Abstract
In this study, a novel donor-acceptor type monomer was designed based on selenophene and benzotriazole with a bulky pendant group and synthesized through Stille coupling reaction. The monomer was polymerized electrochemically by using cyclic voltammetry and also chemically by oxidation in the presence of FeCl3. Both polymers were then compared in terms of their optical properties, electrochemical and spectroelectrochemical behaviors, kinetic and colorimetric properties and surface morphologies. Independent of the polymerization method, both electrochemically (E-PSeBTz) and chemically polymerized (C-PSeBTz) coatings showed quite similar properties. Both polymers have p-doping character and multichromic properties in their oxidized states. The polymers can be fully switched between their oxidized and neutral states in fairly short times with acceptable optical contrast at different wavelengths. Both polymers exhibit a λmax of 505 nm and the optical band gaps of the materials were found to be 1.85 eV and 1.80 eV for E-PSeBTz and C-PSeBTz, respectively.
1. Introduction
In the past decades tremendous amount of work for the synthesis, characterization and application of the conducting polymers has been performed in order to make a collection of materials with various properties in demand for the emerging technologies in various fields of science and technology.[Citation1,Citation2] In order to well-tune the versatile properties of these conducting polymers, many different techniques have been applied, including the Donor–Acceptor (D–A) approach. This approach, also called push–pull method, is based on alternating electron-rich and electron-deficient blocks along the polymer backbone and always enables a re-arrangement of the electron density from D to A. This type of polymers demonstrate a resonance form between D–A and D+–A− which increases the double bond character of the single bonds in the polymer backbone, thus affecting the absorption and the electronic properties.[Citation3,Citation4] Hence, this powerful method enables the ability to finely tune the absorption, modulate the molecular energy levels and the band gap by means of selecting different D–A combinations.[Citation5] The numbers of these combinations could be enhanced even further by the modification of the backbone, π-bridge introduction[Citation6–8] and side chain engineering.[Citation9–14]
Electrochromism is an important phenomenon due to the enormous potential in the rapidly developing area of plastic electronics for the applications of smart windows, organic displays, smart papers.[Citation15–17] Competing with the conventional inorganic devices, electrochromic devices based on electrochromic polymers are likely to be the better alternatives due to their low cost, simple processing, high optical contrast and flexibility.[Citation18] Generally, electrochromic polymers are redox active materials whose optical properties undergo a reversible change upon oxidation and/or reduction. More importantly, some polymers can even have more than two redox states and generate multiple colors (multi-electrochromism).[Citation19] Of the conjugated electrochromic polymers, polythiophene, polypyrrole, polyaniline derivatives are widely studied.[Citation19,Citation20]
In our group, electrochemical synthesis of benzotriazole (BTz) and selenophene (Se) bearing D–A type polymers with linear alkyl chain (PSBT)[Citation21] and branched alkyl chain (PSBTz)[Citation22] were performed (). Both polymers were found to have promising properties for organic solar cell and electrochromic device applications. BTz was chosen as the acceptor due to its well-known hetero-aromaticity and strong electron transporting and accepting properties thanks to its two-electron withdrawing imine nitrogens. Additionally, N–H bond of BTz allows easy incorporation of a convenient alkyl substituent for solution process ability and structural modification for the tuning the electronic properties.[Citation23] Selenophene, on the other hand, is chosen due to its strong electron donating capacity and advanced planarity. In fact, in most cases, Se is chosen as π-bridge between the donor and the acceptor of the D–A copolymers.[Citation24,Citation25] According to literature, Se bearing polymers have increased conductivity and enhanced light absorption. Compared to other chalcogenophene derivatives, selenophene incorporation also lowers the band gap of the polymer since HOMO level was not affected but LUMO level is noticeably lowered.[Citation26–28]
Figure 1. Chemical structures of the Se and BTz containing polymers, PSBT,[Citation21] PSBTz[Citation22] and PSeBTz (this study).
![Figure 1. Chemical structures of the Se and BTz containing polymers, PSBT,[Citation21] PSBTz[Citation22] and PSeBTz (this study).](/cms/asset/a2467a6b-3e5f-4125-8721-7edc43c42977/lmsa_a_1565541_f0001_b.jpg)
For this work, we designed another novel D–A type monomer, based on selenophene and BTz, with a bulky pendant group; (1,3-bis(decyloxy)-5-ethylbenzene) instead of linear or branched alkyl side chains (). The novel monomer, SeBTz, was synthesized through Stille coupling reaction and then polymerized electrochemically using cyclic voltammetry and also chemically using oxidative polymerization in the presence of FeCl3. The electrochemical, spectroelectrochemical, colorimetric, kinetic and morphology properties of the electrochemically polymerized (E-PSeBTz) and chemically polymerized (C-PSeBTz) coatings were comparatively investigated. Additionally, these results were also compared with that of PSBT and PSBTz. Both PSeBTz polymers were found to be a medium band gap polymer (<2.0 eV), having p-dopable and multi-electrochromic properties. Polymers showed switching times in order of couple seconds and good to acceptable optical contrasts.
