Abstract
Histologic transformation to small cell lung cancer (tSCLC) is a rare but increasingly recognised mechanism of acquired resistance to tyrosine kinase inhibitors (TKI) in patients with epidermal growth factor receptor (EGFR)-positive non-small cell lung cancer (NSCLC). Beyond its acknowledged role in TKI resistance, histologic transformation to SCLC might be an important, yet under-recognised, mechanism of resistance in NSCLC treated with immunotherapy. Our review identified 32 studies that investigated tSCLC development in patients with EGFR-mutated NSCLC treated with TKI therapy and 16 case reports of patients treated with immunotherapy. It revealed the rarity of tSCLC, with a predominance of EGFR exon 19 mutations and limited therapeutic options and outcomes. Across all analysed studies in EGFR-mutated NSCLC treated with TKI therapy, the median time to tSCLC development was ∼17 months, with a median overall survival of 10 months. Histologic transformation of EGFR-mutated NSCLC to SCLC is a rare, but challenging clinical problem with a poor prognosis. A small number of documented cases of tSCLC after immunotherapy highlight the need for rebiopsies at progression to diagnose this potential resistance mechanism. Further research is needed to better understand the mechanisms underlying this phenomenon and to develop more effective treatment strategies for patients with tSCLC.
Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, despite advancements in diagnosis and therapy [Citation1]. Two main histologic subtypes of lung cancer are small cell lung cancer (SCLC, up to 15% of total cases) and non-small cell lung cancer (NSCLC, accounting for about 85% of total cases), classified into adenocarcinoma (ADC, the most frequent, about 40% of cases), squamous cell carcinoma (SCC, up to 30% of cases) and large cell carcinoma (up to 10% of cases) [Citation1]. NSCLC is more common and less aggressive than SCLC. SCLC, which exhibits highly malignant behaviour, is derived from cells with neuroendocrine properties [Citation2]. The subtyping of lung cancer plays a pivotal role in guiding molecular analysis within the realm of precision medicine [Citation3,Citation4]. Molecular profiling is indispensable for advanced lung adenocarcinoma patients as it enables the identification of multiple actionable genomic alterations, opening the door to potential benefits from targeted therapies [Citation5]. SCLC molecular subclassifications have also substantially evolved and changed in the last few years [Citation6]. Subclasses include SCLC type A, with high RNA expression of ASCL1; type N, with high expression of NEUROD1; type P, with the expression of POU2F3; and type Y, with high expression of the transcriptional regulator YAP1 [Citation7].
Treatment approaches depending on the stage of the disease significantly diverge between SCLC and NSCLC. In contrast to NSCLC, the absence of predictive and prognostic biomarkers is characteristic of SCLC [Citation6]. According to guidelines, patients with advanced adenocarcinoma should undertake molecular testing for at least receptor tyrosine kinase ROS proto-oncogene 1 (ROS1), ALK receptor tyrosine kinase (ALK), ret proto-oncogene (RET), epidermal growth factor receptor (EGFR), B-Raf proto-oncogene (BRAF), human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma (KRAS), cMET and the neurotrophic receptor tyrosine kinase (NTRK) [Citation8,Citation9]. Alterations of those genes are predictive of response to the corresponding kinase inhibitors that are approved in therapy [Citation10,Citation11]. Likewise, programmed-death ligand 1 (PD-L1) analysis is essential in both NSLCC, ADC, and SCC to select patients for immunotherapy (immune checkpoint inhibitors) compared with SCLC where it does not have a predictive role as a biomarker [Citation6,Citation12].
EGFR mutations are the second most common targetable driver mutations in NSCLC after KRAS gene mutations. They are present in approximately 10% of Caucasian patients and 50% of Asian patients with NSCLC [Citation13]. EGFR-positive NSCLCs are associated with female gender, adenocarcinoma subtype, and a non-smoking or light-smoking history. Clinically significant EGFR mutations in NSCLC are located in exons 18, 19, 20 and 21 [Citation8,Citation9]. The most prevalent mutations are EGFR exon 19 deletions (45%) and EGFR exon 21 L858R substitutions (40%). Common to EGFR exon 19 and 21 mutations is high sensitivity to tyrosine kinase inhibitors (TKI) including first-generation (erlotinib, gefitinib), second-generation (afatinib, dacomitinib), and third-generation (osimertinib) TKI [Citation8,Citation9]. Response to therapy in EGFR 19 and 21 exon mutations and progression-free survival (PFS) is higher in first-line treatment with TKI compared with conventional chemotherapy [Citation8,Citation9]. Despite significant progress in treatment, resistance to TKI-targeted therapy remains a major obstacle in EGFR-mutant NSCLC management. Most patients develop disease resistance after 10 to 13 months of first- or second-generation TKI treatment [Citation8]. EGFR TKI-acquired resistance may be associated with EGFR-dependent molecular mechanisms that include T790M, C797S, L792X, and L718X mutations [Citation14]. Another reason may be EGFR-independent molecular mechanisms that include HER2/ERBB2 and MET gene amplifications, gene rearrangements, disruptions within the PIK3CA, and MAPK signalling pathways as well as the SCLC and the epithelial-mesenchymal transition (EMT) [Citation14].
