Abstract
Translocation, sea ranching, and assisted migration are under scrutiny as methods to augment populations so that harvests can be increased or populations can better adapt to changing environmental conditions. Understanding the ecological effects of any such environmental manipulation is critical to its successful application. One potential ecological effect of any type of stock enhancement is the displacement of either resident or released groups such that finding shelter or foraging habitat is adversely affected. This study examined behavioral interactions of resident and translocated Jasus edwardsii rock lobster after an introduction of 1,961 “small pale” phenotypic morphs to an area populated by the resident “large red” phenotypic morph. This translocation was an experimental stock enhancement conducted as part of a larger study to increase the yield and value of the fishery. Most translocated individuals established a home range within a couple of days of release (generally <2), and these ranges were generally less than 1.0 ha in size. Home-range kernels and foraging ranges overlapped between the two morphs, and there was no evidence of avoidance (Jacob's cohesion index 0.01, Z = 1.06, p = 0.28). This case of translocation for stock enhancement between ecotypes had no detectable adverse effect on either the resident or the translocated population, and in this species, stock enhancement could become part of an integrated conservation and harvest optimization strategy.
INTRODUCTION
Introductions, translocations, and sea ranching are increasingly common in marine and freshwater ecosystems as methods to increase supply or reduce cost of fish for consumption (Hilborn, Citation1998). Fisheries management is progressively turning to land farming practices, translocating and stocking harvested species into “paddocks” or highly productive areas in a process called sea ranching. While the definitions vary widely throughout the literature, the modern term “assisted migration” is a recent incarnation of “translocation” and involves capture and movement of wild organisms. Assisted migration via translocation generally involves transplanting individuals beyond their existing or former range or to a contiguous environment where barriers to dispersal have occurred through habitat fragmentation or to an adjacent poleward environment with lower temperatures (Chapron et al., Citation2008; Mueller and Hellmann, Citation2008; Carroll et al., Citation2009). Assisted migration, or translocation, has been one of the most commonly used tools for biodiversity restoration worldwide (Tavecchia et al., Citation2009), although in terrestrial systems, it has frequently occurred without a clear framework (Hoegh-Guldberg et al., Citation2008), leading to negative impacts (Davidson and Simkanin, Citation2008). In fisheries, following the framework for responsible stock enhancement (Lorenzen et al., Citation2010), assisted migration and translocation also offer a tool for augmenting and increasing supply or reducing cost of production of harvested species.
Translocation to stock enhancement of a non-migratory species was applied with large-scale geographic variation in demographic and market traits to test whether predicted gains in yield could be achieved (Gardner and Van Putten, Citation2008a). The southern rock lobster Jasus edwardsii has two apparent phenotypic morphs—a small slow-growing red and white speckled morph (“small pale” [SP] morph) with longer legs, which resides in deep water, and a larger faster-growing, red morph (“large red” [LR] morph) with shorter legs, which resides in shallow water (Chandrapavan et al., Citation2009a)—but are of one genetic stock (Ovenden et al., Citation1992). Demographic traits of these morphs differ also; the SP phenotypic morph matures at a smaller size (65-mm carapace length [CL]), is slower growing, and tends to have low exploitation rate and high densities (Punt et al., Citation1997). In contrast, the LR phenotypic morph matures at around 105-mm CL, is faster growing, has less dense populations due to the higher exploitation rate (Gardner et al., Citation2006), and is gregarious, often sharing burrows (MacDiarmid, Citation1994). The LR morph is more robust to stress during export (Hawthorne, 2009), and fishers typically receive up to AU$10/kg more for this morph. A large-scale assisted-migration experiment was undertaken, translocating the SP morph from their deep water residence into shallow water with the aim of improving their yield, value, and sustainability.