2. Experimental
2.1. Materials and equipments
N-butyllithium solution (n-BuLi), potassium carbonate, 1-bromodecane, benzotriazole, bromic acid, acetic acid, tri-o-tollyphosphine, bis(triphenylphosphine)palladium(II) dichloride, FeCl3, hydrazine monohydrate THF, DMF, chloroform, hexane, diethyl ether were purchased from Sigma Aldrich Chemical Co. Ltd. 1-Bromomethyl-3,5-dimethoxybenzene was purchased from TCI. Tributyl(selenophen-2-yl)stannane and 4, 7 dibromo-2H-benzo[d[1–3]triazole were synthesized according to earlier described methods.[Citation29,Citation30] The commodity chemicals and solvents were used as received and THF was dried over Na/benzophenone and distilled prior to use. Moisture sensitive reactions were conducted under argon athmosphere, unless mentioned otherwise. For the purification of the materials, Merck Silica Gel 60 was used as the stationary phase with different corresponding mobile phase solvents in the column chromatography. 1H and 13C NMR spectra were recorded on a Bruker Spectrospin Avance DPX-400 Spectrometer with an internal reference of trimethylsilane (TMS). The chemical shifts were reported in ppm relative to CDCl3 at 7.26 ppm and 77 ppm, and DMSO at 2.54 ppm and 39.52 ppm for the 1H and 13C, respectively. Indium tin oxide (ITO) was used as the working electrode, platinum wire was used as the counter electrode and Ag wire was used as the pseudo reference electrode for the electrochemical studies which were carried out in a three-electrode cell using a Gamry 600 potentiostat. The spectroelectrochemical studies were performed by Agilent 8453 UV–Vis spectrophotometer. Colorimetric measurements of polymer were performed at all stages of the oxidation process and the colors of the polymers were identified with using CIE (Commision Internationale de L’Eclairage) coordinates. CIE coordinate system composes of three components including luminance (L), hue (a), and saturation (b). Molecular weight of the polymer was measured by Gel Permeation Chromatography (GPC). The polymer was dissolved in THF (2 mg/mL), stirred for 6 h and filtered through the 0.2 μ filters before running in the universal calibrated PL_GPC 220 instrument. HRMS study was done with a Water SYNAPTM system. Scanning Electron Microscopy (SEM) (JEOL, Model JSM-6400) was used to investigate the surface morphology of the monomer and the polymers.
2.2. Synthesis of the monomer
Electron donating selenophene and electron deficient benzotriazole units were chosen to build the donor–acceptor type monomer. Bulky pendant groups were introduced to enhance the absorption. Additionally, linear alkyl chains were attached to ensure the solubility. The synthesis of the monomer was performed via Stille coupling reaction between 2-(3,5-bis(decyloxy)benzyl)-4,7-dibromo-2H-benzo[d][1–3]triazole and tributyl(selenophen-2-yl)stannane via the palladium with a yield of 99%. The chemical synthesis pathway of the monomer is shown in .
Figure 2. Synthetic pathway for the novel monomer, 2-(3,5-bis(decyloxy)benzyl)-4,7-di(selenophen-2-yl)-2H-benzo[d][1–3]triazole (6).
![Figure 2. Synthetic pathway for the novel monomer, 2-(3,5-bis(decyloxy)benzyl)-4,7-di(selenophen-2-yl)-2H-benzo[d][1–3]triazole (6).](/cms/asset/8f6ab839-02e5-4119-8cf4-5064cc1a4922/lmsa_a_1565541_f0002_b.jpg)
2.2.1. Synthesis of 4,7-dibromo-2-(3,5-dimethoxybenzyl)-2H-benzo[d][1–3]triazole (2)
4,7-Dibromo-2H-benzo[d][1–3]triazole (1) was synthesized according to the previously described method.[Citation30] 4,7-Dibromo-2H-benzo[d][1–3]triazole (1) (5.00 g, 0,02 mol) was dissolved in dry DMF (15 mL) under argon atmosphere at 0 °C and NaH (0.52 g, 0.02 mol) was added at that temperature. The reaction mixture was heated to 60 °C. 1-(Bromomethyl)-3,5-dimethoxybenzene (5.00 g, 0.02 mol) was added and the mixture was refluxed overnight. The product was extracted with chloroform and the organic phase was washed with brine and concentrated to obtain the crude product. Column chromatography (chloroform) gave pure product as a yellowish white solid (2.83 g, 37% yield). 1H NMR (400 MHz, CDCl3) δ 7.74 (s, 2H), 6.6 (d, J = 2.2 Hz, 2H), 6.41 (t, J = 2.2 Hz, 1H), 5.85 (s, 2H), 3.77 (s, 6H). Citation13C NMR (100 MHz, CDCl3) δ 161.0, 144.0, 135.9, 129.8, 110.1, 106.5, 100.6, 60.9, 55.4. HRMS (ESI-TOF-MS, m/z) calculated for C15H13Br2N3O2, 427.9432 found 247.9445 (Supporting Information).
