounded by a thin basement membrane. The gonocytes were comparatively larger cells with large nuclei. The basement membrane separated the testicular cords from the blood vessels (BV). Several Leydig cells were observed in between the testicular cords. × 570. D: Magnified 20 C (box) image showing Leydig cells between the testicular cords. × 1550. E: Semi-thin section of the late gestational period (8 months of age), well-organized testicular cords with the distinctly visible basement membrane. Toluidine blue stain × 1000. F: Electron micrograph of the late gestational period showing testicular cords, gonocyte and pre-Sertoli cells. The nuclei of gonocytes were giant, surrounded by a thin rim of cytoplasm and distinctly visible nucleoli. × 830.
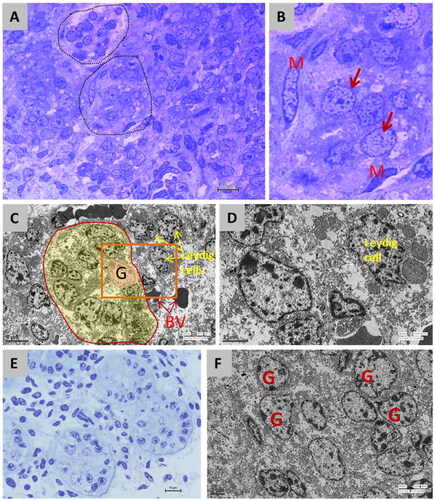
At further development at 7.6 cm CVRL, the testicular cords were seen with gonocytes and Sertoli cells surrounded by a thin basement membrane ( and ). The gonocytes were more giant cells with large nuclei. The basement membrane separated the testicular cords from the blood vessels. Several Leydig cells were observed in between the testicular cords. The gonocytes were rounded cells with centrally located rounded nucleus having prominent nucleolus containing dispersed heterochromatin and euchromatin material. At that stage, some mesenchymal cells showed an elongated nucleus with increased cytoplasmic organelles, which could transform into Leydig cells.
At the late gestational period (8 months of age), the testicular cords were well organized ( and ). The basement membrane was distinctly visible. In testicular cords, gonocyte and pre-Sertoli cells were seen. The nuclei of gonocytes were massive and surrounded by a thin rim of cytoplasm. The distribution of heterochromatin and euchromatin in the nucleus gave them a mottled appearance. The nucleoli were distinctly visible. The gonocytes were primarily seen at the center of the cords, and few were seen toward the periphery. They were round to spindle in shape and occasionally extended cytoplasmic processes. The round nucleus displayed centrally located prominent nucleoli, and the cytoplasm contained abundant glycogen particles, mitochondria of vesicular type, and occasional lipid droplets.
At the neonatal stage, the testicular cords were well organized. They were lined with a well-organized and distinct basement membrane. The cords consisted of one or two gonocytes and pre-Sertoli cells. Gonocytes were primarily located in the center of the cords ( and ). Seminiferous tubules with germ cells and Sertoli cells were seen during the prepubertal period. The germ cells were large cells seen either on the basement membrane or toward the basement membrane. The nuclei were massive, with distinct nucleoli. The transmission electron microscope showed that the heterochromatin materials were dispersed between euchromatin ( and ).
Figure 7. Semi-thin section of neonatal testicular tissue showing well-organized testicular cords lined with distinct basement membrane [A] and electron micrograph showing gonocytes located primarily on the center of the cords with large nuclei and distinct nucleoli [B]. Semi-thin section of the prepubertal period showing seminiferous tubules with large germ cells (G) and Sertoli cells [C] and electron micrograph of the same showing large nuclei of germ cell with the heterochromatin material dispersed in euchromatin [D]. Semi-thin section of the adult period showed spermatogonia (S) which were round, oval to ellipsoidal cells lying over the basement membrane [E]. Electron micrograph of adult testicular tissue showing spermatogonia with a large round nucleus (N) lying over the basement membrane (BM) with a high nucleus-to-cytoplasm ratio [F]. The heterochromatin clumps (Hc) were distributed in between the euchromatic material (Ec), which gave the nucleus a mottled appearance [G]. the cytoplasm was filled with consisted of numerous mitochondria (M) with elongated mitochondrial cristae, strands of smooth endoplasmic reticulum (SER), and parallel strands of rough endoplasmic reticulum (rER) with few vacuoles, Golgi complex and lipid droplets (L) at places [H, I]. Graphically conceptualized view of an SSC and its location [J].