Assisted migration introduces a range of risks to the introduced and resident species (Schlaepfer et al., Citation2009; Araki and Schmid, Citation2010) and must be managed carefully to reduce the risks to ensure the viability of the enhancement. Successful stock enhancement requires a thorough understanding of the ecological processes influenced by stocking in different ecosystems (Stottrup and Sparrevohn, Citation2007), including biological interactions between the resident and inhabitant fish that may influence productivity, genetic diversity, or disease transmission (Hilborn, Citation1998; Araki and Schmid, Citation2010). Of primary concern in the current translocation in this panmictic stock (Ovenden et al., Citation1992) was the displacement of resident animals by the introduced stock, which can occur if space is limited and the carrying capacity is reached or if there is territoriality, dominance, or aggression. As red is inherently intimidating in freshwater fishes and terrestrial species (Barlow, Citation1983; Vercken and Clobert, Citation2008), and size differences can increase agonistic behavior in captive lobsters and catchability in wild J. edwardsii lobsters (Frusher et al., Citation2003; Thomas et al., Citation2003), there was concern that the SP lobsters might be at a disadvantage when introduced into the territory of LR lobsters due to color, size, and prior residence dominance and disorientation upon release. A larger translocation experiment using lobsters from the same source site but translocated to other sites on the Tasmanian coast was effective at changing the color, growth rate, body shape, and size, increasing the value and nutrition of the seafood product (Chandrapavan et al., Citation2009a,Citationb, Citation2010, Citation2011a,Citationb; Green et al., Citation2010). For an assisted migration and restocking to be successful, transplants must survive and prosper in their new environment, establishing residences and home ranges (HRs), thereby increasing productivity (Hilborn, Citation1998; Bell et al., Citation2006, Citation2008). Furthermore, transplants must not displace residents, which would negatively impact the environment or transfer disease (Hoegh-Guldberg et al., Citation2008; Rout et al., Citation2009). Survival of transplanted small pale lobsters was equal to large red residents over a period of 24 months (Green and Gardner, Citation2009). J. edwardsii is a non-migratory lobster, unlike other Palinurids (Panulirus argus and P. cygnus) and was thus unlikely to display directed homing and navigation capabilities observed in P. argus (Boles and Lohmann, Citation2003). The next step was to determine whether they stay in the new location and establish residences and foraging ranges in the short term, as immediate release poses the biggest flight risk (Mills et al., Citation2006).
This article examines the movement and HRs of transplanted and resident lobsters for one month after translocation from deep to shallow water reefs to determine if one morph displaced or influenced the behavior of the other.
MATERIALS AND METHODS
Site and Sampling
One thousand nine hundred sixty-one mature J. edwardsii (1,308 females, 653 males) were captured from a deep water site (65 m) at Maatsuyker Island (43°40′30S 146°12′56E) Tasmania, Australia, and moved north to a shallow water site (9 m) at Emerald Bay, Tasmania (42°36′55S 147°56′35E) in December 2007. Emerald Bay is an inshore temperate site with habitat of rocky reef bounded by large expanses of sand on Tasmania's east coast. Lobsters were caught from Maatsuyker Island using 50 baited metal mesh lobster pots deployed in an area 500 m × 120 m. Pots were emptied twice a day: once at daybreak, then redeployed, and emptied again after midday. At capture, the CL of J. edwardsii was measured.
Translocation
Lobsters were transported in 2 × 4,000 L flow-through tanks on board the RV “Challenger” under ambient water conditions over 14 hr. Lobsters were initially released into an 80-m-diameter net with a height of 1.5 m with braided nylon mesh (stretched mesh size of 21 mm diagonal) set on the sea floor for 24 hr to reduce their initial flight response away from the release site (Mills et al., Citation2006). The base of the net was open and weighted by chains, and the walls were suspended by foam floats. The cage was roofless. Release of translocated lobsters onto the reef within the cage occurred at night to reduce predator mortality (Mills et al., Citation2006).
Acoustic Tracking
To examine impacts of the translocation event, the movement of 11 resident and 29 (of the 1,961) translocated lobsters (20 females, 20 males; ) released lobsters was tracked for 32 days after release using coded V9 and continuous V13 and V16 acoustic transmitters and a VRAP telemetry system (VEMCO Radio-linked Acoustic Positioning, AMRIX, VEMCO Division). Each of the transmitters emits a unique identification code at a random interval of 180–300 sec once activated. Transmitters were attached to the carapace of translocated and resident individuals using standard rapid setting (5 min) epoxy resin. The smallest transmitters were 24 mm long × 9 mm wide and weighed 2.2 g and were attached to the smallest lobsters. The smallest lobster weighed 300 g (78-mm CL) and the average lobster size was 500 g and 95-mm CL (); therefore, the V9 transmitters were 0.7% and 0.4% of the animal's weight, respectively. Continuous tags were 36 mm long × 13 mm wide and weighed 6 g and 54 mm long × 16 mm wide and weighed 9 g for V13 and V16, respectively. These larger tags were used on larger lobsters, weighing 500–900 g. Spiny lobsters of the family Palinuridae, such as Jasus edwardsii, have large spiny antennae and a rostrum protruding from their head, sometimes above the height of the carapace. They are slow moving and not streamlined. The transmitters glued to the dorsal surface of the carapace were very unlikely to increase the costs of locomotion or reduce speed of these slow-moving animals due to their small size and low profile relative to the antennae and rostrum.
