?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Asymmetrical lipid nanoparticles are interesting nanocarriers for charged molecules, like nucleic acids. They promise control over inner and outer charge. High charge density on the inside is favourable for efficient condensation and charge neutralisation of highly charged biopharmaceuticals, while a neutral or slightly negative outer layer promotes biocompatibility. The main goal of this work was the development and characterisation of asymmetric liposomes, prepared using water-in-oil (w/o) nanoemulsions of phospholipids (PLs) and squalene in a centrifugal field. This method enables the control over the lipid composition of each monolayer.
Liposomes were prepared by passing PL w/o nanoemulsions through an oil–water interface previously saturated with PLs. We used N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3-Phosphoethanolamine (NBD-PE) or N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3- phosphocholine (NBD-PC) as a fluorescent marker for either the inner or outer lipid layer and plasmid DNA (pDNA) as nucleic acid payload. The final liposomes had sizes below 200 nm and polydispersity indexes of 0.3 and had a bilayer asymmetry of 70%, thus shielding the charge of positive PLs in the inner bilayer leaflet. Final formulations were examined using negative staining transmission electron microscopy (TEM). Plasmid encapsulation efficiency of the method was 10–15%. Our results indicate that the w/o nanoemulsion-centrifugation method allows the successful production of liposomes with tailored features for encapsulation of nucleic acid therapeutics.
Introduction
Nucleic acids therapeutics has been widely accepted as a cornerstone of future molecular medicine, aiming for precise and targeted intervention in diseases. The main limitation of using such molecules in a therapeutic setting is their inability to enter cells, which is related to their negative charge that impairs the passage over target cells’ membranes. In addition, they tend to be rapidly degraded and cleared [Citation1–4]. Biological and synthetic nanoparticles have been suggested as delivery vehicles for nucleic acids to overcome such problems. Viral vectors that include retroviruses, adeno-associated viruses and lentiviruses, are efficient vehicles. However, they may provoke mutagenesis and carcinogenesis and repeated administration can trigger immune responses that impair payload delivery. Therefore, non-viral vectors (e.g. lipoplexes) are an attractive alternative [Citation5].
Nucleic acids can be efficiently entrapped into lipoplexes. Electrostatic interactions between positively charged lipids and negatively charged nucleic acids are a strong driving force for effective encapsulation. In addition, cationic lipids can play a role in destabilising the endosomal membrane facilitating functional nucleic acid release. However, nanocarriers with a cationic surface charge are rapidly removed and cleared by the reticuloendothelial system (RES) in the primary organs, which constitutes the major obstacle for their therapeutic efficacy. In addition, aggregation with negatively charged blood components may occur and that can produce severe adverse effects such as clogging of capillaries in the lungs. The traditional way of circumventing is by shielding the positive charge of the carrier with DSPE-PEG2000. However, this shielding is often suboptimal as evidenced by the fact that cationic PEG-liposomes never reach circulatory half-lives encountered for neutral or negatively charged PEG-liposomes [Citation4].
Asymmetric nanocarriers with a neutral or negatively charged surface may combine the best of both worlds: protection of the nucleic acid as well as improved biocompatibility [Citation4]. A variety of procedures have been described for preparing formulations with asymmetric bilayers [Citation6]. The choice of the appropriate method is dependent on the required size and characteristics of the nucleic acids during the production process of the nanocarrier, in relation to the composition of the nanocarrier [Citation1].
An innovative method to prepare liposomes, which has attracted attention recently [Citation7–11], is based on the inverted emulsion or phase transfer method. It involves the transfer of water-in-oil (w/o) emulsion droplets through an oil–water interface saturated with a phospholipid (PL) monolayer. The transfer process might occur either spontaneously [Citation11–14], and/or use centrifugal force [Citation7,Citation15–18] and/or use sugar gradients [Citation7,Citation13,Citation17,Citation18], as represented in the scheme in .
Figure 1. Schematic representation of the inverted emulsion process. First, a phospholipid (PL) solution in oil is placed on top of the buffer phase and allowed to equilibrate to form the interface oil–water. After equilibration, the w/o emulsion is gently pipetted on top of the second layer and the system is centrifuged, allowing the w/o droplets to cross the interface. At the end of the process, liposomes are collected from the bottom of the tube.
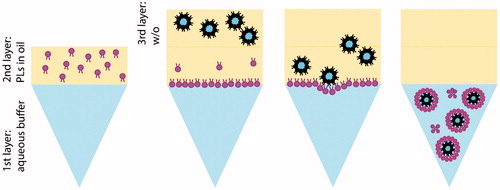
In practice, an oil phase containing dispersed PLs is poured on top of an aqueous phase. The PLs diffuse towards the water–oil interface to form a monolayer, which will serve as the external layer of the liposome. Next, the inverted w/o emulsion can be created using several methods. This emulsion should have appropriate stability to avoid nucleic acid leakage and a suitable size to serve as the inner leaflet of a liposome. This emulsion is carefully placed onto the interface and either spontaneously or external force, the w/o droplets will cross the interface and pick up a second layer of lipids, forming liposomes [Citation7–11,Citation14,Citation15,Citation18–22].