![Figure 7. Semi-thin section of neonatal testicular tissue showing well-organized testicular cords lined with distinct basement membrane [A] and electron micrograph showing gonocytes located primarily on the center of the cords with large nuclei and distinct nucleoli [B]. Semi-thin section of the prepubertal period showing seminiferous tubules with large germ cells (G) and Sertoli cells [C] and electron micrograph of the same showing large nuclei of germ cell with the heterochromatin material dispersed in euchromatin [D]. Semi-thin section of the adult period showed spermatogonia (S) which were round, oval to ellipsoidal cells lying over the basement membrane [E]. Electron micrograph of adult testicular tissue showing spermatogonia with a large round nucleus (N) lying over the basement membrane (BM) with a high nucleus-to-cytoplasm ratio [F]. The heterochromatin clumps (Hc) were distributed in between the euchromatic material (Ec), which gave the nucleus a mottled appearance [G]. the cytoplasm was filled with consisted of numerous mitochondria (M) with elongated mitochondrial cristae, strands of smooth endoplasmic reticulum (SER), and parallel strands of rough endoplasmic reticulum (rER) with few vacuoles, Golgi complex and lipid droplets (L) at places [H, I]. Graphically conceptualized view of an SSC and its location [J].](/cms/asset/f526b8ad-6ea7-473f-910a-19793f61c5c3/labt_a_2285509_f0007_c.jpg)
The spermatogonia were round, oval to ellipsoidal cells lying over the BM (). The nucleus was round () to oval () in shape. The chromatin material was granular. The heterochromatin clumps were distributed between the euchromatic material, giving the nucleus a mottled appearance. The nucleoli were distinct in most of the spermatogonia (). The area occupied by the nucleus was more significant than that occupied by the cytoplasm, thus making the nucleus-to-cytoplasm ratio higher. The cytoplasm consisted of numerous mitochondria with elongated mitochondrial cristae, smooth endoplasmic reticulum (SER), and parallel strands of rough endoplasmic reticulum (RER). At places, the RER was in contact with the nuclear membrane. At places, vacuoles, Golgi complex, and lipid droplets were seen. The free ribosomes were dispersed in the cytoplasm (). The conceptualized TEM view of neonatal testicular tissue is shown ().
Discussion
This study was conducted on fetuses from 38.5 days to 280 days of gestation to analyze the location of SSCs and the development of testicular cords. SSCs have been believed to be the undifferentiated or least differentiated spermatogonia, which remain as single cells on the basement membrane of the seminiferous tubule. In the present study, we observed the position and niche of SSCs to develop from early prenatal life. There were dynamic changes in the SSCs which were most dramatic during the early developmental stage. SSCs were seen as large round, oval to ellipsoidal cells with a round or oval nucleus and a high nucleus-to-cytoplasm ratio. These cells were typically seen in histomorphological staining methods and characteristically marked during immunohistochemical studies with the help of putative markers (OCT4 and PGP9.5).
