ABSTRACT
The scientific literature contains many accounts of application of polymethine dyes, including cyanine dyes, as imaging agents, i.e., “biological stains,” for microscopic investigation of biological materials. Currently, many such dyes are used as probes for living cells, i.e., “fluorescent probes.” Polymethine dyes are defined here by two criteria. First, they possess a conjugated chain of (2n + 1) sp2-hybridized carbon atoms that connect a terminal π-electron-accepting (π-electron withdrawing) group with a terminal π-electron-donating group. Second, they have an odd number (2n + 3) of π-centers and an even number (2n + 4) of π-electrons in this chain, where n equals the number of –CR2=CR3– groups, usually vinylene groups –CH=CH–. Commercialization of diverse chemical types of many polymethine dyes has been attempted. The dyes that have achieved wide application, however, are limited in number and it is these dyes that are emphasized here. Because these polymethine dyes sometimes have been described by confusing, and sometimes confused, names, we clarify here the chemical categories and names of such dyes for the nonchemist, biomedical end user of such imaging agents. Nevertheless, the nomenclature presented here is not intended to replace the traditional “chromophore” categories of dyestuff chemistry, because the latter are held in place both by wide usage and by venerable authorities, such as the Colour Index.
Nature of the problem
Dyes used as imaging agents, sometimes termed “biological stains” or “fluorescent probes,” play a prominent role in microscopic investigation of biological materials (Horobin and Kiernan Citation2002; Stockert and Blázquez-Castro Citation2017). The biomaterial may require dyes for imaging living cells and organisms, as well as fixed tissues or specimens. Many of the staining agents belong to the large class of polymethine dyes.
With these compounds, however, the knowledge gap between biomedical writers concerning dyes and dyestuff chemists can be large, e.g., compare Kiernan (Citation2001) with Mustroph (Citation2022a). Unfortunately, even the accounts of dyestuff chemists can be unsatisfactory. Consequently, the terms, polymethine dye and cyanine dye are often not distinguished clearly and, for example, different classes of dyes that belong to the polymethine category have been designated cyanine dyes (Yaneva et al. Citation2022). Different classifications of the dye classes that belong to the polymethine dyes also add to the confusion. For example, it has been said that only cyanine and hemicyanine dyes belong to the polymethine dye category (Gregory Citation1990) or that only cyanine, hemicyanine, streptocyanine and oxonol dyes should be classified polymethine dyes (Zollinger Citation2003). It also has been stated that styryl dyes do not belong to the class of polymethine dyes and should be classified separately (Gregory Citation1990). Therefore, in the following account, the dye names and structural characteristics are explained briefly.
We do not attempt to be encyclopedic, nor to focus on novel or innovative probes; rather we consider dyes that have found wider use as imaging agents. Chemical structures used as illustrations often are taken from such dyes and the trivial names used in the biomedical literature are appended to these examples. Note that certain polymethine dyes, such as Cy5 and Cy7, possess reactive groups that enable such colorants to act as fluorescent labels of proteins and other biomolecules. Because these reactive dyes are not themselves used directly as stains, they are not described here.
General definition of polymethine dyes
A characteristic feature of many dyes is a conjugated chain with an odd number of methine (–CH=) or substituted methine groups (–CR=) between two terminal groups. König (Citation1922, Citation1926) demonstrated that many classes of dyes meet these criteria, and introduced the term polymethine dyes for them. König (Citation1925) also reported that each additional vinylene (–CH=CH–) group in a polymethine chain resulted in a bathochromic shift of approximately 100 nm in the absorption maximum, the so-called vinylene shift. Because other electronic effects can be transmitted through such a conjugated system and because these are described adequately by the number n of vinylene groups –CH=CH– or substituted vinylene groups –CR2=CR3–, respectively, the representation in 1 is preferred in our account. Based on this general structure the odd number of methine groups is defined as (2n + 1) where n = 0, 1, 2, 3 … are equal to the number of –CR2=CR3– groups in 1.
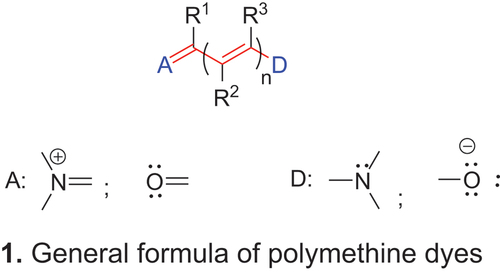
With the development of electronic theory, including valence bond and molecular orbital concepts, König’s definition could be expanded to the number of π-electrons (Herzfeld and Sklar Citation1942; Tyutyulkov et al. Citation1991; Mustroph Citation2022b;). It follows that the polymethine chain in 1 consists of (2n + 1) sp2-hybridized carbon atoms, as indicated by the red color coding and (2n + 1) π-electrons. The two terminal groups, indicated by the blue color coding, are termed π-electron accepting (π-electron withdrawing) group A and π-electron donating group D, where A contributes one and D contributes two π-electrons (a lone pair on the non-ionic atoms) to the conjugated p-system. All atoms contributing a p-atomic orbital are called π-centers. Based on these factors, the following general definition of polymethine dyes can be given (Mustroph Citation2022b):
Polymethine dyes of the general formula 1 are characterized by two features. The first is the presence of a conjugated chain of sp2-hybridized carbon atoms that connects a terminal π-electron-accepting group A and a terminal π-electron-donating group D. The second is an odd number (2n + 3) of π-centers and an even number (2n + 4) of π-electrons, where n = the number of –CR2=CR3– groups (usually vinylene groups –CH=CH–).