Zhang et al. obtained 50–200 nm size liposomes with relatively high encapsulation efficiencies of 6-carboxyfluorescein [Citation22]. Pautot et al. have shown that this technique can yield unilamellar liposomes with sizes ranging from 100 to 1000 nm and encapsulation efficiencies of pyrene-labelled actin that corresponded to 98% [Citation15,Citation16,Citation20]. Later research using the phase transfer method focussed mainly on the production of giant liposomes and the study of droplet-transfer phenomena through the oil–water interface [Citation12,Citation19], encapsulation of nucleic acids [Citation8,Citation23,Citation24], actin reconstitution [Citation17] and other uses as synthetic cell models [Citation7,Citation18]. The technology is also popular to address fundamental aspects in cell biology, since all biological membranes are asymmetric.
Taken together, the inverted emulsion-centrifugation method is a versatile method that allows relatively high encapsulation of water-soluble compounds, allows the use of various buffer conditions, and the inner and outer leaflet compositions of the liposomes can be controlled independently. Despite these advantages, the method is not suitable for industrial applications. The slow equilibration of lipid monolayers at the oil–water interface limits replenishment and thus vesicle yield. This is essentially a diffusion-based process and it depends on several parameters, like the properties of the PLs, oil viscosity and density. In addition, the reported sizes are often in the micrometre range and thus not suitable for intravenous administration.
Our aim was to improve the inverted-emulsion centrifugation technique for the development of nanosized asymmetric liposomes for nucleic acid delivery. We started with nanoemulsions, prepared using PLs and Professor Patrick Couvreur’s favourite molecule: squalene. It adds another avenue of exploration for this remarkable molecule that has been studied intensively as excipient and active ingredient in his laboratory. Initially, we investigated the stability of different w/o nanoemulsions as the initial nanodroplets which form the template for the liposomes. We studied different compositions and emulsification processes on the overall properties and stability of the nanoemulsions. We then incorporated plasmid DNA (pDNA) as a model compound into the most stable nanoemulsions and used the centrifugation technique to prepare liposomes.
Materials and methods
Materials
PLs 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-3-trimethylammonium-propane, chloride salt (DOTAP), 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and DSPE-PEG2000 were obtained from Lipoid (Ludwigshafen, Germany). N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3-Phosphoethanolamine [NBD-PE], Triethylammonium Salt) and N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3-phosphocholine [NBD-PC], Triethylammonium Salt) were obtained from Life technologies (Karlsruhe, Germany). 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt, (HPTS >97%) and squalene (purity ≥98%) were purchased from Sigma-Aldrich (Deisenhofen, Germany).
Methods
Preparation of w/o nanoemulsions
Nano-emulsions (150 µL) were freshly prepared from a PL solution in squalene (typically 1 mg PL/mL squalene) [Citation25,Citation26]. In optimisation studies, we used different water fractions (1, 3, 5 and 10% w/o v/v) for a fixed lipid composition (DPPC/DOTAP (1:1 mol:mol).
After optimisation of the dispersed phase fraction, the lipid ratios and compositions were varied according to . The emulsions were prepared using three different emulsification instruments:
Table 1. Lipid composition and ratios used to screen 1% w/o emulsification conditions. All lipids were previously dissolved in squalene at the indicated concentrations.
The first method required the use of a high energy sonication (HES) method using the ultrasound sonotrode (Branson Sonifier S-450A, Branson Ultrasonics, Eemnesm The Netherlands) using 50% power and five cycles. The emulsification time was varied from 1 up to 10 min, as presented in the results section.
The second method made use of low energy input ultrasound (LES). Samples were pre-sonicated on the sonotrode at 50% power and five cycles for 10 s, followed by emulsification in a water bath (Fisher Scientific FB15047, Waltham, MA)for 15 min.
The last method for downsizing nanoemulsions was vortex-mixing of the nano-emulsion for 5 min followed by hand-extrusion through 400 and 200 nm pore size polycarbonate membranes.
Phospholipid equilibration at oil–water interface
The lipids used to serve as outer leaflet of the liposomes were dissolved in squalene. A 2 mL Eppendorf tube was first filled with 400 µL of HEPES buffered saline (HBS) (HEPES 10 mM, 0.9% NaCl, pH 7.4); then, 200 µL of squalene containing the outer leaflet lipids (190 µL DPPC/10 µL DSPE-PEG2000, mol:mol ratio 72:1) was poured over the aqueous phase, and a ‘milky’ layer formed between the oil–water interface.
