?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lysozymes, efficient alternative supplements to antibiotics, have several benefits in poultry production. In the present study, 120, one–day–old, Ross 308 broiler chickens of mixed sex, were allocated into 2 equal groups, lysozyme treated group (LTG) and lysozyme free group (LFG), to evaluate the efficacy of lysozyme (Lysonir®) usage via both drinking water (thrice) and spray (once). LTG had better (p = 0.042) FCR, and higher European production efficiency factor compared to LFG (p = 0.042). The intestinal integrity score of LTG was decreased (p = 0.242) compared to that of LFG; 0.2 vs. 0.7. Higher (p ≤ 0.001) intestinal Lactobacillus counts were detected in chickens of LTG. Decreased (p ≤ 0.001) IL-1β and CXCL8 values were reported in LTG. The cellular immune modulation showed higher (p ≤ 0.001) opsonic activity (MΦ and phagocytic index) in LTG vs. LFG at 25 and 35 days. Also, higher (p ≤ 0.001) local, IgA, and humoral, HI titers, for both Newcastle, and avian influenza H5 viruses were found in LTG compared to LFG. In conclusion, microbial lysozyme could improve feed efficiency, intestinal integrity, Lactobacillus counts, anti-inflammatory, and immune responses in broiler chickens.
HIGHLIGHTS
Exogenous aqueous and spray microbial lysozyme enhanced growth in commercial broiler chickens
The postbiotic effects of microbial lysozyme modulated intestinal integrity.
Anti-inflammatory, as well as local, cellular, and humoral immune response were stimulated by lysozyme supplementation.
Introduction
For more than 60 years, the poultry industry has utilized antibiotics as growth promoters to boost meat production. Antibiotic-resistant bacteria have been emerged because of this approach, and potentially endangered human health. As a result, non-antibiotic alternatives were urgently needed to sustain poultry health and increase feed benefits ratio.Citation1–9 Thus, many attempts were made to improve gut health, modulate microbiota, enhance intestinal integrity, and manipulate the bacterial cecal community in terms of amending chicken growth performance and body weight. The development of dietary supplements including antimicrobial peptides, bacteriocins, probiotics, prebiotics, herbs and exogenous antimicrobial enzymes such as lysozymes have therefore been continuously increased.Citation10–12
Lysozymes are common antimicrobial enzymes that were discovered 100 years ago by Alexander Fleming.Citation13 These enzymes are widespread in many animal tissues and secretions and the commercial source is obtained from poultry egg white. Different origins of lysozyme exist such as animal, plant, microbial and phage lysozymes.Citation14 The primary chemical structure of lysozyme is a single polypeptide chain with 129 amino acids including 4 pairs of cysteine.Citation15 The antibacterial actions of lysozyme are exerted through both direct bacterioloytic effect by disrupting 1,4-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine of bacterial peptidoglycans in the cell walls of Gram positive and Gram negative bacteriaCitation16 and indirect stimulation of macrophage phagocytic functions.Citation17 Additionally, it supports gut barrier function, thereby improving the growth performance.Citation18 Broiler breeder hens’ growth performance, gut microbiota, antioxidant status, and nonspecific immunity were all enhanced by nutritional supplementation with exogenous lysozyme at a dosage of 90 g ton−1.Citation19 A fusion protein, composed of lysozyme and surfactant protein B, proposed by Akinbi et al.Citation20 for prevention or treatment strategy of cystic fibrosis caused by Pseudomonas aeruginosa in mice led to 6–30 fold higher bacterial clearance compared to wild-type controls. Several records of significant reduction of pneumonia in human following aerosol administration of lysozyme due to the significant reduction in the pneumonia related parameters, such as the bacterial colony-forming in the whole lung and bronchoalveolar lavage fluid (BALF), and the total BALF leukocytes suggesting the effective mitigation of respiratory disorders.Citation21,Citation22 The effects of spraying lysozyme solutions during the food industry on the microbiological stability and organoleptic characteristics of chicken legs with skin have been described with variable degrees of activity. The findings indicated that during cold storage of the legs, there was a significant suppression of the early aerobic bacterial development alongside a limiting of harmful organoleptic alterations. The initial aerobic bacterial count was reduced by 20 times as a result of the larger dosages of lysozyme solution (48,000 U/mL), which may be useful in increasing the shelf life of portioned poultry meat.Citation23
The anti-inflammatory effect mechanisms of lysozymes were proven previously.Citation19,Citation24–27 These studies highlighted the antibacterial, immunomodulatory and systemic anti-inflammatory mechanisms of lysozymes in details. Furthermore, Obmińska-MrukowiczCitation28 studied the immunomodulatory properties of lysozyme dimer in laboratory animals and indicated the pharmacological protection of immunohomeostasis during viral and bacterial infections. Moreover, the researcher mentioned that lysozyme could be applied to enhance the immune response during vaccination and for the compensation of the impaired immune system function due to immunosuppressive factors.