A common mistake that appears in the literature, however, is that only the methine or substituted methine groups between the two terminal unsaturated heterocycles are considered part of the “polymethine chain,” as the following example illustrates: “If in the general cyanine formula n = 0, there is no methine bridge connecting ring A with ring B. Such a dye is sometimes called an apocyanine because of its resemblance to a cyanine” (Kiernan Citation2001). The first sentence is incorrect, because in the diagram provided by Kiernan, when n = 0, one methine group remains between A and D. It is correct that in apocyanines both unsaturated heterocycles are connected directly to each other, but as stated by König and as in the formal definition given above based on his work, the polymethine chain counts from N⊕ to N (see section on apocyanines, below). Often it is not taken into account that sp2-hybridized carbon atoms, which constitute terminal unsaturated heterocycles, form parts of the conjugated chain, and indeed this typically is the case for cyanine dyes.
When the broad definition above is adopted, many dye classes fall into the polymethine category. The structural differences among the individual dye classes lies in the nature of the terminal groups, which will be explained in the following sections.
History of cyanine dyes
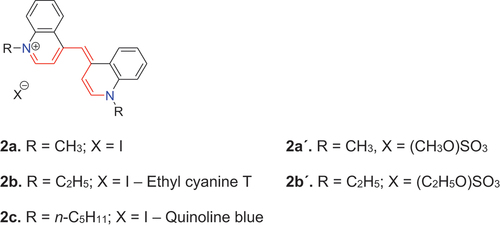
The cyanine class of dyes has a long history, beginning with the laboratory synthesis of the three cyan dyes, 2a–2c, by Williams (Citation1857); he named these dyes iodide of pelamine (Williams Citation1863). von Babo (Citation1857) also described the synthesis of the same basic structures that he named methyl-irisin 2a′ and aethyl-irisin 2b′, respectively. In 1861 Schnitzer (Citation1861) reported the synthesis of 2c on a larger scale and called this dye chinolinblau (chinoline blue, quinoline blue). None of the names, iodide of pelamine, irisin nor chinolinblau prevailed, however, as a name for this dye type. The Ménier company in Paris manufactured large quantities of 2c and exhibited the dye at the London International Exhibition on Industry and Art from May 1 to November 15, 1862. Here, Ménier gave only this single cyan-colored dye 2c the name, cyanine (Hofmann Citation1863). The particular name, cyanine, though given to a single dye eventually became very popular and, perhaps for that reason, evolved into the general label for the whole dye class 2 (Mustroph Citation2020). This is why the N-ethyl derivative 2b initially was named ethyl cyanine T (Hamer Citation1964), a name that has largely been forgotten. Although a dye named quinoline blue has been used as a biological stain, and is still available commercially, this dye includes an isopentyl substituent so it is not identical to 2c.
In 1883 dye 3 was reported (Spalteholz Citation1883). This dye is isomeric with cyanine 2b and, therefore, was named isocyanine. Again, this description applied originally to a single compound, but later provided the name for the entire dye class with this basic structure. Finally, in 1920, a third isomeric compound was described, which is shown as dye 4; this was termed pseudo-isocyanine by Fischer and Scheibe (Citation1920). Today the term pseudo-isocyanine is, analogous to the case of the term cyanine, used for an entire dye class.
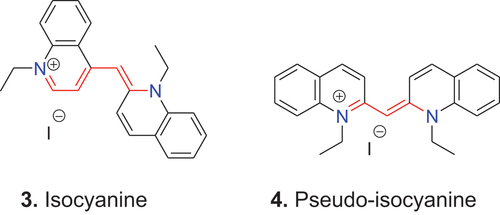
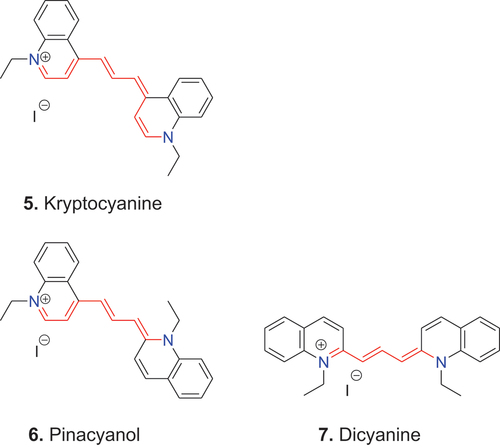
As explained above, each additional vinylene group in the polymethine chain causes a bathochromic shift of approximately 100 nm (König Citation1925). Such structural extension of the conjugated system was carried out with all three basic scaffolds, 2–4, which produced the new dyes, 5–7. These new dyes were called kryptocyanine 5 (Adams and Haller Citation1920; Mills and Braunholtz Citation1923), pinacyanol 6 (Farbwerke Citation1905; Mills and Hamer Citation1920) and dicyanine 7 (Actien-Gesellschaft Citation1903; Mills and Odams Citation1924).