Table 1 Mean size of lobsters used for acoustic tracking
The VRAP system was deployed forming an equilateral triangle spanning 160 m ± 2.5 m on each side above the rocky reef at the research site. The VRAP system successfully tracked the movement of the tagged resident and translocated lobsters with a positional accuracy of ca. 2 m over a 300-m range from the center of the receiver array with a sampling periodicity of approximately 3 min. Resident lobsters were captured from the site, tagged and released, and allowed to re-acclimatize to the habitat for a period of 2.5 days prior to the release of the translocated individuals. After 32 days of tracking lobsters, divers retrieved the tagged lobsters and removed the transmitters from the carapace using a scalpel. No damage was done to the calcified exoskeleton in removing transmitters.
Data Processing
Location estimates were calculated from raw acoustic data collected by the VRAP receivers using VRAP v5.1.2 software (AMRIX, Vemco Division) and the default “position average” algorithm. Location estimates for each acoustic transmitter were exported from the VRAP 5.1.2 software program in standard geodetic format using the (WGS84) and batch loaded into Eonfusion v2.0 (Myriax Pty. Ltd.) for re-projection, processing, and error removal. Post-processing of the data has removed the higher sampling rate of continuous tags. Data from continuous tags was sub-sampled so that the frequency (time between consecutive detections) was similar to those of coded tags (5 min apart). Location estimates were re-projected using the Universal Transverse Mercator (UTM) system and the Geocentric Datum of Australia 1994 (GDA94/MGA [Map Grid of Australia] Zone 55) to allow accurate distance and velocity calculations. Erroneous location estimates were removed by constructing a line feature between consecutive location estimates for each transmitter and examining each segment of each line feature. Location estimates were removed from the data if they resulted in line feature segments where the average velocity exceeded 3 m.min−1, which is the estimated maximum speed of J. edwardsii (Lucieer et al., Citation2007), and the difference in heading from the previous line segment formed an acute angle less than 30. Following the removal of each erroneous location estimate, line features were reconstructed and the process repeated until no further erroneous location estimates were located. The time of day when each location estimate was recorded by the VRAP system was calculated and appended to each location estimate to allow an activity to be calculated for each tagged lobster.
Analyses
An animal's HR is an estimation of the area traveled by an animal in its day-to-day activities of food gathering, mating, and caring for its young (Burt, Citation1943). HRs (95% fixed kernel; Worton, Citation1989) were calculated with a least-square cross-validation method (LSCV), as fixed kernel estimates were least biased in the outer contours (Seaman et al., Citation1999). The kernel method further defines the HR as the area in which there is 95% probability of locating the animal. To assess whether the animals avoided, ignored, or were attracted by one another, Jacob's index of avoidance or cohesion (Jacobs, Citation1974; Brown et al., Citation2000) was calculated with the time window set to 5 min to match the time delay of coded tag transmissions. Jacob's index rises toward 1 if two animals are closer to each other than by chance alone, is close to 0 if the animals ignore each other, and falls towards –1 of the animals avoid each other. This analysis was based on the geometric mean distance (Walls and Kenward, 2001). To test the significance of the association, the values of Jacobs's index were compared with zero in a Wilcoxon signed-ranks test.
RESULTS
Overlap of Resident and Translocated Morph HRs
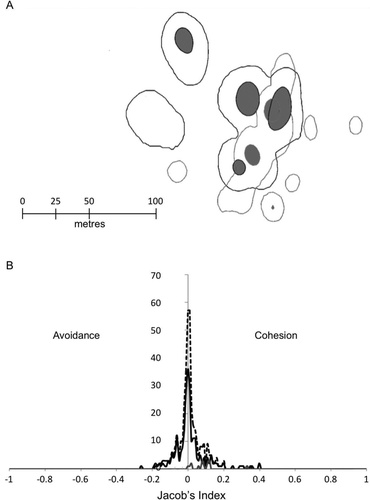
The HRs of residents and translocated lobsters overlapped in time and space (), suggesting no exclusion of individuals within either grouping by the other. The average spatial overlap in kernel HRs (0.95 kernel probability contours) between tagged individuals was 25%, with each tagged lobster co-occupying habitat with one or more other tagged individuals (). There was no distinct preference for cohesion or avoidance between individual lobsters (Z = 1.06, p = 0.28) and no avoidance between resident and translocated lobsters; that is, lobsters generally ignored each other ().
Figure 1 (A) HR kernels of resident and translocated lobsters tracked for 30 days using acoustic telemetry. Black contours represent 95% HR for translocated animals (probability of 0.95 that a positional estimate would have occurred within the contour), and gray lines represent 95% HR contours for resident animals. Contours that are filled (gray shading) represent a 50% probability contour (probability of 0.5 that a positional estimate occurred within the contour) of the activity centre of lobsters. (B) Jacobs index of cohesion/avoidance within and between treatment groups. Solid black line: translocated, solid gray line: residents, broken black line: all lobsters together.
