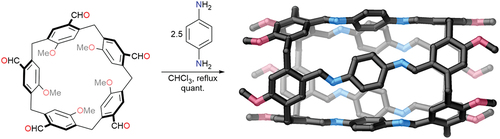
Well-defined chiral nanotubes
The synthesis of pillar[n]arene macrocycles has been one of the most influential developments in the recent history of supramolecular chemistry. These macrocycles have a rich host – guest chemistry and can often be prepared in a single step. Writing in Nature Synthesis, a team led by Andrew Sue (winner of the 2021 Sessler Early Career Research Prize) have shown that pillar[5]arene derivatives can be used to form well-defined chiral nanotubes [Citation1]. The nanotubes are formed in a single-step condensation reaction of a penta-formyl macrocycle and phenylenediamine in quantitative yield (). The penta-formyl compound was previously reported by the same group and was synthesised from tiara[5]arene, which was itself prepared from a rim-differentiated pillar[5]arene [Citation2].
Pillar[5]arenes are chiral but racemise rapidly in solution through rotation of the rings; however, in the nanotubes this rotation is locked and a pair of enantiomeric tubes were formed that could be separated using chiral HPLC. While the binding of chiral guests was not investigated, the nanotubes show strong binding of dihydroxyalkanes and dibromoalkanes with selectivity based on guest size.
Finding the flow
Flow chemistry is hugely important in industrial chemistry, with potential advantages in terms of safety, reproducibility, efficiency and scalability. However, it is often seen as something of an inaccessible dark art by academic researchers. A recent Perspective article in the Journal of the American Chemical Society seeks to change this focusing on potential applications in synthesis and crystallisation [Citation3]. The paper is authored by Andrea Laybourn, Karen Robertson and Anna Slater, one of whom (Slater) has previously demonstrated the use of flow technology to prepare a macrocyclic ‘molecular hinge’ on the gram scale [Citation4]. Refreshingly, the perspective does not try and push the reader towards flow chemistry but rather starts by asking whether flow chemistry should be used at all. It goes on to discuss misconceptions about flow chemistry including the requirement that all components remain dissolved. The authors show that this is not the case, and indeed that flow chemistry can be used to control crystal growth. As the authors highlight, the types of materials being prepared in flow is rapidly expanding and starting to include supramolecular systems. Clearly, there are many opportunities for supramolecular chemists as flow technology becomes more commonplace.
Tackling cancer with controlled self-assembly and disassembly
Self-assembly and disassembly processes are commonplace in biology, but designing synthetic systems to function in the complex cellular environment is a big challenge. Recent work from a team led by Hong Liu, Zijian Guo and Deje Ye has developed a cis-platin prodrug (P-CyPt) that undergoes stepwise chemical transformations and releases both an imaging agent and cis-platin [Citation5].
The first step is the dephosphorylation of an activatable near-IR fluorescent and photoacoustic probe [Citation6] in P-CyPt by membrane-bound alkaline phosphatase enzymes found in tumour cells. This makes the pro-drug scaffold more hydrophobic and fluorescent, triggering self-assembly into fluorescent nanoparticles that can anchor onto the cell membrane and pass into the cell. Once inside, glutathione (abundant in cancer cells) triggers the release of cis-platin and the disassembly of the nanoparticles. The disassembly causes a further increase in near-IR fluorescence as aggregation caused quenching is reduced; thus, the whole process can be visualised using bimodal imaging, enabling imaging-guided therapy.
A big advantage of the stepwise self-assembly and disassembly approach is that the initial pro-drug is a small molecule that can easily penetrate into tumour tissues (which larger nanostructures can struggle to do). The assembly into nanoparticles only happens in the presence of tumour-related stimuli and this triggers cell uptake and subsequent theranostic action.
In brief
Better binding models: Koki Ikemote, Hiroyuki Isobe and co-workers report the use of Akaike’s Information Criterion to determine host–guest stoichiometry in supramolecular complexes [Citation7]. Traditionally, Job plot analysis has been used to determine binding stoichiometry, but as highlighted in 2016 there are serious problems with Job plot analysis for many supramolecular systems [Citation8]. The Akaike Information Criterion is a relatively simple mathematical model commonly used in other scientific fields to measure the appropriateness of a statistical model and avoid over-fitting data. Ikemote, Isobe and colleagues demonstrate its utility in selecting between 1:1, 1:2 and 2:1 guest binding models.
Rings in rings: Xiran Yang and Simin Liu describe the synthesis of new tetra-cationic imidazolium macrocycles that form 1:1 host–guest complexes by binding inside the large cucurbituril macrocycle CB[10] [Citation9]. Formation of these ‘double macrocycle’ complexes is highly favourable (Ka>10 [Citation7] M−1 in water). Interestingly, formation of the double macrocycle complex allows binding of hydroxybenzene derivatives to give ternary complexes in water, but these guests do not bind to the tetra-cationic receptor alone.
Cages capture chloride: Kay Severin and colleagues report the synthesis of dipalladium(II) cages that can bind chloride and bromide with high affinities (>105 Citation5 M−1) in aqueous buffer [Citation10]. The cages contain simple organic ligands and bind the guests in a small interior cavity through eight C–H∙∙∙halide hydrogen bonds, presumably assisted by the 4+ charge of the host. No binding of fluoride, acetate, sulfate, carbonate or phosphate was observed showing a surprising high selectivity for a relatively simple receptor.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Tian Y, Guo Y, Dong X, et al. Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality. Nat Synth. 2023. DOI:10.1038/s44160-022-00235-w
- Yang W, Samanta K, Wan X, et al. Tiara[5]arenes: synthesis, solid-state conformational studies, host–guest properties, and application as nonporous adaptive crystals. Angew Chem Int Ed. 2020;59:3994–3999.
- Laybourn A, Robertson K, Slater AG. Quid pro flow. J Am Chem Soc. 2023;145:4355–4365.
- Jones CD, Kershaw Cook LJ, Marquez-Gamez D, et al. High-yielding flow synthesis of a macrocyclic molecular hinge. J Am Chem Soc. 2021;143:7553–7565.
- Wen X, Zhang R, Hu Y, et al. Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics. Nat Commun. 2023;14:800.
- Yan R, Hu Y, Liu F, et al. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J Am Chem Soc. 2019;141:10331–10341.
- Ikemoto K, Takahashi K, Ozawa T, et al. Akaike’s information criterion for stoichiometry inference of supramolecular complexes. Angew Chem Int Ed. 2023;e202219059. DOI:10.1002/anie.202219059
- Brynn Hibbert D, Thordarson P. The death of the job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem Commun. 2016;52:12792–12805.
- Yang X, Liu S. Cationic cyclophanes-in-cucurbit[10]uril: host-in-host complexes showing cooperative recognition towards neutral phenol guests. Supramol Chem. 2023;1–8. DOI:10.1080/10610278.2023.2170233
- Sudan S, Chen D, Berton C, et al. Synthetic receptors with micromolar affinity for chloride in water. Angew Chem Int Ed. 2023;62:e202218072.