At approximately 38.5 days of gestation (CVRL = 2.2 cm), the genital ridge was seen located medio-ventral to the mesonephric kidney. The genital ridge comprised mesenchymal cells, immature RBCs, differentiating fibroblasts, and primordial germ cells (PGC). The development of genital ridge has been reported in goat embryos by 23 days of gestation, Farooqui et al. (2012)Citation14 in buffalo embryos by 40-43 days of gestation, (Roy et al. 2009 and Singh et al. 2015)Citation15,Citation16 in Shruti buffalo embryos by 38 days (Vaisya and Vyas 1999) and in sheep embryos by 37 days of gestation.Citation17 The PGCs are observed to be located between the mesenchymal cells and nucleated RBCs. The gonadal ridge has been reported as a globular or rectangular thickening on the ventromedial surface of mesonephros in buffalo fetuses at 2.5-3.0 cm CVRL by Singh et al. (2015).Citation16 Basavaiah et al. (2021)Citation17 reported in sheep fetuses that the testicular capsule had begun forming by 44 days of gestation as six to eight layers of mesenchymal cells beneath the germinal epithelium were transformed into tunica albuginea. By this stage, the mesenchymal cells appeared to be making a round whorl-like pattern of testicular cord formation along with the PGCs. Immunostaining at this stage for anti-OCT 4 and PGP 9.5 antibodies showed intense positive reactions; these immunopositive cells had characteristic features of germ cells.
The indifferent gonad was seen a little away from the mesonephric kidney and between the metanephric and mesonephric kidneys at 45 days of gestation. These findings correlated with those of Basavaiah et al. (2021),Citation17 who reported in sheep fetuses that at 46 days of gestation, testes were descended toward the caudal pole of the metanephric kidneys, and the size of the mesonephros had comparatively decreased while the size of the scrotal bud was increased. The PGCs stained intensely positive at this stage for OCT4 and PGP9.5. At 46.6 days of gestation (CVRL = 4 cm), the indifferent gonad had increased in size and was found between the metanephric kidney laterally and the mesonephric kidney medially in the lumbar region. In stained slides, mesenchymal cells are organized around PGCs, similar to cord formation. Carlson (1985) stated that PGCs in the germinal epithelium formed primitive sex cords in the gonadal mesenchyme during sexual differentiation. Immunostaining for OCT4 protein showed the presence of darkly stained PGCs, while that for anti-PGP9.5 also gave strong positive results.
Compared to the expression of OCT-4, which was localized to the nuclei, the expression level of PGP9.5 was very strong throughout the entire cytoplasm of the germ cells. The strong expression of PGP9.5, thus, makes it a much more robust marker of PGCs and SSCs than OCT4 for their identification, enrichment, and in vitro manipulations.
By 52 days of gestation (CVRL = 5.2 cm), the formation of prospective seminiferous cords is noticed. Immunostaining for OCT4 showed a strong positive reaction by PGCs, indicating their undifferentiated state. Immunostaining for anti-PGP9.5 also showed a strong positive reaction by PGCs, which had started colonizing the developing seminiferous cords. By 62.8 days of gestation (CVRL = 7.6 cm), the testis showed testicular cords which enclosed germ cells and supporting cells, similarly reported at 8.0 cm CVRL (65 days) by Kaur et al. (2011)Citation18 in buffalo. Immunostaining with OCT4 gave strong positive results, and PGP9.5 resulted in intensely stained germ cells indicating that from this stage of prenatal life, the indifferent gonad had differentiated into testes of the developing male reproductive system.
Well-organized testicular cords were seen at 114.1 days of gestation (CVRL = 19 cm). Gonocytes were observed in the center and also toward the periphery of the cords, which indicated that the germ cells were moving toward the basement membrane. Kaur et al. (2011)Citation18 reported the formation of seminiferous tubules from preexisting clusters of epitheloid cells at 18.2 cm CVR (110 days) buffalo testis. These findings were also akin to the observations of Abd-Elmaksoud (2005)Citation19 in bovine embryos. The gonocytes, which had increased in number perspectively, were surrounded by Sertoli cells.
By approximately 118.7 days of gestation (CVRL = 20 cm), the size of the organized testicular cords was seen to have increased further. Gonocytes were seen both in the center and toward the periphery, and the extension of their cytoplasmic processes was noted. However, Abd-Elmaksoud (2005)Citation19 reported in 23 cm CRL fetuses of bovine that most spermatogonia are situated in the periphery of the testicular cords. Still, some are more or less located in the center of these cords also. These gonocytes were also stained positively for OCT4 and PGP9.5. At eight months of gestation, seminiferous cords were seen to have become extensive and tortuous compared to early and mid-prenatal life. More gonocytes in this stage are seen toward the periphery, although not in contact with the basement membrane. The proportion of gonocytes in this prenatal stage had increased drastically compared to early and mid-prenatal life. In bovines, Abd-Elmaksoud (2005)Citation19 reported that the seminiferous cords in 63-90cm CRL fetuses are widely separated from each other by the continuously dedifferentiated interstitium and delimited by well-definitive basal lamina as well as two layers of peritubular cells.