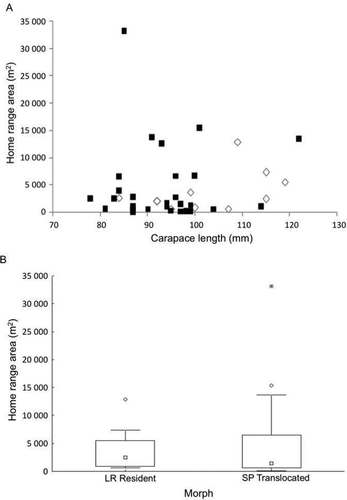
There was no relationship between HR area and lobster size (), and HR areas were variable between individuals of similar size. Most translocated SP individuals established HR within a couple of days of release (generally < 2) and were generally less than 1.0 ha in size. HRs of translocated SP lobsters were similar in size to LR resident animals tagged at the site (; p > 0.1, Kolmogorov–Smirnoff [K-S]). Timing of activity on a daily level were similar between translocated and resident animals (K-S = 0.011, p = 0.117).
Figure 2 (A) HR area (95% probability contour) of resident and translocated lobsters tracked for 30 days using acoustic telemetry; size of lobster is CL at release (▪ SP translocated, ⋄ LR resident). (B) HR size of SP translocated lobsters and LR residents. Boxplots represent median and 25–75%; whiskers illustrate non-outlier ranges (○ outliers, * extreme outliers).
Displacement from Release Site
Residents had a sharp increase in the instantaneous displacement distance from release site at around 2.5–3 days following release (), coinciding with the release of the translocated individuals from the sea cage. Translocated individuals rapidly dispersed from the release site but stayed within 60–80 m of the release site. Individuals who initially dispersed greater distances moved closer to the release site after a couple of days, with several individuals continuing to disperse further from the release site over the 32-day period.
Figure 3 Displacement distance of lobsters from the point of release after tagging: (A) instantaneous displacement distance and (B) total displacement of resident and translocated lobsters. Black lines or markers indicate SP translocated; gray lines or markers indicate LR resident.
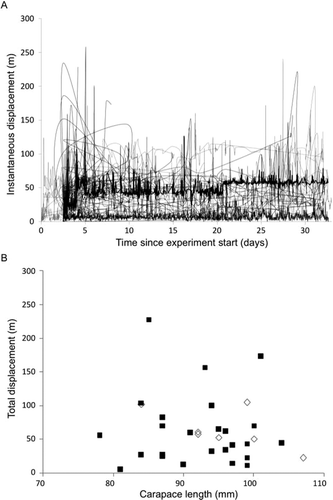
Total displacement (straight line distance between the release location and the final positional coordinates distance) or distance moved did not differ between LR resident and SP translocated lobsters (, Pillai's trace, F 2,28 = 0.07, p > 0.05). Displacement distance was variable between animals, with no clear size effects for either translocated or resident animals (R 2 = 0.01, p < 0.54). The mean displacement distances were 63 m (n = 29) and 65 m (n = 11) for translocated and resident lobsters respectively, and the mean distances moved (± SE) were 1,201 ± 211 m and 1,335 ± 405 m The greatest variability in movement and displacement metrics is observed by intermediate size classes (90–100-mm CL; ).
Tagged Lobster Retention Rates
All 29 SP translocated lobsters were detected during the study period of 32 days. Some individuals were recorded multiple times each day and constantly monitored by the telemetry system, whereas several individuals were positioned on multiple occasions with gaps in detections of several days in duration. The scale and reception range of detection array ensured that tagged lobsters anywhere within the entire study site would be within range of the system and therefore positioned if moving in open habitat. All 29 SP translocated lobsters were positioned at least once within the study area within the last 24 hr of the study. Of the 29 translocated lobsters, only one individual failed to give sufficient data to calculate all metrics described in this summary. Similarly, all 11 tagged resident individuals were retained at the site for the duration of the study.
