Abstract
Colostrum is the initial secretion of the mammary glands following parturition, which offers main food, protection, and biological active substances for the new born. The most threatening episode of neonate’s life is the initial two weeks after birth. This period is associated with high neonatal mortality and morbidity. These worthwhile losses lead to a poor prolificacy rate, low profitability, and ultimately poor performance in animal production. Hence, both diseases and mortality cause valuable losses in terms of production and economic losses. The survival of neonate is correlated with their immune status and passive immune transfer (PIT). Colostrum provides the primary source of nutrition and immunity (PIT) that protects neonates against infections. It must be given as soon as possible after birth since its immunoglobulins are absorbed within the first 16–27 hours after birth, ideally within 2–4 hours. As a result, immunoglobulin (PIT) is the most important component of distressing infectious immunity, and a passable concentration of immunoglobulin in the blood of newborn lambs is linked to their health and survival rate. In this review, we summarized the importance of colostrum in early life and its association with neonatal lamb’s survival, profitability and productivity of sheep farming.
Introduction
The livestock industry plays an essential role in economic development and food security of a country. Around the globe, small ruminants are used to provide meat, milk and animal-by products (wool and skins) products for human consumption. The number of lambs that survive to weaning, as well as the production of fattening and slaughter lambs for meat consumption, are all directly proportional to the efficiency and profitability of sheep farming systems. Almost all the newborns are agammaglobulinemic at birth because they have a non-significant magnitude of antibodies (PIT) at time of birth.Citation1 Colostrum is the initial mammary gland secretion accessible to newborn; it has been amassed from blood to the mammary gland since the last term of pregnancy. Colostrum is a vibrant and primary bioactive reserve of nourishment immunoglobulins (PIT), and its ingestion boosts neonate’s chances of survival in the early ex utero environment. Colostrum is crucial for immune system development, post-natal growth, and thermoregulation, as well as mediating the formation of the ewe-lamb relationship.Citation2 IgG1 and IgG2 immunoglobulins are transported to the mammary gland during this phase. Furthermore, IgG1 immunoglobulins are passively transmitted, whereas IgG2 immunoglobulins are selectively transferred, resulting in significantly higher quantity in colostrum as compared to serum. As pregnancy approaches its end, the entire procedure of transferring immunoglobulins accelerates, and udder involution occurs inside newly formed mammary gland epithelial cells. However, the highest concentration of IgG1 in colostrum arrives a few days before calving and then drops, owing to an increase in the volume of colostrum in mammary gland.Citation3
The administration of colostrum volume and timing is of primary concern after the first 36 hours of life; lambs are unable to digest colostrum, and immunoglobulin G concentration in colostrum falls with @ 3.3 mg/kg-h and becomes zero after 23 hours after parturition.Citation4 The rate and amount of Ig absorption from colostrum are time sensitive, and progressive delays as the colostrum reaches the small intestine could have a deleterious impact on both the rate and amount of absorption.Citation5,Citation6
Colostrum, is nutrient-rich fluid rich in immunological, growth-promoting, tissue-repair and various bioactive peptides along with inhibitory activity toward angiotensin-converting enzyme (ACE) components are released shortly after birth by female mammary glands.Citation7 The source ingredient for immune milk products, colostrum, can be utilized to treat or prevent gastrointestinal illnesses. Colostrum is principal source of nourishment for newborn ruminants and has an important physiologic function in these animals, stimulating transfer of immunoglobulins (PIT) from the dam to the newborn, providing protection against infections in the newborn.Citation8 Furthermore, colostrum is a mixture of miscellaneous constituents, i:e., lactose, fat, vitamins, and minerals, that have a high nutritional importance.Citation9 For millennia, the nutritional worth and technological applicability of sheep’s milk have been highly valued due to its chemical composition. In addition to its nutritive values colostrum encompasses a vibrant combination of proteins that dynamically take part in the neonate’s defence against infections and other postpartum environmental hazards.Citation10 With the exception of lactose, which drastically reduces during the first three days of breastfeeding, colostrum contains more fat, protein, peptides, non-protein nitrogen, ash, vitamins, minerals, hormones, growth factors, and cytokines, which possess vital preventive and protective health benefits effects such as, antimicrobial, anti-carcinogenic, antioxidant, cholesterol-lowering, antioxidant, anti-stress, anti-allergic, lactose intolerance, and ACE inhibitory activities.Citation11
The objective of present review is to summarize the biological value of sheep colostrum and milk and its potential influence of nutrition on immunity, productivity, and survival of neonate in early life of sheep breeding industry. The problems deliberated in this review may be of interest in terms of health and nutrition of humans as well as Veterinary Public health and food safety concerns.
Colostrum and immunity
Colostrum is primary source of energy, nutrition and immunological rich substances for the newborn. Because newborn lambs bore with a limited energy reserves. So, that they need instant acces of colostrum intake, which has sufficient amount of colostrum with respect to quality and quantity.Citation12 Hence, colostrum induced passive immune transfer (PIT) is crucial for the whole life of the lamb. Alongside a strong immunostimulant activity of the colostrum, it is a nutrient rich source for the newborn.Citation13 Recent scientific literature reported that passive immunity in ruminant newborn not only ensure prevent against diseases but also accelerate growth performance.Citation2 Neonatal lamb mortality is a complex problem with no single identifiable cause.Citation7 In addition to its primary role in nutrition absorption and digestion, the gastrointestinal tract also serves as the body’s first line of defense against infections, endotoxins, and antigenic chemicals.Citation14 However, colostrum is primary bioactive reserve of nourishment immunoglobulins, and its ingestion boosts neonate’s chances of survival in the early ex utero environment. Colostrum is crucial for immune system development, post-natal growth, and thermoregulation, as well as mediating the formation of the ewe-lamb relationship.Citation2
All these properties of colostrum make it an irreplaceable life source for the newborn. These unique characteristics of colostrum are directly concerned to the neonatal early life, immunity, survival and ultimately productivity of sheep farming.