Liposomes preparation
To finally obtain the liposomes, 150 µL of w/o nanoemulsion was added onto the intermediate phase that was previously equilibrated for approximately 2 h with PL monolayer. After this, samples were centrifuged for 30 min at 120×g to spin down all the droplets through the oil–water interface to form liposomes. Liposomes were collected from the aqueous phase and kept at room temperature until further analysis.
Characterisation of nano-emulsions and liposomes
The hydrodynamic diameter and polydispersity index of all w/o emulsions (before centrifugation) and liposomes (after centrifugation) were measured by dynamic light scattering (DLS) using a DLS Malvern Zetasizer Nano S (Malvern Instruments, Malvern, UK). The zeta-potential of the liposomes was measured using laser Doppler electrophoresis on a Zetasizer Nano Z (Malvern Instruments) with samples dispersed in 10 mM HEPES buffer pH 7.4 (with no additional salts added). Nanoemulsions were characterised in terms of stability over time. After emulsification, the nanoemulsions were monitored every 5 min for 1 h, and the position of nanoemulsions was measured as a determinant for phase separation/stability as follows:
(1)
(1)
where Ht is the height of the interface between the turbid nanoemulsion in the tube and the clear supernatant measured at time t, and H0 represents the initial height.
Membrane asymmetry of liposomes was determined using a fluorescent quenching assay [Citation27]. The liposomes were prepared by adding either NBD-PE lipid to the inner lipid layer or NBD-PC lipid to the outer lipid layer (1 mol%). The fluorescence of the resultant bilayer vesicles was measured before and after addition of a quenching agent (50µL of sodium hydrosulphite in 1M Tris at pH 10). Inner leaflet quenching was achieved by adding 50µl of Triton-X100 to lyse the bilayer vesicles, allowing access of sodium hydrosulphite and further reduction of fluorescent intensity. The measurement was done by setting the excitation wavelength at 470 nm and the emission wavelength at 550 nm in a Jasco FP8300 spectrofluorometer and calculating as follows:
(2)
(2)
where Intensityt is the fluorescence intensity at time t after quenching, and Intensity0 represents the initial signal.
The overall lipid recovery (yield) was determined by determination of PL in the water phase according to the method of Rouser using sodium dihydrogen phosphate as a standard [Citation28]. The total lipid was then derived from the amount of PL present in the formulation.
The structure of the final nanoparticles was visualised by negative staining transmission electron microscopy (TEM). In brief, the sample was placed on a carbon-coated copper grid (300 mesh; Plano GmbH, Wetzlar, Germany) and allowed to settle for 2 min before being blotted away by filter paper. A 1% ammonium molybdate solution was added to the grid for 2 min before the solution was blotted away and the grid was allowed to dry. Images were recorded at 120 kV on a Philips CM12 transmission electron microscope coupled to a GATAN Multiscan 400HP camera.
Preparation and characterisation of nucleic acid containing emulsions and liposomes
Nano-emulsions B, E and F containing 1 mg/mL PL and ca. 200 ng of pDNA (pCMV-Lacz pDNA, 7164 bp, 2.56 mg/ml; 1% w/o v/v) were freshly prepared by LES emulsification as described above. An aliquot of 150µL of w/o nanoemulsion was added onto 600µL intermediate phase that was previously formed by equilibration of 400µL water phase with 200 µL PL/squalene phase (see Characterisation of nano-emulsions and liposomes section). After this, samples were centrifuged for 30 min at 120×g and liposomes were collected from the aqueous phase and purified by ultracentrifugation (Beckmann ultracentrifuge, Beckman Coulter, Brea, CA, 55,000 rpm, 55 min). The pellet was re-suspended in fresh HBS using the initial volume of water (400 µL).
pDNA encapsulation was determined by gel electrophoresis. Agarose (Roche) 1% w/v was dissolved in 1x Tris-acetate-EDTA buffer (Bio-Rad, Hercules, CA), and after complete dissolution, propidium iodide (Invitrogen, Carlsbad, CA, 16 µL/gel) was added to the agarose. Prior to the experiment, DNA-containing samples were treated with Triton X100 to expose the entrapped pDNA and 8 µL of loading solution (6x solution, Thermo Scientific, Waltham, MA) was added to 20 µL samples. Electrophoresis was carried out in a vertical slab gel apparatus. Fifteen sample-wells were made by use of a comb. The power source was a regulated high-voltage power supply, and electrophoresis was carried out at 100V 1.5 h or until the dye neared the bottom of the gel. The gel was imaged in a Gel Doc XR imager (Bio-Rad, Hercules, CA) and quantified using ImageJ software [Citation29]. The amounts of pDNA detected in the samples were calculated against a standard curve of pDNA in the range of 8.3–132.5 ng, and normalised by the amount used to prepare the emulsions:
(3)
(3)
Results and discussion
Phospholipid equilibration at oil–water interface
One of the requirements to successfully produce liposomes using the inverted emulsions in a centrifugation field is to have a fully equilibrated oil–water interface. Amphiphilic molecules dissolved in the oil phase will diffuse to the water–oil interface and align there with the hydrophilic heads towards the water phase and hydrophobic tails in contact with the oil phase. In this way, the outer leaflet is ready to receive the inner leaflet.