To date, the effect of different routes of lysozyme administration on broiler chickens has not been yet fully investigated. Most of studies often focus on lysozyme administration in feed from birth through market age. However, no studies have also focused on adding lysozyme in drinking water and/or spray that may be appropriate and have the greatest effect on intestinal bacteria populations and broiler performance at exact crucial phases of the broiler growth cycle. Therefore, in this study we aimed to evaluate the postbiotic, anti-inflammatory and immunomodulatory effects of novel aqueous lysozyme (Lysonir®), thrice in drinking water and once as a spray, in commercial broiler chickens.
Material and methods
Ethics statement
All experimental procedures were approved by the Animal care committee of Beni-Sueif University, Egypt (approval number: 021-168).
Experimental design
Chickens and dietary treatments
120, One day old, Ross 308 broiler chickens of mixed sex were assigned to 2 separate (equal) groups that were floor reared in 2 separate units each containing 6 replicates of 10 birds. Balanced rations [corn–soybean based that were formulated based on the nutrient requirements for Ross-308 broiler chickens],Citation29 were provided as shown in . Clean drinking water was ad libitum offered. The vaccination program for both groups was also as follows: bivalent live infectious bronchitis and Newcastle vaccine, MA5 + Clone 30 (Nobilis® Ma5-Clone30, MSD, Intertvet Int., The Netherlands) at 5 days of age via eye drop (ED), bivalent inactivated avian influenza subtype H5 plus Newcastle vaccine (MEFLUVAC® H5 + ND7, MEVAC, Egypt) at 10 days of age through subcutaneous route with a dose of 0.5 mL/bird, Gumboro intermediate plus, Bursine plus® (Zoetis, USA) at 12 days of age via ED and live Newcastle, laSota® (MSD, Intertvet Int., The Netherlands), at 18 days of age via ED.
Table 1. Analysis of the starter and grower diets used in the experiments for the percentage of ingredients and stated composition (%, as-fed basis).
The Lysonir® treated group (LTG) was treated by Lysonir ® (20%) [Microbial lysozyme contains 200 g/L of microbial lysozyme extracted from Acremonium alcalopilum (the only known cellulolytic saprophytic fungus that thrives in alkaline conditions)], produced by MN Trade Industrial Inc., 10th of Ramadan city, Egypt, under registration code in agriculture ministry 2931], for 8 h daily (from 8 AM to 4 PM) during the first 3 days in drinking water using the recommended dose (0.5 mL/L). The same treatment dose was then repeated at days of 11–13 and 18–20 of age, with a daily treating for 8 h in drinking water. Finally, Lysonir® (coarse spray form; coarse spray with droplet size of 100 microns. Spraying was done over the head of birds with 50 cm height from 8 AM for 10 minutes) at 25–27 of age using the recommended dose of 6.5 mL/200 bird was applied. The second group was reared without addition of Lysonir®, free group, (LFG). Birds were exposed to 24 h light throughout the study.
Postbiotic effect assessments
Performance parameters
Average final body weight (AFBW) and feed intake (FI) were weekly determined while feed conversion ratio (FCR) was calculated till the 5th week of age (end of the experiment). The final European production efficiency factor (EPEF) was also estimated using Equation (Equation1(1)
(1) ):
(1)
(1)
Intestinal length and width
The length of intestine (from duodenum to ileum) and width of the ileum center in 18 sacrificed birds from each group were measured at 35th day of age.Citation30 Humanely sacrification through cervical dislocation after the intravenous injection of sodium pentobarbital with a dose of 50 mg/kg was applied.