Initially, cyanine, isocyanine, pseudo-isocyanine, kryptocyanine, pinacyanol and dicyanine were each given their own names. As more and more new dyes were synthesized, such individual naming reached its limits and a new system of nomenclature arose. Although there is now IUPAC nomenclature for cyanine dyes, because this “new” system is still used, it will now be outlined. But first, to illustrate why IUPAC names are opaque for all but experts in chemical nomenclature, consider that dicyanine 7 would be termed (2Z)-1-ethyl-2-[(E)-3-(1-ethylquinolin-1-ium-2-yl)prop-2 enylidene]quinoline; iodide.
In the less systematic, but more intelligible naming system, which is still widely used, the dye name specifies the terminal heterocycles, their positions of linking to the methine chain, and the length of the methine chain that joins them. For example, dyes derived from quinoline 2 are called 4,4′-cyanine. In a more detailed version, the N-substituent and the anion are included as illustrated by the following. Accordingly, dye 2c is named 1,1′-di-n-pentyl-4,4′-cyanine iodide, 3 is named 1,1′-diethyl-2,4′-cyanine iodide, and 4 is named 1,1′-diethyl-2,2′-cyanine iodide. A cyanine dye that contains three methine groups between the unsaturated heterocyclic systems is called a carbocyanine, whereas one containing five methine groups is known as a dicarbocyanine etc. According to this nomenclature, kryptocyanine 5 is named 1,1′-diethyl-4,4′-carbocyanine iodide, pinacyanol 6 is named 1,1′-diethyl-2,4′-carbocyanine iodide and dicyanine 7 is named 1,1′-diethyl-2,2′-carbocyanine iodide. Another naming system addresses such differences by specifying the number of methine groups in the open polymethine chain that links the terminal unsaturated heterocyclic systems as mono-, di- etc.; in such terminology, pinacyanol is a trimethine cyanine.
With the introduction of new heterocycles, dyes with terminal benzthiazoles were named thiacyanine, thiacarbocyanine, thiadicarbocyanine etc. Benzoxazole heterocycles then gave rise to oxacyanine, oxacarbocyanine, oxadicarbocyanine; indolenine/indocyanine and benzimidazole/imidacyanine as further examples. For illustration, 8 is denoted 3,3′-diethyl-2,2′-thiacarbocyanine iodide, 9 is named 3,3′-di(n-pentyl)-2,2′-oxacarbocyanine iodide, 10 is named 1,1′-dimethyl-2,2′-indocarbocyanine chloride and 11 is termed 1,1′,3,3′-tetraethyl-5,5′,6,6′-tetrachloroimidacarbocyanine iodide.
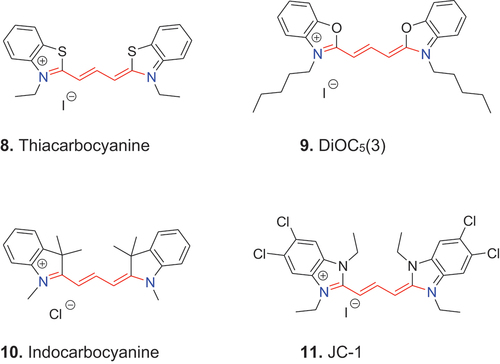
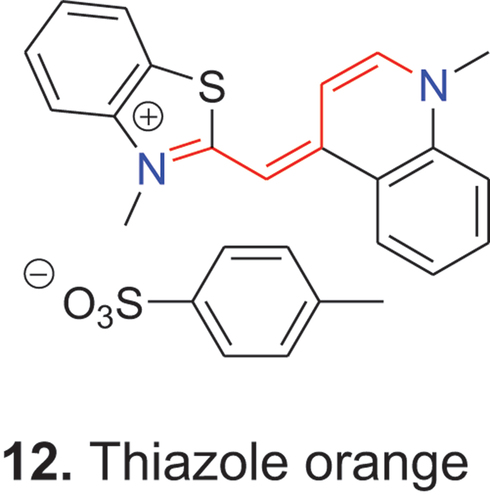
Finally, a cyanine dye is called symmetric when the two terminal heterocycles are the same and asymmetric when they are different. The first asymmetric cyanine dye was the isocyanine 3 (2:4′-cyanine) and for a long time this was the only unsymmetrical cyanine dye. Today, the asymmetric cyanine 12, 3-methyl-2-thiacyanine-1′-methyl-4′-cyanine p-tosylate, better known as thiazole orange, and its derivatives are well known staining agents.
Based on the information given above, all cyanine dyes are defined by the following criteria (Mustroph Citation2022b):
Cyanine dyes are polymethine dyes comprising a conjugated chain of sp2-hybridized carbon atoms that connects two terminal nitrogen atoms, each of which, together with parts of the conjugated chain, constitute unsaturated heterocycles.