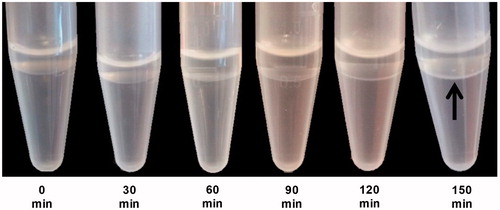
Typically, waiting until a white thin layer is formed is recommended [Citation15]. In the case of DPPC/DSPE-PEG2000, we found that it takes approximately two hours to obtain a visible white thin layer (). However, we might also speculate that this ‘thin layer’ can be composed of multiple layers mainly composed of DPPC, as demonstrated by tensiometric measurements at oil–water interfaces by Hildebrandt et al. [Citation25,Citation30].
Figure 2. Time series of oil–water interface equilibrium images. Each image was captured every 30 min for 2.5 h to allow the interface to be lipid-rich. After 2.5 h at room temperature, a milky thin layer, as pointed by the arrow, was finally formed showing that the interface has already been fully equilibrated.
Stability and phase separation in w/o nanoemulsions
We first evaluated the effect of varying the PL:water ratio on the stability of w/o nanoemulsions. We hypothesised that a higher PL:water ratio (i.e. less water) results in higher stability of the w/o nanodroplets [Citation16]. The water fraction was varied between 1 and 10% v/v. The total volume and lipid composition and emulsification method (HES) were kept constant ().
Figure 3. Lipid composition and ratios used to screen the effect of water fraction in the emulsion stability. (A) composition of nanoemulsions. All lipids were previously dissolved in squalene at the indicated concentrations. (B) Phase stability of nanoemulsions prepared by variations of water content. The measurements were done by observing and measuring the height of phase separation for 60 min after preparation with 5 min interval per observation.
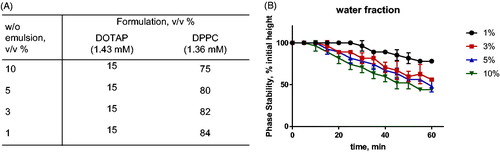
Indeed, the emulsion containing 1% water showed the longest emulsion stability, up to 30 min after emulsification. Higher water contents showed emulsion sedimentation within 10–15 min after emulsification ().
These results are in line with the results of Pautot et al. [Citation16] and confirm that the phase stability of inverted emulsions is improved by minimising the amount of water in the composition. For this reason, formulations containing 1% water were used in the next experiments.
Additional functional lipids and surfactants were added to further improve the stability of the emulsions [Citation15]. Span 80 has a hydrophilic–lipophilic balance (HLB as defined by Griffin) of 4.3 which is ideal for w/o emulsification and stabilisation [Citation15,Citation31–34]. In addition, we introduced DOPE which has been used before for stabilisation of w/o emulsions [Citation35,Citation36] and which is assumed to help in improving endosomal escape of nucleic acids.
shows that the use of both Span 80 and DOPE/DPPC enhanced the stability the emulsions (formulations C and D). Surprisingly, formulations B, E and F showed complete stability even up to 3 h after emulsification. These results support the notion that DOPE is very important for emulsion stabilisation and that it tolerates the additional inclusion of PC and Span 80. The choice of DOPE as stabiliser PL favours the negative curvature as this lipid has a relatively small polar head group [Citation37,Citation38].
Figure 4. Phase stability of w/o emulsions produced with different compositions as provided in . The measurements were done by observing and measuring the height of nanoemulsions for 180 min after preparation.