Intestinal integrity score
The small intestine from 18 sacrificed birds of each group at 35th day of age was opened and scored on a scale from zero to four, based on parameters, such as intestinal ballooning; serosal and/or mucosal redness; reduction of intestinal wall thickness; flaccid and fragile intestinal edges within 3 s after gut dissection; abnormal lumen contents (all previous lesions were inspected at the cranial and caudal from Meckel’s diverticulum) and presence of undigested feed particles caudal from ileo-cecal junction. If any of them were present, they received a score of one; otherwise, they receive a score of 0.Citation31
Intestinal microbiota
Enumeration of lactic acid bacteria (Lactobacillus count): one gram of fresh digesta samples from the crop, ileum, and cecum of 18 sacrificed birds in each group at 15 and 35 days was transferred to 9 mL MRS (De Man, Rogosa and Sharpe) broth and serially diluted in 10-fold increments. 0.1 mL from the last three diluted samples were individually plated on MRS agar (Oxoid Ltd., Hampshire, UK), they were then incubated at 37 °C for 48 h (under microaerophilic conditions).Citation30
Inflammatory mediators screening
Interleukin-1β (IL-1β) assay
IL-1β concentrations were determined by immunoenzymatical assay using chicken IL-1β enzyme linked immunosorbent assay (ELISA) kits (Novatein Bio, Massachusetts, USA). Eighteen serum samples from each group collected at 7, 15, 25, and 35 days. A wavelength of 450 nm was employed to detect the absorptions and by using software, IL-1β concentrations were determined from the standard curve.
RT-qPCR for determination of expression and fold change of CXCL8 and GAPDH genes
Eighteen blood samples from each group, obtained at 7, 15, 25, and 35 days, were examined using RT-qPCR methods to determine changes in the gene expression of CXCL8 in monocyte-derived macrophages. The subsequent primers: CXCL8 F: TAG GAC CAG AGC CAG GAA GA, R: GCT GCA GAA AGC AGG AAA AC, and QuantiTect SybrGreen master mix (Qiagen, Germany) were utilized. Their cycling circumstances were carried out at 95 °C/5 min, 40 cycles of 95 °C/10 s, 60 °C/30 s, and 72 °C/1 min. RT-qPCR results were then analyzed using comparative threshold cycle (CT).Citation32
Immune mediators screening
Local and cellular immunity
Mucosal IgA
Tracheal IgA were measured in 18 serum samples from each chicken group collected at 7, 15, 25, and 35 days using chicken immunoglobulin A (IgA) ELISA kit (catalog No. E33-112, Bethyl Laboratories Inc., Montgomery, TX, USA) according to the method described by Merino-Guzmán et al.Citation33
Opsonic activity assay
CytoSelect™ 96-Well Phagocytosis Assay (Red Blood Cell Substrate, catalog No. CBA-220, Cell Biolabs Inc., San Diego, CA, USA) was used for the detection of MΦ count and phagocytic index in 18 serum samples collected from each chicken group at 7, 15, 25 and 35 days of age according to the method described by Yu et al.Citation34
Humoral immunity
Hemagglutination inhibition (HI) assay
Eighteen serum samples from each group were collected at 25 and 35 days for the detection of HI antibody titers of highly pathogenic avian influenza (HPAI) H5N1 and Newcastle disease (ND). An inactivated AI-H5N1 antigen (A/chicken/Egypt/18-H/2009) was used as the antigens (Ags) to detect AI-H5 antibodies, where LaSota strain for detection of ND antibodies using 8 HA units of both Ags was also used.Citation35
Statistical analysis
The results were presented as means ± SD using independent T-test to determine the significance of differences between LTG and LFG in all mentioned parameters. A probability (p values below 0.05 was considered as statistically significant.
Results
Postbiotic effect of lysozyme
For the performance parameters as indicated in , an insignificant decrease (p = 0.390) in FI (2520 vs. 2530 g), a significant improved (p = 0.042) in FCR (1.36 vs. 1.40), and an insignificant (p = 0.288) numerically higher AFBW (1890 vs. 1850 g) were recorded in LTG compared to LFG. The final EPEF was also significantly higher (p = 0.040) in LTG compared to LFG (397 vs. 379.5).
Table 2. Postbiotic effect of microbial lysozyme on performance.
The length of intestine (from duodenum to ileum) and width of the ileum center was 175 and 1.45 versus 150 and 1.23 cm in LTG and LFG, respectively. The intestinal integrity score was also significantly decreased (p = 0.042) with a value of 0.2 in LTG vs. 0.7 in LFG. Regarding the intestinal Lactobacillus count (log10 CFU/g), significantly greater (p ≤ 0.01) values in the crop, ileum, and cecum were recorded in chickens of LTG at 15 and 35 days, as shown in .
Table 3. Postbiotic effect of microbial lysozyme on the intestinal integrity and intestinal lactobacillus count (log10 CFU/g).