Colostrum and neonatal survival
A major factor affecting ewe productivity and livestock profitability is lamb mortality.Citation12 Mortality rates are mutable by different circumstances (such as failure of PIT, common infections, gestational diseases, environment, management, and other) and since the last decades the average mortality rate of newborn lambs remained relatively constant by 15% around the world. This rate could be to be higher (up to 30%) in small-scale sheep farming systems in developing countries.Citation15 According to recent reports, failure of PIT’s incidence variable from 3.4% to 20%; mortality rates are ranges between 45% and 50% in initial 2 weeks of neonatal life.Citation16 During early transition phase of neonatal lambs low blood Ig concentrations are directly linked with neonatal mortality from infectious causes, and PIT failure in neonatal lambs has a substantial impact on neonatal mortality.Citation14,Citation17 A number of factors can lead to PIT failure, such as inadequate Ig concentration in the colostrum from lack of exposure to a particular pathogen or incapacity to react, inadequate colostrum intake from litter, or inadequate transmural Ig transfer from the newborn intestine to blood. Thus, a number of problems affecting lambs, such as respiratory disorders, diarrhea, septicemia, and frequently omphalophlebitis, have been linked to PIT failure.Citation17 The litter’s entire life would be impacted by all of these factors as well as lack of colostrum intake during the first few hours of neonatal life.Citation16 Intake of colostrum quality and quantity is primary concerned as well as management during the suckling and weaning period; separation of dam caused stress also altered milk quality and suckling frequency, ultimately hindered to induced strong immunity.Citation12 Hence, failure of PIT is the most important cause in newborn lamb mortality.
Colostrum production
The growth and maturation of mammary gland tissues involves various developmental processes, i.e., mammogensis, colostrogenesis, lactogenesis, and involutionCitation18 The transformation of immunoglobulins forms the ewe blood circulation to mammary gland and colostrum is termed colostrogenesisCitation19 There are two specific pathways for the selective transportation of IgG into colostrum.Citation20 The basal cell membrane of plasma secretory cells contained receptors for IgG, located for the channelization of ligand from extracellular fluid. Moreover, mammary glands epithelial cells adopt capability to transcytosis IgG for its transportation into liminal secretion.Citation21 Likewise, some previous studies corroborated the presences of specific receptors. Lactogenesis is a physiochemical mechanism characterized by the synthesis, secretion, ejection and removal of milk.Citation22 The development of mammary glands and milk ejection organized and coordinated by neuroendocrine system. The fundamental function of the endocrine system is to synchronize the development of mammary glands with the reproductive stage of female animals. However, major function of nervous system is to initiate the process of milk let down reflex. These two mechanisms coordinate hypothalamus-pituitary axis and anatomically close connection for the milk synthesis via release of different biochemical products such as prolactin, oxytocin and growth hormone.Citation23 It has been demonstrating that the transportation of IgG into secretion implicate an active specific receptors-mediated mechanism.Citation24 It has been demonstrated that the presences of neonatal Fc receptor (FcRn) in acinar and ductal epithelial cells of the mammary gland in pregnant ewe observed noticeable changes prepartum and postpartum that indicate FcRn has an important role in the transportation of IgG in the course of colostrogenesis.Citation25 Although the localization of FcRn in mammary gland was not deeply studied.Citation26
Composition of colostrum
Physicochemical characteristics of colostrum and milk diverge considerably since colostrum consist of a various mixture of bioactive compounds, dry matter including proteins, lactose, fats, hormones, enzymes, and other peptides, vitamins as well as, amino acids, micro and macro minerals.Citation2 It is a complex biological fluid, and its composition is like that of blood and varies significantly from milk. Colostrum nutrients composition profile differs across species (). Immunoglobulin (PIT), particularly IgG, is a major fundamental contents of colostrum; it’s the amount and availability can directly affect the degree of immunity acquired by the neonate.Citation44–46 Similarly, it contains bacteriostatic and bactericidal substances, including lactoferrin, lysozyme, lacto peroxidase, and proteins peptides,Citation11,Citation47,Citation48 are shown in .
Table 1. Shows the typical chemical composition of colostrum from several domestic and wild animal species and humans.
Table 2. Characteristics of selected protein fractions in colostrum and milk.
Dry matter contents in colostrum
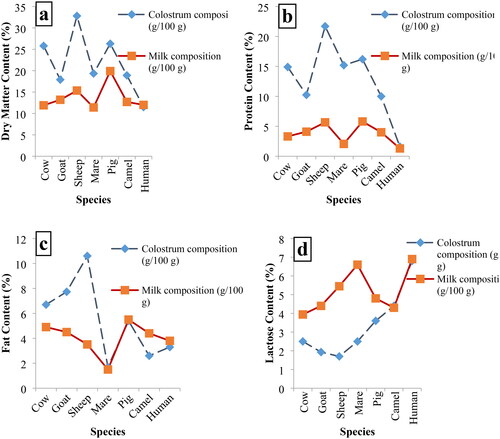
The dry matter contents of animal colostrum and milk consists of all its constituents, excluding water. The colostrum dry matter consists of huge amount of lactose, fat, protein, and various other vibrant components that are of primary concern for fulfilling basic animal nutrition. It provides nutrition and protection to the newborn in crucial first few days of neonatal life. The colostrum DM contents are vary with the passage of time post-parturient and among different species. Since the last many centuries, the importance of sheep’s milk has been acknowledged and appreciated due to its nutritive values and biomedical significances. Sheep milk contains nearly twice as much fat and protein as cow’s milk.Citation51 Ahmadi et al., conducted a comparative study and concluded that in colostrum dry matter concentration percentage proportion in ewes was 24.58, goat 13.0, bovine 12.8, and in human 12.4 during the first 6 hours after parturition. The highest concentration of dry matter was estimated in ewe colostrum, rather than the lowest concentration in human colostrum.Citation52 The similar results were revealed by,Citation11 as shown in .