2.2.2. Synthesis of 5-((4,7-dibromo-2H-benzo[d][1–3]triazole-2-yl)Methyl)benzene-1,3-diol (3)
4,7-dibromo-2-(3,5-dimethoxybenzyl)-2H-benzo[d][1–3]triazole (2) (2.83 g, 6.63 mmol) was dissolved in acetic acid (125 mL). 47% HBr/H2O solution (50 mL) was added to the suspension mixture and heated until clear solution observed and then refluxed overnight at 120 °C. The reaction was cooled to room temperature, poured into cold water and the product was filtered. The filtered material was washed with water a few times and dried. No further purification was performed and the product was obtained as gray/brown solid (2.32 g, 87% yield). 1H NMR (400 MHz, DMSO) δ 9.45 (s, 2H), 7.70 (s, 2H), 6.25 (d, J = 1.9 Hz, 2H), 6.19 (t, J = 1.9 Hz, 1H), 5.90 (s, 2H). Citation13C NMR (100 MHz, DMSO) δ 158.7, 143.2, 136.5, 130.2, 109.43, 106.1, 102.6, 60.3. HRMS (ESI-TOF-MS, m/z) calculated for C13H9Br2N3O2, 397.8963; found 397.8989 (Supporting Info)
2.2.3. Synthesis of 2-(3,5-bis(decyloxy)benzyl)-4,7-dibromo-2H-benzo[d][1–3]triazole (4)
Potassium carbonate (6.59 g, 39.2 mmol) and 1-bromodecane (3.21 g, 14.5 mmol) were added into two-necked flask and dry DMF (8 mL) were added under argon atmosphere. 5-((4,7-Dibromo-2H-benzo[d][1–3]triazol-2-yl)methyl)benzene-1,3-diol (3) (2.32 g, 5.81 mmol) was dissolved in another flask with dry DMF (5 mL) under argon atmosphere. Both solutions were degassed by argon for 30 min. Solution (3) was added dropwise to the first solution. When the addition was completed, the reaction solution was heated to 80 °C and stirred overnight. After the reaction was complete (TLC), the solution was poured into large amount of cold water. The crude product was extracted using diethyl ether and then washed with brine. Further purification was carried out by column chromatography on silica gel using chloroform and hexane (3:1) as the eluent to obtain the pure compound as a white solid (1.78 g, 45% yield). 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 2H), 6.55 (d, J = 1.7 Hz, 2H), 6.37 (t, J = 2.2 Hz, 1H), 5.80 (s, 2H), 3.86 (t, J = 6.5 Hz, 4H), 1.75–1.65 (m, 4H), 1.44–1.17 (m, 28H), 0.90–0.80 (m, 6H). Citation13C NMR (100 MHz, CDCl3) δ 160.6, 144.1, 135.7, 129.7, 110.1, 106.9, 101.5, 67.2, 59.7, 30.9, 28.5, 28.4, 28.3, 28.2, 25.0, 21.7, 13.1. HRMS (ESI-TOF-MS, m/z) calculated for C33H49Br2N3O2, 680.2249; found 680.2283 (Supporting Info)
2.2.4. Synthesis of the monomer, 2-(3,5-bis(Decyloxy)benzyl)-4,7-di(selenophen-2-yl)-2H-benzo[d][1–3]triazole (6)
Tributyl(selenophen-2-yl)stannane (5) was synthesized according to the previously reported method.[Citation29] Tributyl(selenophen-2-yl)stannane (2.10 g, 4.98 mmol) was dissolved in dry THF (8 mL) in a two-necked flask under argon atmosphere. In another flask, 2-(3,5-bis(decyloxy)benzyl)-4,7-dibromo-2H-benzo[d][1–3]triazole (4) (1.65 g, 2.43 mmol) was dissolved with dry THF (5 mL) under argon. Solution (4) was added into two-necked flask slowly and degassed for 1 h. Bis(triphenylphosphine) palladium(II) dichloride (85 mg, 0.12 mmol) and tri-o-tolyphosphine (0.30 g, 0.97 mmol) were added quickly and reaction mixture was refluxed overnight. After the completion of the reaction, the mixture was cooled to room temperature and solvent was removed under reduced pressure. Purification was carried out by column chromatography on silica gel using chloroform and hexane (1:1) as the eluent to obtain the pure compound as orange–yellow liquid (1.89 g, 99%). 