Apocyanine dyes
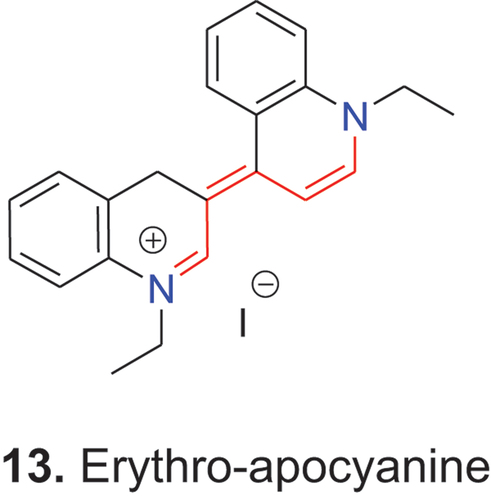
Dyes that lack an open polymethine chain between the two terminal unsaturated heterocycles are called apocyanine dyes (απο = lack). In the classic apocyanine 13 (erythro-apocyanine), five sp2-hybridized carbon atoms lie between the two nitrogen atoms as indicated by the red color coding and the entire conjugated chain is formed by seven π-centers and eight π-electrons.
Apocyanine dyes fulfil the requirements of the polymethine dye definition and are defined by the following criteria (Mustroph Citation2022b):
Apocyanine dyes are polymethine dyes comprising a conjugated chain of sp2-hybridized carbon atoms that connects two terminal nitrogen atoms, each of which, together with the whole conjugated chain, constitute unsaturated heterocycles, and thus lacking an open conjugated chain.
Streptocyanine dyes
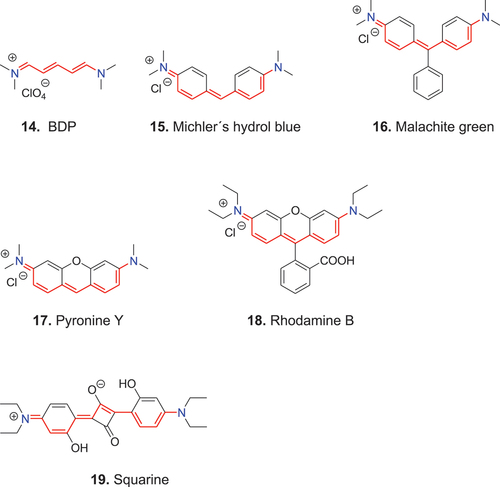
The open-chain polymethine dyes, such as bis-dimethyl pentamethine cyanine 14 (BDP), whose two terminal nitrogen atoms do not form part of an unsaturated heterocyclic system, are the simplest members of the cyanine dye family.
König (Citation1925, Citation1926) termed dyes of this type streptopolymethine dyes. Based on his structural principle that the two terminal nitrogen atoms do not form part of an unsaturated heterocyclic system and the odd number of methine groups, König (Citation1926) classified the diarylmethine dyes, e.g., 15 (Michler′s hydrol blue) and triarylmethine dyes, e.g., 16 (malachite green) as phenylogous streptopolymethine dyes. This classification includes also the heterocyclic derivatives, e.g., 17 (pyronine Y) and 18 (rhodamine B). Furthermore, based on König′s structural principle, squaraine dyes of type 19 belong to the streptopolymethine dyes. For systematic reasons Riester (Citation1963) called König’s streptopolymethine dyes streptocyanine dyes; the latter term better describes their close structural relation to the cyanine dyes (Mustroph Citation2021).
Streptocyanines of the type 14 are widely used as intermediates for preparation of other dyes. Even 15 has seen recent application as a probe, although not for imaging, of subtypes of amyloids (Xiao et al. Citation2020). In addition, many dyes long used as stains and fluorescent probes fall into the streptocyanine category. This is not apparent, however, in the biomedical literature, which uses the dye terms based on traditional chromophore/fluorophore classes. For example, in the standard handbook edited by Horobin and Kiernan (Citation2002), structure 16 is classified as a triarylmethane and structure 18 as a rhodamine. More recently, squaraine dyes, such as 19, also have come into prominence in biomedicine for use as experimental photodynamic therapy agents as well as for bioimaging (Liu et al. Citation2015).
Consequently, the extensive class of streptocyanine dyes is defined as follows (Mustroph Citation2022b):
Streptocyanine dyes are polymethine dyes comprising an open conjugated chain of sp2-hybridized carbon atoms that connects two terminal nitrogen atoms, neither of which forms part of an unsaturated heterocycle.
Hemicyanine dyes
Polymethine dyes in which one terminal nitrogen together with parts of the conjugated chain constitute unsaturated heterocycles, as is typically the case in cyanine dyes, and the second nitrogen is obtained from a terminal nitrogen atom that is not part of an unsaturated heterocyclic system, as typically found in streptocyanine dyes, are named hemicyanine dyes.