Figure 1. Comparative average chemical composition of colostrum and milk of different animal species and humans. (a) Dry matter content (%), (b) protein content (%), (c) fat content (%) and (d) lactose content (%).Citation11,Citation32,Citation47,Citation53–57
Proteins contents in colostrum
Proteins are a highly diverse substance involved in biochemical processes essential for life. These mechanisms are extremely important for animal survival because they boost protection against infections in newborn ruminants. Furthermore, it has been well proven that newborn ruminants who are not administered colostrum during the first hours of birth are more susceptible to illnesses, considerably increasing mortality rates in the first days of life.Citation45 Hence, colostrum is richer in proteins, vitamins, and immunoglobulins.Citation58 These proteins are synthesis in the mammary gland and/or transferred from the bloodstream (or both). However, IgG is directly transported from bloodstream to mammary gland while, IgA is produces in the mammary gland by plasma cells and transferred to the glands.Citation59 As the pregnancy advances toward the final stages in ewe, the gastrointestinal tract changes its digestibility, which results in extra protein availability in the small intestine and enhanced intact protein concentration.Citation28 Intake of low-quality protein results in inferior colostrum quality as well as decreased milk production and development rates. Low protein content has also been linked to impaired immune system function.Citation60 Due to the nutracuticals implication of colostrum, it is used to mitigate various nutritional and biomedical health concerning issues. The immunological significance of colostrum is well documented in scientific research literature.
Lactose contents in colostrum
Lactose is a disaccharide sugar that is composed of two subunits, glucose and galactose. The colostrum sugars are monosaccharides and disaccharides, which are easily digested and absorbed. Lactose is the most abundant sugar in colostrum, which is broken down into simple sugars and absorbed in the small intestine. Colostrum contains a potent mixture of diverse components, especially lactose and other components that have nutritional importance.Citation61 It was reported that the sialic acid content of sheep colostrum was 302.9 ± 42.3 g/L. The amount of energy, especially glucose, available at the end of pregnancy plays a primary role in the synthesis of colostrum.Citation62 The various studies concluded that provision of high nutritional supplementations prior to parturition was beneficial in progesterone clearance and initiation of lacto genesis process. The reduction of progesterone concentration depends on different factors; however, intake of energy supplementation is well documented. Hence, plasma glucose concentration is primary concerning with level of nutrition that directly influences the production of colostrum so that glucose is required in process of lactogenesis.Citation11 The reports concluded that uptake of glucose is about milk production and low blood concentration of glucose leads to reduction in milk production.Citation62 The pregnant ewe was provided with an inadequate feeding program, and their plasma glucose concentration was varied from 2.4 to 4.5 mmol/L. On the other hand, ewe provided with 3 mmol/L glucose produced four-fold more colostrum. The concentration of colostrum lactose was relatively low as compared to the other constituents in ewe colostrumCitation63 (as shown in ).
Fat contents in colostrum
The presence of fat contents in colostrum is one of the primary components for judging quality of colostrum. It is nature’s first gift for neonatal lamb and major source of energy. Among the other bioactive components of sheep colostrum and milk, lipids are vital concern because of their high nutritional value. Because of the smaller size of fat globules, sheep colostrum and milk aid in higher digestibility and more efficient metabolism as compared to the other ruminant species.Citation52 Moreover, lipids can promote the absorption of nutrients to ensure the rapid growth in the early stages of the newborn and the establishment of immunity. Sheep and goat milk contain a higher percentage of smaller fat globules than cow milk; the average diameter of these globules is 3.6 and 3.0 μm, respectively, compared to 4.0 μm in cow milk.Citation64 The various comparative studies between other ruminants’ species, although, it has been strongly evident that sheep colostrum has a higher concentration of gross chemical composition, especially fat contents, were comparatively higher in sheep 9.94% than those measured in goat and cow (6.39%, and 6.66%, respectively), as shown in Citation65.
Factors affecting the colostrum composition
One of the most promising scientific disciplines right now, immune milk and colostrogenesis has considerable promise for enhancing the health and productivity of animals raised for food and ultimately for public health concerns. The composition of colostrum nutrients is strongly correlated with animal species, animal breed and genetic characteristics, feeding program, diet, parity, age of ewe, litter size, sex of lamb, season of parturition, environment, geographical variations, management and husbandry practices. The husbandry and management practices of sheep farming directly influenced the quality and quantity of colostrum production by the ewe.Citation44 Mobilization of body reserve in last trimester of gestation also potentially affects the production of colostrum quality and quantity in sheep.Citation66 This development of the mammary glands as well as mammary cell differentiation is primarily influenced by nutritional level of ewe in the last trimester of pregnancy.Citation63 The Immunoglobulins are primarily circulating in blood circulation of ewe, and near the time of parturition, these Immunoglobulins pass from the ewe blood circulation into the mammary glands.Citation67 Hence, managemental practices during the dry period play a vital role in the progression of colostrogenesis. The production of colostrum is directly influenced by the nutrition level during the prepartum period.