DISCUSSION
Unlike many restocking experiments that do not meet expectations (Hilborn, Citation1998), this translocation experiment has exceeded expectations. There was no evidence of overall displacement or avoidance due to either prior residence and larger size or introduction in large numbers in this translocation of the southern rock lobster Jasus edwardsii. Individuals of the two different morphs—resident LR morph and translocated SP morph—co-existed after translocation with a large amount of HR overlap and with similar daily foraging and movement patterns. A previous study demonstrated that translocated lobsters had similar long-term survival and retention in their new habitat as the resident lobsters (Green and Gardner, Citation2009). Coupled with the other positive outcomes of this large-scale translocation and stock enhancement trial, this evidence of immediate cohabitation and fidelity to the release site furthers confirms that the J. edwardsii fishery can be enhanced through the assisted migration of low-value low-productivity stock to areas of faster growth and improved market traits, as predicted (Gardner and Van Putten, Citation2008a). The economic success of this stock enhancement depends on the mode of translocation, but there is a large scope for improving profit to the industry (Gardner and Van Putten, Citation2008b).
The LR morph of Jasus edwardsii is gregarious (MacDiarmid, Citation1994), and there is no evidence of the conspecific aggression that exist in other lobster species, e.g., clawed lobster Homarus americanus, which can reduce survival of small individuals (Karavanich and Atema, Citation1998). Large and small J. edwardsii commonly share dens, and during the daytime, J. edwardsii occur in aggregations of up to 105 individuals (MacDiarmid, Citation1994), although larger rock lobsters can forage on a broader size range and species composition of prey (Langlois et al., Citation2006) and exclude small lobsters from entering traps (Frusher and Hoenig, Citation2001). This article contains the first observations on the social behaviors of the SP morph, whose small size, pale color, spindly legs, and narrower abdomen are physical characteristics generally associated with being weaker and subordinate. This morph demonstrated similar HRs and significant overlap in HR usage as did the LR morph. The size of the HRs, or preferred habitat area, in this study were similar to those described for the LR morph at a nearby site in 2005 (Lucieer and Pederson, Citation2008), suggesting that the HR and foraging behaviour of resident lobsters have been adequately captured. Also important, no level of avoidance was detected, and in fact, a large amount of overlap in habitat usage of individuals of different morphs was found, indicating the success of this translocation and sea ranching.
Color is frequently a secondary sexual characteristic used to demonstrate fitness or aggression, as it signals better health (Ressel and Schall, Citation1989; Olson and Owens, Citation1998), higher reproductive performance (e.g., access to mates, Evans and Norris [Citation1996]; sperm velocity, Janhunen et al. [Citation2009]), and better access to critical resources (Luchiari et al., Citation2007) in a large range of taxa. In the study species, J. edwardsii, size and morph were not indicative of any dominance hierarchy, and a previous study demonstrated that size and color did not influence survival in translocated SP J. edwardsii compared with resident LRs (Green and Gardner, Citation2009).
In fisheries that are managed using capped quotas, such as individual transferrable quotas (ITQs), which limit the biomass removed by the fishery, a method to increase profits is to increase the value of the catch (Bradshaw, Citation2004). One of the unplanned consequences of the introduction of ITQs is the movement of fishing effort to inshore waters, where the value of the catch is higher, even though catch rates are lower, and thus costs are higher (Linnane and Crosthwaite, Citation2009). Catch price tends to fluctuate with demand and quality of the product (Hundloe, 2002), which presents an opportunity to maximize profits by targeting high-value product or landing it when demand is great. A fishery management strategy that permits both of these possibilities is stock enhancement through sea ranching, and in this species, it had potential to increase production and reduce costs (Gardner and Van Putten, Citation2008b). As translocation increases the productivity of recruits, the economic yield from the fishery is increased, either through lower costs associated with higher catch rates or through larger total allowable catch (TACs) (Dupont et al., Citation2005). Within the adaptive management framework, if the risks are monitored and addressed, then translocation is a real solution to conserving ecosystems (Rout et al., Citation2009; Sutherland et al., Citation2009) and maintaining sustainable fisheries (Stottrup and Sparrevohn, Citation2007).
The translocation experiment described here was part of a large study that increased the yield and value from the translocated SP morphs (Chandrapavan et al., Citation2009a, Citation2010, Citation2011b). The results of the present study on cohabitation show that these benefits came without any apparent cost in terms of short-term displacement or behavioral impacts on resident LR morphs’ sustainability. As demand for seafood increases concurrent with increasing costs of fishing, novel ways of increasing quality and productivity of seafood will become central to maintaining seafood supply. Enhancement regimes, such as assisted migrations and translocation, will become useful tools to ameliorate habitat loss, reduce naturally occurring populations, and bring fish stocks closer to shore. Climate change is only adding to these existing issues, and translocation or assisted migration is one tool advocated as a remedy (Sutherland et al., Citation2009; Vitt et al., Citation2009). Assisted migrations and translocations are increasingly likely to be considered in integrated marine management strategies. Translocation of low productivity stock to areas of higher productivity is one means of overcoming the challenges of improving productivity in wild capture fisheries. This case of assisted migration between ecotypes within a species range had no detectable adverse effect on either the resident or the translocated population, and in J. edwardsii, it could become part of an integrated management strategy to increase economic yield and to reduce ecosystem effects of fishing.