As mentioned earlier, the postnatal life of a buffalo was divided into neonatal, prepubertal, and adult stages. The SSCs are self-renewing population of stem cells in testis and, upon appropriate culture conditions, can acquire multipotency in vitro and grown as germline stem (GS) cells. A group of researchers working on testicular stem cells has also identified pluripotent, VSELs in humans, Bhartiya et al. (2010)Citation2 and mice (Anand et al. 2014; Kaushik and Bhartiya 2020)Citation3,Citation4 as a distinct stem cell population.
Neonatal stage
Gonocytes were observed toward the center of the seminiferous cords loosely supported by Sertoli cells. The SSCs were round cells with distinct spherical to the oval nucleus. Immunostaining for anti-OCT4 and anti-PGP9.5 both yielded strong positive reactions confirming the presence of male germ cells and their pluripotency. The reactivity was nuclear as well as cytoplasmic. As age advanced, the number of GSCs increased and migration of GSCs was noticed toward the periphery of seminiferous cords which kept growing in size. The GSCs, which were located close to the basement membrane, seemed to be held in an adluminal compartment made by cytoplasmic extensions of the Sertoli cells, suggestively for the SSCs to remain pluripotent. With the progression of age and as the SSCs migrated, marked changes were observed, like karyolysis, which made the euchromatin to heterochromatin ratio higher, giving it sort of a mottled appearance.
In the present study, the putative SSCs showed strong immune reactions of OCT4, although weak cytoplasmic reactions were also seen. There could be two possible explanations: 1) due to the lack of buffalo-specific OCT4 monoclonal antibody, we used a polyclonal antibody, which may have given some background cytoplasmic immune reaction; 2) Two isoforms of OCT4 have been reported in the literature for stem cells. Although it is unknown which isoform contributes to the undifferentiated phenotype, isoform A is detected in nuclei, whereas isoform B is detected in the cytoplasm.Citation20,Citation21 Thus, positive cytoplasmic signals may also be due to the presence of isoform B, whose function is unknown. The upregulation of PGP9.5 transcript during non-breeding seasons has been reported in pre-pubertal than in adult caprine male germline stem cells.Citation22
Prepubertal stage
Testes of prepubertal buffalo were relatively large and elongated in shape. An H&E stained section of the testis revealed that lumination had occurred by one year of age due to the liquefaction of the ground substance of seminiferous cords. At this stage of development, few SSCs are seen toward the center of the forming tubules and migrating. At the same time, most SSCs are located toward the periphery of seminiferous tubules where the SSCs had established contact with the basement membrane while settling in between the care of Sertoli cells. A good number of SSCs were immunopositive for PGP9.5, and comparatively fewer SSCs were positive for OCT4. Immunopositive cells expressing OCT4 are seen in moderate amounts compared to in the neonatal stage, with even staining of the nucleus and cytoplasm. In contrast, PGP9.5 expression was reduced in the prepubertal stage.