Effects of nutrition on colostrum composition
The correlation between maternal nutrition in late gestation, the development of mammary glands, and synthesis of colostrum quantity and quality is well established. The results revealed that well-fed animals produce more colostrum than underfed. However, according to recent reports, the effects of maternal nutrition during pregnancy start with the growth of the fetus and may be complicated by or related to postnatal development throughout the nursing phase. One explanation could be that prenatal nutrition, wherein proteins, amino acids, fatty acids, or vitamins can change various metabolic pathways, may also have an impact on colostrum output and mammary gland function.Citation68 Moreover, nutritional management can alter the immune components in colostrum. During the course the last trimester of pregnancy, about 80 to 85% of fetal growth and mammary development have completed high nutritional requirements in pregnant sheep.Citation4 Moreover, supplementation with glucose, carbohydrate, and mannan-oligosaccharides are constituents of yeast cell walls, and these have been revealed to increase the production of antibodies in ewe colostrum and intensify the absorption of colostrum in the newborn lamb.Citation11 According to recent report supplementation with antioxidants like isoflavone aglycones in late pregnancy can effectively reduce the oxidative damage and improve the colostrum’s Ig content. It’s also possible that isoflavone aglycones play a role in estrogen-like effects on the body’s endocrine system and promote breast development during lactation.Citation69 Similarly, feeding with vitamin E and selenium enhances the immune profile of lamb and is helpful for initial stand and suckling, ultimately improving the survival of neonatal lambs.Citation11 It has been illustrated that providing more than required amount of digestible undegradable protein (DUP) to pregnant ewes before 3 weeks of lambing boosts quantity of colostrum as well as protein and fat contents.Citation70,Citation71 However, it has been reported that feeding protected fats (e.g., megalac) to ewes prepartum has increase the fat contents in milk but has no positive effects on rumen activity.Citation72
Effects of genetics on colostrum composition
The quality and quantity of colostrum is are directly correlated with various factors, i: e., animal breed and genetic characteristics, are core factors influencing colostrum IgG contents level.Citation29 Gilbert et al.Citation73 who conducted a comparative analysis between IgG concentration in colostrum verses different sire breeds, litter size, and age of ewe 36 h postpartum., Breeds sampled incorporated Polypay (P), Rambouillet (R), Targhee (T), Columbia (C), Finnish Landrace (F), and Finn crosses (Fx) and their colostrum IgG concentrations were 80, 64, 67, 64, 72, and 69 mg/ml respectively. Moreover, colostrum IgG concentrations were (61, 69 and 77 mg/ml for singles, twins and triplets, respectively). Furthermore, in the ewe age groups were from 1 year to 7 years, the colostrum IgG concentrations were 100, 65, 67,67,66,66, and 53 mg/ml, respectively.
Effects of litter size on colostrum composition
Csapó et al.Citation29 gauged that the colostrum IgG content of twin ewes was higher than that of single ewe (132.4 mg/mL and 112.2 mg/mL, respectively). Similarly,Citation74 evaluated that twin- rearing ewes produced colostrum with greater percentage of fat contents, while triplet -rearing ewes with had decreased protein and Vitamin E contents. In terms of number of lactations, Interestingly,Citation75 noted that triplet-rearing ewes produced 21% more milk per unit of metabolic body weight and were 10% more efficient in the conversion of feed to milk than twin-rearing ewes. The gender of neonate also influences the colostrum components concentration the concentration of colostrum components e.g., IgG contents being higher in the colostrum of cow which that delivered a male calf compared with a female calf. Similarly, in sheep singleton ewes with male lamb had higher colostrum IgG concentrations as compared to female lamb (54.57 ± 5.37 mg-mL vs. 34.66 ± 4.30 mg-mL IgG), respectively.Citation35
Effects of parity on colostrum composition
Zhou et al.Citation36 reported the colostrum Ig content of multiparity goats was higher than that of primiparity goats (153.28 mg/mL and 115.09 mg/mL), it may be because that the primiparity goats is still at a stage of growth and development, the colostrum production, nutrition, and maternal antibody is low. Moreover, when compared to primiparity ewes, the multiparity ewes had significantly higher milk protein, casein, and fat concentrations. In addition, the multiparity ewes were better at renneting milk than the primiparity ewes. Lesser lactations resulted in lesser milk quality. In comparison to multiparity ewes, primiparity ewes’ milk included significantly more mesophilic bacteria, as well as higher concentrations of total coliform and psychrotrophs from their milk.Citation76 A progressive increase of milk protein and fat contents with increasing number of lactations has been reported by Casoli et al.Citation77 The different results of different studies may be due to the different varieties of each trial, because different breeds of nutrients and maternal antibodies are different of in colostrum, and the different individuals of the same breed are still different.
Effects of time on colostrum composition
The composition of colostrum depends on various factors and has a variable correlation with season of parturition, environment, geographical variation, and time after parturition. Total solid was 24.58% in first six hours and 17.32% on third day postpartum, decreases with the passage of time after parturition. Moreover, lactose concentration was 3.74% on day three and it was increased 4.30% at 12th week postpartum that represents increasing with the passage of time. Lactose contents and total solids have a negative correlation with the passage of time.Citation78 Alves et al.Citation79 corroborated the results of previous studies and reveled that protein, fat, total solid (TS) and defatted dry extract content decreased by 29.83, 62.52, 36.58, and 44.72% in the first 36 h after parturition, respectively. Similarly, Ciuryk et al.Citation53 also perceived a decrease of fat content 31.45% and 68.66% for protein when analyzing the first 48 hours following the initial milking, including the colostrum and transitional milk of Merino sheep. Godden et al.Citation80 reported that colostrum from first milking contains maximum nutrients contents especially IgG but with the passage of time its concentration gradually decreases up to the next 6th milking. The composition on 10th day was same. According to Alves et al.Citation79 Similarly, immune-proteins (IgA, IgG, and, IgM), lactoferrin, proline-rich peptides, other high-quality proteins, important enzymes, and minerals (Fe, K, Ca, Mg, Cu, Zn, and others) are substantially higher in the initial hours after parturition, and thereafter decline and vary dramatically over time. The IgG concentrations at 6th, 12th, and 24th hours after lambing were 25.36%, 11.73%, and 0%, respectively. The concentration of other elements like as vitamins, lipids, and lactose in colostrum differs from that of mature milk.Citation81
Effects of climate on colostrum composition
There are various intrinsic and extrinsic factors affecting colostrum quality and quantity, the extrinsic facts include the environment, temperature, geographical variations, noise, etc. Hence, the literature lacks information about the correlation between extrinsic factors. The environmental and thermal stressors can widely affect the productivity of wild and domestic animals. The hot climate has adverse impact on health, reproductive efficiency, and especially colostrum/or milk production in term of quaintly and quality.Citation47 Sevi et al.Citation82 studied the effects of climate changes on the milk composition of ewe milk and concluded that lambing season can significantly influence on milk fat and protein components in ewe milk. Moreover, it is noted that high ambient temperature reduce the fat percentage in colostrum.Citation83 It has been demonstrated that exposure solar radiation has an adverse impact on the milk hygienic quality and causes a high number of polymorphonuclear leukocytes and pathogens.Citation84 Addis et al.Citation85 who conducted a comparative study to analyze the effects of high light -to- dark ratio on milk component contents and concluded that high light-to-dark ratio decrease fat and protein concentrations in colostrum and milk. The reduction of milk fat and protein may be due to higher secretion of prolactin, whose plasma concentration is higher in summer as compared to winter. Moreover, alteration in photo period may causes divergence in quantity and quality of ovine milk. Similarly, longer photo period can lead to more dilution of milk which alters the compositional contents. Ramón et al.Citation86 reported that Calcium (Ca) contents markedly decreases in ewes colostrum and milk that are exposed in high ambient temperatures and suggested that parathyroid hormone may be involved in this phenomenon. It has been observed that in summer season, cow milk mineral contents are significantly decreases, this reduction is probably due to decrease in osmotic pressure in udder in the hot climates.Citation5 Sevi et al.Citation82 reported that in the first hours after exposure to high temperatures, milk production reduced by 1 to 5 and 0.1 to 0.3 g/d per °C above the threshold for milk yield and for fat and protein yields, respectively, in Manchega sheep. It was documented that when the temperatures exceed 35 °C, milk production declines by 20% in the Comisana sheep breed.Citation84 Bernabucci et al.Citation47 reported that markedly influenced the colostrum nutrients components, e.g., IgG lactose and protein, which were much higher as compared to under thermal stressed condition. Probably, small ruminants required more nutrients and bioactive components to combat and compensate the thermal stress.