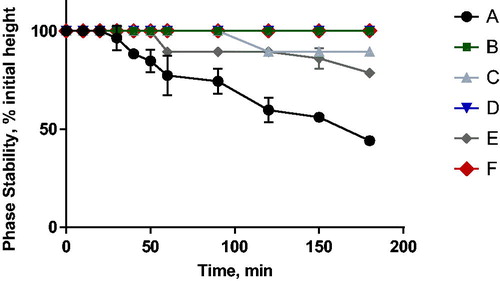
Additionally, DOTAP was chosen as the cationic lipid, not only to increase the encapsulation efficiency of negatively charged payloads like siRNA, but also to stabilise the w/o emulsion in an unsaturated hydrocarbon like squalene. We hypothesised that the self-arrangement of the lipids would affect stability due to the presence of unsaturated bonds, headgroup size and electrostatic interactions. According to Hildebrandt et al. [Citation25], there are large differences between, for example, POPC and DPPC at water–squalene interfaces. The disparities in area per molecule and critical aggregation concentration between different PCs were attributed to fatty acid saturation, where the presence of the double bond in oleoyl chain in POPC led to higher attractive (π–π) interactions between unsaturated oil phase and unsaturated fatty acids of POPC and less attractive interactions between saturated fatty acids chains of DPPC and squalene [Citation25]. Emulsions containing a highly unsaturated hydrocarbon like squalene together with unsaturated (phospho)lipids are, therefore, more stable.
The three formulations with highest stability (B, E, F) were used for further optimisation the emulsification method.
Comparison of emulsification methods
Hand extrusion
Emulsions corresponding to setups B, E and F were first vortexed for 5 min, followed by extrusion over 400 and 200 nm pore filters to reduce size and polydispersity index (PDI) of nanoemulsion droplets. Although extrusion is an excellent method for liposomes preparation, in the case of w/o nanoemulsions large variations were observed in both droplets size and PDI between batches, resulting in low reproducibility (). Thus, for squalene-based w/o emulsions, this method is not appropriate as the viscosity of the oil phase impairs the process.
Table 2. Size and PDI of three different batches of formulations B, E and F. The results suggest high variability between batches.
Stability results of hand-extruded nanoemulsions are shown in Supplemental Figure 1. The size of droplets increased from 150 to 300 nm during the first 15 min after extrusion; the droplets size even reached 600–700 nm 1 h after extrusion. The PDI showed the same trend, increasing four-fold in 1 h. These findings suggest that hand-extruded emulsion had low stability. Whittenton et al. [Citation23] also reported the same phenomena; they suggested to measure and process the emulsion immediately after preparation [Citation23].
High-energy sonication (HES)
The three potential formulations showed very high stability in Stability and phase separation in w/o nanoemulsions section (B, E and F) but displayed very poor reproducibility and stability when processed by hand-extrusion (Hand extrusion section). Thus, a high energy input emulsification method was employed. Ultra-sound sonication offers ease of operation and it is not as time consuming as other techniques [Citation37,Citation38].
The optimal sonication time was investigated by varying the time from 1 up to 10 min. Longer emulsification times were not investigated because increasing energy input may lead to degradation of the loaded biopharmaceutical cargo.
and show that the droplet size decreased to minimally 100 nm, PDI 0.2 with increasing sonication time. This size and PDI was reached after 5 min sonication. Prolonging sonication time to 10 min did not further reduce these parameters. These results are in agreement with the results of Delmas et al. [Citation38].
Figure 5. Size (columns) and PDI (lines) of w/o emulsion produced from formulations B (black), E (blue) and F (green) obtained after 1–10 min of HES exposure. Samples were emulsified using the same energy settings.
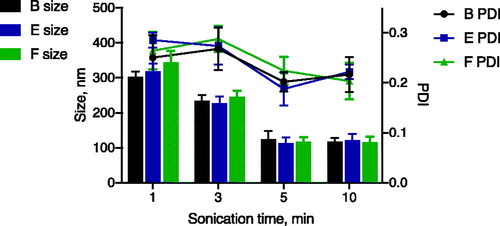
Table 3. Summary of the sizes and PDI of the 1% w/o emulsion provided by the three emulsification methods used in this study.
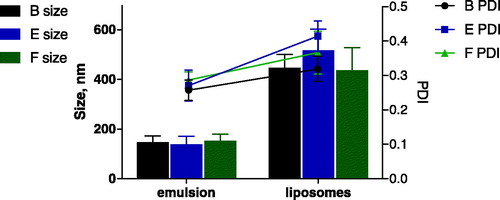
All three nanoemulsions B, E and F were subjected to centrifugation to produce liposomes. We expected to obtain similar sizes and PDI for the final liposomes as observed for the respective nanoemulsions, as the w/o nanoemulsion serves as a template for the final vesicles which would only add a single lipid leaflet.
A comparison between the emulsion and final liposomes is shown in . Astonishingly, the resulting liposomes were much bigger (mean diameter 400–500 nm) than the initial w/o nanoemulsions (mean diameter 130–160 nm). Potential problems that may have resulted in an increased size are fusion of nanoemulsion droplets at the squalene/water interface, or inclusion of squalene oil in the phospholid layer thus not forming a true PL unilamellar bilayer of the liposomes. Similar observations were made previously [Citation23]. Even emulsions stabilised by a surfactant, formulation B and E, resulted in much larger liposomes, indicating processes of either emulsion instability or aggregation during phase transfer.