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 3.9 Hz, 2H), 8.07 (d, J = 5.6 Hz, 2H), 7.60 (s, 2H), 7.43–7.38 (m, 2H), 6.65 (d, J = 2.1 Hz, 2H), 6.40 (t, J = 2.1 Hz, 1H), 5.87 (s, 2H), 3.91 (t, J = 6.6 Hz, 4H), 1.78–1.67 (m, 4H), 1.44–1.17 (m, 28H), 0.88 (t, J = 6.7 Hz, 6H). Citation13C NMR (100 MHz, CDCl3) δ 160.5, 145.1, 142.3, 136.5, 131.4, 130.5, 128.3, 125.6, 123.2, 106.9, 101.4, 67.2, 59.7, 30.9, 28.5, 28.4, 28.3, 28.2, 25.0, 21.7, 13.1. HRMS (ESI-TOF-MS, m/z) calculated for C41H56N3Se2O2, 782.2694; found 782.2703.
3. Results and discussion
3.1. Synthesis of the polymer by electrochemical polymerization
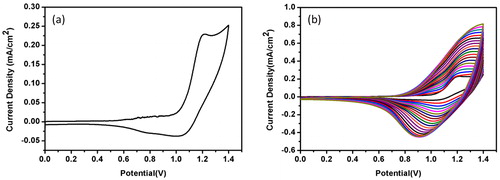
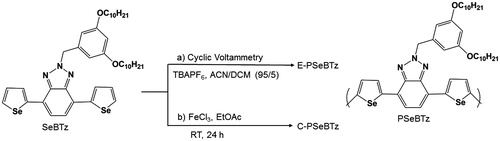
As schematically presented in , electrochemical polymerization of the monomer was carried out in the presence of 0.01 M monomer, 0.1 M TBAPF6 in ACN/DCM (95/5 v/v) in a three-electrode cell equipped with Pt counter electrode and Ag wire pseudo reference electrode. E-PSeBTz films were coated on an ITO glass by scanning potentio dynamically wherein the potential was cycled between 0 V and +1.4 V with 100 mV/s scan rate of 20 cycles. The coated E-PSeBTz film was washed using ACN to remove unreacted monomer and excess TBAPF6 after the electropolymerization.
Figure 3. Schematic presentation of (a) electrochemical polymerization and (b) chemical polymerization of SeBTz.
As seen in , in the first cycle of the electrochemical polymerization, an irreversible oxidation peak emerges at a potential of (Eoxm) 1.21 V indicating the formation of a reactive intermediate for the monomer, SeBTz. With the consecutive cycles (), the peak current increases, indicating the formation of thin film of PSeBTz on ITO coated glass slide revealing an oxidation potential of 1.19 V (Eoxdoping). The oxidation potential of the monomer SeBTz was found to be almost the same as the previously reported SBT[Citation21] and SBTz[Citation22] due to the same main backbone of the three monomers.
3.2. Synthesis of the polymer by chemical oxidation
Anhydrous FeCl3 (43.7 mg, 269 μmol) was taken into the reaction balloon which was filled with argon and dissolved in a minimum amount of EtOAc. 2-(3,5-bis(decyloxy)benzyl)-4,7-di(selenophen-2-yl)-2H-benzo[d][1–3]triazole (60 mg, 79,94 μmol) dissolved in a minimum amount of EtOAc was prepared in a vial and dropwise introduced to the reaction balloon under dark environment. The reaction was stirred at room temperature for 24h. Cold MeOH was added to the reaction balloon to precipitate all polymers. To eliminate the excess amount of FeCl3, the polymer was washed with large amount of MeOH continuously using suction filtration. The polymer was then taken into a balloon and 5% hydrazine monohydrate solution was introduced and stirred for 30 min in order to stop the polymerization. Then, chloroform and hydrazine monohydrate were removed under reduced vacuum. The polymer was precipitated with MeOH, filtrated by suction filtration and washed with MeOH and acetone respectively until no color change was observed. After dried under hot vacuum to remove the solvents, pure polymers of about 24.4 mg were finally collected with a yield of about 40%. GPC: number average molecular weight (Mn): 5980, molecular average molecular weight (Mw): 8641, polydispersity index (PDI): 1.4. 1H NMR (400 MHz, CDCl3) δ 8.2–6.3 (br, aromatic protons), 6.0–5.5 (br, −N-CH2), 4.1–3.5 (br, −O-CH2), 2.0–1.0 (br, −CH2), 1.0–0.7 (br, −CH3).