Absorption of colostrum
The colostrum is first nature’s gift for the newborn lamb, having been accumulated since the last term of gestation from the blood to mammary gland secretion of ewe. As the neonatal lamb suckles and ingests colostrum, it takes into gastrointestinal tract.Citation45 The neonatal lamb gastrointestinal tract proteolytic activity is very low, which is further minimized due to the presence of trypsin inhibitors in colostrum. This inhibitory enzyme intercept colostrum proteins from degradation and bypasses it into small intestine for the use of beneficial food.Citation87 Colostrum immunoglobulin binds to the specialized FcRn receptors on the intestinal epithelium cells of neonatal lambs. However, when once immunoglobulin attaches to the epithelial cells, they actively pinocytose into intestinal capillaries, where the ultimately absorbed immunoglobulins reaches in systemic circulation. In this way, neonatal lamb attains a huge amount of maternal immunoglobulin.Citation88 The intestinal route seems to be a nonspecific pathway, and ultimately a significant amount IgG is absorbed and again attains the intestinal lumen.Citation88 Mayer et al.Citation25 conducted an immunohistological approach in neonatal lamb to investigate the location and detection of FcRn expression pattern in small intestine and concluded that FcRn was strongly expressed at the top of neonatal lamb duodenal crypt cells while weakly expressed at the base of neonatal lamb duodenal crypt cells, which was previously demonstrated to secret IgG1 in newborn ruminants. Later on, through advanced and novel research work, it has been elucidated that FcRn transfer of passive humoral immunity from dam to fetus, cattle,Citation89 sheep,Citation90 pig,Citation91 and human.Citation92 Almost all mammalian species have FcRn receptors, and their multifaceted activity depends on their capacity to prevent IgG from being degraded by intracellular lysosomes and to release attached cargo during exocytic processes at the plasma membrane.Citation93 Moreover, expression of FcRn were transferred to the apical membrane location in sheep and cows after parturition.Citation25,Citation89 The transportation of IgG1 is selective, and those that have structural resemblance with immunoglobulin A (IgA) are comparatively more resistant to proteolysis.Citation94 Neonatal Fc receptor (FcRn), the transport receptor for maternal IgG in humans, mice, and rats, is made up of a heterodimer of an integral membrane glycoprotein, such as the MHC class I-chains and 2-microglobulin. FcRn serves as the receptor in charge of maintaining the prolonged half-life and high concentration of IgG in serum, preventing IgG from degrading.Citation95,Citation96 Provided biochemical evidence of IgG transport in the neonatal rat intestinal epithelium with pH binding dependency in both its transport and protection receptors roles. FcRn binds IgG in intracellular transport vesicles with Nano molar affinity at acidic pH (6.5) and releases IgG when exposed to bloodstream pH (7.4).Citation86
Conclusions and future directions
In this review, the leading emphasis is how quality and quantity of colostrum can enhance maternal antibodies and immunoglobulin magnitude in colostrum for the acquisition of passive transfer of immunity in newborn lambs. Sheep rearing and farming is an integral part of human cultures around the world since the ancient times. Livestock farming play a crucial role in the national economy of country as well as human health beneficial concerns. Current research on sheep colostrum and milk will be beneficial to this underprivileged section of human society. Sheep anatomical placenta endometrium structure creates synepitheliochorial interhemal barrier. Due to which neonatal lambs are born agammaglobulinemic (non-significant magnitude of antibodies) at time of birth. The non-availability of defensive immunity enhances the chances of neonatal mortality. Colostrum is primary source of nutrition, energy and immunity (passive immune transfer) in early life threating phase. Hence, in time intake of adequate amount of colostrum in early life significantly improves survival rate of neonatal lambs, and it also mitigate the effects of risk factors for lamb mortality such as infectious diseases, low birth weight, and stress reaction. Thus neonates need immediate access to intake colostrum which has enough quality and quantity. According to literature data, passive immunity in ruminant neonates not only ensure prevent against diseases but also accelerate growth performance. Therefore, there is dire needs to improve the colostrum quality and quantity. Hereinafter, in this review we have emphasized the enrichment of colostrum quality and quantity with reference to passive immune transfer, neonatal survival, livestock productivity and profitability, ultimately, veterinary public health and food safety.
In order to exploit on molecular data basses for more efficient genetic selection, contemporary genetic research on genetic polymorphism, mapping and identifying the genes and markers that regulate productivity and quality attributes will be more fruitful in future.
Authors’ contributors
J XP and L GQ: contributed conception and design of this review; UF and SA: initial and revised manuscript preparation; JD. HY and M.A revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
The authors are thankful to anonymous, for their valuable comments, suggestion and proofreading of the manuscript.
Disclosure statement
All authors declare no conflict and controversy regarding the application of current study.