The transformation of NSCLC to SCLC is a relatively rare event present in 3–14% of cases. This transformation is a clinically significant phenomenon due to poor outcomes with a median survival of only 11 months, attracting substantial consideration in the ongoing oncology research [Citation15–17]. Understanding the molecular mechanisms underlying the transformation from NSCLC to SCLC is critical for improving diagnosis, treatment, and patient outcomes [Citation18]. The exact molecular mechanisms of this transformation are not yet fully elucidated, but several hypotheses regarding the molecular mechanisms associated with the transformation from NSCLC to SCLC have been proposed [Citation19,Citation20]. Among possible molecular mechanisms of transformation, we emphasise genetic alterations and mutations, especially TP53 and RB1 alterations, whose gene mutations are frequently observed in both NSCLC and SCLC. These tumour suppressor genes are believed to play a pivotal role in the transformation process [Citation15]. In addition, the activation of certain transcription factors (NEUROD1 and ASCL1) is associated with SCLC and may contribute to the transition from NSCLC to SCLC phenotype [Citation21]. The next factor influencing possibly the transformation from NSCLC to SCLC is cellular plasticity and lineage switching, especially EMT, a process where cells lose their epithelial characteristics and gain more mesenchymal-like properties. It is suggested that the transition from NSCLC to SCLC might involve EMT, allowing cells to acquire characteristics typical of SCLC [Citation22]. Regarding lineage plasticity, increasing scientific evidence indicates that certain NSCLC cells might possess inherent plasticity, allowing them to switch between different cell lineages, including acquiring neuroendocrine features associated with the SCLC phenotype [Citation23,Citation24]. Another plausible mechanism of transformation from NSCLC to SCLC would be related to the microenvironment and signalling pathways. Indeed, factors within the tumour microenvironment, such as signalling molecules and interactions with stromal cells, might influence the transformation process. Dysregulation of signalling pathways like Notch and Wnt pathways has been implicated in the switch between different lung cancer subtypes [Citation25,Citation26]. Likewise, the significance of therapeutic pressure and resistance has been accentuated in the literature. Prolonged exposure to specific treatments, such as targeted therapies or chemotherapy, might contribute to the emergence of resistant clones with altered characteristics resembling SCLC [Citation27]. Tumour cells might adapt and evolve under therapeutic pressure, leading to a more aggressive phenotype resembling SCLC [Citation24].
Immune checkpoint inhibitors (ICI) targeting program death 1 (PD-1) and its ligand PD-L1 have revolutionised first and second-line treatment for advanced NSCLC [Citation8,Citation9]. Resistance to immunotherapy can be categorised as intrinsic, relating to the tumour itself, and extrinsic, encompassing the tumour microenvironment and aberrant vasculature. Unlike EGFR-positive NSCLC treated with TKI, where transformation to SCLC is a well-documented resistance mechanism, such conversion after immunotherapy remains exceptionally rare [Citation28,Citation29]. One contributing factor to the infrequent detection of SCLC during immunotherapy treatment for NSCLC is the limited occurrence of biopsies following progression on immunotherapy.
Advancement in understanding the mechanisms of resistance to EGFR TKI therapy and immunotherapy of NSCLC to SCLC transformation is crucial for developing personalised therapeutic strategies and improving patient outcomes.
In this literature review, we explore the molecular and clinical characteristics of EGFR-mutated NSCLC and NSCLC treated with immunotherapy that transforms into SCLC. Additionally, we provide a detailed case presentation of a patient with transformed SCLC to illustrate the clinical picture and challenges in diagnosis and management.
Materials and methods
Literature search
We conducted a comprehensive search of the PubMed/MEDLINE/PMC database, focusing on studies published between 2011 and 2023, with the latest search conducted in late December 2023. Our search strategy included keywords related to NSCLC transformation to SCLC and EGFR TKI and immunotherapy (immune checkpoint inhibitors). We further supplemented our search by reviewing existing systematic reviews and reviews focused on transformed SCLC, identifying relevant studies not captured in the initial database search [Citation30].
Inclusion and exclusion criteria
We included all studies that investigated the transformation of NSCLC to SCLC, enrolled patients receiving EGFR TKI, and were published in the English language. In our review, we included case report studies of patients with tSCLC who received immunotherapy for NSCLC. We excluded case reports that included patients with tSCLC receiving EGFR TKI and studies published in languages other than English.
Data analysis
For each included study, we extracted and analysed the basic, molecular, and clinical characteristics. Basic characteristics included patient demographics, baseline clinical characteristics, and TKI treatment before tSCLC. Molecular characteristics included EGFR, RB1, TP53 and PIK3CA mutational status before and after tSCLC. Clinical characteristics included treatment details, time to tSCLC, and survival outcomes. We employed a similar analytical approach to extract and analyse the same data points from our case report.
Results
NSCLC transformation to SCLC under the EGFR tyrosine kinase inhibitors
Basic characteristics
Our comprehensive literature search identified 32 studies reporting cases of NSCLC transformation to SCLC during treatment with EGFR TKI therapy. summarises the basic characteristics of these patients.
Table 1. Basic characteristics of patients with EGFR-mutated NSCLC before transformation to SCLC.
The largest study, encompassing 3600 patients, identified 3% (107 patients) with tSCLC [Citation15]. The median age of patients in the analysed studies was 60 years, ranging from 51 to 71 years. The proportion of non-smokers and smokers in the analysed studies varied widely, ranging from 25% to 83% and 14 to 75%. An analysis of the two studies with the highest numbers of patients indicates the predominance of the non-smoking population (58% and 69% vs. 29% of smokers) [Citation17,Citation44]. In the aforementioned two studies, women represented the majority of patients accounting for 69% and 57% compared with 31% and 43% of men. First-generation TKI was the most commonly used (84%), followed by third-generation (33%) and second-generation (23%) TKI [Citation17].
Molecular characteristics
summarises the molecular characteristics of patients with tSCLC.
Table 2. Molecular characteristics of patients with tSCLC.