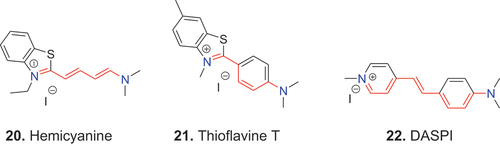
As in streptocyanine dyes, the terminal nitrogen atom, which is not part of an unsaturated heterocyclic system, can be connected directly to the open conjugated chain 20. Alternatively, the connection may be via a phenyl group, which forms part of the chain, as in 21 (thioflavin T), and 22 (DASPI).
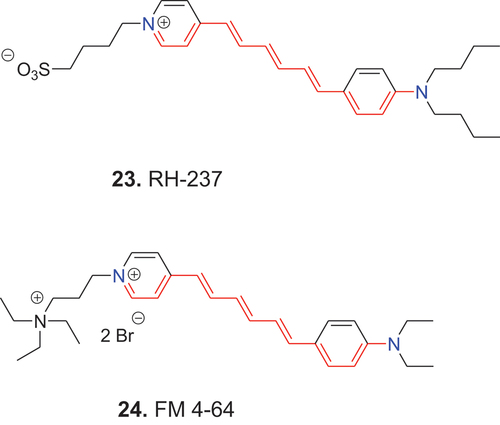
Because of the positive charge, fewer lipophilic substituents and the relatively small molecular size, 22 (DASPI) is highly soluble in aqueous systems. With increasing molecular size and more lipophilic substituents, such as in 23 (RH-237), water solubility decreases, but this effect often can be reversed by substitution with a sulfonic acid group. The combination of a positively charged dye and a negatively charged substituent, however, results in formation of an inner salt, and poor water solubility. Less lipophilic substituents, such as those of the exocyclic amino group in 24 (FM 4-64) combined with a positively charged substituent, produce a double positively charged dye and increased water solubility. For investigation of membrane potential it is significant that such dyes, even when hydrophilic, are nevertheless often amphiphilic.
In the first electronic excited state the open-chain dyes 23 and 24 undergo twisting vibration around the C–C single bonds and photoisomerism around the C=C double bonds. These processes can affect voltage sensitivity. To eliminate this effect, the open polymethine chain dyes 23 and 24 have been bridged to form dyes such as 25 (ANNINE-6) and 26 (ANNINE-6plus) (Fromherz et al. Citation2008).
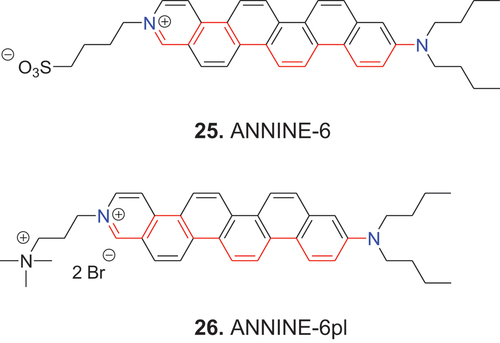
In the “stiff” hemicyanine dyes 25 and 26, twisting vibration around the C–C single bonds is restricted, and photo-isomerism around the C=C double bonds is hindered by annelated benzene rings, which improves the photophysical properties. Paralleling the examples of 23 and 24 discussed above, 25 (ANNINE-6) is insoluble in water, while 26 (ANNINE-6plus) exhibits high water solubility.
The examples, 20–26, illustrate how the hemicyanine dye class includes a wide range of chemical structures. Consequently, special terms have been applied to the individual substructures. For example, dyes whose terminal nitrogen atom is coupled directly to the polymethine chain, as in 20, are called enamines. Phenylogous zeromethine hemicyanine dyes, e.g., 21, often are referred to by the incorrect term, apocyanine (see section on apocyanine dyes). The term styryl dye often is used to refer to hemicyanine dyes that contain a styryl group, e.g., 22. Alternatively, as in the case of 22, the dyes are termed stilbazolium dyes. All of these dyes, however, belong to the hemicyanine dye class.
Use of the term hemicyanine dye describes the close structural relation to the cyanine dyes, and is defined as follows (Mustroph Citation2022b):
Hemicyanine dyes are polymethine dyes comprising a conjugated chain of sp2-hybridized carbon atoms that connects two terminal nitrogen atoms, of which one nitrogen atom and part of the conjugated chain constitute an unsaturated heterocycle as in cyanine dyes, and the second terminal nitrogen atom does not form part of an unsaturated heterocycle as in streptocyanine dyes.
Oxonol dyes
Anionic polymethine dyes in which both terminal oxygen atoms are exocyclic constituents of unsaturated heterocycles are named oxonol dyes, e.g., 27, 28 and 29.
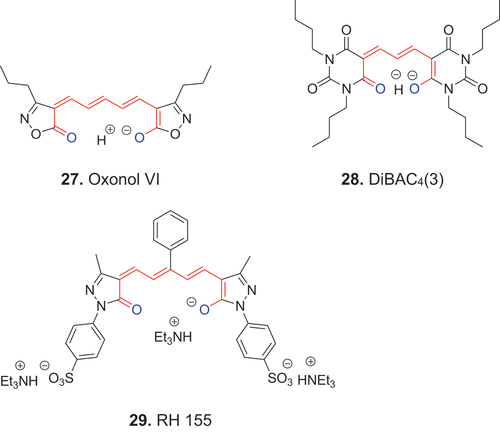
Dyes such as 27 (oxonol VI), 28 (DiBAC4(3)) and 29 (RH 155) have been widely used as voltage sensitive imaging dyes in many eukaryotic and prokaryotic cell lines as well as with tissue slices. Such dyes have also been applied to flow cytometry (Shapiro Citation2000).