Additional information
Funding
References
- Alves AC, Alves NG, Ascari IJ, Junqueira FB, Coutinho AS, Lima RR, Pérez JR, De Paula SO, Furusho-Garcia IF, Abreu LR. Colostrum composition of Santa Inês sheep and passive transfer of immunity to lambs. Journal of Dairy Sci. 2015;98(6):1–13.
- Agenbag B, Swinbourne AM, Petrovski K, van Wettere WHEJ. Lambs need colostrum: a review. Livest Sci. 2021;251:1.
- Korhonen H, Marnila P, Gill HS. Milk immunoglobulins and complement factors. Br J Nutr. 2000;84 Suppl 1(S1):S75–12.
- Hinde D, Woodhouse M. Ewe nutrition and colostrum. Livestock. 2019;24(Sup2):9–14.
- Bahga CS, Gangwar PC, Mehta SN, Dhingra DP. Effect of cooling on concentration of electrolytes in milk of buffaloes (Bos Bubalis) during summer. Indian J Dairy Sci. 1985 38(1):36–40
- Fischer-Tlustos AJ, Lopez A, Hare KS, Wood KM, Steele MA. Effects of colostrum management on transfer of passive immunity and the potential role of colostral bioactive components on neonatal calf development and metabolism. Can J Anim Sci. 2021;101(3):405–426.
- Puppel K, Gołębiewski M, Grodkowski G, et al. Composition and factors affecting quality of bovine colostrum: a review. Animals. 2019;9(12):1070.
- Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156(2 Suppl):S8–S15.
- Ontsouka CE, Bruckmaier RM, Blum JW. Fractionized milk composition during removal of colostrum and mature milk. J Dairy Sci. 2003;86(6):2005–2011.
- Bendixen E, Danielsen M, Hollung K, Gianazza E, Miller I. Farm animal proteomics - A review. J Proteomics. 2011;74(3):282–293.
- Pecka-Kiełb E, Zachwieja A, Wojtas E, Zawadzki W. Influence of nutrition on the quality of colostrum and milk of ruminants. Mljekarstvo. 2018;68(3):169–181.
- Shiels D, Loughrey J, Dwyer CM, Hanrahan K, Mee JF, Keady TWJ. A survey of farm management practices relating to the risk factors, prevalence, and causes of lamb mortality in Ireland. Animals. 2021;12(1):30.
- Nowak R, Poindron P. From birth to colostrum: early steps leading to lamb survival. Reprod Nutr Dev. 2006;46(4):431–446.
- Sallam AM. Risk factors and genetic analysis of pre-weaning mortality in Barki lambs. Livest Sci. 2019;230:103818.
- Santos JDCd, Saraiva EP, Pimenta Filho EC, et al. Neonatal mortality of lambs in production systems in a semi-arid environment: main risk factors. J Agric Sci. 2023;161(3):438–449.
- An J, Liu Y, Wang Y, et al. The role of intestinal mucosal barrier in autoimmune disease: a potential target. Front Immunol. 2022;13:871713.
- Hernández-Castellano LE, Suárez-Trujillo A, Martell-Jaizme D, Cugno G, Argüello A, Castro N. The effect of colostrum period management on BW and immune system in lambs: from birth to weaning. Animal. 2015;9(10):1672–1679.
- Sahu S, Albaugh ME, Martin BK, et al. Growth factor dependency in mammary organoids regulates ductal morphogenesis during organ regeneration. Sci Rep. 2022;12(1):7200.
- Fleet IR, Goode JA, Hamon MH, Laurie MS, Linzell JL, Peaker M. Secretory activity of goat mammary glands during pregnancy and the onset of lactation. J Physiol. 1975;251(3):763–773.
- Mondeshka L, Dimitrova T, Markov N, Hristov M. Goat colostrum – composition and impact. Sci Pap Ser D Anim Sci. 2022;LXV(1):400–407.
- Barrington GM, Besser TE, Gay CC, Davis WC, Reeves JJ, McFadden TB. Effect of prolactin on in vitro expression of the bovine mammary immunoglobulin G1 receptor. J Dairy Sci. 1997;80(1):94–100.
- Lérias JR, Hernández-Castellano LE, Suárez-Trujillo A, Castro N, Pourlis A, Almeida AM. The mammary gland in small ruminants: major morphological and functional events underlying milk production - A review. J Dairy Res. 2014;81(3):304–318.
- Lechan RM, Toni R. Functional anatomy of the hypothalamus and pituitary - endotext - NCBI Bookshelf. Endotext (Internet). 2016.
- Butler JE. Bovine immunoglobulins: an augmented review. Vet Immunol Immunopathol. 1983;4(1–2):43–152.
- Mayer B, Zolnai A, Frenyó LV, et al. Localization of the sheep FcRn in the mammary gland. Vet Immunol Immunopathol. 2002;87(3-4):327–330.
- Brinkhaus M, Pannecoucke E, van der Kooi EJ, et al. The Fab region of IgG impairs the internalization pathway of FcRn upon Fc engagement. Nat Commun. 2022;13(1):6073.
- Jones KHL, Richard E. Human Reproductive Biology (Third Edition). Academic Press (USA). 2006:231–243.
- Trotta RJ, Vasquez-Hidalgo MA, Vonnahme KA, Swanson KC. Effects of nutrient restriction during midgestation to late gestation on maternal and fetal postruminal carbohydrase activities in sheep. J Anim Sci. 2020;98(1):skz393.
- Csapó J, Csapó-Kiss Z, Martin TG, Szentpeteri J, Wolf G. Composition of colostrum from goats, ewes and cows producing twins. Int Dairy J. 1994;4(5):445–458.
- Yang XY, Chen JP, Zhang FX. Research on the chemical composition of Saanen goat colostrum. Int J Dairy Tech. 2009;62(4):500–504.
- Arain HH, Khaskheli M, Arain MA, Soomro AH, Nizamani AH. Heat stability and quality characteristics of postpartum buffalo milk. Pakistan J Nutr. 2008;7(2):303–307.