3.3. Electrochemical properties of the polymers
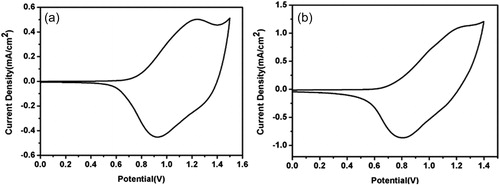
As shown in , both E-PSeBTz and C-PSeBTz have only p-doping property with a reversible redox couple at 1.19 V/1.05 V and 1.27 V/0.81 V, respectively. It is worth to note that, p-doping property means that the positive charges are formed on the polymer backbone upon oxidation, balanced by the negatively charged counter ions. These positive charges can easily migrate throughout the polymer backbone and to nearby chains being responsible for conductivity of the doped polymer.[Citation31] The HOMO energy level of the polymers were estimated as −5.52 eV and −5.44 eV for E-PSeBTz and C-PSeBTz, respectively using the onset of the corresponding oxidation (Eoxonset) 0.77 V and 0.69 V by calculating the energy levels based on to the vacuum level from the given equation HOMO = −(4.75 + Eoxonset) (eV).
3.4. Spectroelectrochemical properties of the polymers
In order to investigate the spectroelectrochemical properties of the electrochemically and chemically synthesized polymer films, UV–Vis spectra were performed in a monomer free solution at different potentials sequentially to observe the changes in absorbance as the polymer film was oxidized in stepwise manner. reveals the spectroelectrochemistry of the electrochemically and chemically prepared films at neutral state and doped states. E-PSeBTz and C-PSeBTz films revealed similar absorption spectra for both neutral and oxidized states. Maximum absorption peak of the neutral PSeBTz films corresponding to distinctive π–π* transition were observed at 505 nm.
Figure 6. Normalized electronic absorption spectra of the (a) electrochemically and (b) chemically synthesized polymer in 0.1M TBAPF6/ACN solution.
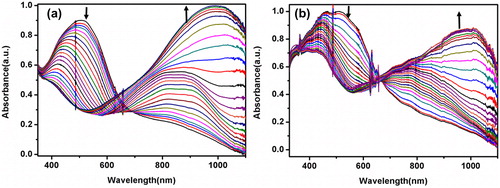
The band gaps of E-PSeBTz and C-PSeBTz were calculated as 1.85 eV and 1.80 eV, respectively from the onset of the π–π* transition for the neutral films (Egop = 1241/ʎonset). As polymer films do not show any n-doping character, the LUMO energy level could not be calculated from the cyclic voltammogram. Instead, they were estimated using the optical band gap value (Egel = |HOMO-LUMO|) to be −3.60 eV and −3.57 eV for E-PSeBTz and C-PSeBTz, respectively. All corresponding data were summarized in . The band gap of both E-PSeBTz and C-PSeBTz were found to be higher than those of PSBT (1.67 eV) and PSBTz (1.82 eV). Such an increase might be explained by relatively shorter polymer chains and depressed conjugation caused by the steric hindrance due to the comparatively bulky alkyl chain of PSeBTz films.
Table 1. Summary of electrochemical and spectroelectrochemical properties of PSBT,[21] PSBTz[22] and PSeBTz (this work) synthesized both electrochemical and chemical methods.
Upon increasing oxidation of E-PSeBTz film, the absorbance transitions corresponding to the π–π* transitions of around 500 nm decreased in intensity and typical evolution of peaks around 1000 nm generated corresponding to polaronic and bipolaronic bands due to the formation of free charge carriers. Similarly for C-PSeBTz film, increased oxidation decreased the intensity of absorption transitions at 400 nm and 500 nm for π–π* transitions and increased that of polaron–bipolaron bands at 1000 nm.