Acknowledgments
© Bridget S. Green, Hugh Pederson, and Caleb Gardner
REFERENCES
- Araki , H. and Schmid , C. 2010 . Is hatchery stocking a help or harm? . Aquaculture , 308: : 2 – 11 .
- Barlow , G. 1983 . Do Gold Midas cichlid fish win fights because of their color, or because they lack normal coloration? A logistic solution . Behav. Ecol. Sociobiol. , 13: : 197 – 204 .
- Bell , J. D. , Bartley , D. M. , Lorenzen , K. and Loneragan , N. R. 2006 . Restocking and stock enhancement of coastal fisheries: Potential, problems and progress . Fisheries Research Restocking and Stock Enhancement of Coastal Fisheries—Potential, Problems and Progress, Special Symposium, 7th Asian Fisheries Forum , 80: : 1 – 8 .
- Bell , J. D. , Leber , K. M. , Blankenship , H. L. , Loneragan , N. R. and Masuda , R. 2008 . A new era for restocking, stock enhancement and sea ranching of coastal fisheries resources . Rev. Fish. Sci. , 16: : 1 – 9 .
- Boles , L. C. and Lohmann , K. J. 2003 . True navigation and magnetic maps in spiny lobsters . Nature , 421: : 60 – 63 .
- Bradshaw , M. 2004 . A combination of state and market through ITQs in the Tasmanian commercial rock lobster fishery: The tail wagging the dog? . Fish. Res. , 67: : 99 – 109 .
- Brown , D. R. , Stouffer , P. C. and Strong , C. M. 2000 . Movement and territoriality of wintering Hermit Thrushes in southeastern Louisiana . Wilson Bull. , 112: : 347 – 353 .
- Burt , W. H. 1943 . Territoriality and home range concepts as applied to mammals . J. Mammal. , 24: : 346 – 352 .
- Carroll , M. J. , Anderson , B. J. , Brereton , T. M. , Knight , S. J. , Kudrna , O. and Thomas , C. D. 2009 . Climate change and translocations: The potential to re-establish two regionally-extinct butterfly species in Britain . Biol. Conserv. , 142: : 2114 – 2121 .
- Chandrapavan , A. , Gardner , C. and Green , B. S. 2010 . Growth rate of adult rock lobsters Jasus edwardsii increased through translocation . Fish. Res , 105: : 244 – 247 .
- Chandrapavan , A. , Gardner , C. and Green , B. S. 2011a . Haemolymph condition of deep-water southern rock lobsters (Jasus edwardsii) translocated to inshore reefs . Mar. Freshwat. Behav. Physiol. , 44: : 21 – 32 .
- Chandrapavan , A. , Gardner , C. , Green , B. S. , Linnane , A. and Hobday , D. 2011b . Improving marketability through translocation: A lobster case study from southern Australia . ICES J. Mar. Sci. , 68: : 1842 – 1851 .
- Chandrapavan , A. , Gardner , C. , Linnane , A. and Hobday , D. 2009a . Colour variation in the southern rock lobster Jasus edwardsii and its economic impact on the commercial Industry . NZ J. Mar. Freshwat. Res. , 43: : 537 – 545 .
- Chandrapavan , A. , Guest , M. , Nichols , P. and Gardner , C. 2009b . Translocating southern rock lobsters (Jasus edwardsii) from deep-water to shallow inshore water enhances nutritional condition through omega-3 long-chain polyunsaturated fatty acid content . J. Exp. Mar. Biol. Ecol. , 375: : 9 – 15 .
- Chapron , G. , Samelius , G. , Hoegh-Guldberg , O. , Hughes , L. , McIntyre , S. , Lindenmayer , D. B. , Parmesan , C. , Possingham , H. P. and Thomas , C. D. 2008 . Reply to skeptical of assisted colonization . Science , 322: : 1049 – 1050 .
- Davidson , I. and Simkanin , C. 2008 . Skeptical of assisted colonization . Science , 322: : 1048 – 1049 .
- Dupont , D. P. , Fox , K. J. , Gordon , D. V. and Grafton , R. Q. 2005 . “ Profit and price effects of multi-species individual transferable quotas ” . In J. Agr. Econ. Vol. 56 , 31 – 57 .
- Evans , M. R. and Norris , K. 1996 . The importance of carotenoids in signaling during aggressive interactions between male firemouth cichlids (Cichlasoma meeki) . Behav. Ecol. , 7 : 1 – 6 .