All samples included in the analysed studies had EGFR mutation. Among the three studies with the largest cohorts, EGFR exon 19 mutations were detected in 64%, 75% and 72% of patients, while EGFR exon 21 mutations were identified in 24%, 21% and 22% of patients, respectively [Citation15,Citation17,Citation44]. In the examination of EGFR status following the transformation from NSCLC to SCLC, the analysis revealed EGFR exon 19 mutations in 80% and 62%, alongside EGFR exon 21 mutations in 86% and 24% of patients, respectively [Citation17,Citation44]. The majority of studies did not perform genetic analysis of RB1, TP53, and PIK3CA mutations before and after NSCLC transformation to SCLC. Before tSCLC diagnosis, the largest study identified TP53 as the most frequent mutation, detected in 95% of patients, followed by RB1 mutation at 83%, and PIK3CA mutation at 25% [Citation15].
Clinical characteristics
The clinical features of the tSCLC patient are summarised in .
Table 3. Clinical characteristics of patients with tSCLC.
Across all analysed studies with available data, the median time for tSCLC development was 17 months. The analysed studies revealed a range in the median time tSCLC development, with the shortest observed at three months and the longest at 49 months following the treatment [Citation52,Citation58]. Platinum (cisplatin or carboplatin) and etoposide-based chemotherapy were the primary treatment regimens for most patients diagnosed with tSCLC. A limited number of analysed studies provided data on overall survival (OS) following tSCLC diagnosis. Analysis of this data revealed a poor median OS of 10 months.
NSCLC transformation to SCLC under the therapy with immune checkpoint inhibitors
Basic and clinical characteristics of patients with tSCLC after immunotherapy for NSCLC are shown in .
Table 4. Basic and clinical characteristics of patients with tSCLC under the therapy with immune checkpoint inhibitors.
Through a literature search, we found 16 patients with a diagnosis of tSCLC after progression on immunotherapy for NSCLC with the addition of our case report as a total of 17 patients with tSCLC. The median age for the presented cases was 65 years, of which four (23%) were women and thirteen (77%) were men. All patients whose smoking status was known were smokers. According to the pathological findings, most patients were diagnosed with squamous cell carcinoma, eight (47%), six (35%) patients had a diagnosis of adenocarcinoma, two (12%) patients with a diagnosis of poorly differentiated NSCLC, and one (6%) a diagnosis of combined adenocarcinoma with high-grade neuroendocrine carcinoma (HGNEC). While TP53 analysis was limited at initial NSCLC diagnosis in three (18%) patients, subsequent testing after tSCLC was performed in a larger group and all tests showed TP53 positivity in six (35%) patients. Only one (6%) of patients had RB1 testing at the initial NSCLC diagnosis, and it was negative. After tSCLC diagnosis, two (12%) patients have reported testing for RB1, both with positive results. Nivolumab was the most frequently used drug, prescribed in over half (53%) of patients receiving immunotherapy for NSCLC. In all cases, it was prescribed as part of second-line treatment for NSCLC. The median time to tSCLC after NSCLC diagnosis was 22.5 months, while from immunotherapy to tSCLC was 16 months. Following tSCLC diagnosis, 65% of patients received platinum-etoposide-based chemotherapy. Patients diagnosed with tSCLC had a median overall survival of 9 months.
Case presentation
A 65-year-old woman, a smoker (20 pack per year) underwent a neurological examination due to mild left hemiparesis in January 2018. An urgent brain CT scan revealed three metastatic lesions in the right middle and lower frontal gyrus (). The further diagnostic approach included a chest X-ray, revealing a 5 cm pulmonary mass right in the apical segment of the lower lobe with numerous smaller pulmonary nodules, without mediastinal and hilar lymph node involvement. These findings were further confirmed by the subsequent lung computed tomography (). A subsequent transbronchial biopsy of the identified lung mass revealed NSCLC, subtype adenocarcinoma (), with the tumour cells positive for TTF-1 and negative for p40. The tumour cells did not harbour EGFR and ALK genomic alterations. PD-L1 expression by immunohistochemistry revealed diffuse and strong expression in >90% of tumour cells (SP263 clone, Ventana).
Figure 1. (A) The initial CT scan of the lungs showed the presence of a tumour mass in the right lower lung lobe. (B, C) The MRI scan of the brain during the initial presentation of the patient with symptoms of left hemiparesis revealed the presence of three metastases in the brain. (D) CT scan of transformed SCLC cancer after progression on immunotherapy with pembrolizumab. (E, F) The MRI scan of the brain reveals new metastatic deposits (arrows in the MRI scans indicate metastatic deposits).
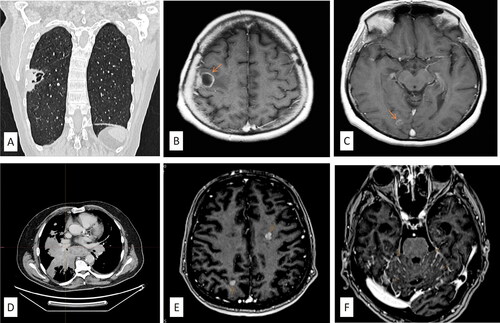
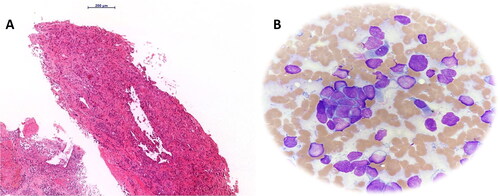
Figure 2. (A) Transbronchial biopsy of the identified lung mass revealed a cellular neoplasm composed of the large neoplastic cells with moderate to marked nuclear atypia, arranged in glandular, acinar and solid growth patterns (hematoxylin and eosin stain, magnification 10×); (B) a smear of the punctate showing aggregates and single small neoplastic cells with scanty basophilic cytoplasm and hyperchromatic nuclei without prominent nucleoli, consistent with SCLC morphology (May Grunwald-Giemsa stain, magnification, 40×).