Anti-inflammatory effects
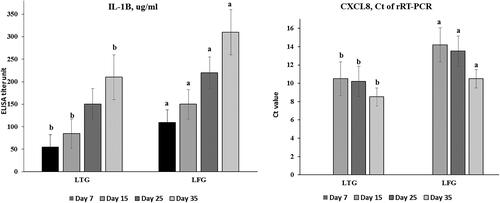
The anti-inflammatory effects of lysozyme were evident through the significant lower (p ≤ 0.001) IL-1β and CXCL8 (pro-inflammation indicators) values in LTG rather than LFG which were reported throughout the experiment, (), e.g., at 35 days, results were 210 vs. 310 and 8.5 vs. 10.5 for IL-1β and CXCL8 in LTG and LFG, respectively.
Cellular and local immunity
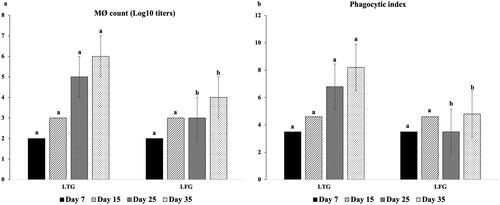
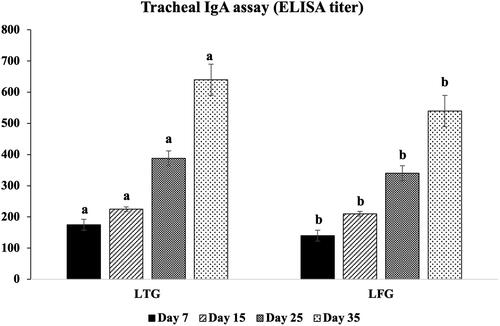
The opsonic activity (MΦ and phagocytic index) was significantly greater (p ≤ 0.001) in LTG compared to LFG, with MΦ counts of 105,106 vs.103, 104 and phagocytic index as 6.8, 8.2 vs. 3.5, 4.8 at 25 and 35 days, respectively (). Significantly higher (p ≤ 0.001) local IgA was recorded (640 vs. 540 ELISA units at 35 days), indicating a high immune modulation in LTG in relation to LFG ().
Humoral immunity
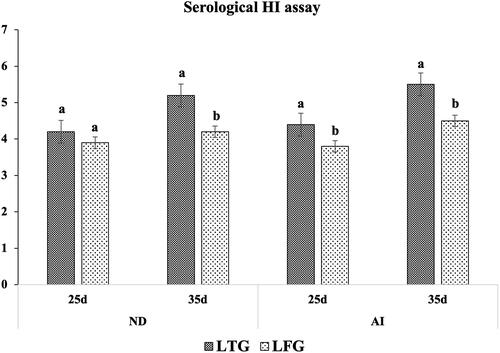
Higher HI titers for both ND and HPAI-H5N1 were reported, indicating a significantly higher (p ≤ 0.01) immune modulation in LTG. At 35 days, the HI titers for ND and H5N1 reached 5.2 and 5.5 vs. 4.2 and 4.5 in LTG and LFG, respectively ().
Discussion
Lysozyme has a significant antibacterial effect on both Gram negative and Gram positive bacteria by impairing DNA and RNA synthesis, activating autolysin production, permeabilizing cell walls and membranes, causing their depolarization, and ultimately cytosol leakage.Citation36,Citation37 By altering the intestinal histology and exerting an influence on the enzyme expression and metabolism of the cecal microbiota, the use of lysozyme as a feed supplement enhanced the growth performance of broiler chickens.Citation38 According to an in-vitro study by Zhang et al.Citation39 200 μg/ml of lysozyme prevented Clostridium perfringens from growing as well as the synthesis of the toxin that results in the lesions linked to necrotic enteritis (NE) in chickens. According to certain in-vivo investigations, lysozyme could be a good option to get rid of Clostridium perfringens and boost broiler chicken development.Citation40 However, extended in vivo research are urgently required to determine the best doses of lysozyme to use in poultry instead of antibiotics and to identify the critical times of the broiler growth cycle when lysozyme may have the biggest effects on growth performance and the microbiota populations of the gastrointestinal tract (GIT) of broiler chickens.Citation41 Generally, the supplementation with lysozyme could support poultry industry with several benefits such as providing antibiotic free poultry products, enhancing innate and adaptive immunity, gut integrity, alongside with anti-inflammatory and antioxidant effects in broiler chickens.Citation42
In the same direction, the use of a microbial lysozyme (Lysonir®) in commercial broiler chickens through both drinking water (thrice) and spray (once), novel methods of application was evaluated in this study. Our results obviously indicated significant high (p ≤ 0.05) performance parameters regarding FCR,and EPEF which could be related to better intestinal integrity score and significant higher (p ≤ 0.05) intestinal Lactobacillus counts in lysozyme treated group (LTG) compared to lysozyme free group (LFG). Similar results were obtained by Abdel-Latif et al.Citation19 who reported significant (p < 0.05) improvements with the dietary supplementation of exogenous lysozyme (with a dose of 90 g/ton) for broiler chickens such as 5.39% in a growth rate or AFBWG (without difference in feed intake), 6.1% in FCR, and 17.2% in EPEF. The same results were supported by Gong et al.Citation43 who determined the effect of 100 ppm LYZ as feed additive on growth performance and intestinal microbiota of broiler chickens in each period of the growth cycle using new or used litter and concluded that LYZ supplementation had changed the intestinal microbiota of broiler chickens through reducing the number of harmful bacteria.