- Pecka-Kiełb E, Vasil M, Zachwieja A, et al. An effect of mammary gland infection caused by Streptococcus uberis on composition and physicochemical changes of cows’ milk. Pol J Vet Sci. 2016;19(1):49–55.
- Van Hese I, Goossens K, Vandaele L, Opsomer G. Invited review: MicroRNAs in bovine colostrum—Focus on their origin and potential health benefits for the calf. J Dairy Sci. 2020;103(1):1–15.
- El-Hatmi H, Girardet JM, Gaillard JL, Yahyaoui MH, Attia H. Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin Res. 2007;70(2-3):267–271.
- Abecia J-A, Garrido C, Gave M, et al. Exogenous melatonin and male foetuses improve the quality of sheep colostrum. J Anim Physiol Anim Nutr (Berl). 2020;104(5):1305–1309.
- Zhou A, Liu G, Jiang X. Characteristic of the components and the metabolism mechanism of goat colostrum: a review. Anim Biotechnol. 2023;34(8):4135–4146.
- Park YW, Zhang H, Zhang B, Zhang L. Mare milk. In Hand-Book of Milk of Non-Bovine Mammals. Blackwell Pubishing (UK); 2006:275S296.
- Haenlein GFW, Park YW. Handbook of Milk of Non-Bovine Mammals. Blackwell Publishing (UK); 2006.
- Oyeniyi OO, Hunter AG. Colostral constituents including immunoglobulins in the first three milkings postpartum. J Dairy Sci. 1978;61(1):44–48.
- McManaman JL. Lipid transport across the mammary gland. Ion Transp Across Epithel Tissues Dis Ion Channels Transp Ep Heal Dis. 2020;2:241–277.
- Ballard O, and Morrow AL. Human milk composition: nutrients and bioactive factors. Pediat Clin N Amer. 2013;60(1):49–74.
- Jain G, Singh A, Aslam NJS, Auddy N, Verma PS. Comparison between chemical compositions of colostrum and milk in different livestock species. Environ Agric Heal. 2020:62.
- Merin U, Bernstein S, Van Creveld C, Yagil R, Gollop N. Camel (Camelus dromedarius) colostrum and milk composition during the lactation. Milchwissenschaft. 2001;56(2):70–74.
- Castro N, Capote J, Bruckmaier RM, Argüello A. Management effects on colostrogenesis in small ruminants: a review. J Appl Anim Res. 2011;39(2):85–93.
- Hernández-Castellano LE, Morales-delaNuez A, Sánchez-Macías D, et al. The effect of colostrum source (goat vs. sheep) and timing of the first colostrum feeding (2h vs. 14h after birth) on body weight and immune status of artificially reared newborn lambs. J Dairy Sci. 2015;98(1):204–210.
- Pisello L, Forte C, D’Avino N, et al. Evaluation of Brix refractometer as an on-farm tool for colostrum IgG evaluation in Italian beef and dairy cattle. J Dairy Res. 2021;88(2):189–193.
- Bernabucci U, Basiricó L, Morera P. Impact of hot environment on colostrum and milk composition. Cell. Mol. Biol. 2013;59(1):67–83.
- Barrington GM, McFadden TB, Huyler MT, Besser TE. Regulation of colostrogenesis in cattle. Livest Prod Sci. 2001;70(1–2):95–104.
- Rizzello CG, Losito I, Gobbetti M, Carbonara T, De Bari MD, Zambonin PG. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J Dairy Sci. 2005;88(7):2348–2360.
- Qian ZY, Jollès P, Migliore-Samour D, Schoentgen F, Fiat AM. Sheep κ-casein peptides inhibit platelet aggregation. Biochim Biophys Acta. 1995;1244(2–3):411–417.
- Pustkowiak H, Ebrowska AŻ. Wp ł yw Substytucji Mleka Owczego Mlekiem Krowim na w ł a ś Ciwo ś ci Bundzu. ŻYWNOŚĆ Nauka Technologia Jakość. 2009;5(66):96–106.
- Ahmadi M, et al. Colostrum from different animal species – a product for health status enhancement. Bull Univ Agric Sci Vet Med Cluj-Napoca Anim Sci Biotechnol. 2016;73(1):1–7.
- Ciuryk S, Molik E, Kaczor U, Bonczar G. Chemical composition of colostrum and milk of Polish Merino sheep lambing at different times. Arch Tierz Dummerstorf. 2004;47:129–134.
- Zachwieja A, Szulc T, Potkański A, Mikuła R, Kruszyński W, Dobicki A. Effect of different fat supplements used during dry period of cows on colostrum physicochemical properties. Bio Anim Husb. 2007;23(5–2):67–75.
- Pecka E, Dobrzański Z, Zachwieja A, Szulc T, Czyz K. Studies of composition and major protein level in milk and colostrum of mares. Anim Sci J. 2012;83(2):162–168.
- Nowak W, Mikuła R, Kasprowicz-Potocka M, et al. Effect of cow nutrition in the far-off period on colostrum quality and immune response of calves. Bull Vet Inst Pulawy. 2012;56(2):241–246.
- Czerniawska-Piątkowska E, Czerniawska-Piątkowska E, Kowalewska-Łuczak I, Pecka-Kiełb E. Investigation on relationships of the FABP3 and SLC27A3 genes with milk production traits in sheep. J Elem. 2017;22(4/2017):1485–1493.
- Abdel-Salam ZA, Abdel-Mageed II, Harith MA, Abdel-Salam SAM. Evaluation of proteins in sheep colostrum via laser-induced breakdown spectroscopy and multivariate analysis. J Adv Res. 2019;15:19–25.
- Wheeler TT, Hodgkinson AJ, Prosser CG, Davis SR. Immune components of colostrum and milk - A historical perspective. J Mammary Gland Biol Neoplasia. 2007;12(4):237–247.
- Rushton J. The Economics of Animal Health and Production.pdf. 2009.
- Keskin M, Güler Z, Gül S, Biçer O. Changes in gross chemical compositions of ewe and goat colostrum during ten days postpartum. J Appl Anim Res. 2007;32(1):25–28.