- Frusher , S. D. and Hoenig , J. M. 2001 . Impact of lobster size on selectivity of traps for southern rock lobster (Jasus edwardsii) . Can. J. Fish.Aquat. Sci. , 58: : 2482 – 2489 .
- Frusher , S. D. , Hoenig , J. M. and Gardner , C. 2003 . Have changes in selectivity masked recruitment declines in crustacean trap fisheries? . Fish. Res. , 65: : 467 – 474 .
- Gardner , C. , Frusher , S. , Barrett , N. S. , Haddon , M. and Buxton , C. 2006 . Spatial variation in size at onset of maturity of female southern rock lobster Jasus edwardsii around Tasmania, Australia . Scientia Marina , 70: : 423 – 430 .
- Gardner , C. and Van Putten , E. I. 2008a . Biological modelling of translocation as a management tool for a rock lobster fishery . Rev. Fish. Sci. , 16: : 81 – 90 .
- Gardner , C. and Van Putten , E. I. 2008b . The economic feasibility of translocating rock lobsters to increase yield . Rev. Fish. Sci. , 16: : 154 – 163 .
- Green , B. S. and Gardner , C. 2009 . Surviving a sea-change: Survival of southern rock lobster (Jasus edwardsii) translocated to a site of fast growth . ICES J. Mar. Sci. , 66: : 656 – 664 .
- Green , B. S. , Gardner , C. , Linnane , A. and Hawthorne , P. J. 2010 . The good, the bad and the recovery in an assisted migration . PLoS One , 5: : e14160
- Hawthorne , P . 2009 . “ Effects of deep water hauling and translocation from deep water to shallow water inshore reefs on the physiological condition of southern rock lobster (Jasus edwardsii) ” . In Honours thesis , Adelaide , , South Australia : Flinders University .
- Hilborn , R. 1998 . The economic performance of marine stock enhancement projects . Bull. Mar. Sci. , 62: : 661 – 674 .
- Hoegh-Guldberg , O. , Hughes , L. , McIntyre , S. , Lindenmayer , D. B. , Parmesan , C. , Possingham , H. P. and Thomas , C. D. 2008 . Assisted colonization and rapid climate change . Science , 321: : 345 – 346 .
- Hundloe , T. 2002 . Valuing Fisheries: An Economic Framework , St. Lucia : University of Queensland Press .
- Jacobs , J. 1974 . Quantitative measurement of food selection . Oecologia , 14: : 413 – 417 .
- Janhunen , M. , Rudolfsen , G. , Kekalainen , J. , Figenschou , L. , Peuhkuri , N. and Kortet , R. 2009 . Spawning coloration and sperm quality in a large lake population of Arctic charr (Salmonidae: Salvelinus alpinus L.) . Biol. J. Linn. Soc. , 98: : 794 – 802 .
- Karavanich , C. and Atema , J. 1998 . Olfactory recognition of urine signals in dominance fights between male lobster, Homarus americanus . Behaviour , 135: : 719 – 730 .
- Langlois , T. J. , Anderson , M. J. , Brock , M. and Murman , G. 2006 . Importance of rock lobster size-structure for trophic interactions: choice of soft-sediment bivalve prey . Mar. Biol. , 149: : 447 – 454 .
- Linnane , A. and Crosthwaite , K. 2009 . Spatial dynamics of the South Australian rock lobster (Jasus edwardsii) fishery under a quota-based system . NZ J. Mar. Freshwat. Res. , 43: : 475 – 484 .
- Lorenzen , K. , Leber , K. M. and Blankenship , H. L. 2010 . Responsible approach to marine stock enhancement: An update . Rev. Fish. Sci. , 18: : 189 – 210 .
- Luchiari , A. C. , Duarte , C. R. D. , Freire , F. A. D. and Nissinen , K. 2007 . Hierarchical status and colour preference in Nile tilapia (Oreochromis niloticus) . J. Ethol. , 25: : 169 – 175 .
- Lucieer , V. , Lawler , M. , Morffew , M. and Pender , A. 2007 . “ Estuarine habitat mapping in the Derwent—2007: A resurvey of marine habitats ” . In SeaMap Tasmania Hobart , Tasmania
- Lucieer , V. and Pederson , H. 2008 . Linking morphometric characterisation of rocky reef with fine scale lobster movement . J. Photogrammetry Rem. Sens. , 63: : 496 – 509 .
- MacDiarmid , A. B. 1994 . Cohabitation in the spiny lobster Jasus-Edwardsii (Hutton, 1875) . Crustaceana , 66: : 341 – 355 .