Low-energy sonication (LES)
As an alternative to HES, the water bath sonicator is a milder technique in terms of mechanical stress, circumventing the possibility of denaturing sensitive APIs [Citation39]. In a bath sonicator, the force is transferred indirectly through the medium so that the wave generated is milder, yet still able to downsize the particle with less destructive potential. Furthermore, a bath sonicator offers a more homogenous distribution of the wave resulting in the formation of more homogenous droplets sizes, whereas a probe tip sonicator relies mainly on the depth of the tip submerged which determines the distribution of the wave into the samples.
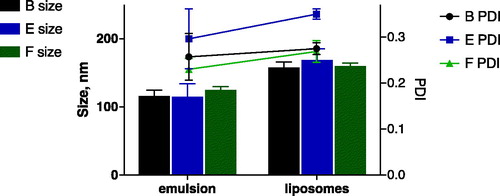
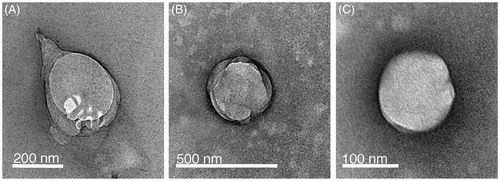
As shown in and , we could obtain w/o nanoemulsions with a size below 150 nm after 15 min of ultrasound exposure. These were subjected to centrifugation to produce liposomes which had an acceptable size (below 200 nm) and PDI (PDI 0.2–0.3). Although subject to artefacts introduced by sample preparation, negative staining TEM images () suggest that the liposomes resemble the size measured by DLS. Moreover, it appears that addition of Span 80 or DPPC/Span 80 is not necessary. All three formulations were investigated for asymmetry and pDNA encapsulation.
Asymmetry of lipid bilayers of liposomes
For the bilayer asymmetry studies, 1 mol% of fluorescent NBD-PE was added to the nano-emulsions that formed the inner lipid leaflets, or 1 mol% of NBD-PC was added to the outer lipid composition that was used to saturate the oil–water interface. The addition of NBD-labeled lipids did not influence the size of the vesicles. Sodium hydrosulphite (1M Na2S2O4 in Tris pH 10) was employed as a quencher. Since this molecule does not diffuse across lipid bilayers, it will only quench NBD in the outer lipid leaflet, thus allowing determination of the NBD residing in the inner lipid leaflet. Total quenching of the NBD signal was achieved after addition of Triton X-100 in order to lyse the bilayer, allowing excess sodium hydrosulphite to further quench NBD dye inside the vesicles.
As shown in , when NBD-PE was incorporated in the inner leaflet, only about 20–30% of total fluorescence intensity decreased upon addition of quencher while the remaining fluorescence was only quenched after adding Triton X-100. This suggests that 80–70% of the lipid marker was located in the inner leaflet of the bilayer. Similarly, when NBD-PC was added to outer leaflet, 65–75% of the fluorescence intensity was quenched immediately, i.e. once the quencher was offered to the outer lipid leaflet; the remaining signal was quenched after TritonX-100 was added to the cuvette (). Again, this trend was observed in the vesicles from all formulations. These results confirm that the produced bilayers from the three formulations (B, E and F) contain 70–80% asymmetry ().
Figure 9. Fluorescence quenching assay for determination of asymmetry degree of liposomes produced from the compositions B (black), E (blue) and F (green).
A) NBD-PE was added to the inner layer; B) NBD-PC was added to the outer layer.
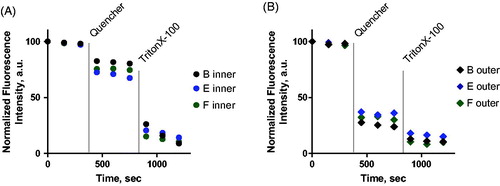
Table 4. Main features of the liposomes produced from LES-nanoemulsions.
The asymmetry assay showed uneven distributions for all the formulations. These results are in line with previous research [Citation16] where the authors also reported negligible flip-flop over a 24 h period.
Characterisation of pDNA-loaded asymmetric liposomes
Following the successful production of liposomes with a high asymmetry degree, the next step was to encapsulate pDNA as a model biopharmaceutical drug. The purified liposomes were characterised for particle size, PDI, zeta potential, PL recovery and pDNA encapsulation efficiency (see ).