- Banchero GE, Quintans G, Martin GB, Milton JTB, Lindsay DR. Nutrition and colostrum production in sheep. 2. Metabolic and hormonal responses to different energy sources in the final stages of pregnancy. Reprod Fertil Dev. 2004;16(6):645–653.
- Banchero GE, Milton JTB, Lindsay DR, Martin GB, Quintans G. Colostrum production in ewes: a review of regulation mechanisms and of energy supply. Animal. 2015;9(5):831–837.
- Biadała A, Konieczny P. Goat’s milk-derived bioactive components - A review. Mljekarstvo. 2018;68(4):239–253.
- Kessler EC, Bruckmaier RM, Gross JJ. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J Dairy Sci. 2019;102(6):5542–5549.
- Al-Sabbagh TA, Swanson LV, Thompson JM. The effect of ewe body condition at lambing on colostral immunoglobulin G concentration and lamb performance. J Anim Sci. 1995;73(10):2860–2864.
- Guha S, Sharma H, Deshwal GK, Rao PS. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process. Nutr. 2021;3(1).
- Marshall NE, Abrams B, Barbour LA, et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am J Obstet Gynecol. 2022;226(5):607–632.
- Wu H, Yang J, Wang S, et al. Effects of soybean isoflavone and astragalus polysaccharide mixture on colostrum components, serum antioxidant, immune and hormone levels of lactating sows. Animals. 2021;11(1):132.
- Amanlou H, Karimi A, Mahjoubi E, Milis C. Effects of supplementation with digestible undegradable protein in late pregnancy on ewe colostrums production and lamb output to weaning. J Anim Physiol Anim Nutr (Berl). 2011;95(5):616–622.
- Khalili M, et al. The effect of feeding inorganic and organic selenium sources on the hematological blood parameters, reproduction and health of dairy cows in the transition period. Acta Sci - Anim Sci. 2020;42(1):1–10.
- Behan AA, Loh TC, Fakurazi S, Kaka U, Kaka A, Samsudin AA. Effects of supplementation of rumen protected fats on rumen ecology and digestibility of nutrients in sheep. Animals. 2019;9(7):400.
- Gilbert RP, Gaskins CT, Hillers JK, Parker CF, McGuire TC. Genetic and environmental factors affecting immunoglobulin G1 concentrations in ewe colostrum and lamb serum. J Anim Sci. 1988;66(4):855–863.
- Dwyer CM, Morgan CA. Maintenance of body temperature in the neonatal lamb: effects of breed, birth weight, and litter size. J Anim Sci. 2006;84(5):1093–1101.
- Loerch SC, Mcclure KE, Parker CF. Effects of number of lambs suckled and supplemental protein source on lactating ewe performance. J Anim Sci. 1985;1:2.
- Sevi A, Taibi L, Albenzio M, Muscio A, Annicchiarico G. Effect of parity on milk yield, composition, somatic cell count, renneting parameters and bacteria counts of Comisana ewes. Small Rumin Res. 2000;37(1–2):99–107.
- Kacskovics I, Kis Z, Mayer B, et al. FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol. 2006;18(4):525–536.
- Udeh NE, Oguike MA. Influence of Spondias mombin L (hog plum) on colostrum, milk composition and growth in West African dwarf sheep. Anim Prod Res Adv. 2008;4(3–4). DOI: 10.4314/apra.v4i3-4.49779.
- Alves AC, Alves NG, Ascari IJ, et al. Colostrum composition of Santa Inês sheep and passive transfer of immunity to lambs. J Dairy Sci. 2015;98(6):3706–3716.
- Godden S. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract. 2008;24(1):19–39.
- Khan A, et al. Prevalence and aetiopathology of infectious kerato-conjunctivitis ‘ Pink Eye ‘ in neonatal Pak-Karakul lambs. Level Immunogl Relation Neonatal. 1998;(2018).
- Sevi A, Taibi L, Albenzio M, Muscio A, Dell’Aquila S, Napolitano F. Behavioral, adrenal, immune, and productive responses of lactating ewes to regrouping and relocation. J Anim Sci. 2001;79(6):1457–1465.
- Nardone A, Lacetera N, Bernabucci U, Ronchi B. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J Dairy Sci. 1997;80(5):838–844.
- Sevi A, Annicchiarico G, Albenzio M, Taibi L, Muscio A, Dell’Aquila S. Effects of solar radiation and feeding time on behavior, immune response and production of lactating ewes under high ambient temperature. J Dairy Sci. 2001;84(3):629–640.
- Dahl GE, Buchanan BA, Tucker HA. Photoperiodic effects on dairy cattle: a review. J Dairy Sci. 2000;83(4):885–893.
- Ramón M, Díaz C, Pérez-Guzman MD, Carabaño MJ. Effect of exposure to adverse climatic conditions on production in Manchega dairy sheep. J Dairy Sci. 2016;99(7):5764–5779.
- Trzebiatowski L, Georgiev P, Wehrend A. Trypsin-inhibitor activity in colostrum-an overview. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2022;50(4):258–264.
- Mayer B, Zolnai A, Frenyó LV, et al. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107(3):288–296.
- Casoli C, Duranti E, Morbidini L, Panella F, Vizioli V. Quantitative and compositional variations of Massese sheep milk by parity and stage of lactation. Small Rumin Res. 1989;2(1):47–62.
- Yan MJ, Humphreys J, Holden NM. Life cycle assessment of milk production from commercial dairy farms: the influence of management tactics. J Dairy Sci. 2013;96(7):4112–4124.
- Guo J, Li F, Qian S, et al. TGEV infection up-regulates FcRn expression via activation of NF-κ B signaling. Sci Rep. 2016;6:32154.
- Antohe F, Rădulescu L, Gafencu A, Gheţie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol. 2001;62(2):93–105.
- Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci U S A. 2004;101(30):11076–11081.
- Newby TJ, Bourne FJ. The nature of the local immune system of the bovine small intestine. Immunol Commun. 1976;31(3):475–480.
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337(6203):184–187.
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–725.