Oxonol dyes are defined as follows (Mustroph Citation2022b):
Oxonol dyes are polymethine dyes comprising a conjugated chain of sp2-hybridized carbon atoms that connects two terminal oxygen atoms, each of which is an exocyclic constituent of an unsaturated heterocycle.
Streptooxonol dyes
Anionic polymethines, whose two terminal oxygen atoms are not exocyclic constituents of unsaturated heterocycles, are named streptooxonol dyes. The simplest are the anions of conjugated dialdehydes such as glutacondialdehyde, which is a pentamethine oxonol 30 (POx).
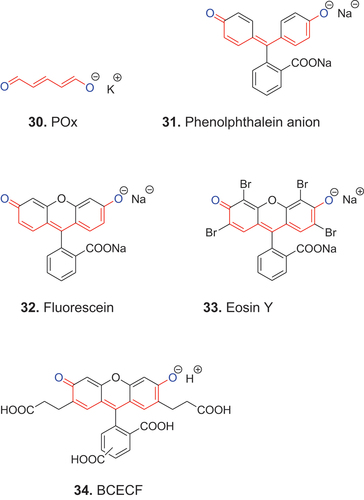
Based on König’s structural principle (König Citation1926; Tyutyulkov et al. Citation1991), the phenylogous streptooxonol dyes such as the phenolphthalein anion 31 and heterocyclic derivatives such as 32 (fluorescein), 33 (eosin Y) and 34 (BCECF), are also members of this group. Some of these dyes have biomedical imaging applications. Eosin Y is a component of “hematoxylin and eosin,” a widely used routine stain for sections of normal and diseased human and animal tissues (Suvarna et al. Citation2019); BCECF is used as a pH indicator within living cells (Han and Burgess Citation2009); and fluorescein is used as a tracer in plants and animals (Pfautsch et al. Citation2015). Phenolphthalein is a pH indicator for aqueous solutions.
Streptooxonol dyes are defined as follows (Mustroph Citation2022b):
Streptooxonol dyes are polymethine dyes comprising an open conjugated chain of sp2-hybridized carbon atoms that connects two terminal oxygen atoms, each of which is an exocyclic constituent of an unsaturated heterocycle.
Merocyanine dyes
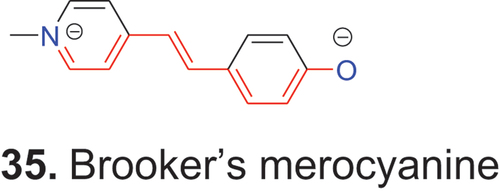
In addition to the cationic and anionic polymethines described so far, there are also neutral polymethines. Neutral organic compounds that have their formal unit electrical charges of opposite sign are called zwitterionic compounds. If one terminal component is typically as found in cyanine dyes and the second part is an unsaturated heterocycle with an exocyclic constituent as in oxonol dyes, then such compounds are called merocyanine (μεροσ = part) dyes. Perhaps the best-known merocyanine is 35 (Brooker’s merocyanine) (Brooker et al. Citation1951), which often is used as model compound for merocyanine dyes.
Merocyanine 540, 36, was among the first fluorescent dyes used to determine membrane potentials in eukaryotic and prokaryotic cells.
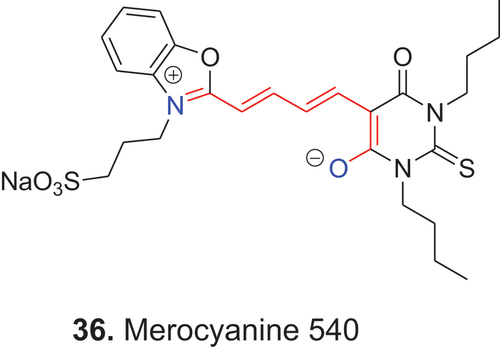
Measurement of membrane potential in this way, however, has declined due to the phototoxicity of the dye and the development of superior probes. Because irradiation of merocyanine 540 produces both singlet oxygen and other reactive oxygen species, including oxygen radicals, the dye now is more commonly used as a photosensitizer in investigations of photodynamic therapy (D’Alessandro and Priefer Citation2020).
Merocyanine dyes have been defined as follows (Mustroph Citation2022b):
Merocyanine dyes are polymethine dyes comprising a conjugated chain of sp2-hybridized carbon atoms that connects a terminal nitrogen atom, where the nitrogen atom and part of the conjugated chain constitute an unsaturated heterocycle as in cyanine dyes, and the second terminal atom is an oxygen atom, which is an exocyclic constituent of an unsaturated heterocycle as in oxonol dyes.