Adult stage
The testes in adult buffalo were ovoid and larger. It was covered by a serosal layer called tunica vaginalis. Histomorphology of adult testis revealed large-sized seminiferous tubules, and spermatogenesis was noticed to be fully established by the presence of a good number of spermatids in varied phases of spermiogenesis. Basally located SSCs were seen partly flattened from the point of contact with the basement membrane dispersed between Sertoli cells. The expression of OCT4 was drastically reduced in adult buffaloes. In contrast, the expression of PGP9.5 was increased compared to the results of prepubertal buffalo’s testes expression. However, it was downregulated compared with the neonatal immunopositive reaction of cells. This suggests that the population of SSCs keeps increasing in adult life also.Citation23 reported in monkeys that PGP9.5 is expressed in the cytoplasm of 1) all of the type Ad spermatogonia; 2) a group of the type Ap spermatogonia, and 3) none of the type B spermatogonia. In the present study, the putative SSCs showed strong immune reaction of OCT4, although weak cytoplasmic localization were also seen. As elaborated above, the weak cytoplasmic immune reaction could either be due to background fluoresce caused by the use of a polyclonal antibody or due to the presence of OCT4 isoform B, whose function is not yet known. Nonetheless, the presence of the nuclear signal of OCT4 in our study correlates with the pluripotent state. The expression of PGP 9.5 in testicular cells appears to vary between rodents and non-rodent animals. While in mice, it is reported to be expressed by both spermatogonia and Sertoli cells,Citation24–26 it is specifically expressed in gonocytes and spermatogonia of cattle,Citation27 boarsCitation28 goatsCitation22,Citation29 and monkeys (Tokunaga et al., 2012). In the present study, the expression of PGP9.5 was observed in all the stages of testicular development; however, a lower proportion of PGP9.5 positive cells were observed in prepubertal testes as compared to the neonatal and adult stages. The distribution pattern of the PGP 9.5 – positive cells was consistent with the gonocyte migration and location of spermatogonia in the seminiferous tubules of neonatal, prepubertal, and mature buffalo testis, which is consistent with those reported earlier in other non-rodent species such as cattle and pigs.
The mean relative expression (delta Ct) of genes–varied among the different stages of development. The relative expression of the OCT4 gene was down-regulated in the adult testes about its expression during prepubertal and neonatal life. The relative expression of the UCHL1 (PGP9.5) gene expressed in terms of fold chain was down-regulated in the prepubertal testes. It was up-regulated in the neonatal testes about its expression during adult life.
Transmission electron microscopic studies
At the early stage of development (5.2 cm CVRL), the primordial germ cells were seen in between the mesenchymal cells, fibroblasts developing into peritubular myoid cells. At places, they looked to form testicular cords. The PGCs were large, round cells with large nuclei and a thin rim of cytoplasm. Heterochromatin was dispersed in between the euchromatin and the nucleus. The developing myoid cells had a sizeable elongated nucleus that occupied most parts of the cell.
At further development at 7.6 cm CVRL, the testicular cords were seen with gonocytes and Sertoli cells surrounded by a thin basement membrane. The gonocytes were more giant cells with large nuclei. The basement membrane separated the testicular cords from the blood vessels. Several Leydig cells were observed in between the testicular cords.Citation16 reported three types of germ cells gonocytes, intermediate, and prespermatogonia cells at 17.5 cm CVRL. The gonocytes were rounded cells with centrally located rounded nucleus having prominent nucleolus containing dispersed heterochromatin and euchromatin material. At that stage, some mesenchymal cells showed elongated nuclei with increased cytoplasmic organelles, which could be transforming into Leydig cells.
The testicular cords were well organized at the late gestational period (8 months of age). The basement membrane was distinctly visible. In testicular cords, gonocyte and pre-Sertoli cells were seen. The nucleoli were distinctly visible. The gonocytes were seen mainly at the center of the cords and few were seen toward the periphery.Citation30 performed studies on the electron microscopy of the primordial germ cells on six human embryos between 26 and 31 days post-conception. They reported from their results that primordial germ cells were found in the interstitial tissue of the dorsal mesentery or the genital ridge. They were round to spindle in shape and occasionally extended cytoplasmic processes. The round nucleus displayed centrally located prominent nucleoli, and the cytoplasm contained abundant glycogen particles, mitochondria of vesicular type, bundles of monofilaments, and occasional lipid droplets.