These results show pDNA loaded liposomes had similar size and PDI as empty liposomes prepared by LES method, as presented in . Final size (<200 nm) and charge (slightly negative zeta-potential) of the liposomes is in good agreement with their intended application as nanocarrier for in vivo applications. Some authors have discussed that droplets with small sizes provide good transfer, contrary to the larger droplets that may cross the interface oil–water, but most of them break up and release the encapsulated APIs [Citation10,Citation19,Citation20,Citation23,Citation40]. It has also been described that some of the droplets stay stationary at the interface while others may fuse with the interface [Citation23] or most of the liposomes stay anchored to the interface [Citation21]. Accordingly, a low transfer yield is obtained after centrifugation previously reported to be around 4% [Citation41]. Consequently, the encapsulation of an API in the w/o is compromised during the droplets crossing the interface.
Our results set a turning point to these observations. We show that it is possible to obtain liposomes with narrow (nano)size distributions and accordingly the transfer of intact nanoemulsion droplets was ∼10–15%, as can be deduced from recovery of encapsulated pDNA. Even higher PL recoveries were found of ca. 45%, which can be related to transfer of empty nanoemulsion droplets besides pDNA filled nanoemulsion droplets. Since the zeta-potential of the pDNA-loaded lipid nanoparticles was negative for all three formulations, we concluded that the cationic DOTAP lipid had been retained within the inner lipid leaflet. The presence of pDNA thus did not perturb the asymmetry of the liposomes.
It is worth to stress that, to the best of our knowledge, this is the first report in which the centrifugation technology is employed for production of nanosized liposomes that actually contain a payload like pDNA. Our results are encouraging and indicate that the positive charge of the inner leaflet is fully masked by 95% (v/v) of zwitterionic lipids (DPPC) and 5% of anionic DSPE-PEG2000 in the outer leaflet of small liposomes.
Conclusions
The inverted emulsion technique is based on the premise that stable inverted emulsions cross the interface oil–water and are enwrapped by a second monolayer of PLs during transfer over the interface. We have improved protocols for preparation of w/o nanoemulsions and have collected liposomes from the water phase that are similar in size and dispersity as the initial nanoemulsions. The simplicity of the protocol, the opportunity to create asymmetric bilayers and the improvements in encapsulation efficiency make this technique attractive for the formulation of nucleic acids. Encouraged by these results, research is ongoing to improve lipid and drug recovery rates, liposome yield and encapsulation efficiency as well as to investigate the oil–lipid interactions, scale-up and the bioactivity of the encapsulated cargo.
Supplemental Figure 1
Download PDF (128.7 KB)Acknowledgements
We acknowledge Lipoid for kindly providing the phospholipids used.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- de Jesus MB, Zuhorn IS. Solid lipid nanoparticles as nucleic acid delivery system: properties and molecular mechanisms. J Control Release. 2015;201:1–13.
- Wei J, Jones J, Kang J, et al. RNA-induced silencing complex-bound small interfering RNA is a determinant of RNA interference-mediated gene silencing in mice. Mol Pharmacol. 2011;79:953–963.
- Lobovkina T, Jacobson GB, Gonzalez-Gonzalez E, et al. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano. 2011;5:9977–9983.
- Mokhtarieh AA, Cheong S, Kim S, et al. Asymmetric liposome particles with highly efficient encapsulation of siRNA and without nonspecific cell penetration suitable for target-specific delivery. Biochim Biophys Acta Biomembr. 2012;1818:1633–1641.
- Liu F, Huang L. Development of non-viral vectors for systemic gene delivery. J Control Release. 2002;78:259–266.
- Li W, Szoka FC. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449.
- Chiba M, Miyazaki M, Ishiwata S. Quantitative analysis of the lamellarity of giant liposomes prepared by the inverted emulsion method. Biophys J. 2014;107:346–354.
- Fujii S, Matsuura T, Sunami T, et al. Liposome display for in vitro selection and evolution of membrane proteins. Nat Protoc. 2014;9:1578–1591.
- Hadorn M, Boenzli E, Eggenberger Hotz P, et al. Hierarchical unilamellar vesicles of controlled compositional heterogeneity. PLoS One. 2012;7:1–7.
- Nishimura K, Suzuki H, Toyota T, et al. Size control of giant unilamellar vesicles prepared from inverted emulsion droplets. J Colloid Interface Sci. 2012;376:119–125.
- Saha A, Mondal G, Biswas A, et al. In vitro reconstitution of a cell-like environment using liposomes for amyloid beta peptide aggregation and its propagation. Chem Commun. 2013;49:6119–6121.
- Yamada A, Yamanaka T, Hamada T, et al. Spontaneous transfer of phospholipid-coated oil-in-oil and water-in-oil micro-droplets through an oil/water interface. Langmuir. 2006;22:9824–9828.
- Hamada T, Miura Y, Komatsu Y, et al. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J Phys Chem B. 2008;112:14678–14681.
- Kubatta EA, Rehage H. Characterization of giant vesicles formed by phase transfer processes. Colloid Polym Sci. 2009;287:1117–1122.