Conclusion
After synthesis of the first organic dyes, many different dye classes have been prepared and used in a wide variety of industrial applications (Gregory Citation1990; Zollinger Citation2003; Towns Citation2019; Pfaff Citation2022). Many of them are part of the polymethine dye class. This is not obvious at first glance, however, because the polymethine class includes a wide range of dyes with diverse chemical structures. In addition, due to long history, a dye class sometimes has more than one name. The first common criterion for polymethine dyes is a conjugated chain of sp2-hybridized carbon atoms that connects a terminal π-electron-accepting group with a terminal π-electron-donating group. The second common and most important criterion is an odd number, 2n + 3, of π-centers and an even number of 2n + 4 π-electrons, where n = the number of –CR2=CR3– groups, usually vinylene groups (–CH=CH–).
We can now make a couple of intriguing general points. The first is the 19th century character of the categories typically used to describe dyes and fluorochromes in the biomedical literature. One of us (RWH) was particularly aware of this, having been the co-author of a handbook (Horobin and Kiernan Citation2002) that describes such agents. Saying that a dye is “an acridine” or “a thiazine” or “a xanthene” is both commonplace and normal in the biomedical literature, including that handbook (Horobin and Kiernan Citation2002). On the other hand, as shown by the sections of this paper that describe streptocyanine and streptooxonol dyes, the traditional categories tell us little about a dye’s electronic character. Such traditional terms will not disappear, however, because they remain in wide use by venerable authorities including the Colour Index.
A second, and perhaps more surprising, point was how many dyestuff chemists, including the renowned authority Zollinger (Citation2003), also generated errors of nomenclature.
Although the style of nomenclature espoused by the account presented here is not going to replace the approach favored by the Colour Index among others, it can provide a novel and bracing chemical viewpoint for those (e.g., RWH) who have been up until now, without awareness, immersed in tradition. It also is apparent that obtaining accurate information is not straightforward, and appeals to authorities can be an unreliable strategy; after all, the investigators cited in the initial “nature of the problem” section of the article presented here were both knowledgeable and often well known.
Acknowledgment
RWH thanks Justin Hargreaves in the School of Chemistry, Glasgow University, for providing facilities.
Disclosure statement
The authors declare no conflict of interest.
References
- Actien-Gesellschaft für Anilin-Fabrikation in Berlin. 1903 Jun 5. Verfahren zur Darstellung sensibilisierend wirkender Farbstoffe der Cyaninenreihe. DE 155541.
- Adams EQ, Haller HL. 1920. Kryptocyanines. A new series of photosensitizing dyes. J Am Chem Soc. 42:2661–2663. doi: 10.1021/ja01457a026.
- Brooker LGS, Keyes GH, Heseltine DW. 1951. Color and constitution. XI. Anhydronium bases of p-hydroxystyryl dyes as solvent polarity indicators. J Am Chem Soc. 73:5350–5356. doi: 10.1021/ja01155a097.
- D’Alessandro S, Priefer R. 2020. Non-porphyrin dyes used as photosensitizers in photodynamic therapy. J Drug Deliv Sci Technol. 60:101979. doi: 10.1016/j.jddst.2020.101979.
- Farbwerke H. 1905 Jul 22. Verfahren zur Darstellung von blauen Farbstoffen der Chinolingruppe. DE 172118.
- Fischer O, Scheibe G. 1920. Beitrag zur Kenntnis der Chinocyanine. J Prakt Chem. 100:86–90. doi: 10.1002/prac.19201000107.
- Fromherz P, Hübener G, Kuhn B, Hinner MJ. 2008. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur Biophys J. 37:509–514. doi: 10.1007/s00249-007-0210-y.
- Gregory P. 1990. Classification of dyes by chemical structure. In: Waring D, Hallas G, Eds. The chemistry and application of dyes. Plenum Press, New York, NY; p. 36–38. doi: 10.1007/978-1-4684-7715-3_2.
- Hamer FM. 1964. The cyanine dyes and related compounds. In: Weissberger A, Ed. The chemistry of heterocyclic compounds. Vol. 18. Interscience, New York, NY; p. 34. doi: 10.1002/9780470186794.
- Han J, Burgess K. 2009. Fluorescent indicators for intracellular pH. Chem Rev. 110:2709–2728. doi: 10.1021/cr900249z.
- Herzfeld KF, Sklar AL. 1942. Colour and constitution of polymethine dyes. Rev Mod Phys. 14:294–302. doi: 10.1103/RevModPhys.14.294.
- Hofmann AW. 1863. Researches on some of the artificial colouring matters. – No. I. On the composition of the blue derivatives of the tertiary monoamines derived from cinchonine. Proc Roy Soc. 12:410–418. doi: 10.1098/rspl.1862.0085.
- Horobin RW, Kiernan JA, Eds. 2002. Conn’s biological stains: a handbook of dyes, stains and fluorochromes for use in biology and medicine. 10th ed. Bios, Oxford.
- Kiernan JA. 2001. Classification and naming of dyes, stains and fluorochromes. Biotech Histochem. 76:261–277. doi: 10.1080/bih.76.5-6.261.278.
- König W. 1922. Über die Konstitution der Pinacyanole, ein Beitrag zur Chemie der Chinocyanine. Ber Dtsch Chem Ges. 55:3293–3313. doi: 10.1002/cber.19220550942.