3.5. Colorimetric properties of the polymers
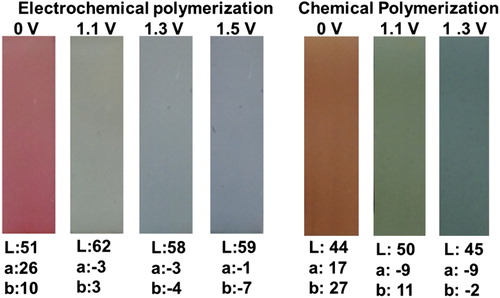
Colorimetry measurements of the polymer films were performed at all stages of the oxidation processes and the corresponding colors were identified as L, a, b values. Polymers displayed red and orange colors in their neutral states and multi-chromism (green, gray, and bluish gray for E-PSeBTz) (light and dark green for C-PSeBTz) in different oxidized states as summarized in . It is worth to compare the colors of the polymers with different side chains. PSBT with linear alkyl chain were reported to be purple in its neutral state while in its oxidized states blue color with green intermediates was observed.[Citation21] PSBTz with the branched alkyl chain, on the other hand, displayed red purple color in its neutral state and transmissive blue in its oxidized state.[Citation22] Such differences show that side chain engineering is crucial in terms of their effect on the electrochromic properties of the resulting polymers with the same conjugated main chain.
3.6. Kinetic properties of the polymers
C-PSeBTz showed 9% transmittance change at 505 nm and 44% at 995 nm (). Switching times were recorded as 2.2 s and 1.3 s for E-PSeBTz and 1.7 s and 1.4 s for C-PSeBTz for the corresponding wavelengths, as summarized and compared with PSBT,[Citation21] and PSBTz[Citation22] ().
Figure 8. Percent transmittance change monitored at maximum wavelengths of the electrochemically synthesized polymer (top) and chemically synthesized polymer (bottom) in 0.1 M TBAPF6/ACN electrolyte solution.
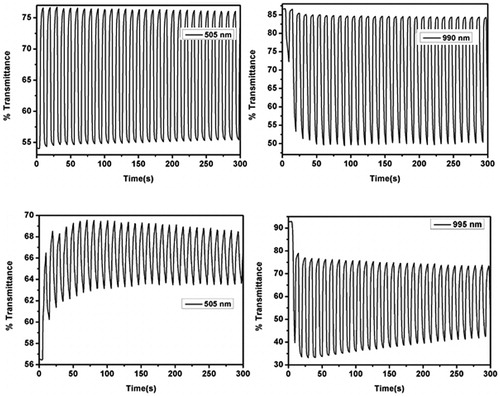
Table 2. Optical contrast and switching times of PSBTCitation[21], PSBTzCitation[22] and E-PSeBTz and C-PSeBTz from this work.
3.7. Morphological properties of the polymers
The surface morphological properties of the monomer (SeBTz) and the electrochemically (E-PSeBTz) and chemically (C-PSeBTz) synthesized polymers were investigated using SEM. The surface morphologies are quite different among each other (). The monomer surface seems to have filament like hairy, E-PSeBTz has cauliflower like structure while C-PSeBTz has compact, uniform and smooth surface.
4. Conclusion
A novel monomer, consisting of selenophene and benzotriazole with a bulky pendant group, was designed using D–A approach, synthesized through Stille coupling and polymerized both electrochemically and chemically. Optical properties, electrochemical and spectroelectrochemical behaviors, kinetic and colorimetric properties and surface morphologies of E-PSeBTz and C-PSeBTz were investigated and compared. The electrochemical and optical studies revealed that both polymer films have p-doping character, can be reversibly oxidized and reduced, displays red color in their neutral states and multichromic properties in their oxidized states. Additionally, the polymers have considerably short switching times and moderate contrast ratios. Both polymers can be regarded as medium band gap polymers with band gaps less than 2.0 V. These results show that PSeBTz and its derivatives can be satisfactory candidates for many research fields including organic solar cell applications and electrochromic devices. Besides, in order to tune these properties, many other potential acceptor units can be introduced to the monomer structure. Also the monomer can be used as a co-monomer for the synthesis of copolymers with better properties. Within our ongoing project studies, we are currently studying on a series of D–A type copolymers containing of SeBTz and benzodithiophene derivatives for organic solar cell studies.
Supplemental Material
Download MS Word (2.3 MB)Additional information
Funding
References
- Heeger, A.-J. Semiconducting Polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. DOI: 10.1039/b914956m.
- Gunbas, G.-E.; Durmus, A.; Toppare, L. Could Green be Greener? Novel Donor–Acceptor‐Type Electrochromic Polymers: Towards Excellent Neutral Green Materials with Exceptional Transmissive Oxidized States for Completion of RGB Color Space. Adv. Mater. 2008, 20, 691–695. DOI: 10.1002/adma.200890012.