During the diagnostic work-up in February 2018, gamma knife radiosurgery for three brain metastases was performed, with no complications. Due to PD-L1 positivity, the patient started immunotherapy in the same month with intravenous pembrolizumab 200 mg every three weeks. No significant adverse events were observed during pembrolizumab therapy. The patient received 32 cycles of pembrolizumab in two years, the last cycle was in February 2020, achieving a complete remission.
Due to the COVID-19 outbreak, the regular follow-up of the patient was interrupted. Her first check-up after the COVID-19 pandemic in our Department was in March 2021 due to dyspnoea and progressive cough. A diagnostic work-up was performed, including MRI and CT scans of the brain, CT scan of the thorax and abdomen (), revealing the disease progression in the lung and mediastinum, and new metastatic deposits in the brain. Biopsy imprint of the new lung mass revealed single clusters of small malignant cells with scanty basophilic cytoplasm, with finely granular nuclear chromatin, without prominent nucleoli. Fine needle cytology aspiration revealed similar morphologic findings, consistent with SCLC, with neuroendocrine morphology ().
The whole brain radiotherapy was conducted. Given her clinical status (ECOG 0-1), cisplatin-etoposide chemotherapy was initiated. The patient received four cycles after which a CT scan showed partial regression of the primary tumour without any distant metastasis. Two additional cycles of chemotherapy were given. Seven months later, her clinical status rapidly deteriorated and no further oncologic treatment was initiated. Our patient died 11.5 months after the diagnosis of tSCLC.
Discussion
tSCLC represents a rare but potentially treatment-limiting mechanism of resistance to various therapeutic interventions in NSCLC, including targeted therapies with TKI and immunotherapy. In our review, we comprehensively surveyed the literature of existing studies exploring the transformation of NSCLC to tSCLC after resistance to EGFR TKI and immunotherapy. Two recent large studies challenged earlier estimates of the tSCLC from NSCLC following EGFR TKI, highlighting a considerably lower incidence of 3% compared to the 14% originally reported [Citation15,Citation16,Citation34]. However, the incidence of tSCLC after immunotherapy in NSCLC remains mainly unknown. This limited understanding can be attributed to two key factors. Firstly, current guidelines do not routinely recommend rebiopsies upon disease progression with ICI therapy [Citation70]. Secondly, unlike EGFR TKI resistance where targeted therapy options remain viable in roughly 50% of rebiopsy cases, established target therapeutic pathways for post-immunotherapy tSCLC are currently lacking [Citation70]. To the best of our knowledge, only 16 well-documented cases of tSCLC after immunotherapy for NSCLC have been published so far, supplemented by our case report.
Based on clinical characteristics, most patients with EGFR-mutated NSCLC are predominantly women, non-smokers, or light smokers [Citation37,Citation71]. In contrast, in the subset of patients who develop tSCLC after immunotherapy for NSCLC, the majority of patients are men and smokers. Our female patient was a smoker. The median age with tSCLC in patients treated with EGFR TKI was 52 years, whereas for patients with immunotherapy, it ranged from 56 to 75 [Citation28,Citation29,Citation36,Citation60]. Our patient was also diagnosed at a similar age. The diagnostic work-up confirmed that our patient had oligometastatic brain disease without EGFR and ALK alterations. Patients with tSCLC tend to have lower PD-L1 expression than before NSCLC transformation [Citation34,Citation72]. In our patient, PD-L1 expression was >90% at the initial diagnosis of NSCLC, with no subsequent PD-L1 analysis conducted following the diagnosis of tSCLC. Following brain gamma knife radiosurgery, pembrolizumab was initiated due to high PD-L1 expression. Our patient demonstrated a robust response to immunotherapy, achieving a complete response. Subsequently, after completing a two-year course of pembrolizumab, the patient underwent clinical follow-up.
Patients who have undergone multiple lines of EGFR TKI therapy face an elevated risk of developing tSCLC (Ferrer) [Citation44]. The time for the diagnosis of tSCLC in patients treated with EGFR TKI for NSCLC can vary unpredictably, with the time to diagnosis of tSCLC occurring at any point during treatment. Multiple studies have reported diverse median times until the identification of tSCLC following NSCLC. Two large studies revealed surprisingly late median times for tSCLC diagnosis (18 and 22 months) in EGFR TKI-treated NSCLC, exceeding the typical PFS of 10-13 months and suggesting the potential for late-onset transformation [Citation8,Citation17,Citation34]. In contrast, in published case reports, patients treated with immune checkpoint inhibitors may experience tSCLC diagnosis at diverse intervals ranging from two to 35 months [Citation28,Citation29,Citation67]. The coexistence of triple TP53 and RB1 mutations detected in 5% of EGFR-mutated NSCLC patients is associated with shorter PFS on EGFR TKI and significantly increased risk for developing tSCLC [Citation49]. Lee et al. reported a substantial 43-fold increase in the risk of developing tSCLC with triple EGFR, TP53, and RB1 mutations in comparison with non-transformed EGFR NSCLC [Citation55]. This underscores the importance of rebiopsy in these patients, particularly those with poor response to EGFR TKI (PFS ≤12 months), to ensure early detection of tSCLC and optimise therapeutic strategies with a minimal delay [Citation37]. After 13 months of clinical follow-up, our patient exhibited disease progression in the lungs, mediastinum, and brain, with cytologic findings after bronchoscopy confirming tSCLC. Cytopathologic verification of tSCLC after NSCLC immunotherapy with pembrolizumab was also published in a case report by Okeya et al. [Citation67]. To the best of our knowledge, our case is the first case of verification of tSCLC during clinical follow-up after a complete response to immunotherapy with pembrolizumab for NSCLC.