At the neonatal stage, the testicular cords were well organized. They were lined with a well-organized and distinct basement membrane. The cords consisted of one or two gonocytes and pre-Sertoli cells. Gonocytes were primarily located in the center of the cords. Seminiferous tubules with germ cells and Sertoli cells were seen during the prepubertal period. The germ cells were large cells seen either on the basement membrane or toward the basement membrane. The nuclei were huge with distinct nucleoli. The transmission electron microscope showed that the heterochromatin material was dispersed in euchromatin.
The spermatogonia were round, oval to ellipsoidal cells lying over the BM. The chromatin material was granular. The heterochromatin clumps were distributed between the euchromatic material, giving the nucleus a mottled appearance. The cytoplasm consisted of numerous mitochondria with elongated mitochondrial cristae, smooth endoplasmic reticulum (SER), and parallel strands of rough endoplasmic reticulum (RER). At places, the RER was in contact with the nuclear membrane. These cells might be actively dividing progenitor cells. Abd-Elmaksoud (2005)Citation19 in their study on the ultrastructure of the adult bovine testis, reported that the basal lamina is surrounded by 3-5 layers of partially overlapping myofibroblasts covered on both sides by an inconstant basal lamina. The cytoplasmic matrix of myofibroblasts appeared appreciably electron-dense and contained few mitochondria and some elongated profiles of rER. A peculiar and frequent feature of the cell membrane was round or pear-shaped micro-pinocytotic vesicles.
Conclusion
The findings of the present study are summarized as: SSCs migrate from the center to the periphery, from after-birth until adulthood in buffalo when they finally settle down on the basement membrane supported by Sertoli cells. The stemness of SSCs remains until maturity, but it was noticed that the proportion of SSCs significantly decreased by adult life when observed both at the protein level and gene level. The number of SSCs increased with the development of the testis and its tubules, with the approximate maximum proportion being present in neonatal and prepubertal life. The immunoexpression of putative SSC markers, OCT4 and PGP9.5, concluded that stem cell proportion was highest in the neonatal stage, followed by prepubertal and prenatal stages. Based on the recent work reporting pluripotent VSELs in humans and mice, its existence in buffaloes will be worth investigating. Neonatal testes will be the choice of tissue for harvesting SSCs for its use in testicular regenerative medicine.
In conclusion, our study contributes to the understanding of buffalo SSCs and their behavior throughout development. These findings have implications for both basic research and the potential clinical applications of SSCs in reproductive medicine and regenerative therapies. Further investigations into VSELs in buffaloes hold promise for expanding our knowledge of stem cell biology and its translational applications.
Compliance with ethical standards
The research was conducted following ethical standards and guidelines of the Institutional Animal Ethics Committee (IAEC), Guru Angad Dev Veterinary and Animal Sciences, Ludhiana, Punjab, India. It was duly approved by the Dean of Post-graduate Studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21(6):776–798.
- Bhartiya D, Kasiviswanathan S, Unni SK, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as a stem cell marker. J Histochem Cytochem. 2010;58(12):1093–1106.
- Anand S, Bhartiya D, Sriraman K, Patel H, Manjramkar DD. Very small embryonic-like stem cells survive and restore spermatogenesis after busulphan treatment in mouse testis. J Stem Cell Res Ther. 2014;4:216.
- Kaushik A, Bhartiya D. Additional evidence to establish existence of two stem cell populations including VSELs and SSCs in adult mouse testes. Stem Cell Rev Rep. 2020;16(5):992–1004.
- Devi L, Goel S. Spermatogonial stem cells and testis-tissue cryopreservation as a tool for conservation of buffalo germplasm. In: Biotechnological Applications in Buffalo Research. Singapore: Springer Singapore; 2022:413–438.
- Soliman MK. Studies on the physiological chemistry of the allantoic and amniotic fluids of buffalo at various periods of pregnancy. Indian Vet J. 1975;52:106–111.
- Pathak D, Bansal N. Histomorphological studies on the different nuclei of hypothalamus of Indian buffalo. Indian J. Anim. Res. 2021;55(8):973–978.