Lactobacillus and Bifidobacterium are the two key helpful genus of bacteria in the GIT of birds (Fooks and Gibson, 2002), while the main pathogenic gut bacteria of poultry are Clostridium, Escherichia coli, Salmonella, and Camplylobacter.Citation44 Abdel-Latif et al.Citation19 and GongCitation42 recorded an enhancement in the gut microbiota through significant decreases (p < 0.01) in the damaging fecal Coliform and Clostridia and an increase (p < 0.05) in the valuable Lactobacillus counts. Similar results were indicated not only in chickens but also in rabbits by El-Deep et al.Citation45 who reported that LYZ (200 mg per kg diet) enhanced the growth performance (p < 0.05), reduced feed intake and FCR, improved the hematological and serum biochemical parameters, linearly reduced (p < 0.05) the total count of Escherichia coli and Clostridium was decreased and considerably increased the Lactobacilli count in rabbits.
The maintenance of a healthy microbiota within the GIT environment has a strong relationship with the bird’s health, well-being, and productivity.Citation46 This might be the actual situation of the broiler chickens in LTG in our study as the hydrolytic enzyme, lysozyme, could protect the birds against these harmful bacteria through its hydrolysis compromising direct action of the integrity of the bacterial cell wall, causing its lysis.Citation16,Citation18 Liu et al.Citation41 also mentioned that exogenous lysozyme declined C. perfringens colonization and increased the intestinal barrier function and growth performance of chickens. In another study by Xia et al.Citation47, the lysozyme supplementation led to a significant (p < 0.05) enrichment of genes involved in the synthesis/degradation of bacterial outer membranes and cell walls, cross-cell substrate transport, and carbohydrate metabolic processes, promoting the cecal microbiota carbon and energy metabolism. However, this did not contribute significantly (p > 0.05) to the growth, and different compositions of the bacterial and fungal communities of cecal microbiota in broiler chickens. In this study, the improvement in intestinal integrity score between LTG vs. LFG was a direct result of lysozyme treatment (p ≤ 0.05) as recorded previously by Du and GuoCitation48 who proved that lysozyme or essential oils decreased the mortality, improved the intestinal integrity, alleviated the gut lesions, and significantly reduced the ileal concentration of sialic acid and the Mucin2 mRNA expression following C. perfringens infection in chickens.