Histologic transformation is one of the potential mechanisms of resistance to EGFR TKI in NSCLC. This phenomenon is not exclusive to lung cancer. Similarly, prostate cancer has also revealed histologic transformation as a resistance mechanism to androgen therapy, underscoring its association with therapy resistance [Citation73]. The finding from Wang et al. highlights the high prevalence (88%) of initial EGFR mutations in patients with tSCLC originating from NSCLC adenocarcinoma [Citation36]. This suggests that tSCLC is not a new tumour arising after treatment, but rather a transformed subtype of the original NSCLC adenocarcinoma [Citation38,Citation51]. A parallel pattern is evident in cases of tSCLC after immunotherapy for NSCLC, where essential genetic alterations, such as the TP53 mutation, remain conserved. Indeed, two case reports indicated identical molecular characteristics with TP53 mutations present before and after diagnosis of tSCLC [Citation63,Citation66]. According to Zeng et al. the transformation of NSCLC to SCLC could be attributed to tumour heterogeneity, specifically the coexistence in 1–3% of all SCLC cases of combined NSCLC and SCLC components initially [Citation70,Citation74]. Therapeutic inhibition, such as during immunotherapy treatment, may lead to a reduction or disappearance of the initially dominant NSCLC component. In response, the immunotherapy-resistant SCLC component becomes predominant, potentially explaining the transition from NSCLC to SCLC. Diagnosis of combined NSCLC and SCLC histologies may be challenging after biopsy due to insufficient pathological material [Citation18]. Combined NSCLC-SCLC histology with tSCLC appears to be more prevalent in EGFR mutation-positive NSCLC compared to EGFR wild-type tumours, as noted by Oser et al. [Citation18]. In the study by Yu et al. a poor differentiation status at diagnosis was described in the majority of patients, suggesting that these tumours have a greater predisposition to the development of tSCLC [Citation33]. The link between this tumour characteristic and potential resistance mechanisms is further underscored by two case reports confirming the transformation of poorly differentiated NSCLC to tSCLC after treatment with immunotherapy [Citation28,Citation69]. TP53 (59%), RB1 (58%), and PIK3CA (27%) are the most common mutations in tSCLC after EGFR TKI therapy [Citation17]. For the origin of tSCLC, two hypotheses are proposed. The first hypothesis is about the origins of SCLC from NSCLC, and the second hypothesis is related to the NSCLC heterogeneity and transformation to SCLC due to the NSCLC inhibition with some of the modalities of systemic therapy [Citation30]. The first hypothesis can be explained by the inactivation of TP53 and RB1 in alveolar cell type 2, which can be precursors of NSCLC and potentially lead to the transformation of NSCLC into SCLC [Citation36,Citation39]. tSCLC as a mechanism of resistance to immunotherapy in NSCLC was first described in 2017 by Imakita [Citation28]. The presence of TP53 mutations identified both at the initial diagnosis of NSCLC and later in tSCLC suggests that these alterations might play a driving role in the transformation process. Interestingly, a case report of an adenocarcinoma patient treated with nivolumab revealed a TP53 mutation at both stages, alongside other potential contributing factors like PIK3CA and SOX2 amplification, further supporting this hypothesis [Citation63]. Although our case report presents valuable clinical observations, the interpretation of potential drivers of transformation is unfortunately limited. Crucial information about TP53, RB1, and PIK3CA gene status was missing due to the lack of comprehensive analysis at the initial diagnosis of NSCLC and after diagnosis of tSCLC.
Due to the lack of therapeutic guidelines for the treatment of tSCLC, current treatment modalities are based on retrospective studies and case reports [Citation30]. Platinum and etoposide-based chemotherapy remains the standard treatment for tSCLC with the response to this regimen similar to that observed in classic SCLC. In the absence of specific guidelines tailored to tSCLC, current treatment approaches after progression on immunotherapy for NSCLC are the same as for classical SCLC and are based on platinum-etoposide chemotherapy. In our case, the patient underwent chemotherapy with cisplatin and etoposide, resulting in a partial response in the primary lung cancer and a complete response in brain metastasis of tSCLC. The remission persisted for seven months, which is a substantially longer PFS than the one reported by Marcoux et al. (3.4 months) [Citation17]. Therapeutic alternatives for tSCLC following resistance to EGFR TKI, with demonstrated efficacy in terms of objective response rate (ORR) and PFS, encompass taxane chemotherapy, anti-angiogenic therapy using anlotinib, and local radiotherapy. However, immunotherapy has not shown significant benefits in this clinical setting [Citation17,Citation37]. While a considerable proportion of tSCLC patients harbour an initial EGFR mutation in their NSCLC, its reduced expression results in a lower sensitivity to TKI therapy [Citation30,Citation75]. The ongoing phase I study (NCT03567642) involving osimertinib, platinum, and etoposide chemotherapy aims to address the prevention of tSCLC development in metastatic EGFR mutant NSCLC patients with concurrent RB1 and TP53 alterations [Citation15].
The prognosis for tSCLC is significantly worse compared with other lung cancer subtypes due to its aggressive nature and limited treatment options. The OS after diagnosis of tSCLC shows no significant difference between patients treated with EGFR mutant or immunotherapy for NSCLC [Citation44]. The median survival for NSCLC patients undergoing EGFR TKI treatment and subsequently diagnosed with tSCLC is poor and comparable to classic SCLC, averaging approximately 11 months [Citation17]. Among NSCLC patients treated with immunotherapy and diagnosed with tSCLC, survival times varied considerably, ranging from two to 16 months [Citation29,Citation68]. Regrettably, due to a substantial deterioration in the general condition of our patient, further oncologic treatment was not feasible. The patient survived for ∼11 months following the diagnosis of tSCLC.