- Pautot S, Frisken BJ, Weitz DA. Production of unilamellar vesicles using an inverted emulsion. Langmuir. 2003;19:2870–2879.
- Pautot S, Frisken BJ, Weitz DA. Engineering asymmetric vesicles. Proc Natl Acad Sci USA. 2003;100:10718–10721.
- Pontani LL, Van Der Gucht J, Salbreux G, et al. Reconstitution of an actin cortex inside a liposome. Biophys J. 2009;96:192–198.
- Yanagisawa M, Iwamoto M, Kato A, et al. Oriented reconstitution of a membrane protein in a giant unilamellar vesicle: experimental verification with the potassium channel KcsA. J Am Chem Soc. 2011;133:11774–11779.
- Hase M, Yamada A, Hamada T, et al. Transport of a cell-sized phospholipid micro-container across water/oil interface. Chem Phys Lett. 2006;426:441–444.
- Pautot S, Frisken BJ, Cheng JX, et al. Spontaneous formation of lipid structures at oil/water lipid interfaces. Langmuir. 2003;19:10281–10287.
- Takiguchi K, Negishi M, Tanaka-Takiguchi Y, et al. Transformation of ActoHMM assembly confined in cell-sized liposome. Langmuir. 2011;27:11528–11535.
- Zhang L, Hu J, Xiao Z, et al. Preparation of liposomes by a controlled assembly method. Mol Cryst Liq Cryst Sci Technol Sect A Mol Cryst Liq Cryst. 1997;295:125–128.
- Whittenton J, Harendra S, Pitchumani R, et al. Evaluation of asymmetric liposomal nanoparticles for encapsulation of polynucleotides. Langmuir. 2008;24:8533–8540.
- Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci USA. 2004;101:17669–17674.
- Hildebrandt E, Dessy A, Sommerling JH, et al. Interactions between phospholipids and organic phases: insights into lipoproteins and nanoemulsions. Langmuir. 2016;32:5821–5829.
- Hildebrandt E, Vrânceanu M, Nirschl H, et al. Phospholipids as emulsifiers for micro/nano droplets suitable for biotechnological systems integration. La Houille Blanche. 2013;2:68–73.
- McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30:11819–11827.
- Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496.
- Rasband WS. ImageJ, Bethesda, Maryland, USA: U. S. National Institutes of Health; 1997–2018. Available from: https://imagej.nih.gov/ij/
- Hildebrandt E, Heyder C, de Matos MBC. et al. Liposomal formulations of mistletoe produced by centrifugal technologies and cell proliferation analysis of both mistletoe extracts and isolated mistletoe lectin I. In: Die Mistel in der Tumortherapie 4. Aktueller Stand der Forschung und klinische Anwendung. Essen, KVC Verlag, 2016. p. 97–112
- Politova N, Tcholakova S, Denkov ND. Factors affecting the stability of water-oil-water emulsion films. Colloids Surf A Physicochem Eng Asp. 2017;522:608–620.
- Koneva AS, Safonova EA, Kondrakhina PS, et al. Effect of water content on structural and phase behavior of water-in-oil (n-decane) microemulsion system stabilized by mixed nonionic surfactants SPAN 80/TWEEN 80. Colloids Surf A Physicochem Eng Asp. 2017;518:273–282.
- Capdevila M, Maestro A, Porras M, et al. Preparation of span 80/oil/water highly concentrated emulsions: influence of composition and formation variables and scale-up. J Colloid Interface Sci. 2010;345:27–33.
- Lv G, Wang F, Cai W, et al. Influences of addition of hydrophilic surfactants on the W/O emulsions stabilized by lipophilic surfactants. Colloids Surf A Physicochem Eng Asp. 2014;457:441–448.
- Knoth A, Scherze I, Muschiolik G. Stability of water-in-oil-emulsions containing phosphatidylcholine-depleted lecithin. Food Hydrocoll. 2005;19:635–640.
- Ushikubo FY, Cunha RL. Stability mechanisms of liquid water-in-oil emulsions. Food Hydrocoll. 2014;34:145–153.
- Mahdi Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization—a comparison. Int J Food Prop. 2006;9:475–485.
- Delmas T, Piraux H, Couffin AC, et al. How to prepare and stabilize very small nanoemulsions. Langmuir. 2011;27:1683–1692.
- Lapinski MM, Castro-Forero A, Greiner AJ, et al. Comparison of liposomes formed by sonication and extrusion: rotational and translational diffusion of an embedded chromophore. Langmuir. 2007;23:11677–11683.
- Hu PC, Li S, Malmstadt N. Microfluidic fabrication of giant lipid vesicles. ACS Appl Mater Interfaces. 2011;3:1434–1440.
- Abkarian M, Loiseau E, Massiera G. Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter. 2011;7:4610.