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.-M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. DOI: 10.1021/acs.chemrev.5b00098.
- Kutkan, S.; Goker, S.; Hacioglu, S.-O.; Toppare, L. Syntheses, Electrochemical and Spectroelectrochemical Characterization of Benzothiadiazole and Benzoselenadiazole Based Random Copolymers. J. Macromol. Sci. Pure Appl. Chem 2016, 53, 475–483. DOI: 10.1080/10601325.2016.1189280.
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. DOI: 10.1021/ar2002446.
- Dou, L.; Chang, W.-H.; Gao, J.; Chen, C.-C.; You, J.; Yang, Y. A Selenium-substituted Low-Bandgap Polymer with Versatile Photovoltaic Applications. Adv. Mater. 2013, 25, 825–831. DOI: 10.1002/adma.201203827.
- Bin, H.; Xiao, L.; Liu, Y.; Shen, P.; Li, Y. Effects of Donor Unit and π‐Bridge On Photovoltaic Properties of D–A Copolymers Based on Benzo[1,2‐b:4,5‐c']‐dithiophene‐4,8‐dione Acceptor Unit. J. Polym. Sci. Part A: Polym. Chem. 2014, 52, 1929–1940. DOI: 10.1002/pola.27209.
- Hacioglu, S.-O.; Toksabay, S.; Sendur, M.; Toppare, L. Synthesis and Electrochromic Properties of Triphenylamine Containing Copolymers: Effect of π‐Bridge on Electrochemical Properties. J. Polym. Sci. Part A: Polym. Chem. 2014, 52, 537–544. DOI: 10.1002/pola.27030..
- Zhang, S.; Uddin, M.-A.; Zhao, W.; Ye, L.; Woo, H.-Y.; Liu, D.; Yang, B.; Yao, H.; Cui, Y.; Hou, J. Optimization of Side Chains in Alkylthiothiophene-Substituted Benzo[1,2-b:4,5-b′]dithiophene-Based Photovoltaic Polymers. Polym. Chem. 2015, 6, 2752–2760. DOI: 10.1039/C5PY00071H.
- Yao, H.; Ye, L.; Fan, B.; Huo, L.; Hou, J. Influence of the Alkyl Substitution Position on Photovoltaic Properties of 2D-BDT-Based Conjugated Polymers. Sci. China Mater. 2015, 58, 213–222. DOI: 10.1007/s40843-015-0036-3.
- Zhang, S.; Ye, L.; Zhao, W.; Liu, D.; Yao, H.; Hou, J. Side Chain Selection for Designing Highly Efficient Photovoltaic Polymers with 2D-Conjugated Structure. Macromolecules 2014, 47, 4653–4659. DOI: 10.1021/ma500829r.
- Jiang, J.-M.; Lin, H.-K.; Lin, Y.-C.; Chen, H.-C.; Lan, S.-C.; Chang, C.-K.; Wei, K.-H. Side Chain Structure Affects the Photovoltaic Performance of Two-Dimensional Conjugated Polymers. Macromolecules 2014, 47, 70–78. DOI: 10.1021/ma401897b.
- Mei, J.; Bao, Z. Side Chain Engineering in Solution-Processable Conjugated Polymers. Chem. Mater. 2014, 26, 604–615. DOI: 10.1021/cm4020805.
- Lee, J.; Kim, M.; Kang, B.; Jo, S.-B.; Kim, H.-G.; Shin, J.; Cho, K. Side‐Chain Engineering for Fine‐Tuning of Energy Levels and Nanoscale Morphology in Polymer Solar Cells. Adv. Energy Mater. 2014, 4, 1400087. DOI: 10.1002/aenm.201400087.
- Thakur, V.-K.; Ding, G.-Q.; Ma, J.; Lee, P.-S.; Lu, X.-H. Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications. Adv. Mater 2012, 24, 4071–4096. DOI: 10.1002/adma.201200213.
- Neo, W.-T.; Loo, L.-M.; Song, J.; Wang, X.-B.; Cho, C.-M.; Chan, H.-S.-O.; Zong, Y.; Xu, J.-W. Solution-Processable Blue-to-Transmissive Electrochromic Benzotriazole-containing Conjugated Polymers. Polym. Chem. 2013, 4, 4663–4675. DOI: 10.1039/c3py00677h.
- Neo, W.-T.; Ye, Q.; Chua, S.-J.; Xu, J.-W. Conjugated Polymer-based Electrochromics: Materials, Device Fabrication and Application Prospects. J. Mater. Chem. C 2016, 4, 7364–7376. DOI: 10.1039/C6TC01150K.