Our case presentation is limited by the absence of detailed genetic analysis at the initial diagnosis of NSCLC for a better understanding of the disease origin and subsequent histologic transformation. Additionally, with only biopsy confirmation of NSCLC, the possibility of combined SCLC cannot be definitively ruled out. However, the favourable response to pembrolizumab immunotherapy suggests that the combined histology of NSCLC and SCLC is less likely. The dependence on cytopathologic analysis for tSCLC confirmation and the subsequent unavailability of adequate tissue for both histopathological verification and comprehensive genomic profiling limits our understanding of the underlying genetic drivers of the disease transformation.
In conclusion, our review confirmed the rarity of tSCLC, particularly when treated with immunotherapy. Regardless of prior treatment or resistance mechanisms, tSCLC is associated with a poor prognosis, emphasising the importance of rebiopsy after disease progression in all subtypes of NSCLC to ensure accurate diagnosis of transformed SCLC. The limitations in current diagnostic and therapeutic strategies highlight the ongoing need for improved guidelines and clinical practices for tSCLC. The evolving landscape of tSCLC requires ongoing research efforts, increased awareness, and a multidisciplinary approach for better understanding and tailored therapeutic strategies of tSCLC.
Author contributions
Kresimir Tomic and Semir Vranic wrote the original draft. Kresimir Tomic, Kristina Krpina and Semir Vranic prepared the figures. Kresimir Tomic, Kristina Krpina, Lara Baticic, Miroslav Samarzija and Semir Vranic edited and critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgement
The authors are grateful to Ana Mataić, MD and Silvana Smojver-Ježek, MD, PhD, from the University Hospital Center Zagreb for providing the microscopic images of the case.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Perez-Moreno P, Brambilla E, Thomas R, et al. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18(9):2443–2451. doi: 10.1158/1078-0432.CCR-11-2370.
- Bruno R, Alì G, Poma AM, et al. Non-small cell lung cancer molecular characterization of advanced disease with focus on sex differences: a narrative review. Precis Cancer Med. 2021;4:14–14. doi: 10.21037/pcm-20-72.
- Edsjö A, Holmquist L, Geoerger B, et al. Precision cancer medicine: concepts, current practice, and future developments. J Intern Med. 2023;294(4):455–481. doi: 10.1111/joim.13709.
- Mehmood S, Aslam S, Dilshad E, et al. Transforming diagnosis and therapeutics using cancer genomics. Cancer Treat Res. 2023;185:15–47. doi: 10.1007/978-3-031-27156-4_2.
- Krpina K, Vranić S, Tomić K, et al. Small cell lung carcinoma: current diagnosis, biomarkers, and treatment options with future perspectives. Biomedicines. 2023;11(7):1982. doi: 10.3390/biomedicines11071982.
- Ding XL, Su YG, Yu L, et al. Clinical characteristics and patient outcomes of molecular subtypes of small cell lung cancer (SCLC). World J Surg Oncol. 2022;20(1):54. doi: 10.1186/s12957-022-02528-y.
- NCCN Clinical Practice Guidelines in Oncology. Updated non-small cell lung cancer version 5.2023; 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–357. doi: 10.1016/j.annonc.2022.12.009.
- Pennell NA, Arcila ME, Gandara DR, et al. Biomarker testing for patients with advanced non-small cell lung cancer: real-world issues and tough choices. Am Soc Clin Oncol Educ Book. 2019;39:531–542. doi: 10.1200/EDBK_237863.
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, The International Association for the study of lung cancer, and the association for molecular pathology. Arch Pathol Lab Med. 2018;142(3):321–346. doi: 10.5858/arpa.2017-0388-CP.
- Vranic S, Gatalica Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol Biomed. 2023;23(1):15–25. doi: 10.17305/bjbms.2022.7953.
- Hirsch FR, Bunn PA.Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10(5):432–433. doi: 10.1016/S1470-2045(09)70110-X.
- Koulouris A, Tsagkaris C, Corriero AC, et al. Resistance to TKIs in EGFR-Mutated non-small cell lung cancer: from mechanisms to new therapeutic strategies. Cancers. 2022;14(14):3337. doi: 10.3390/cancers14143337.
- Sivakumar S, Moore JA, Montesion M, et al. Integrative analysis of a large Real-World cohort of small cell lung cancer identifies distinct genetic subtypes and insights into histologic transformation. Cancer Discov. 2023;13(7):1572–1591. doi: 10.1158/2159-8290.CD-22-0620.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003.
- Marcoux N, Gettinger SN, O’Kane G, et al. EGFR-Mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278–285. doi: 10.1200/JCO.18.01585.
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165-72–e172. doi: 10.1016/S1470-2045(14)71180-5.
- Kolesar J, Peh S, Thomas L, et al. Integration of liquid biopsy and pharmacogenomics for precision therapy of EGFR mutant and resistant lung cancers. Mol Cancer. 2022;21(1):61. doi: 10.1186/s12943-022-01534-8.
- Reita D, Pabst L, Pencreach E, et al. Molecular mechanism of EGFR-TKI resistance in EGFR-Mutated non-small cell lung cancer: application to biological diagnostic and monitoring. Cancers . 2021;13(19):4926. doi: 10.3390/cancers13194926.
- Baine MK, Hsieh MS, Lai WV, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15(12):1823–1835. doi: 10.1016/j.jtho.2020.09.009.
- Chan JM, Quintanal-Villalonga A, Gao VR, et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell. 2021;39(11):1479–1496 e18. doi: 10.1016/j.ccell.2021.09.008.