Additionally, according to several studies,Citation17,Citation42,Citation49,Citation50 lysozymes are linked to indirect bacteriolytic activity through stimulating macrophage phagocytic function. The immunoglobulins IgG, IgM, and IgA found in poultry peripheral blood at the greatest levels are significant indicators of the humoral immune system’s functionality.Citation51 Herein, greater immunomodulations were demonstrated in LTG compared to LFG by raising cellular (opsonic activity through MΦ and phagocytic index), local (IgA) and humoral (HI titers for ND and HPAI-H5N1) immune responses. Abdel-Latif et al.Citation19 indicated that the gut nonspecific immunity biomarkers expression (INF-γ, IL-10, and IL-18), and serum globulin levels were significantly elevated in lysozyme-supplemented groups (especially LYZ90; 90 mg lysozyme/Kg diet) which confirmed the enhancement of the innate (nonspecific) immunity that was reflected on the increase of HI titers for ND. Several research evidences showed that lysozyme plays an important role not only in defense mechanism but also in regulation and/or activation of immune response mitigating the inflammatory response.Citation14,Citation27,Citation52–57 The enhancement of macrophage-dependent innate immunity through the activation of specific cathelicidins eliciting a pronounced immune response.Citation56,Citation58
Lysozyme has anti-inflammatory properties in addition to its antibacterial activity through a gene-regulatory system involving inflammatory pathway proteins such IL-1β and tumor necrosis factor alpha (TNF-α). Ibrahim et al.Citation24 recorded, for the first time, that lysozyme peptides could antagonize the pathogen-induced inflammatory response through the significant reduction in pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6. Also, the in-vitro supplementation of lysozyme in a monocyte cell line, a kind of cell that interacts with lysozyme, supported the evidence the anti-inflammatory action of lysozyme on the basis of transcriptomic regulation data resulting from the broad perspective of a whole-transcriptome profiling.Citation25 Lysozyme, used orally, induced an anti-inflammatory effect in-vitro and in-vivo through mitigating the phosphorylation of JNK, and significant reduction in the amounts of IL-6 and TNF-α in sera.Citation26 The obtained anti-inflammatory effect appeared without inhibiting innate immune responses of macrophages. Not only in animals, but also in human, the use of lysozyme was effective in the treatment of inflammation through minimizing the pro-inflammatory mediators pathways such as IL-1β, IL-6 TNF-α and induction of immunomodulation.Citation25,Citation26,Citation59–61 Herein, a considerable reduction in pro-inflammatory cytokine levels of IL-1β and CXCL8 throughout the experimental period suggested that the inflammatory status of the birds had been minimized. This is another way by which the lysozyme (Lysonir®) treated birds performed better.
Conclusion
Exogenous aqueous microbial lysozyme (Lysonir®) prophylactic protocol in drinking water and spray, as innovative methods, had multiple beneficial effects in commercial broiler chickens such as postbiotic enhancement of feed efficiency, intestinal integrity, and intestinal Lactobacillus counts as well as improvement of the anti-inflammatory, local, cellular, and humoral immune, responses.
Ethical approval
All experimental procedures were approved by the Animal care committee of Beni-Sueif University, Egypt (approval number: 021-168).
Acknowledgments
Authors gratefully thank Dr. Moatasem Nowara, the chairman of MN trade veterinary pharmaceutical company for his support. The authors acknowledge the financial support of Princess Nourah bint Abdulrahman University, Researchers Supporting Project number (PNURSP2024R30), by Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. They also acknowledge their institutes and universities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data of this study are available from the corresponding authors, [MAA & AO], upon reasonable request.
Additional information
Funding
References
- Robredo B, Singh KV, Baquero F, Murray BE, Torres C. Vancomycin-resistant Enterococci isolated from animals and food. Int J Food Microbiol. 2000;54(3):1–11.
- Gambarotto K, Ploy M-C, Dupron F, Giangiobbe M, Denis F. Occurrence of vancomycin-resistant enterococci in pork and poultry products from a cattle-rearing area of France. J Clin Microbiol. 2001;39(6):2354–2355.
- Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol. 2003;6(5):439–445.
- Castanon J. History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci. 2007;86(11):2466–2471.
- Abd El-Ghany WA, Abdel-Latif MA, Hosny F, et al. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult Sci. 2022;101(8):101988.
- Abdel-Latif MA, Abd El-Hack ME, Swelum AA, et al. Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals. 2018;8(10):184.
- Abdel-Latif MA, Elbestawy AR, El-Far AH, et al. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal MRNA expression genes in broiler chickens. Animals. 2021;11(8):2302.
- Manaa EA, Abdel-Latif MA, Ibraheim SE, et al. Impacts of Macleaya cordata on productive performance, expression of growth-related genes, hematological, and biochemical parameters in Turkey. Front Vet Sci. 2022;9:873951.
- Landy N, Kheiri F. Effects of hydrolyzed cottonseed protein on growth performances, carcass traits, immunity, microbial and morphological responses of the small intestine and total antioxidant capacity of serum, and small intestine in broiler chickens. Iran J Appl Anim Sci. 2023;13(1):121–132.
- Landy N, Kheiri F, Faghani M. Effects of periodical application of bioactive peptides derived from cottonseed on performance, immunity, total antioxidant activity of serum and intestinal development of broilers. Anim Nutr. 2021;7(1):134–141.
- Oakley BB, Lillehoj HS, Kogut MH, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. 2014;360(2):100–112.
- Gadde U, Kim W, Oh S, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18(1):26–45.
- Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc Royal Soc London Series B, Contain Papers of Biol Character. 1922;93(653):306–317.