- Mills , D. J. , Gardner , C. and Johnson , C. R. 2006 . Experimental reseeding of juvenile spiny lobsters (Jasus edwardsii): Comparing survival and movement of wild and naive lobsters at multiple sites . Aquaculture , 254: : 256 – 268 .
- Mueller , J. M. and Hellmann , J. J. 2008 . An assessment of invasion risk from assisted migration . Conserv. Biol. , 22: : 562 – 567 .
- Olson , V. A. and Owens , I. P. F. 1998 . Costly sexual signals: Are carotenoids rare, risky or required? . Trends Ecol. Evol. , 13: : 510 – 514 .
- Ovenden , J. R. , Brasher , D. J. and White , R. W. G. 1992 . Mitochondrial-DNA analyses of the red rock lobster Jasus-Edwardsii supports an apparent absence of population subdivision throughout Australasia . Mar. Biol. , 112: : 319 – 326 .
- Punt , A. E. , Kennedy , R. B. and Frusher , S. D. 1997 . Estimating the size-transition matrix for Tasmanian rock lobster, Jasus edwardsii . Mar. Freshwat. Res. , 48: : 981 – 992 .
- Ressel , S. and Schall , J. J. 1989 . Parasites and showy males—malarial infection and color variation in fence lizards . Oecologia , 78: : 158 – 164 .
- Rout , T. M. , Hauser , C. E. and Possingham , H. P. 2009 . Optimal adaptive management for the translocation of a threatened species . Ecol. Appl. , 19: : 515 – 526 .
- Schlaepfer , M. A. , Helenbrook , W. D. , Searing , K. B. and Shoemaker , K. T. 2009 . Assisted colonization: Evaluating contrasting management actions (and values) in the face of uncertainty . Trends Ecol. Evol. , 24: : 471 – 472 .
- Seaman , D. E. , Millspaugh , J. J. , Kernohan , B. J. , Brundige , G. C. , Raedeke , K. J. and Gitzen , R. A. 1999 . Effects of sample size on kernel home range estimates . J. Wildl. Manag. , 63: : 739 – 747 .
- Stottrup , J. G. and Sparrevohn , C. R. 2007 . Can stock enhancement enhance stocks? . J. Sea Res. , 57: : 104 – 113 .
- Sutherland , W. J. , Adams , W. M. , Aronson , R. B. , Aveling , R. , Blackburn , T. M. , Broad , S. , Ceballos , G. , Cote , I. M. , Cowling , R. M. , Da Fonseca , G. A. B. , Dinerstein , E. , Ferraro , P. J. , Fleishman , E. , Gascon , C. , Hunter , M. , Hutton , J. , Kareiva , P. , Kuria , A. , MacDonald , D. W. , MacKinnon , K. , Madgwick , F. J. , Mascia , M. B. , McNeely , J. , Milner-Gulland , E. J. , Moon , S. , Morley , C. G. , Nelson , S. , Osborn , D. , Pai , M. , Parsons , E. C. M. , Peck , L. S. , Possingham , H. , Prior , S. V. , Pullin , A. S. , Rands , M. R. W. , Ranganathan , J. , Redford , K. H. , Rodriguez , J. P. , Seymour , F. , Sobel , J. , Sodhi , N. S. , Stott , A. , Vance-Borland , K. and Watkinson , A. R. 2009 . One hundred questions of importance to the conservation of global biological diversity . Conserv. Biol. , 23: : 557 – 567 .
- Tavecchia , G. , Viedma , C. , Martinez-Abrain , A. , Bartolome , M. A. , Gomez , J. A. and Oro , D. 2009 . Maximizing re-introduction success: Assessing the immediate cost of release in a threatened waterfowl . Biol. Conserv. , 142: : 3005 – 3012 .
- Thomas , C. W. , Carter , C. G. and Crear , B. J. 2003 . Feed availability and its relationship to survival, growth, dominance and the agonistic behaviour of the southern rock lobster, Jasus edwardsii in captivity . Aquaculture , 215: : 45 – 65 .
- Vercken , E. and Clobert , J. 2008 . The role of colour polymorphism in social encounters among female common lizards . Herpetolog. J. , 18: : 223 – 230 .
- Vitt , P. , Havens , K. and Hoegh-Guldberg , O. 2009 . Assisted migration: Part of an integrated conservation strategy . Trends Ecol. Evol. , 24: : 473 – 474 .
- Walls , S. S. and Kenward , R. E. 2001 . Spatial consequences of relatedness and age in buzzards . Anim. Behav. , 61: : 1069 – 1078 .
- Worton , B. J. 1989 . Kernel methods for estimating the utilization distribution in home-range studies . Ecology , 70: : 164 – 168 .