- Testa U, Castelli G, Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers. 2018;10(8):248. doi: 10.3390/cancers10080248.
- Shaurova T, Zhang L, Goodrich DW, et al. Understanding lineage plasticity as a path to targeted therapy failure in EGFR-mutant non-small cell lung cancer. Front Genet. 2020;11:281. doi: 10.3389/fgene.2020.00281.
- Xue W, Cai L, Li S, et al. WNT ligands in non-small cell lung cancer: from pathogenesis to clinical practice. Discov Oncol. 2023;14(1):136. doi: 10.1007/s12672-023-00739-7.
- Sharif A, Shaji A, Chammaa M, et al. Notch transduction in non-small cell lung cancer. Int J Mol Sci. 2020;21(16):5691. doi: 10.3390/ijms21165691.
- Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):40. doi: 10.1186/s12943-023-01740-y.
- Imakita T, Fujita K, Kanai O, et al. Small cell lung cancer transformation during immunotherapy with nivolumab: a case report. Respir Med Case Rep. 2017;21:52–55. doi: 10.1016/j.rmcr.2017.03.019.
- Imakita T, Fujita K, Kanai O, et al. Small cell transformation of non-small cell lung cancer under immunotherapy: case series and literature review. Thorac Cancer. 2021;12(22):3062–3067. doi: 10.1111/1759-7714.14180.
- Yin X, Li Y, Wang H, et al. Small cell lung cancer transformation: from pathogenesis to treatment. Semin Cancer Biol. 2022;86(Pt 2):595–606. doi: 10.1016/j.semcancer.2022.03.006.
- Chen Y, He M, Dai Z, et al. Clinical and molecular profiling of EGFR-mutant lung adenocarcinomas transformation to small cell lung cancer during TKI treatment. Front Oncol. 2023;13:1308313. doi: 10.3389/fonc.2023.1308313.
- Mambetsariev I, Arvanitis L, Fricke J, et al. Small cell lung cancer transformation following treatment in EGFR-mutated non-small cell lung cancer. J Clin Med. 2022;11(5):1429.
- Yu L, Bazhenova L, Gold K, et al. Clinicopathologic and molecular characteristics of EGFR-mutant lung adenocarcinomas that transform to small cell lung cancer after TKI therapy. Transl Lung Cancer Res. 2022;11(3):452–461. doi: 10.21037/tlcr-21-665.
- Fujimoto D, Akamatsu H, Morimoto T, et al. Histologic transformation of epidermal growth factor receptor-mutated lung cancer. Eur J Cancer. 2022;166:41–50. doi: 10.1016/j.ejca.2022.02.006.
- Jin CB, Yang L. Histological transformation of non-small cell lung cancer: clinical analysis of nine cases. World J Clin Cases. 2021;9(18):4617–4626. doi: 10.12998/wjcc.v9.i18.4617.
- Wang W, Xu C, Chen H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: a multicenter retrospective study. Lung Cancer. 2021;155:20–27. doi: 10.1016/j.lungcan.2021.03.006.
- Wang S, Xie T, Hao X, et al. Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma. Thorac Cancer. 2021;12(19):2585–2593. doi: 10.1111/1759-7714.14144.
- Bai W, Zhen C, Zhang R, et al. Clinicopathological features of patients with transformation from EGFR mutant lung adenocarcinoma to small cell lung cancer. Transl Cancer Res. 2021;10(8):3694–3704. doi: 10.21037/tcr-21-653.
- Xie T, Li Y, Ying J, et al. Whole exome sequencing (WES) analysis of transformed small cell lung cancer (SCLC) from lung adenocarcinoma (LUAD). Transl Lung Cancer Res. 2020;9(6):2428–2439. doi: 10.21037/tlcr-20-1278.
- Mu Y, Hao X, Xing P, et al. Acquired resistance to osimertinib in patients with non-small-cell lung cancer: mechanisms and clinical outcomes. J Cancer Res Clin Oncol. 2020;146(9):2427–2433. doi: 10.1007/s00432-020-03239-1.
- Vendrell JA, Quantin X, Serre I, et al. Combination of tissue and liquid biopsy molecular profiling to detect transformation to small cell lung carcinoma during osimertinib treatment. Ther Adv Med Oncol. 2020;12:1758835920974192. doi: 10.1177/1758835920974192.
- Zeng L, Xiao L, Jiang W, et al. Investigation of efficacy and acquired resistance for EGFR-TKI plus bevacizumab as first-line treatment in patients with EGFR sensitive mutant non-small cell lung cancer in a real world population. Lung Cancer. 2020;141:82–88. doi: 10.1016/j.lungcan.2020.01.009.
- Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res. 2020;26(11):2654–2663. doi: 10.1158/1078-0432.CCR-19-3563.
- Ferrer L, Giaj Levra M, Brevet M, et al. A brief report of transformation from NSCLC to SCLC: molecular and therapeutic characteristics. J Thorac Oncol. 2019;14(1):130–134. doi: 10.1016/j.jtho.2018.08.2028.
- Iacono D, Osman GA, Migliorino MR, et al. Intrapatient molecular and histologic heterogeneity after first-generation or second-generation TKI therapy of NSCLC patients: potential clinical impact on subsequent third-generation TKI treatment. Am J Clin Oncol. 2019;42(11):845–850. doi: 10.1097/COC.0000000000000615.
- Yang H, Liu L, Zhou C, et al. The clinicopathologic of pulmonary adenocarcinoma transformation to small cell lung cancer. Medicine. 2019;98(12):e14893.). doi: 10.1097/MD.0000000000014893.
- Lee K, Kim Y, Jung HA, et al. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer. 2019;130:87–92. doi: 10.1016/j.lungcan.2019.01.012.