- Jiang L, Li Y, Wang L, et al. Recent insights into the prognostic and therapeutic applications of lysozymes. Front Pharmacol. 2021;12:767642.
- Proctor VA, Cunningham F, Fung DY. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr. 1988;26(4):359–395.
- Ibrahim HR, Higashiguchi S, Koketsu M, et al. Partially unfolded lysozyme at neutral pH agglutinates and kills Gram-negative and Gram-positive bacteria through membrane damage mechanism. J Agric Food Chem. 1996;44(12):3799–3806.
- Biggar WD, Sturgess J. Role of lysozyme in the microbicidal activity of rat alveolar macrophages. Infect Immun. 1977;16(3):974–982.
- May K, Wells J, Maxwell C, Oliver W. Granulated lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology of 10-day-old pigs. J Anim Sci. 2012;90(4):1118–1125.
- Abdel-Latif MA, El-Far AH, Elbestawy AR, Ghanem R, Mousa SA, Abd El-Hamid HS. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLOS One. 2017;12(10):e0185153.
- Akinbi HT, Epaud R, Bhatt H, Weaver TE. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol. 2000;165(10):5760–5766.
- Bhavsar T, Liu M, Hardej D, Liu X, Cantor J. Aerosolized recombinant human lysozyme ameliorates Pseudomonas aeruginosa–induced pneumonia in hamsters. Exp Lung Res. 2010;36(2):94–100.
- Ferraboschi P, Ciceri S, Grisenti P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics. 2021;10(12):1534.
- Kijowski J, Marciszewska C, Cegielska-Radziejewska R, Popiół A. Effect of lysozyme treatment on quality and bacterial contamination of chilled chicken legs. J Vet Res. 2013;57(1):79–84.
- Ibrahim HR, Hamasaki K, Miyata T. Novel peptide motifs from lysozyme suppress pro-inflammatory cytokines in macrophages by antagonizing toll-like receptor and LPS-scavenging action. Eur J Pharm Sci. 2017;107:240–248.
- Bergamo A, Gerdol M, Pallavicini A, et al. Lysozyme-induced transcriptional regulation of TNF-α pathway genes in cells of the monocyte lineage. Int J Mol Sci. 2019;20(21):5502.
- Tagashira A, Nishi K, Matsumoto S, Sugahara T. Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages. Cytotechnology. 2018;70(3):929–938.
- Ragland SA, Criss AK. From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLOS Pathog. 2017;13(9):e1006512.
- Obmińska-Mrukowicz B. Immunomodulatory properties of lysozyme dimer under conditions of stimulation or suppression of the immune system – preclinical trials. Journal of Biomedical Research and Therapeutics. 2022;1(1):23–31.
- Aviagen R. Ross 308 Nutrition Specifications. Scotland (UK): Aviagen. 2014.
- Dahiya J, Hoehler D, Wilkie D, Van Kessel A, Drew M. Dietary glycine concentration affects intestinal Clostridium perfringens and lactobacilli populations in broiler chickens1. Poult Sci. 2005;84(12):1875–1885.
- De Gussem M, editor. Macroscopic scoring system for bacterial enteritis in broiler chickens and turkeys. WVPA Meeting, Merelbeke, Belgium. 2010.
- Moges R. The immune modulatory effects of tylvalosin in porcine neutrophils and macrophages in vitro. Master,s thesis, Calgary University, Calgary, Canada. Retrieved from https://prism.ucalgary.ca. doi:10.11575/PRISM/28175. 2017;
- Merino-Guzmán R, Latorre JD, Delgado R, et al. Comparison of total immunoglobulin A levels in different samples in Leghorn and broiler chickens. Asian Pacific J Trop Biomed. 2017;7(2):116–120.
- Yu Z, Ono C, Aiba S, et al. Therapeutic concentration of lithium stimulates complement C 3 production in dendritic cells and microglia via GSK‐3 inhibition. Glia. 2015;63(2):257–270.
- World Organization for Animal Health (WOAH). Avian Influenza (Including Infection with High Pathogenicity Avian Influenza Viruses). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE: Paris, France. Chapter 3.3.4. 2021.
- Baksi S, Chauhan P, Rao N, Chauhan A. Effect of lysozymes, antibiotics and probiotics on growth performance and biochemical parameters in broiler chickens. Int J Livest Res. 2019;9(0):1.
- Derde M, Vié V, Walrant A, et al. Antimicrobial activity of lysozyme isoforms: key molecular features. Biopolymers. 2017;107(12):e23040.