- Pathak D, Bansal N, Singh O, Gupta K, Ghuman SPS. Immunohistochemical localization of estrogen receptor alpha (Erα) in the oviduct of Indian buffalo during follicular and luteal phases of estrous cycle. Trop Anim Health Prod. 2019;51(6):1601–1609.
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675.
- Pathak D, Bansal N. A transmission electron microscopic study on the buffalo uterus during follicular and luteal phases of estrous cycle. Indian J. Anim Sc. 2011;81(7):704–707.
- Choudhary RK, Pathak D, Choudhary S, Verma R. Immunolocalization of estrogen alpha and progesterone beta receptors in the goat mammary gland. Indian J. Anim. Sci. 2018;88:31.
- Mahla RS, Reddy N, Goel S. Spermatogonial stem cells (SSCs) in Buffalo (Bubalus bubalis) testis. PLoS One. 2012;7(4):e36020.
- Choudhary RK, Choudhary S, Mukhopadhyay CS, Pathak D, Verma R. Deciphering the transcriptome of prepubertal buffalo mammary glands using RNA sequencing. Funct Integr Genomics. 2019;19(2):349–362.
- Farooqui MM, Chandrapal A, Prakash A. Histological and histochemical studies on the prenatal development of testis in goats (Capra hircus). Int J Morphol. 2012;30(4):1408–1421.
- Roy KS, Bhatia H, Pathak D, Kumar A. Prenatal development of testis in buffalo (Bubalus bubalis): A histomorphological study. Indian J. Anim. Sci. 2009;79(9):30–32.
- Singh I, Bansal N, Uppal V, Anuradha A. Differentiation of bipotential gonads in buffalo foetus: A histomorphological study. Indian J Anim Sci. 2015;85(1):46–48.
- Basavaiah M, Nagamalleswari Y, Raju NKB, Raghunath M. Prenatal morphogenesis of testis in sheep (Ovis aries). Indian J Vet. Anat. 2021;33(2):94–96.
- Kaur M, Bansal N, Uppal V. Histogenesis of testicular parenchyma during prenatal life in buffalo. Int J Morphol. 2011;29(4):1109–1114.
- Abd-Elmaksoud A. Morphological, Glycohistochemical, and Immunohistochemical Studies on the Embryonic and Adult Bovine Testis [PhD thesis]. Munich, Germany: Ludwig Maximilian University; 2005:70–101.
- Cauffman G, Liebaers I, Van Steirteghem A, Van de Velde H. POU5F1 isoforms show different expression patterns in human embryonic stem cells and preimplantation embryos. Stem Cells. 2006;24(12):2685–2691.
- Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26(12):3068–3074.
- Singh SP, Kharche SD, Pathak M, et al. Reproductive stage-and season-dependent culture characteristics of enriched caprine male germline stem cells. Cytotechnology. 2022;74(1):123–140.
- Tokunaga Y, Imai S, Torii R, Maeda T. Cytoplasmic liberation of protein gene product 9.5 during the seasonal regulation of spermatogenesis in the monkey (Macaca fuscata). Endocrinology. 1999;140(4):1875–1883.
- Kwon J, Wang YL, Setsuie R, et al. Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice. Biol Reprod. 2004;71(2):515–521.
- Wang YL, Liu W, Sun YJ, et al. Overexpression of ubiquitin carboxyl‐terminal hydrolase L1 arrests spermatogenesis in transgenic mice. Mol Reprod Dev. 2006;73(1):40–49.
- Kwon J, Sekiguchi S, Wang YL, Setsuie R, Yoshikawa Y, Wada K. The region-specific functions of two ubiquitin C-terminal hydrolase isozymes along the epididymis. Exp Anim. 2006;55(1):35–43.
- Wrobel KH. Prespermatogenesis and spermatogoniogenesis in the bovine testis. Anat Embryol (Berl). 2000;202(3):209–222.
- Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia‐specific marker in the pig testis: Application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73(12):1531–1540.
- Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9. 5 markers. J Assist Reprod Genet. 2012;29(10):1029–1038.
- Fukuda T, Hedinger C, Groscurth P. Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res. 1975;161(1):55–70.