- Mehlman C, Cadranel J, Rousseau-Bussac G, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: a multicentric retrospective French study. Lung Cancer. 2019;137:149–156. doi: 10.1016/j.lungcan.2019.09.019.
- Offin M, Chan JM, Tenet M, et al. Concurrent RB1 and TP53 alterations define a subset of EGFR-mutant lung cancers at risk for histologic transformation and inferior clinical outcomes. J Thorac Oncol. 2019;14(10):1784–1793. doi: 10.1016/j.jtho.2019.06.002.
- Ahmed T, Vial MR, Ost D, et al. Non-small cell lung cancer transdifferentiation into small cell lung cancer: a case series. Lung Cancer. 2018;122:220–223. doi: 10.1016/j.lungcan.2018.06.024.
- Minari R, Bordi P, Del Re M, et al. Primary resistance to osimertinib due to SCLC transformation: issue of T790M determination on liquid re-biopsy. Lung Cancer. 2018;115:21–27. doi: 10.1016/j.lungcan.2017.11.011.
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-Positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527–1534. doi: 10.1001/jamaoncol.2018.2969.
- Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov. 2018;8(12):1529–1539. doi: 10.1158/2159-8290.CD-18-1022.
- Lin CC, Shih JY, Yu CJ, et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med. 2018;6(2):107–116. doi: 10.1016/S2213-2600(17)30480-0.
- Lee JK, Lee J, Kim S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35(26):3065–3074. doi: 10.1200/JCO.2016.71.9096.
- Ahn S, Hwang SH, Han J, et al. Transformation to small cell lung cancer of pulmonary adenocarcinoma: clinicopathologic analysis of six cases. J Pathol Transl Med. 2016;50(4):258–263. doi: 10.4132/jptm.2016.04.19.
- Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. doi: 10.1016/j.lungcan.2016.07.007.
- Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol. 2013;8(10):1265–1271. doi: 10.1097/JTO.0b013e3182a407fa.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246.
- Wang D, Ye W, Chen D, et al. Transformation of lung squamous cell carcinoma to small cell lung cancer after immunotherapy resistance: a case report. Cancer Manag Res. 2023;15:803–808. doi: 10.2147/CMAR.S420485.
- Liu H, Chen LH, Zhang ZH, et al. Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: a report of two cases. Front Oncol. 2022;12:1022705. doi: 10.3389/fonc.2022.1022705.
- Arakawa S, Yoshida T, Shirasawa M, et al. RB1 loss induced small cell lung cancer transformation as acquired resistance to pembrolizumab in an advanced NSCLC patient. Lung Cancer. 2021;151:101–103. doi: 10.1016/j.lungcan.2020.11.016.
- Sehgal K, Varkaris A, Viray H, et al. Small cell transformation of non-small cell lung cancer on immune checkpoint inhibitors: uncommon or under-recognized? J Immunother Cancer. 2020;8(1):e000697. doi: 10.1136/jitc-2020-000697.
- Si X, You Y, Zhang X, et al. Histologic transformation of lung cancer during pembrolizumab therapy: a case report. Thorac Cancer. 2020;11(3):793–796. doi: 10.1111/1759-7714.13312.
- Miura N, Matsubara T, Takamori S, et al. Histological conversion from adenocarcinoma to small cell carcinoma of the lung after treatment with an immune checkpoint inhibitor: a case report. Oxf Med Case Reports. 2020;2020(4):omaa026.
- Bar J, Ofek E, Barshack I, et al. Transformation to small cell lung cancer as a mechanism of resistance to immunotherapy in non-small cell lung cancer. Lung Cancer. 2019;138:109–115. doi: 10.1016/j.lungcan.2019.09.025.
- Okeya K, Kawagishi Y, Muranaka E, et al. Hyperprogressive disease in lung cancer with transformation of adenocarcinoma to small-cell carcinoma during pembrolizumab therapy. Intern Med. 2019;58(22):3295–3298. doi: 10.2169/internalmedicine.2892-19.
- Iams WT, Beckermann KE, Almodovar K, et al. Small cell lung cancer transformation as a mechanism of resistance to PD-1 therapy in KRAS-Mutant lung adenocarcinoma: a report of two cases. J Thorac Oncol. 2019;14(3):e45–e48. doi: 10.1016/j.jtho.2018.11.031.
- Abdallah N, Nagasaka M, Abdulfatah E, et al. Non-small cell to small cell lung cancer on PD-1 inhibitors: two cases on potential histologic transformation. Lung Cancer. 2018;9:85–90. doi: 10.2147/LCTT.S173724.
- Zeng J, Ding X, Ding J, et al. Histological transformation into SCLC: an important resistance mechanism of NSCLC upon immunotherapy. Front Immunol. 2023;14:1275957. doi: 10.3389/fimmu.2023.1275957.
- Roca E, Gurizzan C, Amoroso V, et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treat Rev. 2017;59:117–122. doi: 10.1016/j.ctrv.2017.07.007.
- Guleria P, Kumar S, Malik PS, et al. PD-L1 expression in small cell and large cell neuroendocrine carcinomas of lung: an immunohistochemical study with review of literature. Pathol Oncol Res. 2020;26(4):2363–2370. doi: 10.1007/s12253-020-00832-0.
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059.
- Dagogo-Jack I, Saltos A, Shaw AT, et al. Pathology issues in thoracic oncology: histologic characterization and tissue/plasma genotyping may resolve diagnostic dilemmas. Am Soc Clin Oncol Educ Book. 2017;37(37):619–629. doi: 10.1200/EDBK_175197.
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6(1):6377. doi: 10.1038/ncomms7377.