- König W. 1925. Über “vinylenhomologe” Indol- und Pyrrol-Farbstoffe. Angew Chem. 38:743–748. doi: 10.1002/ange.19250383504.
- König W. 1926. Über den Begriff der “Polymethinfarbstoffe” und eine davon ableitbare allgemeine Farbstoff-Formel als Grundlage einer neuen Systematik der Farbenchemie. J Prakt Chem. 112:1–36. doi: 10.1002/prac.19261120101.
- Liu T, Huo F, Yin C, Li JF, Niu L. 2015. A highly selective fluorescence sensor for cysteine/homocysteine and its application in bioimaging. RSC Adv. 5:28713–28716. doi: 10.1039/C5RA03011K.
- Mills WH, Braunholtz WT. 1923. The cyanine dyes. Part VII. A new method of formation of the carbocyanines. The constitution of the thioisocyanines and of kryptocyanine. J Chem Soc Trans. 123:2804–2813. doi: 10.1039/CT9232302804.
- Mills WH, Hamer FM. 1920. The cyanine dyes. Part III. The constitution of pinacyanol. J Chem Soc Trans. 117:1550–1562. doi: 10.1039/CT9201701550.
- Mills WH, Odams RC. 1924. The cyanine dyes. Part VIII. Synthesis of a 2 : 4′-carbocyanine. Constitution of the dicyanines. J Chem Soc Trans. 125:1913–1921. doi: 10.1039/CT9242501913.
- Mustroph H. 2020. Cyanine dyes. Phys Sci Rev. 5:20190145. doi: 10.1515/psr-2019-0145.
- Mustroph H. 2021. Streptocyanine dyes. Phys Sci Rev. 6:137–147. doi: 10.1515/psr-2020-0198.
- Mustroph H. 2022a. Correspondence on “cyanine dyes containing quinolinemoieties: history, synthesis, optical properties and applications”. Chem Eur J. 28:e202103714. doi: 10.1002/chem.202103714.
- Mustroph H. 2022b. Bring back order in the polymethine dye medley: classification, structure and spectra. Dyes Pigm. 208:110783. doi: 10.1016/j.dyepig.2022.110783.
- Pfaff G, Ed. 2022. Encyclopedia of color, dyes, pigments. De Gruyter, Berlin.
- Pfautsch S, Renard J, Tjoilker MG, Salih A. 2015. Phloem as capacitor: radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol. 167:963–971. doi: 10.1104/pp.114.254581.
- Riester O. 1963. Polymethinfarbstoffe. In: Foerst W, Ed. Ullmanns Encyklopädie der technischen Chemie. 3rd ed. Urban & Schwarzenberg, München; p. 315.
- Schnitzer G. 1861. Ueber Anilinroth und Chinolinblau. Chem Centr NF. 6:636–638.
- Shapiro HM. 2000. Membrane potential estimation by flow cytometry. Methods. 21:271–279. doi: 10.1006/meth.2000.1007.
- Spalteholz W. 1883. Ueber Farbstoffe aus dem Steinkohlentheerchinolin. Ber Dtsch Chem Ges. 16:1847–1852. doi: 10.1002/cber.18830160267.
- Stockert JC, Blázquez-Castro A. 2017. Fluorescence microscopy in life sciences. Bentham Books, Sharjah. doi: 10.2174/97816810851801170101.
- Suvarna SK, Layton T, Bancroft JD. 2019. Bancroft’s theory and practice of histological techniques. 8th ed. Elsevier, Amsterdam.
- Towns A. 2019. Colorants: general survey. Phys Sci Rev. 4:20190008. doi: 10.1515/psr-2019-0008.
- Tyutyulkov N, Fabian J, Mehlhorn A, Dietz F, Tadjer A. 1991. Polymethine dyes – structure and properties. St. Kliment Ohridski University Press, Sofia; p. 18.
- von Babo LH. 1857. Ueber einige Zersetzungsprodukte des Cinchonins. J Prakt Chem. 72:73–88. doi: 10.1002/prac.18570720110.
- Williams CG. 1857. Researches on chinoline and its homologues. Trans R Soc Edinb. 21:377–401. doi: 10.1017/S0080456800032208.
- Williams CG. 1863. On the chinoline and leukoline series. J Chem Soc. 16:375–378. doi: 10.1039/JS8631600375.
- Xiao Y, Rocha S, Kitts CC, Reymerd A, Beke-Somfaie T, Fredericka KK, Nordén B. 2020. Michler’s hydrol blue elucidates structural differences in prion strains. Proc Natl Acad Sci USA. 117:29677–29683. doi: 10.1073/pnas.2001732117.
- Yaneva Z, Iva Nova D, Nevena Nikolova N, Toneva M. 2022. Organic dyes in contemporary medicinal chemistry and biomedicine. I. From the chromophore to the bioimaging/bioassay agent. Biotechnol Biotechnol Equip. 36:1–14. doi: 10.1080/13102818.2022.2039077.
- Zollinger H. 2003. Color chemistry. 3rd ed. Wiley-VCH, Weinheim; p. 74.