- Beaujuge, P.-M.; Reynolds, J.-R. Color Control in π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. DOI: 10.1021/cr900129a.
- Argun, A.-A.; Aubert, P.-H.; Thompson, B.-C.; Schwendeman, I.; Gaupp, C.-L.; Hwang, J.; Pinto, N.-J.; Tanner, D.-B.; MacDiarmid, A.-G.; Reynolds, J.-R. Multicolored Electrochromism in Polymers: Structures and Devices. Chem. Mater. 2004, 16, 4401–4412. DOI: 10.1021/cm049669l.
- Mortimer, R.-J. Organic Electrochromic Materials. Electrochim. Acta 1999, 44, 2971–2981. DOI: 10.1016/S0013-4686(99)00046-8.
- Cetin, G.-A.; Balan, A.; Durmus, A.; Gunbas, G.-E.; Toppare, L. A New p-and n-Dopable Selenophene Derivative and its Electrochromic Properties. Org. Electron 2009, 10, 34–41. DOI: 10.1016/j.orgel.2008.09.004.
- Soylemez, S.; Hacioglu, S.-O.; Kesik, M.; Unay, H.; Cirpan, A.; Toppare, L. A Novel and Effective Surface Design: Conducting Polymer/β-Cyclodextrin Host-Guest System for Cholesterol Biosensor. ACS Appl. Mater. Interfaces 2014, 6, 18290–18300. DOI: 10.1021/am5054493.
- Balan, A.; Baran, D.; Toppare, L. Benzotriazole Containing Conjugated Polymers for Multipurpose Organic Electronic Applications. Polym. Chem. 2011, 2, 1029–1043. DOI: 10.1039/c1py00007a.
- Unay, H.; Unlu, N.-A.; Hizalan, G.; Hacioglu, S.-O.; Yildiz, D.-E.; Toppare, L.; Cirpan, A. Benzotriazole and Benzodithiophene Containing Medium Band Gap Polymer for Bulk Heterojunction Polymer Solar Cell Applications. J. Polym. Sci. Part A: Polym. Chem. 2015, 53, 528–535. DOI: 10.1002/pola.27467.
- Onk, I.; Hizalan, G.; Cevher, S.-C.; Hacioglu, S.-O.; Toppare, L.; Cirpan, A. Multipurpose Selenophene Containing Conjugated Polymers for Optoelectronic Applications. J. Macromol. Sci.Pure Appl. Chem 2017, 54, 133–139. DOI: 10.1080/10601325.2017.1265396.
- Patra, A.; Bendikov, M. Polyselenophenes. J. Mater. Chem 2010, 20, 422–433. DOI: 10.1039/B908983G.
- Kim, B.; Yeom, H.-R.; Yun, M.-H.; Kim, J.-Y.; Yang, C. A Selenophene Analogue of PCDTBT: Selective Fine-Tuning of LUMO to Lower of the Bandgap for Efficient Polymer Solar Cells. Macromolecules 2012, 45, 8658–8664. DOI: 10.1021/ma302133h.
- Wang, D.-H.; Pron, A.; Leclerc, M.; Heeger, A. Additive‐Free Bulk‐Heterojuction Solar Cells with Enhanced Power Conversion Efficiency, Comprising a Newly Designed Selenophene‐Thienopyrrolodione Copolyme. J. Adv. Funct. Mater. 2013, 23, 1297–1304. DOI: 10.1002/adfm.201202541.
- Aydemir, K.; Tarkuc, S.; Durmus, A.; Gunbas, G.-E.; Toppare, L. Synthesis, Characterization And Electrochromic Properties of a Near İnfrared Active Conducting Polymer of 1,4-di(Selenophen-2-yl)-Benzene. Polymer 2008, 49, 2029–2032. DOI: 10.1016/j.polymer.2008.03.004.
- Cevher, S.-C.; Unlu, N.-A.; Ozelcaglayan, A.-C.; Apaydin, D.-H.; Udum, Y.-A.; Toppare, L.; Cirpan, A. Fused Structures in the Polymer Backbone to İnvestigate the Photovoltaic and Electrochromic Properties of Donor–Acceptor‐Type Conjugated Polymers. J. Polym. Sci. A Polym. Chem. 2013, 51, 1933–1941. DOI: 10.1002/pola.26553.
- Beverina, L.; Pagani, G.-A.; Sassi, M. Multichromophoric Electrochromic Polymers: Colour Tuning of Conjugated Polymers through the Side Chain Functionalization Approach. Chem. Comm 2014, 50, 5413–5430. DOI: 10.1039/C4CC00163J.