- Humphrey BD, Huang N, Klasing KC. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J Nutr. 2002;132(6):1214–1218.
- Zhang G, Darius S, Smith S, Ritchie S. In vitro inhibitory effect of hen egg white lysozyme on Clostridium perfringens type A associated with broiler necrotic enteritis and its α‐toxin production. Lett Appl Microbiol. 2006;42(2):138–143.
- Zhang G, Mathis GF, Hofacre CL, Yaghmaee P, Holley RA, Duranc TD. Effect of a radiant energy–treated lysozyme antimicrobial blend on the control of Clostridial necrotic enteritis in broiler chickens. Avian Dis. 2010;54(4):1298–1300.
- Liu D, Guo Y, Wang Z, Yuan J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010;39(1):17–24.
- Gong M. Efficacy of lysozyme as an alternative to antibiotics for broiler chickens. Master,s thesis, Faculty of Agriculture, University of Dalhousie, Halifax, Nova Scotia. Retrieved from http://hdl.handle.net/10222/48594. 2014.
- Gong M, Anderson D, Rathgeber B, MacIsaac J. The effect of dietary lysozyme with EDTA on growth performance and intestinal microbiota of broiler chickens in each period of the growth cycle. J Appl Poult Res. 2017;26(1):1–8.
- Flickinger EA, Loo JV, Fahey GC. Nutritional responses to the presence of inulin and oligofructose in the diets of domesticated animals: a review. Crit Rev Food Sci Nutr. 2003;43(1):19–60.
- El-Deep MH, Amber KA, Eid YZ, et al. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits’ cecum. Front Vet Sci. 2020;7:579576.
- Mitsch P, Zitterl-Eglseer K, Köhler B, Gabler C, Losa R, Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult Sci. 2004;83(4):669–675.
- Xia Y, Kong J, Zhang G, Zhang X, Seviour R, Kong Y. Effects of dietary supplementation with lysozyme on the structure and function of the cecal microbiota in broiler chickens. PLOS One. 2019;14(6):e0216748.
- Du E, Guo Y. Dietary supplementation of essential oils and lysozyme reduces mortality and improves intestinal integrity of broiler chickens with necrotic enteritis. Anim Sci J. 2021;92(1):e13499.
- Thacore H, Willett HP. The formation of spheroplasts of Mycobacterium tuberculosis in tissue culture cells. Am Rev Respir Dis. 1966;93(5):786–796.
- Osserman E. Postulated relationships between lysozyme and immunoglobulins as mediators of macrophage and plasma cell functions. Adv Pathobiol. 1976;4:98–105.
- Wei F, Hu X, Xu B, et al. Ammonia concentration and relative humidity in poultry houses affect the immune response of broilers. Genet Mol Res. 2015;14(2):3160–3169.
- Namba Y, Hidaka Y, Taki K, Morimoto T. Effect of oral administration of lysozyme or digested bacterial cell walls on immunostimulation in guinea pigs. Infect Immun. 1981;31(2):580–583.
- Ganz T, Gabayan V, Liao H-I, et al. Increased inflammation in lysozyme M–deficient mice in response to Micrococcus luteus and its peptidoglycan. Blood J Am Soc Hematol. 2003;101(6):2388–2392.
- Nash JA, Ballard TNS, Weaver TE, Akinbi HT. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J Immunol. 2006;177(1):519–526.
- Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci. 2010;35(1):127–160.
- Ragland SA, Schaub RE, Hackett KT, Dillard JP, Criss AK. Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. Cell Microbiol. 2017;19(3):e12662.
- Bergamo A, Sava G. Pharmacological modulation of host immunity with hen egg white lysozyme (HEWL)—a review. Molecules. 2023;28(13):5027.
- Bu H-F, Wang X, Zhu Y-Q, et al. Lysozyme-modified probiotic components protect rats against polymicrobial sepsis: role of macrophages and cathelicidin-related innate immunity. J Immunol. 2006;177(12):8767–8776.
- Cartel F, Cartei G, Ceschia V, Pacor S, Sava G. Recovery of lymphocyte CD4+: CD8+ ratio in patients treated with lysozyme. Drug Invest. 1992;4(1):51–57.
- Lee M, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem. 2009;57(6):2233–2240.
- Glamočlija U, Mehić M, Šukalo A, Avdić AT, Jaganjac JD. Lysozyme in the treatment of non-infectious sore throat. Bosn J Basic Med Sci. 2020;20(2):281–282.